Abstract
Density functional theory and time-dependent density functional theory have been enacted to investigate the effects of donor and acceptor on the first hyperpolarizability of Lindquist-type organo-imido polyoxometalates (POMs). These calculations employ a range-separated hybrid exchange-correlation functional (ωB97X-D), account for solvent effects using the implicit polarizable continuum model, and analyze the first hyperpolarizabilities by using the two-state approximation. They highlight the beneficial role of strong donors as well as of π-conjugated spacers (CH=CH rather than C≡C) on the first hyperpolarizabilities. Analysis based on the unit sphere representation confirms the one-dimensional push-pull π-conjugated character of the POMs substituted by donor groups and the corresponding value of the depolarization ratios close to 5. Furthermore, the use of the two-state approximation is demonstrated to be suitable for explaining the origin of the variations of the first hyperpolarizabilities as a function of the characteristics of a unique low-energy charge-transfer excited state and to attribute most of the first hyperpolarizability changes to the difference of dipole moment between the ground and that charge-transfer excited state.
1. Introduction
Polyoxometalates (POMs) are nanomolecular metal-oxides made of metal atoms (M) from groups VB (often, V) and VIB (often Mo or W) in a high oxidation state. These anionic nanoclusters are built from MOxy- oxyanion polyhedra linked together by shared O atoms. Much is known about their redox properties, leading to interesting catalytic activities [1], while they also found applications in life sciences [2]. Moreover, recent experimental investigations have demonstrated that POM units can be combined with organic moieties, leading to hybrid organic-inorganic compounds that exhibit nonlinear optical (NLO) properties [3,4,5,6,7]. Though reference [6] deals with third-order NLO effects, the other investigations as well as the current contribution focus on the second-order NLO effects and, more precisely, on second harmonic generation (SHG). These experimental studies [3,4,5,7] extended the field of organic and organometallic compounds, which can present large second-order NLO responses combined with short response times [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25]. In particular, refs. [3,4] demonstrated that POMs may provide a new generation of high performance, high transparency, and potentially redox-switchable NLO materials. In the case of Lindqvist-type organo-imido-substituted hexamolybdates bearing π-conjugated ligands, these investigations demonstrated that the polyanion clusters act as electron acceptors because the first hyperpolarizability (β), which characterizes the SHG response at the molecular scale, is enhanced when these organic linkers are substituted by electron donors, in other words, when they present a strong non-centrosymmetry. These experimental characterizations have been carried out using the hyper-Rayleigh scattering (HRS) technique [26]. Though these interpretations were in contradiction with previous quantum chemical calculations [27,28], a recent contribution by the authors [29] showed that the polyanions of these Lindqvist-type POMs play the role of electron acceptor and that the use of an inappropriate density functional theory (DFT) exchange-correlation functional (XCF) was responsible for the incorrect structure-property relationships in refs. [27,28]. In reference [29], a striking difference with respect to the previous theoretical studies results from the use of a range-separated hybrid XCF, known to describe reliably the hyperpolarizabilities. Indeed, (linear and) nonlinear electric-field-induced polarization effects, which determine the β responses, are intrinsically nonlocal and require the use of XCFs that contain nonlocal (Hartree-Fock) exchange [30]. On the contrary, local density approximation (LDA) and generalized-gradient approximation (GGA) suffer from shortsightedness to the electric field perturbations, as previously demonstrated and analyzed [31,32,33].
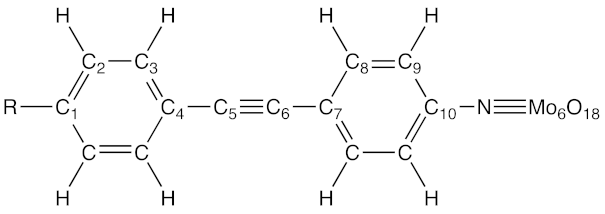
Following reference [4], 11 hexamolybdate compounds (labeled 0–10) have been selected in reference [29] and a detailed quantum chemical investigation has been enacted. Besides the selection of reliable methods to predict their geometrical structures, linear and nonlinear optical responses, that work provided an interpretation of the second-order NLO responses in agreement with the experimental data, bringing additional tools to deduce structure-property relationships and to support the design of new POM derivatives with large NLO responses. In this work, we extend our previous investigation by addressing one aspect of the structure-NLO property relationships in Lindqvist-type POMs: the modulation of the donor/acceptor character of the organic linker either by changing the substituent on the terminal phenyl ring or by changing the π-conjugated character by replacing the CC triple bond by a CC double bond (Figure 1).
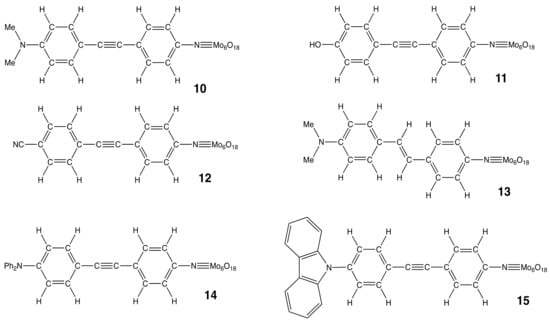
Figure 1.
Structure of POM derivatives 10–15. POMs 0–10 have been studied in our previous work (reference [29]), and a consistent notation has been adopted for easing the discussion.
The first point is tackled by starting from compound 10 and by changing its dimethylamino substituent, either by small groups, a hydroxyl substituent (11), or a cyano one (12). This will further confirm the contrasted role of acceptor and donor groups (CN versus OH and NMe2). Then, the comparison between 10 and 13 aims at comparing the role of the linker, containing an ethynyl (10) or an ethenyl (13) spacer. Finally, based on reference [5], other donor groups are considered. These are bulkier than in 10, a diphenylamino (14) or a carbazole (15) substituent.
2. Computational Methods
DFT and TDDFT calculations were carried out using the Gaussian16 package [34]. Geometry optimizations were performed using ωB97X-D XCF [35], which includes 100% of long-range exact exchange, a small fraction (about 22%) of short-range exact exchange, a modified B97 XCF for short-range interaction, the B97 correlation density functional [36] and empirical atom−atom London dispersion corrections. The default range-separating parameter ω = 0.2 Bohr−1 was used. The atomic basis set consists of 6-311+G(d,p) for C, H, N, O, and LANL2TZ for the Mo atoms. The reliability of this ωB97X-D/6-311+G(d,p)/LANL2TZ method for the geometry optimization of POM derivatives was demonstrated in comparison with other XC functionals in our previous work [29] and was confirmed here by comparison with experimental data from reference [3]. To better describe the impact of the solvent effects, geometry optimizations were performed in solution using the integral equation formalism (IEF) of the polarizable continuum model (PCM) (IEF-PCM), which represents the solvent by a dielectric continuum characterized by its dielectric permittivity (ε) [37]. Using the optimized geometries, the SHG β tensor components were calculated employing the time-dependent density functional theory (TDDFT) method [38,39] with the ωB97X-D XC functional, the 6-311G(d)/LanL2TZ basis set, and the IEF-PCM scheme to account for solvent effects. Both static and dynamic responses were evaluated for an incident wavelength of 1064 nm. Computing β has always been a challenge, in particular, for large compounds and for compounds having donor and/or acceptor substituents because of the intrinsic nonlocal nature of the response. ωB97X-D falls in a new class of DFT functional known as range-separated functionals, which are capable of capturing both short-range and long-range interactions.
To allow comparisons with the experiment, the HRS first hyperpolarizabilities, , were evaluated from the tensor components. These are reported as well as the depolarization ratios (DR), which reflects the NLOphore shape. Full expressions for and DR are available from refs. [40,41]. In the case of one-dimensional push-pull π-conjugated systems, the tensor is dominated by a single diagonal component, , where z is the charge-transfer axis. In that case, there is a simple relationship between and that component [4]:
To describe the first hyperpolarizability, the unit sphere representation (USR) was adopted [42]. It consists (i) in evaluating an effective induced dipole:
where is the first hyperpolarizability tensor and is a unit vector of the electric field, of which the polarization is defined in spherical coordinates by the and angles, and (ii) then in representing the induced dipoles on a sphere centered at the molecule center of mass. The interpretation of the values can further be performed by resorting to perturbation theory, where is expressed under the form of a summation over the excited states (SOS) [43,44]. In particular, the reliability of the two-state approximation (TSA) has been demonstrated for push-pull π-conjugated systems [45]. In that scheme, the response is dominated by a single low-energy charge-transfer excited state and, for the diagonal component along the charge-transfer axis, :
where , the excitation energy from the ground state g to the excited state e, is the lowest-energy dipole-allowed excited state, is the corresponding difference in dipole moment, and is the transition dipole moment, related to the oscillator strength of the excitation:
3. Results and Discussion
3.1. Ground State Equilibrium Geometries
Selected ground state equilibrium geometrical parameters of compounds 10–15, which have been optimized with the IEF-PCM/ωB97X-D/6-311G*/LanL2TZ method, are displayed in Table 1 and compared with the experimental results from X-ray diffraction (XRD) (10, 14, and 15).

Table 1.
Selected equilibrium bond lengths (in Å) and valence angles (deg.) of POMs derivatives as obtained at the IEF-PCM/B97X-D/6-311G*/LanL2TZ level in comparison with experimental results from X-ray diffraction 1,2.
The agreement is globally suitable, though the medium effects are different, i.e., the calculations do not account for crystal packing effects but for an isotropic dielectric medium (which is consistent with the calculations of the NLO responses, compared to measurements carried out in solution). Differences in bond lengths are generally of the order of 0.02 Å or less. For the C10-N-Mo valence angle, the difference can attain 4–8°, which is attributed to the crystal environment.
Comparing 10 and 13, besides the ethenyl versus ethynyl linker, the differences of geometry are very small (0.005 Å or less on the bond lengths and the same BLA value). This is consistent with their similar charge distributions in the ground state (vide infra). Then, comparing the optimized geometries of compounds with a CC triple bond as a spacer, differences in BLA are obvious. In the case of 10, the BLA amounts to 0.024 Å and decreases to 0.018 Å in 14 (R = NPh2) and 0.012 Å in 15 (R = carbazole). In fact, the BLA is a probe of the donor or acceptor strength of the substituent. When the substituent is a H atom (compound 0 from reference [29]), the BLA reduces to 0.008 Å while with the dimethylamino group (10), which is a strong donor, the quinoid character of the ring increases, and the BLA increases to 0.024 Å. So, on the basis of the BLA, the donor character decreases in the order NMe2 > NPh2 > carbazole. When R = OH (11), the BLA also amounts to 0.012 Å. When the substituent is a cyano group (12), it is slightly larger (0.016 Å), highlighting the acceptor character of that group. Note that in the case of 15, the BLA is similar to that found in compounds 4 and 8 of reference [29], which involve a pyrrolyl substituent. Indeed, such as the pyrrolyl substituent, the carbazole of 15 is tilted (by 56°) with respect to the plane of the attached phenyl ring, reducing the donor character of the N atom. Moreover, the phenyl rings of the NPh2 substituent in 14 are also tilted with respect to the phenyl of the π-conjugated linker, but the tilt angle is smaller than in 15 and goes down to 33°.
These BLA variations and the subsequent interpretation of the donor strengths are corroborated by the C1-N bond lengths, which increase in the order NMe2 < NPh2 < carbazole (both from XRD data and from calculations). The substituent effects on the other geometrical parameters are much smaller because these units (CC triple bond, the other phenyl ring, and the POM moiety) are farther away.
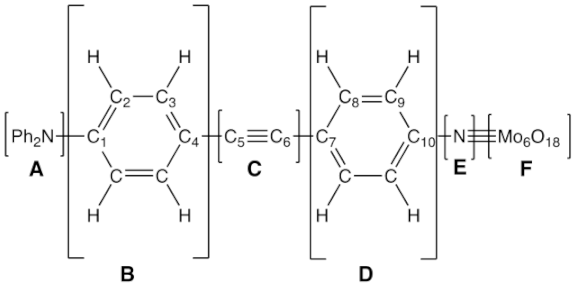
3.2. Natural Population Analysis (NPA) Charge Distributions
Natural population analysis (NPA) was carried out for the different segments of the POMs, as defined in Table 2. The NPA charge on the POM moiety is always close to −1.9 e, while for the linking N atom (E moiety) and the nearby phenyl ring (D moiety), they amount to −0.3 e and 0.2 e. These sum up to −2 e, which is the global anion charge. The ethynyl/ethenyl spacer can be considered neutral, with negligible impact on the substituent. Thus, the global charge of the A and B units is also zero, and the differences refer to the donor/acceptor strengths. In the case of 10, both A and B bear a quasi-zero charge. When replacing the NMe2 substituent with NPh2 and then a carbazole, the substituent becomes more negative. This can be explained by the fact that its donor (mesomer) character does not compensate anymore for the acceptor (inductive) effect of the N atom. In the case of 11, the donor character of the hydroxyl group is smaller than that of the NMe2 in 10, while the acceptor character of the cyano group appears small, with a charge of −0.04 e.

Table 2.
Natural Population Analysis charge distributions (in e) in the A–F moieties of compounds 10–15, as obtained from calculations performed at the IEF-PCM/B97X-D/6-311G*/LanL2TZ level of approximation. The A–F moieties are defined in the scheme below.
3.3. First Hyperpolarizabilities
The first hyperpolarizabilities of compounds 10−15 are listed in Table 3. Besides the values and their depolarization ratios, the values are reported to allow straightforward comparisons with the experiment. To a suitable extent, variations of the values are consistent with the BLA values: a large BLA leads to a large value. This is substantiated by the relative values of the compounds bearing an amino substituent: (10, NMe2) > (14, NPh2) > (15, carbazole). On this basis, the dimethylamino group is a better donor than the diphenylamino one, both being stronger than carbazole. The weaker donor character of carbazole can be associated with its steric interactions with the phenyl ring, leading to a twist by about 56° and a subsequent reduction in π-conjugation. The intermediate value of 14 is then associated with the twist of the phenyl rings (33°). This BLA- relationship is also verified by the differences between compounds 11 (R = OH) and 10 (R = NMe2). Nevertheless, the BLA is not the only criterion to take into account. So, compound 12 bearing a cyano group has a much smaller value than reference compound 10 in comparison to compound 11. This is attributed to the acceptor character of the cyano substituent, which, although it affects the BLA of the phenyl ring, is poorly conjugated with the POM acceptor moiety (vide infra). In addition, compounds 10 and 13 have similar BLA values, but the value of compound 13 is 21% (41%) larger than that of compound 10 in the static limit (at 1064 nm). This is explained by the nature of the spacer between the phenyl rings: a single-double-single bond pattern enables better the propagation of the donor effect than a single-triple-single bond pattern.

Table 3.
HRS first hyperpolarizabilities (in 103 au) of the POM derivatives as calculated at the IEF-PCM(solvent = acetonitrile)/TDDFT/ωB97X-D/6-311G*/LanL2TZ level in comparison with experimental data from reference [5]. Depolarization ratios are given in parentheses. Columns 4,5 report the values (in 10−30 esu, B convention, i.e., the values are divided by two with respect to the T convention values) while column 6 reports the corresponding experimental values.
The DR values are generally close to 5, which is the typical value for one-dimensional push-pull π-conjugated systems, which whom the tensor is dominated by a single diagonal component (along the charge-transfer axis, called here ). This ratio gets also closer to 5 when going from the static value to the response at 1064 nm. The DR is also slightly smaller for compounds 14 and 15, which is attributed to the more extended nature of the donor substituents. The USRs of the tensor further describe the second-order responses of these POM derivatives (Figure 2). All USRs highlight the non-centrosymmetry of the second-order NLO response as well as the strong one-dimensional push-pull character of these compounds. The arrows go from the acceptor (POM moiety) to the donor groups. Their lengths are proportional to the amplitude of the response in a given direction. Note that in the case of the cyano group, the arrows are much smaller but still oriented in the same direction, which attributes to the POM a stronger acceptor character than to the cyano group.
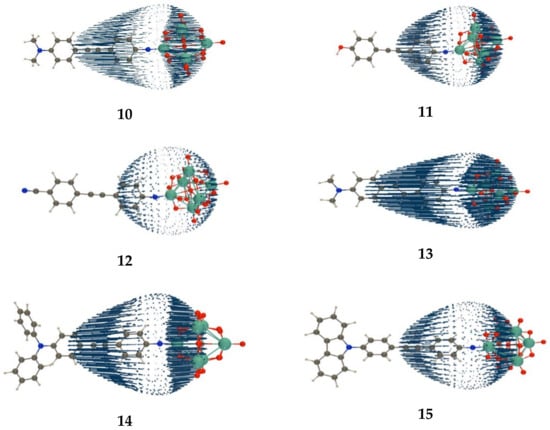
Figure 2.
Unit sphere representation of the static first hyperpolarizability tensor of compounds 10–15, as calculated at the TDDFT/ωB97X-D level of approximation (USR factor of 0.0001).
Comparisons to the experiment confirm that the carbazole group is the weakest donor among the three amino substituents because the response is substantially smaller for compound 15 than for 10 and 14. On the other hand, there is an inversion between the relative values of compounds 10 and 15. First, the quantitative agreement between theory and experiment is very suitable for 10, but for 15, calculations predict a decrease by 14%, while experiments show an increase by 34% with respect to 10. This difference might result from the combination of the limitations of the method of calculation (the XCF and the treatment of the solvent are approximate), together with the experimental error bars.
3.4. UV-Absorption Spectra and Interpretation of the β Values
The UV/visible absorption spectra of POMs 10–15 were simulated at the TDDFT level and are drawn in Figure 3. At large wavelengths (330–450 nm), they are dominated by a single transition, which corresponds to the second excited state. The characteristics of that state are listed in Table 4. Besides the excitation energy and oscillator strength, the charge-transfer distance (dCT) and amplitude (qCT), together with the change of dipole moment upon excitation () are listed.
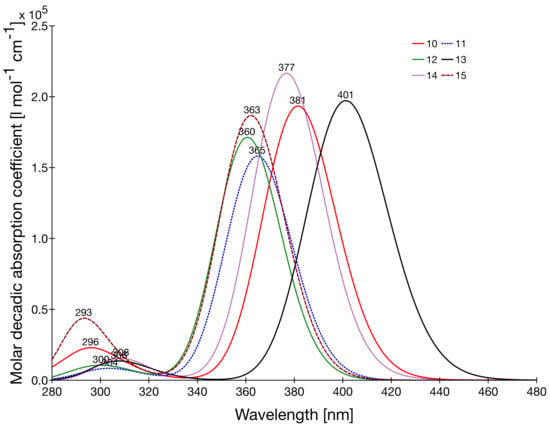
Figure 3.
UV/visible absorption spectra of compounds 10–15 as obtained at the IEF-PCM/TDDFT/ωB97X-D/6-311G*/LanL2TZ level of approximation. Each transition is described by a Gaussian having an HWHM = 0.3 eV.

Table 4.
UV/visible spectra characteristics of compounds 10–15 as determined at the IEF-PCM(solvent = acetonitrile)/TDDFT/ωB97X-D/6-311G*/LanL2TZ level of approximation. The and quantities have been evaluated using the two-state approximation (in 103 a.u.) and the CPKS values are also given for comparison.
Starting from 10, the lowest excitation energy band is blue-shifted by ~0.2 eV for compounds 11 and 12 while it is red-shifted by almost 0.2 eV for compound 13, characterized by a better π-conjugated spacer between the phenyl rings. In the case of the amino-like substituents, comparison with the experiment is possible. Their calculated vertical excitation energies are systematically larger than the photon energies corresponding to the maximum of absorption (by 0.22–0.31 eV). This is consistent with the difference of nature between these two quantities and, subsequently, the fact that the maximum absorption is lowered in energy by about 0.2 eV with respect to the vertical excitation energy, as calculated recently for fluorescent protein chromophores [46]. Among these three amino-like compounds, POM 10 presents the smallest calculated (experimental) excitation energy, 2.98 eV. It is blue-shifted by 0.04 eV (experimentally, 0.04 eV as well) when the substituent is NPh2 (14) and by 0.17 eV (experimentally, 0.26 eV) when it is a carbazole (15). Among these, 14 presents the largest calculated oscillator strength () and experimental extinction coefficient. 10 and 13, which differ by the π-spacer, present similar oscillator strengths, which are larger than those of POMs 11 and 12.
For the dominant electronic excitation, the change of electron density, , was calculated (Figure 4). The patterns are similar: they highlight the acceptor character of the POM moiety and the donor character of the terminal organic moiety. Moreover, their extents differ among the compounds. For 12, the hardly spreads over the phenyl ring that bears the acceptor cyano group. For the other compounds, the terminal phenyl ring is involved. For 15, only the N atom of the carbazole takes part as donating moiety. The donating contribution of the amino groups is larger in 14 and even more in 10 and 13. By integration of the volumes with positive or negative , the changes of dipole moment upon excitation, , were evaluated. Their ordering is opposite to the corresponding excitation energies: 13 < 10 < 14 < 11 < 15 < 12. This change of dipole moment, which can be expressed as the product between a charge-transfer distance () and the amount of charge transfer (), is dominated by the former. Indeed, the range of values is narrow, 0.50–0.66 e, while the range of values is much broader, from 1.97 Å in 12 to more than twice as much in 10, 14, and 15.
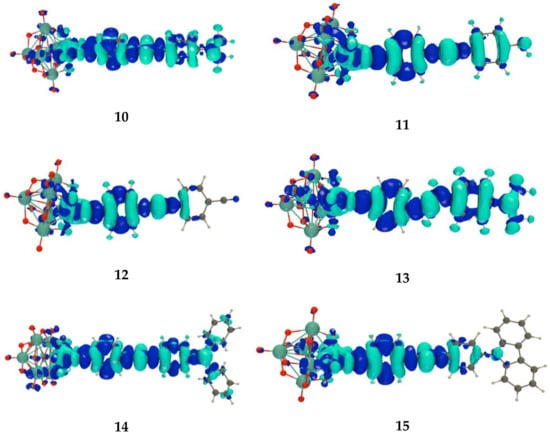
Figure 4.
Excitation-induced electron density difference () as calculated for the key excited state of POMs 10–15 at the IEF-PCM/TDDFT/ωB97X-D/6-311G*/LanL2TZ level of approximation (iso-value = 0.0005 a.u.; light/dark blue corresponds to negative/positive so that the excitation-induced electron transfer goes from the light to the dark blue).
In the case of 10, 14, and 15, these TDDFT/ωB97X-D UV/vis absorption characteristics are not straightforward to compare with those reported in reference [5], obtained at the TDDFT level with the SAOP (statistical average of orbital potential) XCF because their lowest excitation energy absorption bands result from more than one electronic transition with non-negligible oscillator strength, while in our case, there is only one transition. The comparison with the Stark spectroscopy data of reference [5] is also difficult for the same reason (more than one excited state) as well as owing to the specific experimental conditions (butyronitrile glasses at 77 K).
The linear optical properties were then employed to calculate the first hyperpolarizabilities within the TSA, [45] in fact the assumed dominant diagonal component of the tensor in the direction of the CT axis, , and from these the values (Table 4). This assumption has been demonstrated to be valid because the DRs are close to the reference value of 5. A comparison between the TSA and CPKS static values is provided in Figure 5. The agreement between the two sets of values is excellent, with a correlation factor of 0.97. The slope of the least-squares fit linear relationship amounts to 1.31, indicating that the TSA overestimates, on average, the CPKS values by 31%. Such an overestimation of the TSA approach has been observed in other quantum chemistry investigations [47]. For our target compounds, the TSA is, therefore, a reliable model to explain the variations of values and, the data of Table 4 points out that their variations are mostly governed by (and specifically, ), then, on an equal foot, by the excitation energies and oscillator strengths (transition dipoles).
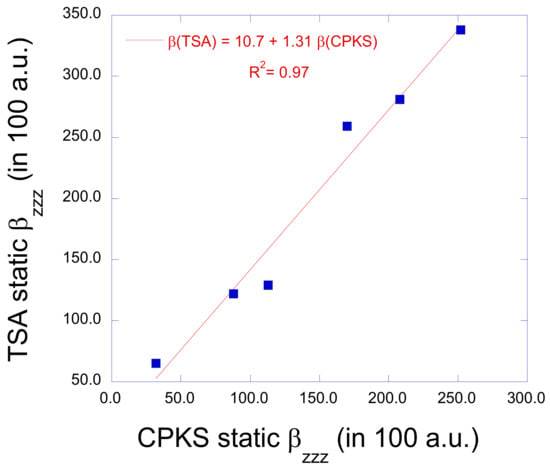
Figure 5.
Comparison between static values obtained within the two-state approximation and the full values calculated at the response TDDFT level (CPKS in the static limit). All quantities have been obtained at the IEF-PCM/TDDFT/ωB97X-D/6-311G*/LanL2TZ level of approximation and are expressed in 102 a.u.
4. Conclusions and Outlook
Following reference [29], density functional theory and time-dependent calculations have been performed on an extended series of Lindquist-type organo-imido polyoxometalates derivatized with ligands bearing donor or acceptor groups to unravel the relationships between their structures and first hyperpolarizabilities. The electron acceptor character of the hexamolybdate moiety has been confirmed by these new quantum chemical analyses, highlighting that when associated with a donor substituent, its first hyperpolarizability increases, and stronger donor leads to larger first hyperpolarizabilities. By adopting a range-separated hybrid XC functional (ωB97X-D) and by describing solvent effects with the polarizable continuum model, a set of consistent data has been obtained: a stronger donor substituent on the terminal phenyl ring (i) increases its quinoid character (larger bond length alternation), (ii) augments the charge transfer between the substituent and that phenyl ring in the electronic ground state, (iii) decreases the excitation energy corresponding to the dominant low-energy excitation band, (iv) increases the oscillator strength, and (v) strongly enhances the excitation-induced change of dipole moment. In particular, in comparison to an ethynyl, the presence of an ethenyl π-spacer between the phenyl rings is favorable to achieve a large first hyperpolarizability. A larger first hyperpolarizability and corresponding consistent differences in the linear optical responses are also achieved in the case of a dimethylamino donor group, in comparison to a hydroxyl substituent, and even more in comparison to a cyano acceptor group.
Calculations have also enabled comparisons with recent experimental investigations [5], involving dimethylamino, diphenylamino, and carbazole moieties as donor groups. Calculations and experimental data highlight the weaker donor character of the carbazole moiety, which is attributed to the torsion angle between the carbazole and the attached phenyl ring, leading to a reduction in π-conjugation. On the other hand, calculations and experiments provide different conclusions on the comparison between POM bearing the dimethylamino or diphenylamino donor group. This difference is associated with the combination of the limitations of the method of calculation (the XCF and the treatment of the solvent are approximate) and of the experimental error bars.
Other structural effects of the POM on the first hyperpolarizability can be considered, such as the combination of two POM moieties with one organic linker, as proposed in reference [6], or the opposite, two organic ligands on one POM moiety. In both cases, the NLO performance of these Λ-shape compounds could be compared to those of their push-pull one-dimensional derivatives. Another direction would be to consider POM containing two types of metal atoms, such as the combination of W and Mo atoms in reference [48].
Author Contributions
Conceptualization, B.C.; formal analysis, E.R. and B.C.; investigation, E.R.; writing—original draft preparation, E.R. and B.C.; writing—review and editing, B.C.; supervision, B.C.; project administration, B.C.; funding acquisition, B.C. Both authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University of Namur, the FNRS-FRFC (Conventions No. GEQ U.G006.15, U.G018.19, and RW/GEQ2016), and the Walloon Region (Convention RW/GEQ2016).
Data Availability Statement
Data are stored on the computers of the HPC platform of UNamur.
Acknowledgments
The calculations were performed on the computers of the Consortium des Équipements de Calcul Intensif and particularly those of the High-Performance Computing Platform, which are supported by the FNRS-FRFC, the Walloon Region, and the University of Namur (Conventions No. GEQ U.G006.15, U.G018.19, 1610468, and RW/GEQ2016).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- Pope, M.T.; Müller, A. Polyoxometalate Chemistry: An Old Field with New Dimensions in Several Disciplines. Angew. Chem. Int. Ed. Engl. 1991, 30, 34–48. [Google Scholar] [CrossRef]
- Anyushin, A.V.; Kondinski, A.; Parac-Vogt, T.N. Hybrid Polyoxometalates as Post-functionalization Platforms: From Fundamentals to Emerging Applications. Chem. Soc. Rev. 2020, 49, 382–432. [Google Scholar] [CrossRef]
- Al-Yasari, A.; Van Steerteghem, N.; El Moll, H.; Clays, K.; Fielden, J. Donor—Acceptor Organo-Imido Polyoxometalates: High Transparency, High Activity Redox-Active NLO Chromophores. Dalton Trans. 2016, 45, 2818–2822. [Google Scholar] [CrossRef] [Green Version]
- Al-Yasari, A.; Van Steerteghem, N.; Kearns, H.; El Moll, H.; Faulds, K.; Wright, J.A.; Brunschwig, B.S.; Clays, K.; Fielden, J. Organoimido-Polyoxometalate Nonlinear Optical Chromophores: A Structural, Spectroscopic, and Computational Study. Inorg. Chem. 2017, 56, 10181–10194. [Google Scholar] [CrossRef] [Green Version]
- Al-Yasari, A.; Spence, P.; El Moll, H.; Van Steerteghem, N.; Horton, P.N.; Brunschwig, B.S.; Fielden, J. Fine-Tuning Polyoxometalate Non-Linear Optical Chromophores: A Molecular Electronic “Goldilocks” Effect. Dalton Trans. 2018, 47, 10415–10419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Yasari, A.; El Moll, H.; Purdy, R.; Vincent, B.K.; Spence, P.; Malval, J.P.; Fielden, J. Optical Third Order Non-Linear Optical And Electrochemical Properties Of Dipolar, Centrosymmetric and C2v Organoimido Polyoxometalate Derivatives. Phys. Chem. Chem. Phys. 2021, 23, 11807–11817. [Google Scholar] [CrossRef]
- Boulmier, A.; Vacher, A.; Zang, D.; Yang, S.; Saad, A.; Marrot, J.; Oms, O.; Mialane, P.; Ledoux, I.; Ruhlmann, L.; et al. Anderson-Type Polyoxometalates Functionalized by Tetrathiafulvalene Groups: Synthesis, Electrochemical Studies, and NLO Properties. Inorg. Chem. 2018, 57, 3742–3752. [Google Scholar] [CrossRef]
- Perez-Moreno, J.; Zhao, Y.; Clays, K.; Kuzyk, M.G.; Shen, Y.; Qiu, L.; Hao, J.; Guo, K. Modulated Conjugation as a Means of Improving the Intrinsic Hyperpolarizability. J. Am. Chem. Soc. 2009, 131, 5084–5093. [Google Scholar] [CrossRef]
- Castet, F.; Rodriguez, V.; Pozzo, J.L.; Ducasse, L.; Plaquet, A.; Champagne, B. Design and Characterization of Molecular Nonlinear Optical Switches. Acc. Chem. Res. 2013, 46, 2656–2665. [Google Scholar] [CrossRef] [PubMed]
- Karamanis, P.; Otero, N.; Pouchan, C. Unleashing the Quadratic Nonlinear Optical Responses of Graphene by Confining White-Graphene (h-BN) Sections in Its Framework. J. Am. Chem. Soc. 2014, 136, 7464–7473. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.; Park, J.; De Mey, K.; Hu, X.; Duncan, T.V.; Beratan, D.N.; Therien, M.J. Large Hyperpolarizabilities at Telecommunication-Relevant Wavelengths in Donor-Acceptor-Donor Nonlinear Optical Chromophores. ACS Cent. Sci. 2016, 2, 954–966. [Google Scholar] [CrossRef]
- Coe, B.J.; Rusanova, D.; Joshi, V.D.; Sanchez, S.; Vavra, J.; Khobragade, D.; Severa, L.; Cisarova, I.; Saman, D.; Pohl, R.; et al. Helquat Dyes: Helicene-like Push−Pull Systems with Large Second-Order Nonlinear Optical Responses. J. Org. Chem. 2016, 81, 1912–1920. [Google Scholar] [CrossRef]
- Lacroix, P.G.; Malfant, I.; Lepetit, C. Second-Order Nonlinear Optics in Coordination Chemistry: An Open Door towards Multi-Functional Materials and Molecular Switches. Coord. Chem. Rev. 2016, 308, 381–394. [Google Scholar] [CrossRef]
- Knoppe, S.; Hakkinen, H.; Verbiest, T.; Clays, K. Role of Donor and Acceptor Substituents on the Nonlinear Optical Properties of Gold Nanoclusters. J. Phys. Chem. C 2018, 122, 4019–4028. [Google Scholar] [CrossRef]
- Van Bezouw, S.; Koo, M.J.; Lee, S.C.; Lee, S.H.; Campo, J.; Kwon, O.P.; Wenseleers, W. Three-Stage pH-Switchable Organic Chromophores with Large Nonlinear Optical Responses and Switching Contrasts. Chem. Commun. 2018, 54, 7842–7845. [Google Scholar] [CrossRef] [Green Version]
- Tonnelé, C.; Champagne, B.; Muccioli, L.; Castet, F. Second-Order Nonlinear Optical Properties of Stenhouse Photoswitches: Insights from Density Functional Theory. Phys. Chem. Chem. Phys. 2018, 20, 27658–27667. [Google Scholar] [CrossRef] [PubMed]
- Lou, A.J.T.; Marks, T.J.A. Twist on Nonlinear Optics: Understanding the Unique Response of π-Twisted Chromophores. Acc. Chem. Res. 2019, 52, 1428–1438. [Google Scholar] [CrossRef] [PubMed]
- Rigamonti, L.; Forni, A.; Cariati, E.; Malavasi, G.; Pasini, A. Solid-State Nonlinear Optical Properties of Mononuclear Copper (II) Complexes with Chiral Tridentate and Tetradentate Schiff Base Ligands. Materials 2019, 12, 3595. [Google Scholar] [CrossRef] [Green Version]
- Rothe, C.; Neusser, D.; Hoppe, N.; Dirnberger, K.; Vogel, W.; Gámez-Valenzuela, S.; Ludwigs, S. Push-Pull Chromophores for Electro-Optic Applications: From 1D Linear to β-branched Structures. Phys. Chem. Chem. Phys. 2020, 22, 2283–2294. [Google Scholar] [CrossRef]
- Qiu, S.; Morshedi, M.; Kodikara, M.S.; Du, J.; de Coene, Y.; Zhang, C.; Humphrey, M.G. Organometallic complexes for nonlinear optics. 66. Synthesis and quadratic nonlinear optical studies of trans-[Ru{C C {2, 5-C4H2S-(E)-CHCH} n-2, 5-C4H2S (NO2)} Cl (dppe) 2](n = 0–2). J. Organomet. Chem. 2020, 919, 121306. [Google Scholar] [CrossRef]
- Cesaretti, A.; Foggi, P.; Fortuna, C.G.; Elisei, F.; Spalletti, A.; Carlotti, B. Uncovering Structure—Property Relationships in Push–Pull Chromophores: A Promising Route to Large Hyperpolarizability and Two-Photon Absorption. J. Phys. Chem. 2020, 124, 15739–15748. [Google Scholar] [CrossRef]
- Ramos, T.N.; Canuto, S.; Champagne, B. Unraveling the Electric Field-Induced Second Harmonic Generation Responses of Stilbazolium Ion Pairs Complexes in Solution Using a Multiscale Simulation Method. J. Chem. Inf. Model. 2020, 60, 4817–4826. [Google Scholar] [CrossRef] [PubMed]
- Idney, B.; Tertius, L.F.; Leandro, R.F.; Herbert, C.G.; Marcos, A.C. Applicability of DFT Functionals for Evaluating the First Hyperpolarizability of Phenol Blue in Solution. J. Chem. Phys. 2021, 154, 094501. [Google Scholar] [CrossRef]
- Moris, M.; Van Den Eede, M.P.; Koeckelberghs, G.; Deshaume, O.; Bartic, C.; Clays, K.; Cleuvenbergen, S.; Verbiest, T. Solvent Role in the Self-Assembly of Poly (3-alkylthiophene): A Harmonic Light Scattering Study. Macromolecules 2021, 54, 2477–2484. [Google Scholar] [CrossRef]
- Castet, F.; Gillet, A.; Bureš, F.; Plaquet, A.; Rodriguez, V.; Champagne, B. Second-Order Nonlinear Optical Properties of Λ-Shaped Pyrazine Derivatives. Dye. Pigment. 2021, 184, 108850. [Google Scholar] [CrossRef]
- Verbiest, T.; Clays, K.; Rodriguez, V. Second-Order Nonlinear Optical Characterizations Techniques: An Introduction; CRC Press: New York, NY, USA, 2009. [Google Scholar]
- Yan, L.; Yang, G.; Guan, W.; Su, Z.; Wang, R. Density Functional Theory Study on the First Hyperpolarizabilities of Organoimido Derivatives of Hexamolybdates. J. Phys. Chem. B 2005, 109, 22332–22336. [Google Scholar] [CrossRef]
