Get a Grip: Variation in Human Hand Grip Strength and Implications for Human Evolution
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample
2.2. Data Collection Procedure
2.2.1. Questionnaire
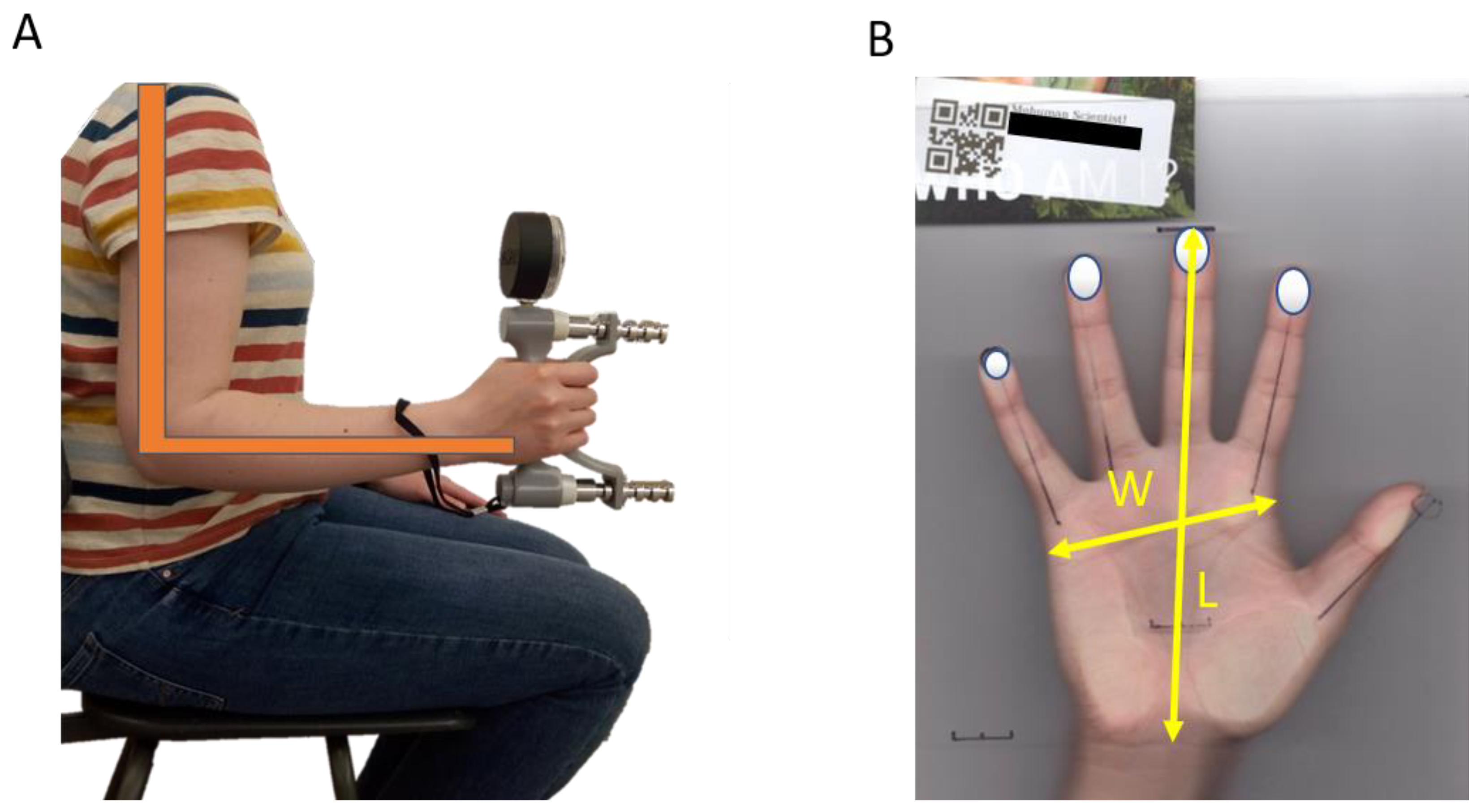
2.2.2. Grip Strength
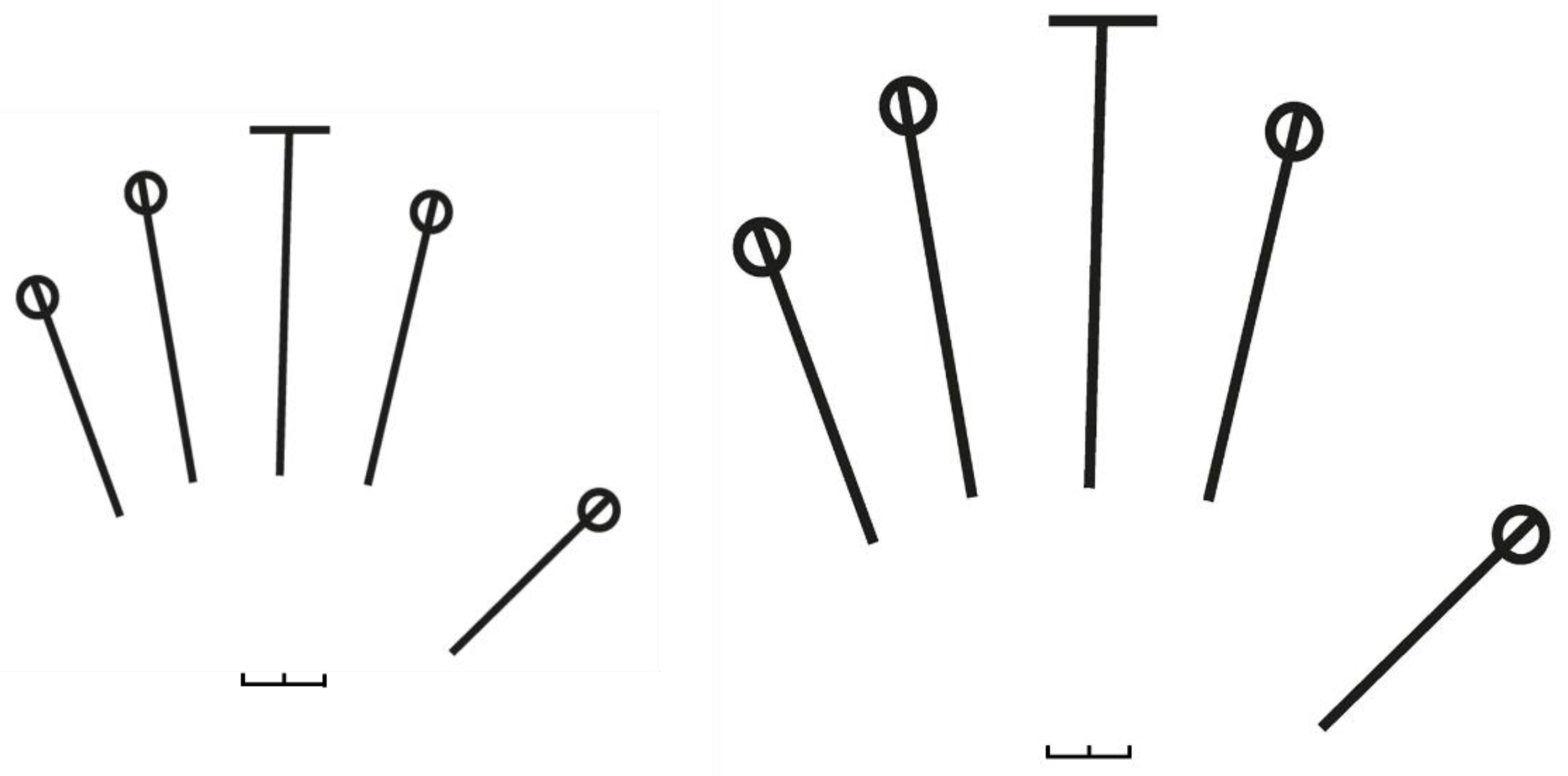
2.2.3. Hand Size and Shape
2.3. Statistical Analyses
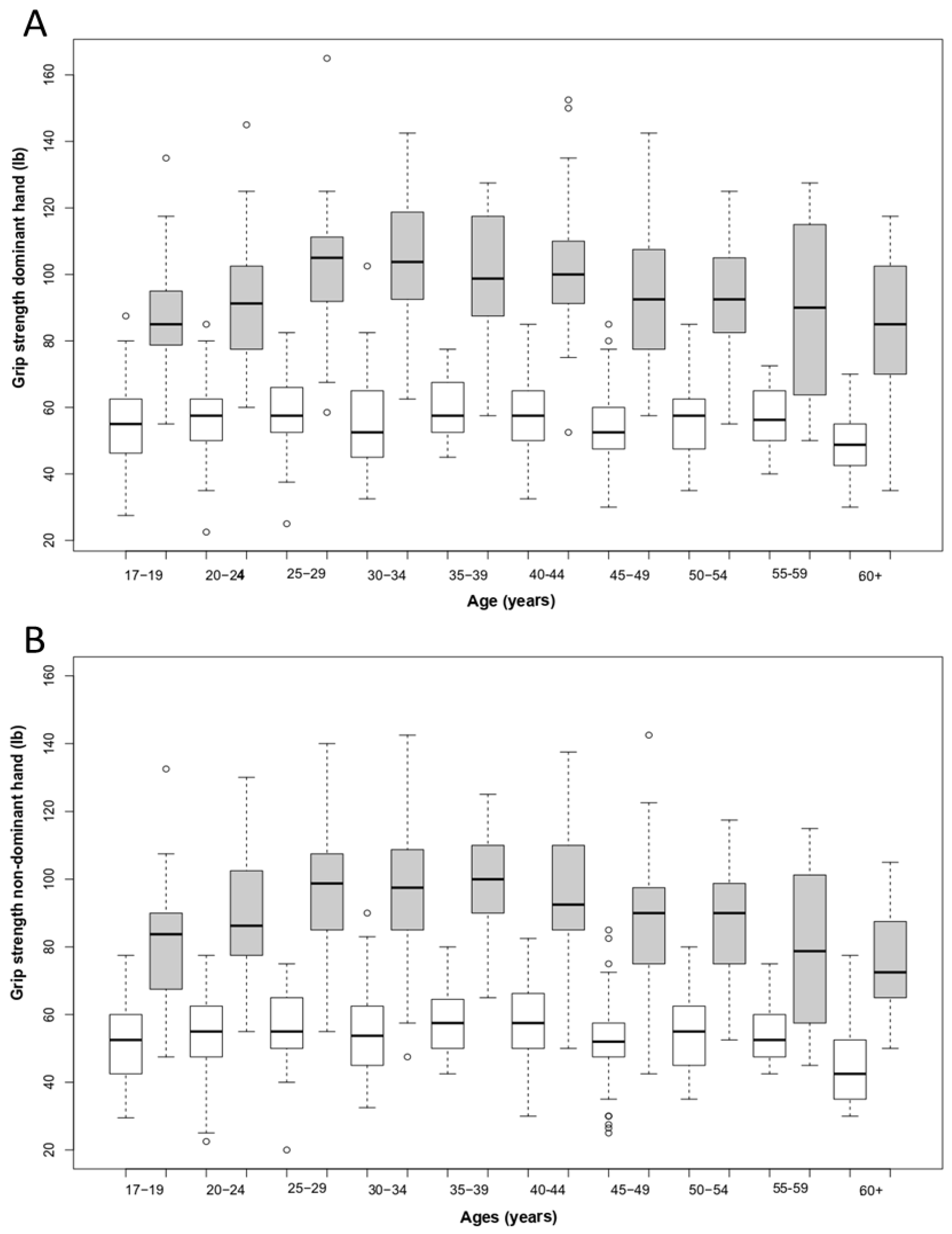
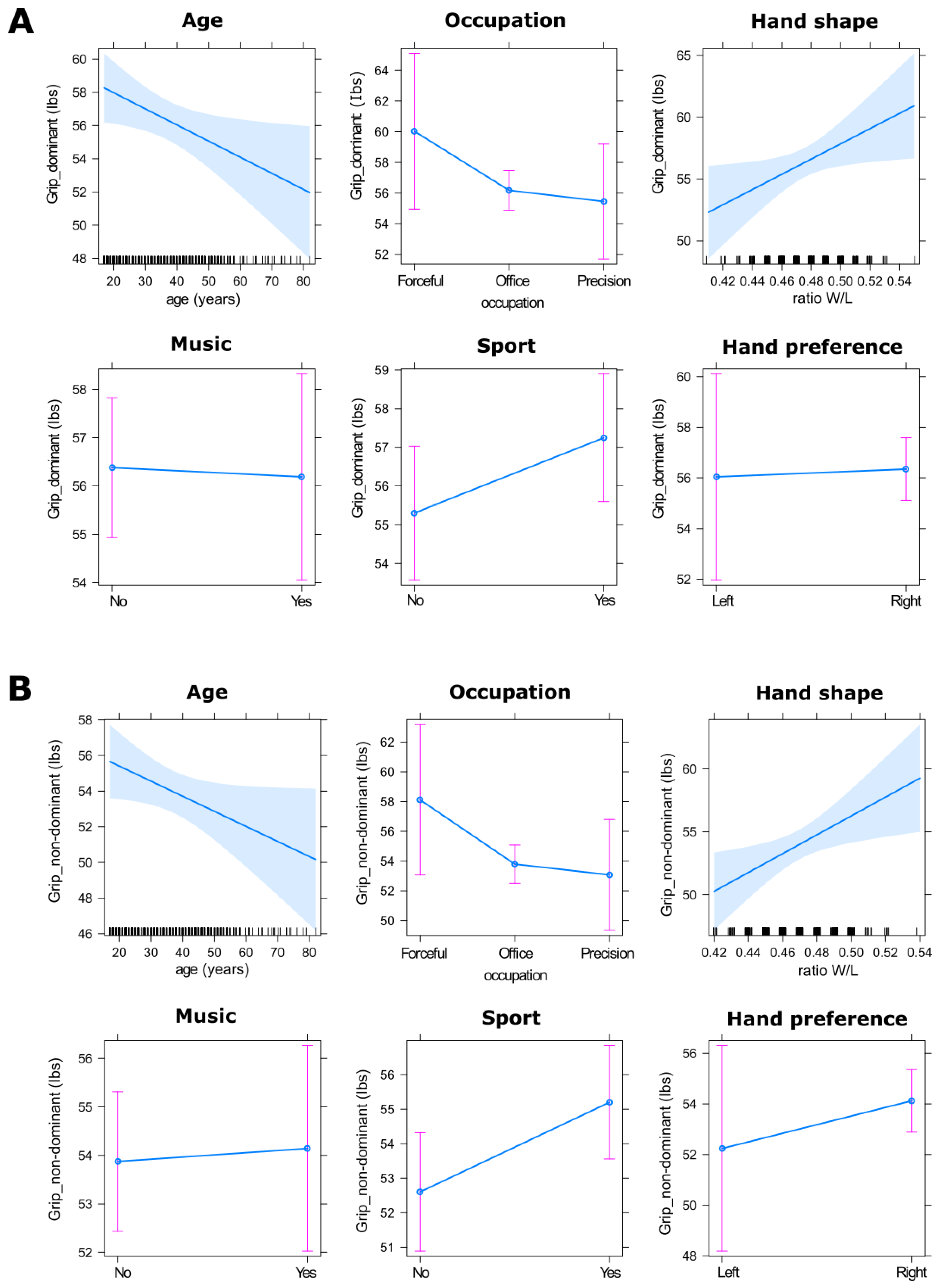
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Marzke, M.W.; Wullstein, K.L.; Viegas, S.F. Evolution of the power (squeeze) grip and its morphological correlates in hominids. Am. J. Phys. Anthropol. 1992, 89, 283–298. [Google Scholar] [CrossRef]
- Marzke, M.W. Tool making, hand morphology and fossil hominins. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2013, 368, 20120414. [Google Scholar] [CrossRef]
- Toth, N.; Schick, K. Early stone industries and inferences regarding language and cognition. In Tools, Language and Cognition in Human Evolution; Gibson, K.R., Ingold, T., Eds.; Cambridge University Press: Cambridge, UK, 1993; pp. 346–362. [Google Scholar]
- McPherron, S.P.; Alemseged, Z.; Marean, C.W.; Wynn, J.G.; Reed, D.; Geraads, D.; Bobe, R.; Béarat, H.A. Evidence for stone-tool-assisted consumption of animal tissues before 3.39 million years ago at Dikika, Ethiopia. Nature 2010, 466, 857–860. [Google Scholar] [CrossRef] [PubMed]
- Toth, N.; Schick, K. The Oldowan: The tool making of early hominins and chimpanzees compared. Annu. Rev. Anthropol. 2009, 38, 289–305. [Google Scholar] [CrossRef]
- Braun, D.R.; Aldeias, V.; Archer, W.; Arrowsmith, J.R.; Baraki, N.; Campisano, C.J.; Deino, A.L.; DiMaggio, E.N.; Dupont-Nivet, G.; Engda, B.; et al. Earliest known Oldowan artifacts at> 2.58 Ma from Ledi-Geraru, Ethiopia, highlight early technological diversity. Proc. Natl. Acad. Sci. USA 2019, 116, 11712–11717. [Google Scholar] [CrossRef]
- Stout, D.; Toth, N.; Schick, K.; Chaminade, T. Neural correlates of Early Stone Age toolmaking: Technology, language and cognition in human evolution. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008, 363, 1939–1949. [Google Scholar] [CrossRef]
- Toth, N.; Schick, K. An overview of the cognitive implications of the Oldowan Industrial Complex. Azania Archaeol. Res. Afr. 2018, 53, 3–39. [Google Scholar] [CrossRef]
- Williams, E.M.S.; Gordon, A.D.; Richmond, B.G. Upper limb kinematics and the role of the wrist during stone tool production. Am. J. Phys. Anthropol. 2010, 43, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.M.S.; Gordon, A.D.; Richmond, B.G. Hand pressure distribution during Oldowan stone tool production. J. Hum. Evol. 2012, 62, 520–532. [Google Scholar] [CrossRef]
- Williams-Hatala, E.M.; Hatala, K.G.; Gordon, M.; Key, A.; Kasper, M.; Kivell, T.L. The manual pressures of stone tool behaviors and their implications for the evolution of the human hand. J. Hum. Evol. 2018, 119, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Stout, D.; Hecht, E.E. Evolutionary neuroscience of cumulative culture. Proc. Natl. Acad. Sci. USA 2017, 114, 7861–7868. [Google Scholar] [CrossRef] [PubMed]
- