Abstract
The layered oxides LiNixMnyCozO2 (NMCs, x + y + z = 1) with high nickel content (x ≥ 0.6, Ni-rich NMCs) are promising high-energy density-positive electrode materials for Li-ion batteries. Their electrochemical properties depend on Li+/Ni2+ cation disordering originating from the proximity of the Li+ and Ni2+ ionic radii. We synthesized a series of the LiNi0.8Mn0.1Co0.1O2 NMC811 adopting two different disordering schemes: Ni for Li substitution at the Li site in the samples finally annealed in air, and close to Ni↔Li antisite disorder in the oxygen-annealed samples. The defect formation scenario was revealed with Rietveld refinement from powder X-ray diffraction data, and then the reliability of semi-quantitative parameters, such as I003/I104 integral intensity ratio and c/(2√6a) ratio of pseudocubic subcell parameters, was verified against the refined defect concentrations. The I003/I104 ratio can serve as a quantitative measure of g(NiLi) only after explicit correction of intensities for preferred orientation. Being normalized by the total scattering power of the unit cell, the I003/I104 ratio depends linearly on g(NiLi) for each disordering scheme. The c/(2√6a) ratio appears to be not reliable and cannot be used for a quantitative estimate of g(NiLi). In turn, the volume of the Rm unit cell correlates linearly with g(NiLi), at least for defect concentrations not exceeding 5%. The microscopy techniques such as high-resolution high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) and electron diffraction tomography (EDT) allow us to study the materials locally, still, there is no proper quantitative approach for comprehensive analysis of defects. In the present work, the TEM-assisted quantitative Li+/Ni2+ disordering analysis with EDT and HAADF-STEM in six Ni-rich NMC samples with various defects content is demonstrated. Noteworthy, while PXRD and EDT methods demonstrate overall defect amounts, HAADF-STEM allows us to quantitatively distinguish regions with various disordering extents. Therefore, the combination of mentioned PXRD and TEM methods gives the full picture of Li+/Ni2+ mixing defects in Ni-rich NMCs.
1. Introduction
Owing to higher specific capacity, higher energy density and reduced use of high-cost and relatively scarce cobalt compared to conventional LiCoO2 positive electrode (cathode) for Li-ion batteries (LIBs), layered mixed lithium and transition metals (TM) oxides LiNixMnyCozO2 (NMCs, x + y + z = 1) are considered to be the most promising candidates for positive electrodes (cathodes) for next-generation LIBs [1,2]. NMCs with high nickel content (termed Ni-rich NMCs, where x ≥ 0.6) are of particularly great interest, as such materials can provide high specific capacity (up to 220 mAh/g in the 2.7–4.3 V vs. Li/Li+ potential window), and the energy density of such materials can reach 800 Wh/kg [3].
The crystal structure of NMCs is of α-NaFeO2 type (space group Rm) consisting of TMO2 layers built up with edge-sharing TMO6 octahedra and separated by layers of octahedrally coordinated Li cations, being an ordered derivative of the rock-salt structure. In an ideal case, the Li and TM cations are located in the octahedral Wyckoff 3a and 3b sites, respectively, thus occupying the octahedral voids in the underlying “cubic” (“ccc” or ABCABC) close packing of oxygen atoms along the [001] direction (also denoted as the O3 structure highlighting the octahedral coordination of Li and presence of three Li layers per repeat period along the c-axis [4]). This layered structure provides a two-dimensional space for the transport of lithium ions while the material undergoes electrochemical Li (de)intercalation.
In the Ni-rich NMC compounds, the Ni cations are in the mixed-valent Ni2+-Ni3+ state. It is generally known that there is strong tendency of Ni2+ to occupy the Li+ 3b sites because of similar ionic radii of Ni2+ and Li+ in contrast to larger difference with Ni3+ (r(Ni3+) = 0.56 Å, r(Ni2+) = 0.69 Å, r(Li+) = 0.76 Å for CN = 6 [5]) and lower energy barrier of Ni2+ migration from their native 3b to Li+ 3a sites, promoting Ni2+/Li+ exchange, compared to lower probability of such exchange for Co3+ and Mn4+ [6]. The point defects due to the Ni2+/Li+ exchange are widely held in the literature on NMC cathode materials as anti-site defects, or cation disordering. The Ni2+/Li+ mixing degree is found to increase with increasing the nickel content in NMCs [7,8]. In general, the Ni2+/Li+ anti-site defects are believed to negatively affect the electrochemical properties of Ni-rich NMCs. The disordered phases are known to have a smaller distance between the TM layers and, therefore, a higher Li+ migration energy barrier compared to the well-ordered phase, leading to a drop of Li diffusivity by blocking Li+ migration paths [9,10,11].
The Ni2+/Li+ anti-site defects are not the sole reason for the appearance of the Ni cations at the Li site and/or Li cations at the TM site. The pure anti-site disorder can be described using Kröger–Vink notations as
This exchange preserves the chemical composition, but in fact, the Li/TM ratio in Ni-rich NMCs is not constant and depends on partial oxygen pressure and temperature during the last stage of the solid-state synthesis [12]:
Here the oxygen molecule creates two cationic 3a and 3b sites which are both populated with the Li cations () from the Li source (Li(s)) whereas the charge compensation is achieved by oxidation of the Ni2+ to Ni3+ cation at its native site (). In the electrochemically delithiated material partial loss of oxygen is possible accompanied by migration of the Ni cations to the Li sites that is expressed with a different equation:
Here the Li vacancy is created by electrochemical deintercalation resulting in adding a single positive charge to the Ni site. The resulting Ni and O vacancies annihilate causing gradual transformation of a well-ordered layered structure into a spinel-like structure, and ultimately into a rock-salt type structure. This structure “densification” leads to loss of the electrochemical capacity and may increase flammability hazard via evolved oxygen reacting with organic components of electrolyte [13,14,15,16]. Moreover, the different structural organization of the initial (layered) and the resulting disordered structures leads to a strong microstrain in the primary particles during multiple charge/discharge cycles and, as a result, a formation of microcracks in the secondary particles, that in turn results in battery failure due to loss of electronic conduction [10,17,18]. On the contrary, a number of studies, including theoretical ones, showed the positive influence of small Ni2+/Li+ mixing, demonstrating that certain concentrations of anti-site defects in the volume of the sample can benefit the thermal and structural stability of Ni-rich NMCs during electrochemical cycling at high states of charge [7,18,19,20]. Thus, the exact role of Ni2+/Li+ exchange in the cathode material’s properties is still under debate.
There is no doubt that whatever the origin of Ni2+ at the Li site is (i.e., Equations (1) or (2)), these defects appear primarily at the surface [15,21,22,23]. This is not surprising as the surface might be enriched with Ni2+ comparing to the bulk as a consequence of partial reduction due to insufficiently high partial oxygen pressure during the synthesis or due to reductive interactions with the electrolyte components. Thus, apart from the total content of Ni2+ at the Li site, its local quantification at the crystal surface is of particular interest as it can be directly related to the synthesis conditions and electrochemical behavior of Ni-rich NMCs. However, to our knowledge, systematic investigations of quantitative distribution of cationic defects from bulk to the surface of the NMC particles have not been reported yet.
Powder X-ray diffraction (PXRD) is the most common technique used for the assessment of the Ni2+/Li+ disorder, both quantitatively and semi-quantitatively. The ratio of the integrated intensities of the 003 and 104 reflections (I003/I104) and the c/(2√6a) ratio (indicating the trigonal distortion of the f.c.c. cubic subcell) are the two most extensively used semi-quantitative parameters, while the fractional occupancies of Li and Ni ions at 3b and 3a sites, respectively, directly determined via Rietveld refinement, are considered to be reliable quantitative parameters. Still, PXRD has limitations such as low sensitivity towards such “light” atoms as Li. Neutron powder diffraction (NPD) is a more powerful technique for quantification of the Ni2+/Li+ disordering, particularly in a joint Rietveld refinement with synchrotron X-ray powder diffraction data [24,25,26]. However, NPD usually requires a substantial amount of the material, which is not easily available from the electrochemical cell where the quantity of the cathode material is limited to dozens of mg. Moreover, both PXRD and NPD are essentially bulk methods, which cannot differentiate the effects originating from the surface and the bulk, inhomogeneous composition, different particle size and shape, etc. Transmission electron microscopy (TEM) is an alternative approach, very suitable for local area analysis [27]. High-resolution high angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) and annular bright-field STEM (ABF-STEM) imaging techniques are widely used to obtain the local structural information, but in most cases in a rather empirical and less quantitative manner [15,28,29,30,31]. Nevertheless, the tools based on statistical parameter estimation approaches for retrieving local crystallographic information from the TEM images are already available and can be potentially applied to measuring the cationic disorder in Ni-rich NMCs at an atomic scale [32,33].
Another quantitative technique that was successfully applied to anti-site defects evaluation is electron diffraction tomography (EDT) or 3D-electron diffraction (3D-ED) [34]. Electron diffraction is more sensitive to the “light” atoms than PXRD [35], for example, in NMCs the “visibility” of Li cations with electrons is around three times higher, compared to X-rays [36]. Indeed, EDT has already been used successfully for the quantification of Li and TM exchange in polyanion Li-ion battery cathodes [37]. EDT reduces the locality of structural characterization down to the crystals not larger than 200–500 nm closing the gap between bulk diffraction techniques and TEM images [38].
Thus, comprehensive consideration of various approaches to proper quantification of Ni2+/Li+ disorder is required for better understanding and control over the crystal structure of Ni-rich NMCs and, therefore, their electrochemical properties. In the present work, we tried to utilize the capabilities of TEM imaging and diffraction techniques for quantification of the occupancy factors of the Ni and Li sites in a typical Ni-rich NMC material LiNi0.8Mn0.1Co0.1O2 (NMC811) and provide the comparison of the obtained quantitative results with those extracted from various quantitative and semi-quantitative treatments of laboratory powder X-ray diffraction patterns. EDT is demonstrated to be a reliable technique for measuring defect concentration at the level of a single crystallite. We suggest the quantitative analysis of high-resolution HAADF-STEM images via statistical parameter estimation theory as a tool for discriminating the local regions with different degrees of anti-site disorder.
2. Materials and Methods
Six NMC811 samples presented in Table 1 with various degrees of Ni2+/Li+ disorder were obtained by co-precipitation of either hydroxide or carbonate transition metal (TM) precursors from sulfates and acetates as transition metals sources followed by annealing of the precursors with LiOH·H2O in either air or oxygen atmosphere. Typically, 2M TM sulfate solution (NiSO4·6H2O, CoSO4·7H2O and MnSO4·H2O, Sigma Aldrich, St. Louis, MO, USA, ≥99%) or 0.5M TM acetate solution ((CH3COO)2Ni·4H2O, (CH3COO)2Mn·4H2O, and (CH3COO)2Co·4H2O, Sigma Aldrich, St. Louis, MO, USA, ≥99%) with the Ni:Mn:Co = 0.8:0.1:0.1 molar ratio, 4M (for sulfates) and 1M (for acetates) NaOH or 2M (for sulfates) and 0.5M (for acetates) Na2CO3 with the appropriate amount of NH3·H2O were used (Table 1). The sulfate or acetate solution and sodium carbonate or hydroxide were separately pumped into a continuously stirred batch reactor under Ar atmosphere. The pH and temperature of the mixed solution were maintained at 11–11.5 and 50 °C for the hydroxide precipitation and 7.8 and 56 °C for the carbonate one. The resulting precipitates were filtered and washed with deionized water several times to remove residual ions. The washed precursors were dried in a vacuum oven at 90 °C for 12 h. The obtained TM hydroxides or carbonates were mixed with LiOH·H2O with a molar ratio of 1:1.06 or 1:1.03, respectively, and annealed for 12 h at 750 °C in oxygen flow or for 5 h at 500 °C and then for 12 h at 850 °C in air.

Table 1.
Synthesis conditions of the NMC811 samples.
Powder X-ray diffraction (PXRD) patterns were collected with a Huber G670 Guinier diffractometer with Co-Kα1 radiation (λ = 1.78892Å, curved Ge (111) monochromator, transmission geometry image plate detector) at room temperature in the 4–100° 2θ range with the angular step of 0.005°. Rietveld refinement was carried out using the JANA2006 program package [39].
TEM samples were prepared in air by crushing the crystals with an agate mortar and pestle in ethanol and depositing drops of suspension onto a carbon film supported by a copper grid. High angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) images were acquired on a probe aberration-corrected Thermo Fisher Titan Themis Z electron microscope at 200 kV (the probe convergence semiangle is 30 mrad, the inner and outer acceptance angles of the HAADF detector are 57 and 200 mrad, respectively). The quantitative analysis of Ni2+/Li+ mixing from high-resolution HAADF-STEM images was performed with the StatSTEM software [33].
Electron diffraction tomography (EDT) data were collected using Thermo Fisher Tecnai G2 (Eindhoven, The Netherlands) microscope at 200 kV by measuring series of selected area electron diffraction patterns taken with an angular interval of 1° in the highest achievable for each crystal angular range. The exact ranges for corresponding crystals are provided in Table S1 of Supporting Information. No electron beam precession was used. Acquisition was started from a random crystal orientation far from the low-index zone axes to minimize multiple scattering effects. The obtained series of diffraction patterns were treated using PETS and JANA 2006 software [33,40] for the reflection search, cluster analysis, indexing, refinement of the unit cell parameters and orientation matrix and final integration of the reflection intensities. The obtained set of intensities and hkl values was used for the kinematical structure refinement that is assumed to be reasonable approximation as the materials do not contain atomic entities with high scattering power [36].
3. Results and Discussion
The crystallographic parameters of the NMC811 materials were first obtained through the Rietveld refinement from PXRD data. The PXRD patterns of all six samples (Figure 1) demonstrate well-crystallized single-phase compounds with the layered α-NaFeO2-type structure. The patterns were completely indexed with the hexagonal cell and Rm space group with the unit cell parameters and volumes listed in Table 2. The crystal structure refinement was conducted using the following scheme. The 3a (0,0,0) site was jointly populated by Li and Ni, whereas Li, Ni, Mn and Co were placed at the 3b (0,0,1/2) site. The occupancy factors gLi and gNi at the 3a site were refined with the gLi + gNi = 1 constrain. The occupancy factors gMn and gCo at the 3b site were fixed to 0.1 each according to the NMC811 chemical composition, and gLi and gNi were refined with gLi + gNi = 0.8. For the S1air, S2air and S3air samples the refined gLi at the 3b site was equal to zero within one standard deviation. Thus, it was further assumed that Li does not populate this crystallographic position. Common atomic displacement parameter (ADP) was refined for all cationic and oxygen sites. Finally, preferred orientation along the [001] direction was taken into account with the March–Dollase function. The resulting low-reliability factors and good agreement between the experimental and calculated PXRD profiles (Figures S1–S6) indicate high reliability of the Rietveld refinements. The crystallographic data, after the Rietveld refinement and parameters of the Ni2+/Li+ mixing, are shown in Table 2.
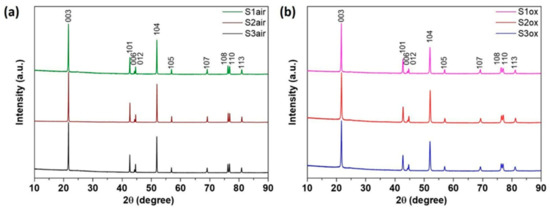
Figure 1.
PXRD patterns of the NMC811 (a) air-annealed and (b) oxygen-annealed samples.

Table 2.
Unit cell parameters, refined atomic coordinates, ADPs, March–Dollase preferred orientation parameter (τ), interatomic distances, parameters of the Ni/Li mixing at the 3a and 3b sites and reliability factors for the NMC811 materials after Rietveld refinement.
The refined structures and chemical compositions are in general agreement with the sample preparation conditions. Annealing of the samples at higher temperature and lower partial oxygen pressure (850 °C, air, p(O2) = 0.21 atm) is favorable for a lower oxidation state of Ni that results in introducing Ni into the Li site similar to the Equation (3). Indeed, all air-prepared samples demonstrate certain Ni for Li substitution at the 3a site and full TM occupation of the 3b site. Larger unit cell volume for the air-annealed samples (<V> = 102.01 Å3) compared to that of the oxygen-annealed ones (<V> = 101.46 Å3) indicates more reduced Ni. Annealing at lower temperature and higher partial oxygen pressure (750 °C, air, p(O2) = 1 atm) introduces Li into the TM site according to Equation (2) that raises the Ni oxidation state. It should be noted that in neither of the sample the defect concentrations correspond to the pure anti-site scenario as defined with Equation (1).
Furthermore, the semi-quantitative parameters, I003/I104 and c/(2√6a) ratios were calculated from the PXRD profiles and compared with the Ni fraction at the Li site obtained via the Rietveld refinement (Table 3). The ratio of integral intensities of the 003 and 104 reflections, after polarization, geometry and Lorentz corrections, and (ideally) corrections for absorption and temperature factors, can be considered as proportional to the squared ratio of the corresponding structure factors F003 and F104:

Table 3.
Semi-quantitative parameters for assessing the Li/Ni disorder in the NMC811 samples.
Here, f3a = gNifNi + (1 − gNi)fLi, f3b = gNifNi + (0.8 − gNi)fLi + 0.1(fMn + fCo), zO—z-coordinate of the oxygen atom. This equation highlights the different nature of the 003 and 104 reflections: the former is the superlattice reflection arising from the layered ordering at the 3a and 3b sites with the structure factor proportional to the difference in scattering power at these positions, whereas the latter is the basic reflection of the parent rock-salt Fmm structure with the structure factor correlating with F000 which counts the total number of electrons in the unit cell and depends on the overall Li and Ni content. The I003/I104 intensity ratio also depends on the z-coordinate of the oxygen position, but it does not differ substantially if the violation of the layered ordering is relatively weak (Table 2) and can be safely neglected. The I003/I104 intensity ratio has frequently been used for the assessment of the Li/Ni disorder [41,42,43,44,45].
Indeed, the I003/I104 ratio generally correlates with the concentration of the cationic defects. The lowest (1.208) and highest (1.382) I003/I104 ratios were obtained for the S3air and S2ox samples with the highest (5.02%) and lowest (2.24%) Ni fractions in the Li position, respectively. However, the dependence of I003/I104 on the Ni content in the 3a site lacks systematic dependence for all six samples. Careful analysis of the PXRD patterns and residuals after the Rietveld refinement revealed that the origin of this discrepancy is in intensity distortion due to preferred orientation which is albeit small but different for all studied samples. The preferred intensity contribution was modeled with the March–Dollase function:
where Thkl is the intensity correction factor, τ is the preferred orientation parameter obtained in the Rietveld refinement (Table 2) and φhkl is the angle between the reciprocal lattice vector Hhkl and preferred orientation direction (0° and 55° for the 003 and 104 reflections, respectively). After preferred orientation (po) correction, the I003/I104 ratio follows the hyperbolic curve being plotted against the Ni content in the Li site, as expected from Equation (4) (Figure 2a). However, the air-annealed and oxygen-annealed samples cannot be distinguished from this dependence because it is governed by two factors simultaneously: f3a − f3b difference and f3a + f3b dependence on the overall Li and TM content. The two sets of samples with essentially different defect formation scenarios, as discussed above, can be discriminated by plotting the po-corrected I003/I104 ratio normalized by squared F000 for each structure (Figure 2b), where the air- and oxygen-annealed samples follow two separate straight lines with different slopes.
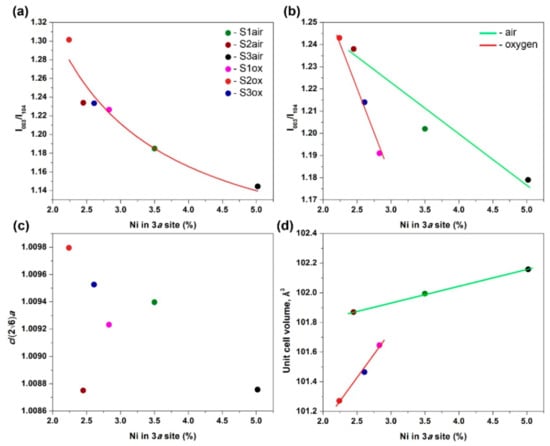
Figure 2.
Correlation of semi-quantitative parameters with the Ni content in the Li site: (a) I003/I104 ratio corrected for preferred orientation (red line show fitted hyperbolic curve, R2 = 0.907); (b) I003/I104 ratio corrected for preferred orientation and normalized by (F000)2 (lines are guide to the eye, R2 = 0.954 for the air-annealed samples, R2 = 0.989 for the oxygen-annealed ones); (c) c/(2√6a); (d) unit cell volume (lines are guide to the eye, R2 = 0.999 for the air-annealed samples, R2 = 0.969 for the oxygen-annealed ones).
To summarize, the I003/I104 ratio can reflect the concentration of cationic point defects only qualitatively being sensitive even to small preferred orientation which is always present in the Ni-rich NMCs due to their layered structure. Sensible defect quantification with the I003/I104 ratio requires knowing the defect formation model (as given with the Equations (1)–(3)), correction for preferred orientation and normalization per total scattering power of the unit cell. In addition, the I003/I104 ratio cannot be used for a direct comparison of the materials if the PXRD patterns were measured with different experimental setups without corrections for polarization, Lorentz and geometry factors, and absorption corrections as well (that is particularly difficult as it is rarely measured and reported). The Rietveld method is the preferable technique for extracting the defect concentration from PXRD data.
The c/(2√6a) parameter reflects the Li/Ni disorder only indirectly being a geometric measure of the deviation of the hexagonal unit cell of Ni-rich NMCs from their parent cubic rock-salt subcell in which c/(2√6a) = 1. Layered ordering of larger Li (r(Li+) = 0.76 Å) and smaller Ni (r(Ni3+) = 0.56 Å, r(Ni2+) = 0.69 Å) at the 3a and 3b sites leads to anisotropic expansion of the cubic subcell along {111}c = [001]h direction and increase in c/(2√6a) above 1. According to that, the largest c/(2√6a) is observed for the less defect S2ox sample, whereas the most defect S3air sample demonstrates one of the smallest values. However, in general, the correlation between c/(2√6a) and the Ni fraction in the Li position is surprisingly poor (Figure 2c). In fact, it is difficult to expect that the c/(2√6a) ratio will behave monotonically with the Ni fraction at the 3a site as this ratio is determined by the distribution of three atomic species with different ionic radii (Li+, Ni2+ and Ni3+) over two crystallographic sites. Unit cell volume appears to be more precise geometrical parameter which linearly correlates with the defect concentration according to Vegard law [46,47] (Figure 2d), but the slope of this linear dependence is essentially different for the air-annealed and oxygen-annealed samples (V = p + qgNi, V − unit cell volume (Å3), gNi − Ni fraction in the 3a site (%); p = 101.60(1), q = 0.112(3), R2 = 0.999 for the air-annealed samples; p = 99.9(2), q = 0.62(8), R2 = 0.969 for the oxygen-annealed samples).
In order to perform a more local analysis of Ni2+/Li+ disorder, the crystal structure of all NMC811 samples was examined via the EDT technique. Additionally, single-crystal EDT experiments are not dependent on preferred orientation, in contrast to the PXRD data. For this, 3D-ED sets were acquired from three single crystals for each sample to provide relevant statistics. The crystal structure refinement from the 3D-ED data was performed in a similar scheme as described for PXRD data treatment. The refinement parameters and parameters of the Ni2+/Li+ mixing at the cationic sites are listed in Table S1. The structure refinement from the EDT data revealed reliability factors RF within the range of 14–32% (Table S1). The higher RF for the EDT data compared to PXRD ones are known to originate from the unavoidable dynamical scattering effects. As a result, electron diffraction conditions in the 3D-ED deviate from kinematic approximation, commonly applied while treating the diffraction data, even for 100–200 nm crystallites from which the 3D-ED data were collected. Nevertheless, the obtained reliability factors are generally considered credible for this method [36,37].
The obtained results from EDT data demonstrate the absence of Li+ at the 3b sites for air-annealed samples, thus reaffirming the results of Rietveld refinement. For all three air-annealed samples, gLi at 3b sites turned out to have zero value within one standard deviation. Regarding the oxygen-annealed materials, for S3ox sample gLi at the 3b sites estimated with 3D-ED is very similar to that refined from PXRD data within the statistical error, while for S1ox and S2ox samples 3D-ED pointed to slightly smaller Li+ concentration at the 3b site than those obtained from Rietveld refinement. However, for all oxygen-annealed materials gLi at 3b sites is statistically significant, which means that switching the annealing atmosphere from air to oxygen changes the defect formation mechanism. The comparison of the Ni fraction in the Li site determined with the Rietveld refinement from PXRD data and from EDT demonstrates a solid correlation (Figure 3) that renders the EDT technique a reliable method for the determination of cationic point defects in the crystal structure of layered oxides. The most important issue is the validity of kinematical structure refinement from EDT data due to the strong dynamic scattering effects, which can be partially mitigated by selecting sufficiently thin crystals for data collection but could also be more rigorously addressed in the dynamical effects are reduced of fully taken into account [48,49,50,51]. Although being not superior compared to the Rietveld refinement (at least within kinematical approximation) and intrinsically lower in representativity, EDT might become indispensable if the Ni/Li disorder should be determined in the electrochemically-treated electrodes which often demonstrate poor PXRD patterns deteriorated by amorphous additives, current collector and multiphase nature.
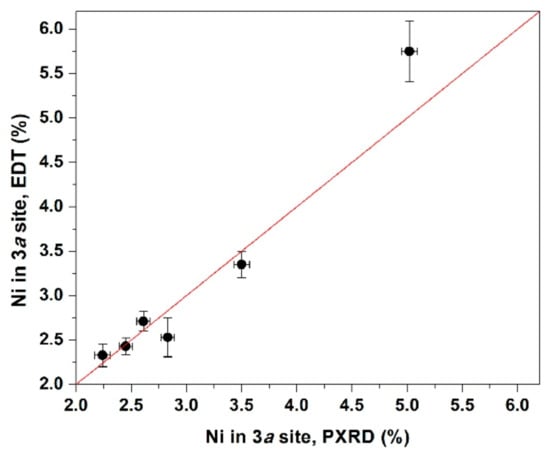
Figure 3.
Correlation of the Ni fraction at the Li site as refined from PXRD and EDT data. The bars indicate standard deviations obtained in each method.
Although both PXRD and EDT provide a reliable characterization of the cation disorder, they are not capable of delivering local information, such as defect concentration at the surface and in the bulk of the NMC crystallites. Atomic-resolution HAADF-STEM imaging has been frequently used for assessing local Ni2+/Li+ disorder, but only in a qualitative manner. The [010] and [10] HAADF-STEM images, in which the Li and TM slabs can be easily distinguished, were interpreted visually, sometimes supporting the conclusions by plotting the intensity profiles. The local structure of the disordered regions was analyzed by estimation of the brightness of the dots, associated with Ni ions migrated to Li slabs, and followed by tentative assignment of the local areas to layered, spinel, or rock-salt structure types [15,27,28,29,30,31]. The development of state-of-the-art techniques, aimed at the quantitative analysis of high-resolution STEM images, based on the application of statistical parameter estimation theory [33,52,53,54], makes it possible to directly quantify the number of atoms in each projected atomic column in the HAADF-STEM image. Until recently, the HAADF-STEM image processing was applied predominantly to the metallic nanoparticles [55], while within the present work we attempted to apply such an approach to cation disordering in Ni-rich NMCs.
Several assumptions were made to apply the statistical parameter estimation theory. First, the scattering power of Ni, Mn and Co was considered equal as their atomic numbers are close to each other, meaning that the TMs were considered as one type of atom. Second, due to the strong dependence of HAADF signal on average atomic number Z along the atomic column (≈Zn, where 1.4 ≤ n ≤ 2) [56], the Li-ions impact on the scattering cross-sections can be considered as negligible. Thus, all the atomic columns in the HAADF-STEM image of Ni-rich NMCs virtually consist of TM ions exclusively and only TM cations were counted while performing calculations. Oxygen atoms weakly contribute to the HAADF-STEM images, but they do not mix with the cationic columns if the structure is viewed along [010]/[100] direction of the Rm structure selected for imaging. This direction also provides well-separated images of the atomic columns corresponding to the 3a and 3b sites.
For further investigation, the S1air, S1ox and S3ox NMC811 samples were sorted to demonstrate the correlation between the distribution of disordered cation and synthesis conditions. Such choice is attributed to the similar synthesis route for these samples via hydroxide co-precipitation from TM sulfates with the difference in the pH at the first step of the synthesis procedure and annealing atmosphere at the second stage. In addition, the S3air sample, unlike the others prepared by carbonate routine, was selected as a comparative example. The [010] HAADF-STEM images were collected for S1air, S1ox, S3air, and S3ox NMC811 samples (Figure 4), followed by the quantitative analysis of a fraction of the Ni ions, migrated to the Li+ 3a sites via statistical parameter estimation theory, employed into the StatSTEM software [33]. Figure 4b,e,h,k demonstrates the color-coded number of TM atoms in the atomic columns of the 3a and 3b sites. The parameter (N is the number of TM cations in the atomic column of the corresponding site) was retrieved from the atom number map using a dedicated clusterization procedure described in more detail in Supplementary Materials. This parameter is related to the ratio of the TM occupancies at the 3a and 3b sites as . The clusterization color maps, shown in Figure 4c,f,i,l illustrate the spatial distribution of regions with various disordering levels. The amount of disorder in each sample is generally decreasing from the edge to the bulk with the major part located at the surface. It is worth noting that Ni in Li sites is distributed non-uniformly, forming domains rather than a continuous layer with definite thickness, as was stated in earlier works. This observation is quite unexpected and confronts the conjecture on a formation of a “blocking” surface layer that impedes the Li diffusion due to the high Ni fraction in the Li site. In fact, the discontinuous nature of the disordered surface region might create percolated diffusion pathways, thus maintaining high Li permeability. Therefore, the precise structure of the “blocking” layer deserves further in-depth investigations.
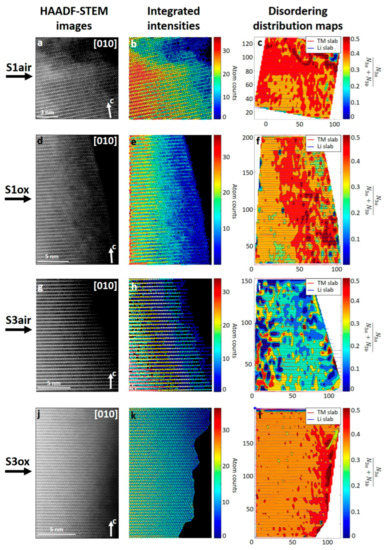
Figure 4.
Quantitative analysis of atomic-resolution HAADF-STEM images of S1air, S1ox, S3air, and S3ox NMC811 samples with statistical parameter estimation theory. For each sample [010] HAADF-STEM images (left column, a,d,g,j), corresponding color-coded number of TM atoms in each atomic column (middle column, b,e,h,k), and the Ni ions cation disordering distribution color map of the regions with various disordering levels in the near-surface region (right column, c,f,i,l) are shown.
Concerning the particular disordering level at different regions, the maps of hydroxide-prepared samples S1air, S1ox and S3ox clearly show that, despite the relative closeness of the disordering level, their distribution varies significantly from the S1air sample, where defects are propagating towards the bulk of the crystal, to the S3ox, where defects are segregated at the 20–25 Å surface layer. Meanwhile, similar maps for the carbonate-prepared S3air sample demonstrate the uniform distribution of distinct disordered regions in both surface and bulk, as opposed to the hydroxide-prepared ones with a gradual changing of disordering level. Thus, the quantitative analysis demonstrates that even the slightest change in synthesis conditions leads to the significant redistribution of the cation disordering.
Indeed, the proposed method opens doors for the proper monitoring of the disordered cation concentration and their localization at the atomic scale, which could help to shed light on the impact of the cation interslab exchange on electrochemical properties of cathode materials. However, like every method, quantitative HAADF-STEM imaging also possesses certain limitations and should be applied carefully in order not to avoid artifacts and overinterpretations. Foremost, the HAADF-STEM technique requires a relatively high electron dose to maintain a suitable signal-to-noise ratio, but prolonged beam exposure can lead to the formation of beam-induced cationic point defects formation in layered NMCs cathode materials. These defects are similar to those formed during synthesis and/or battery operation that may result in misleading image interpretation [57,58]. The last but not the least problem of the quantitative HAADF-STEM imaging is representativity, which is here even more acute than for the EDT method since no more than a few dozens of nm2 space can be scanned in a single image. The depth of field of the electron probe, limited to ≈100–150 nm, and strong -beam interaction with the matter also dictates the possibility of studying only the near-surface area of the crystallite, while its deeper inner part remains virtually inaccessible. This disadvantage, however, can be mitigated by the preparation of uniformly thin slices with a focused ion beam (FIB) technique, but mobility and migration probability of the Ni and Li cations under high energy ion beam must be perfectly understood first.
4. Conclusions
The comprehensive quantitative and semi-quantitative analysis of Ni2+/Li+ cation disordering in Ni-rich NMC (LiNi0.8Mn0.1Co0.1O2) samples were performed by PXRD, 3D-ED (EDT), and quantitative HAADF-STEM on various spatial scales. Rietveld refinement from PXRD data provides primary information on the cation disorder schemes, which strongly depend on the synthesis conditions. Estimation of defect concentrations in a semi-quantitative manner is also possible using the I003/I104 integral intensity ratio but only after correction for preferred orientation, whereas the c/(2√6a) ratio of pseudocubic subcell parameters does not quantitatively correlate with the cation disorder and must be used with extreme care. EDT appears to measure the cation disorder reliably correlating well with the Rietveld refinement results while delivering the information about defects distribution on the level of separate crystallites. The work highlights a new computational approach for the cation disordering study, based on quantitative analysis of high-resolution HAADF-STEM images, which provides an unprecedented opportunity to identify and distinguish regions with various Ni2+/Li+ disorder levels. The proposed technique could potentially serve as an efficient tool for the investigation of Ni2+/Li+ defect structure at the atomic scale, providing detailed information about its formation, localization and evolution during electrochemical cycling.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/sym13091628/s1, Figure S1–S6: Experimental, calculated and difference PXRD profiles after the Rietveld refinement of the NMC811 structure in the S1air, S1ox, S2air, S2ox, S3air, S3ox samples. Table S1: Results of the 3D ED study of Ni2+/Li+ anti-site defects. Table S1. Results of the 3D-ED study of Ni2+/Li+ anti-site defects. Clusterization procedure description.
Author Contributions
Conceptualization, A.M.A.; methodology, A.A.S., E.D.O., S.A.A. and A.M.A.; validation, E.D.O. and A.A.S.; formal analysis, A.A.S. and E.D.O.; investigation, A.M.A., A.A.S., E.D.O. and A.V.M.; writing—original draft preparation, E.D.O.; writing—review and editing, A.M.A. and A.A.S.; supervision, A.M.A.; funding acquisition, A.M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation, grant 20-13-00233.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Access to the Transmission Electron Microscopy (TEM) facilities was granted by the Advanced Imaging Core Facility (AICF) of Skoltech.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Whittingham, M.S. Ultimate limits to intercalation reactions for lithium batteries. Chem. Rev. 2014, 114, 11414–11443. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for rechargeable Li batteries. Chem. Mater. 2009, 22, 587–603. [Google Scholar] [CrossRef]
- Manthiram, A.; Knight, J.C.; Myung, S.-T.; Oh, S.-M.; Sun, Y.-K. Nickel-rich and lithium-rich layered oxide cathodes: Progress and perspectives. Adv. Energy Mater. 2015, 6. [Google Scholar] [CrossRef]
- Delmas, C.; Fouassier, C.; Hagenmuller, P. Structural classification and properties of the layered oxides. Physica 1980, 99, 81–85. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A Cryst. Phys. Diffr. Theor. Gen. Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Gao, A.; Sun, Y.; Zhang, Q.; Zheng, J.; Lu, X. Evolution of Ni/Li antisites under the phase transition of a layered LiNi1/3Co1/3Mn1/3O2 cathode. J. Mater. Chem. A 2020, 8, 6337–6348. [Google Scholar] [CrossRef]
- Zheng, J.; Ye, Y.; Liu, T.; Xiao, Y.; Wang, C.; Wang, F.; Pan, F. Ni/Li disordering in layered transition metal oxide: Electrochemical impact, origin, and control. Acc. Chem. Res. 2019, 52, 2201–2209. [Google Scholar] [CrossRef]
- Xu, J.; Lin, F.; Doeff, M.; Tong, W. A review of Ni-based layered oxides for rechargeable Li-ion batteries. J. Mater. Chem. A 2016, 5, 874–901. [Google Scholar] [CrossRef] [Green Version]
- Kang, K.; Meng, Y.S.; Bréger, J.; Grey, C.P.; Ceder, G. Electrodes with high power and high capacity for rechargeable lithium batteries. Science 2006, 311, 977–980. [Google Scholar] [CrossRef]
- Yu, H.; Qian, Y.; Otani, M.; Tang, D.; Guo, S.; Zhu, Y.; Zhou, H. Study of the lithium/nickel ions exchange in the layered LiNi0.42Mn0.42Co0.16O2 cathode material for lithium ion batteries: Experimental and first-principles calculations. Energy Environ. Sci. 2014, 7, 1068–1078. [Google Scholar] [CrossRef]
- Zhang, S.S. Problems and their origins of Ni-rich layered oxide cathode materials. Energy Storage Mater. 2019, 24, 247–254. [Google Scholar] [CrossRef]
- Bianchini, M.; Roca-Ayats, M.; Hartmann, P.; Brezesinski, T.; Janek, J. There and back again—The journey of LiNiO 2 as a cathode active material. Angew. Chem. Int. Ed. 2018, 58, 10434–10458. [Google Scholar] [CrossRef]
- Yan, P.; Nie, A.; Zheng, J.; Zhou, Y.; Lu, D.; Zhang, X.; Xu, R.; Belharouak, I.; Zu, X.; Xiao, J.; et al. Evolution of lattice structure and chemical composition of the surface reconstruction layer in Li1.2Ni0.2Mn0.6O2 cathode material for lithium ion batteries. Nano Lett. 2014, 15, 514–522. [Google Scholar] [CrossRef]
- Yan, P.; Zheng, J.; Lv, D.; Wei, Y.; Zheng, J.; Wang, Z.; Kuppan, S.; Yu, J.; Luo, L.; Edwards, D.; et al. Atomic-resolution visualization of distinctive chemical mixing behavior of Ni, Co, and Mn with Li in layered lithium transition-metal oxide cathode materials. Chem. Mater. 2015, 27, 5393–5401. [Google Scholar] [CrossRef]
- Lin, F.; Markus, I.M.; Nordlund, D.; Weng, T.-C.; Asta, M.D.; Xin, H.; Doeff, M. Surface reconstruction and chemical evolution of stoichiometric layered cathode materials for lithium-ion batteries. Nat. Commun. 2014, 5, 3529. [Google Scholar] [CrossRef]
- Li, T.; Yuan, X.-Z.; Zhang, L.; Song, D.; Shi, K.; Bock, C. Degradation mechanisms and mitigation strategies of nickel-rich NMC-based lithium-ion batteries. Electrochem. Energy Rev. 2019, 3, 43–80. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Oh, P.; Liu, X.; Lee, M.-J.; Cho, W.; Chae, S.; Kim, Y.; Cho, J. Nickel-rich layered lithium transition-metal oxide for high-energy lithium-ion batteries. Angew. Chem. Int. Ed. 2015, 54, 4440–4457. [Google Scholar] [CrossRef]
- Zheng, J.X.; Liu, T.C.; Hu, Z.X.; Wei, Y.; Song, X.H.; Ren, Y.; Wang, W.D.; Rao, M.M.; Lin, Y.; Chen, Z.H.; et al. Tuning of thermal stability in layered Li(NixMnyCoz)O2. J. Am. Chem. Soc. 2016, 138, 13326–13334. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Ceder, G.; Grey, C.; Yoon, W.-S.; Jiang, M.; Breger, J.; Shao-Horn, Y. Cation ordering in layered O3 Li[NixLi1/3−2 x/3Mn2/3-x/3]O2 (0 ⩽ x⩽ 1/2) compounds. Chem. Mater. 2005, 17, 2386–2394. [Google Scholar] [CrossRef]
- Sun, G.; Yin, X.; Yang, W.; Song, A.; Jia, C.; Yang, W.; Du, Q.; Ma, Z.; Shao, G. The effect of cation mixing controlled by thermal treatment duration on the electrochemical stability of lithium transition-metal oxides. Phys. Chem. Chem. Phys. 2017, 19, 29886–29894. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Liang, C.; Wang, L.; Zheng, Y.; Perananthan, S.; Longo, R.C.; Ferraris, J.P.; Kim, M.; Cho, K. Kinetic stability of bulk LiNiO2 and surface degradation by oxygen evolution in LiNiO2 based cathode materials. Adv. Energy Mater. 2018, 9. [Google Scholar] [CrossRef]
- Tian, C.; Nordlund, D.; Xin, H.; Xu, Y.; Liu, Y.; Sokaras, D.; Lin, F.; Doeff, M.M. Depth-dependent redox behavior of LiNi0.6Mn0.2Co0.2O2. J. Electrochem. Soc. 2018, 165, A696–A704. [Google Scholar] [CrossRef] [Green Version]
- Zou, L.; Zhao, W.; Liu, Z.; Jia, H.; Zheng, J.; Wang, G.; Yang, Y.; Zhang, J.-G.; Wang, C. Revealing cycling rate-dependent structure evolution in ni-rich layered cathode materials. ACS Energy Lett. 2018, 3, 2433–2440. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, T.; Liu, J.; He, L.; Chen, J.; Zhang, J.; Luo, P.; Lu, H.; Wang, R.; Zhu, W.; et al. Insight into the origin of lithium/nickel ions exchange in layered Li(NixMnyCoz)O2 cathode materials. Nano Energy 2018, 49, 77–85. [Google Scholar] [CrossRef] [Green Version]
- Yin, L.; Mattei, G.S.; Li, Z.; Zheng, J.; Zhao, W.; Omenya, F.; Fang, C.; Li, W.; Li, J.; Xie, Q.; et al. Extending the limits of powder diffraction analysis: Diffraction parameter space, occupancy defects, and atomic form factors. Rev. Sci. Instrum. 2018, 89, 093002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, L.; Li, Z.; Mattei, G.S.; Zheng, J.; Zhao, W.; Omenya, F.; Fang, C.; Li, W.; Li, J.; Xie, Q.; et al. Thermodynamics of antisite defects in layered NMC cathodes: Systematic insights from high-precision powder diffraction analyses. Chem. Mater. 2019, 32, 1002–1010. [Google Scholar] [CrossRef] [Green Version]
- Hwang, S.; Su, D. TEM studies on electrode materials for secondary ion batteries. Inorg. Battery Mater. 2019, 311, 1–27. [Google Scholar] [CrossRef]
- Zhu, J.; Sharifi-Asl, S.; Garcia, J.C.; Iddir, H.H.; Croy, J.R.; Shahbazian-Yassar, R.; Chen, G. Atomic-level understanding of surface reconstruction based on Li[NixMnyCo1−x−y]O2 single-crystal studies. ACS Appl. Energy Mater. 2020, 3, 4799–4811. [Google Scholar] [CrossRef]
- Kim, U.-H.; Park, G.-T.; Conlin, P.; Ashburn, N.; Cho, K.; Yu, Y.-S.; Shapiro, D.A.; Maglia, F.; Kim, S.-J.; Lamp, P.; et al. Cation ordered Ni-rich layered cathode for ultra-long battery life. Energy Environ. Sci. 2021, 14, 1573–1583. [Google Scholar] [CrossRef]
- Jung, S.-K.; Gwon, H.; Hong, J.; Park, K.-Y.; Seo, D.-H.; Kim, H.; Hyun, J.; Yang, W.; Kang, K. Understanding the degradation mechanisms of LiNi0.5 Co0.2 Mn0.3 O2 cathode material in lithium ion batteries. Adv. Energy Mater. 2013, 4. [Google Scholar] [CrossRef]
- Schweidler, S.; De Biasi, L.; Garcia, G.; Mazilkin, A.; Hartmann, P.; Brezesinski, T.; Janek, J. Investigation into mechanical degradation and fatigue of high-Ni NCM cathode material: A long-term cycling study of full cells. ACS Appl. Energy Mater. 2019, 2, 7375–7384. [Google Scholar] [CrossRef]
- Van Aert, S.; De Backer, A.; Martinez, G.T.; Dekker, A.D.; Van Dyck, D.; Bals, S.; Van Tendeloo, G. Advanced electron crystallography through model-based imaging. IUCrJ 2016, 3, 71–83. [Google Scholar] [CrossRef] [Green Version]
- De Backer, A.; Bos, K.H.W.V.D.; Broek, W.V.D.; Sijbers, J.; Van Aert, S. StatSTEM: An efficient approach for accurate and precise model-based quantification of atomic resolution electron microscopy images. Ultramicroscopy 2016, 171, 104–116. [Google Scholar] [CrossRef]
- Gemmi, M.; Mugnaioli, E.; Gorelik, T.E.; Kolb, U.; Palatinus, L.; Boullay, P.; Hovmöller, S.; Abrahams, J.P. 3D electron diffraction: The nanocrystallography revolution. ACS Cent. Sci. 2019, 5, 1315–1329. [Google Scholar] [CrossRef] [Green Version]
- Vainshtein, B.K.; Zvyagin, B.B.; Avilov, A.S. Diffraction Structure Analysis in Electron—Diffraction Techniques; Cowley, J.M., Ed.; Oxford University Press: Oxford, UK, 1992; pp. 216–312. [Google Scholar]
- Hadermann, J.; Abakumov, A.M. Structure solution and refinement of metal-ion battery cathode materials using electron diffraction tomography. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2019, 75, 485–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karakulina, O.M.; Khasanova, N.R.; Drozhzhin, O.A.; Tsirlin, A.A.; Hadermann, J.; Antipov, E.V.; Abakumov, A.M. Antisite disorder and bond valence compensation in Li2FePO4F cathode for Li-Ion batteries. Chem. Mater. 2016, 28, 7578–7581. [Google Scholar] [CrossRef]
- Mugnaioli, E. Closing the gap between electron and X-ray crystallography. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2015, 71, 737–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petříček, V.; Dušek, M.; Palatinus, L. Crystallographic computing system JANA2006: General features. Z. Krist. Cryst. Mater. 2014, 229, 345–352. [Google Scholar] [CrossRef]
- Palatinus, L.; Brázda, P.; Jelínek, M.; Hrda, J.; Steciuk, G.; Klementova, M. Specifics of the data processing of precession electron diffraction tomography data and their implementation in the program PETS2.0. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2019, 75, 512–522. [Google Scholar] [CrossRef]
- Nam, K.-W.; Bak, S.-M.; Hu, E.; Yu, X.; Zhou, Y.; Wang, X.; Wu, L.; Zhu, Y.; Chung, K.Y.; Yang, X.-Q. Combining in situ synchrotron X-ray diffraction and absorption techniques with transmission electron microscopy to study the origin of thermal instability in overcharged cathode materials for Lithium-Ion batteries. Adv. Funct. Mater. 2012, 23, 1047–1063. [Google Scholar] [CrossRef]
- Morales, J.; Vicente, C.P.; Tirado, J.L. Cation distribution and chemical deintercalation of Li1-xNi1+xO2. Mater. Res. Bull. 1990, 25, 623–630. [Google Scholar] [CrossRef]
- Ohzuku, T.; Ueda, A.; Nagayama, M. Electrochemistry and structural chemistry of LiNiO2 (R3m) for 4 volt secondary lithium Cells. J. Electrochem. Soc. 1993, 140, 1862–1870. [Google Scholar] [CrossRef]
- Hua, W.; Zhang, J.; Zheng, Z.; Liu, W.; Peng, X.; Guo, X.-D.; Zhong, B.; Wang, Y.-J.; Wang, X. Na-doped Ni-rich LiNi0.5Co0.2Mn0.3O2 cathode material with both high rate capability and high tap density for lithium ion batteries. Dalton Trans. 2014, 43, 14824–14832. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, W.; Mauger, A.; Qilu, A.; Gendron, F.; Julien, C. Minimization of the cation mixing in Li1+x(NMC)1−xO2 as cathode material. J. Power Sources 2010, 195, 1292–1301. [Google Scholar] [CrossRef]
- Vegard, L. Die konstitution der mischkristalle und die raumfüllung der atome. Z. Phys. 1921, 5, 17–26. [Google Scholar] [CrossRef]
- Denton, A.R.; Ashcroft, N.W. Vegard’s law. Phys. Rev. A 1991, 43, 3161–3164. [Google Scholar] [CrossRef] [PubMed]
- Mugnaioli, E.; Gemmi, M. Single-crystal analysis of nanodomains by electron diffraction tomography: Mineralogy at the order-disorder borderline. Z. Kristallogr. Cryst. Mater. 2018, 233, 163–178. [Google Scholar] [CrossRef]
- Palatinus, L.; Jacob, D.; Cuvillier, P.; Klementova, M.; Sinkler, W.; Marks, L.D. Structure refinement from precession electron diffraction data. Acta Crystallogr. Sect. A Found. Crystallogr. 2013, 69, 171–188. [Google Scholar] [CrossRef]
- Palatinus, L.; Petříček, V.; Corrêa, C.A. Structure refinement using precession electron diffraction tomography and dynamical diffraction: Theory and implementation. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 235–244. [Google Scholar] [CrossRef]
- Palatinus, L.; Corrêa, C.A.; Steciuk, G.; Jacob, D.; Roussel, P.; Boullay, P.; Klementová, M.; Gemmi, M.; Kopeček, J.; Domeneghetti, M.C.; et al. Structure refinement using precession electron diffraction tomography and dynamical diffraction: Tests on experimental data. Acta Cryst. B 2015, 71, 740–751. [Google Scholar] [CrossRef]
- LeBeau, J.M.; Findlay, S.D.; Allen, L.J.; Stemmer, S. Standardless atom counting in scanning transmission electron microscopy. Nano Lett. 2010, 10, 4405–4408. [Google Scholar] [CrossRef] [PubMed]
- Van Aert, S.; Batenburg, K.J.; Rossell, M.D.; Erni, R.; Van Tendeloo, G. Three-dimensional atomic imaging of crystalline nanoparticles. Nature 2011, 470, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Bals, S.; Van Aert, S.; Van Tendeloo, G.; Brande, D.A. Statistical estimation of atomic positions from exit wave reconstruction with a precision in the picometer range. Phys. Rev. Lett. 2006, 96, 096106. [Google Scholar] [CrossRef] [Green Version]
- De Backer, A.; Jones, L.; Lobato, I.; Altantzis, T.; Goris, B.; Nellist, P.D.; Bals, S.; Van Aert, S. Three-dimensional atomic models from a single projection using Z-contrast imaging: Verification by electron tomography and opportunities. Nanoscale 2017, 9, 8791–8798. [Google Scholar] [CrossRef] [PubMed]
- Guzzinati, G.; Altantzis, T.; Batuk, M.; De Backer, A.; Lumbeeck, G.; Samaee, V.; Batuk, D.; Idrissi, H.; Hadermann, J.; Van Aert, S.; et al. Recent advances in transmission electron microscopy for materials science at the EMAT lab of the University of Antwerp. Materials 2018, 11, 1304. [Google Scholar] [CrossRef] [Green Version]
- Lin, F.; Markus, I.M.; Doeff, M.; Xin, H.L. Chemical and structural stability of lithium-ion battery electrode materials under electron beam. Sci. Rep. 2014, 4, 5694. [Google Scholar] [CrossRef] [Green Version]
- Hobbs, L.W. Electron-beam sensitivity in inorganic specimens. Ultramicroscopy 1987, 23, 339–344. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).