- Janjua, M.R.S.A.; Liu, C.G.; Guan, W.; Zhuang, J.; Muhammad, S.; Yan, L.K.; Su, Z.M. Prediction of Remarkably Large Second-Order Nonlinear Optical Properties of Organoimido-Substituted Hexamolybdates. J. Phys. Chem. 2009, 113, 3576–3587. [Google Scholar] [CrossRef]
- Rtibi, E.; Abderrabba, M.; Ayadi, S.; Champagne, B. Theoretical Assessment of the Second-Order Nonlinear Optical Responses of Lindqvist-Type Organoimido Polyoxometalates. Inorg. Chem. 2019, 58, 11210–11219. [Google Scholar] [CrossRef]
- Lescos, L.; Sitkiewicz, S.; Beaujean, P.; Blanchard-Desce, M.; Champagne, B.; Matito, R.E.; Castet, F. Performance of DFT Functionals for Calculating the Second-Order Nonlinear Optical Properties of Dipolar Merocyanines. Phys. Chem. Chem. Phys. 2020, 22, 16579–16594. [Google Scholar] [CrossRef]
- Champagne, B.; Perpète, E.A.; Jacquemin, D.; van Gisbergen, S.J.A.; Baerends, E.J.; Soubra-Ghaoui, C.; Kirtman, B. Assessment of Conventional Density Functional Schemes for Computing the Dipole Moment and (Hyper)polarizabilities of Push–Pull π-Conjugated Systems. J. Phys. Chem. 2000, 104, 4755–4763. [Google Scholar] [CrossRef]
- Bulat, F.A.; Toro-Labbé, A.; Champagne, B.; Kirtman, B.; Yang, W. Density-Functional Theory (hyper) polarizabilities of Push-Pull π-Conjugated Systems: Treatment of Exact Exchange and Role of Correlation. J. Chem. Phys. 2005, 123, 014319. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Autschbach, J. Influence of the Delocalization Error and Applicability of Optimal Functional Tuning in Density Functional Calculations of Nonlinear Optical Properties of Organic Donor-Acceptor Chromophores. ChemPhysChem 2013, 14, 2450–2461. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revis. C.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Chai, J.D.; Head-Gordon, M. Long-range Corrected Hybrid Density Functionals with Damped Atom-Atom Dispersion Corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becke, A.D. Density-Functional Thermochemistry. V. Systematic Optimization of Exchange-Correlation Functionals. J. Chem. Phys. 1997, 107, 8554–8560. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef]
- Van Gisbergen, S.J.A.; Snijders, J.G.; Baerends, E.J. Calculating Frequency-Dependent Hyperpolarizabilities Using Time-Dependent Density Functional Theory. J. Chem. Phys. 1998, 109, 10644–10656. [Google Scholar] [CrossRef] [Green Version]
- Helgaker, T.; Coriani, S.; Jørgensen, P.; Kristensen, K.; Olsen, J.; Ruud, K. Recent Advances in Wave Function-Based Methods of Molecular-Property Calculations. Chem. Rev. 2012, 112, 543–631. [Google Scholar] [CrossRef]
- Bersohn, R.; Pao, Y.H.; Frisch, H.L. Double-Quantum Light Scattering by Molecules. J. Chem. Phys. 1966, 45, 3184–3198. [Google Scholar] [CrossRef]
- Castet, F.; Bogdan, E.; Plaquet, A.; Ducasse, L.; Champagne, B.; Rodriguez, V. Reference Molecules for Nonlinear Optics: A Joint Experimental and Theoretical Investigation. J. Chem. Phys. 2012, 136, 024506. [Google Scholar] [CrossRef]
- Tuer, A.; Krouglov, S.; Cisek, R.; Tokarz, D.; Barzda, V. Three-Dimensional Visualization of the First Hyperpolarizability Tensor. J. Comput. Chem. 2011, 32, 1128–1134. [Google Scholar] [CrossRef]
- Orr, B.J.; Ward, J.F. Perturbation Theory of the Non-Linear Optical Polarization of an Isolated System. Mol. Phys. 1971, 20, 513–526. [Google Scholar] [CrossRef]
- Bishop, D.M. Explicit Non-divergent Formulas for Atomic and Molecular Dynamic Hyperpolarizabilities. J. Chem. Phys. 1994, 100, 6535–6542. [Google Scholar] [CrossRef]
- Oudar, J.L.; Chemla, D.S. Hyperpolarizabilities of the Nitroanilines and Their Relations to the Excited State Dipole Moment. J. Chem. Phys. 1977, 66, 2664–2668. [Google Scholar] [CrossRef]
- Zutterman, F.; Liégeois, V.; Champagne, B. Simulation of UV/visible Absorption Spectra of Fluorescent Protein Chromophore Models. ChemPhotoChem 2017, 1, 281–297. [Google Scholar] [CrossRef]
- Champagne, B.; Kirtman, B. Alternative Sum-Over-States Expressions for the First Hyperpolarizability of Push-Pull π-Conjugated Systems. J. Chem. Phys. 2006, 125, 024101. [Google Scholar] [CrossRef]
- Wei, Y.; Lu, M.; Cheung, C.F.-C.; Barnes, C.L.; Peng, Z. Functionalization of [MoW5O19]2- with Aromatic Amines: Synthesis of the First Arylimido Derivatives of Mixed-Metal Polyoxometalates. Inorg. Chem. 2001, 40, 5489–5490. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).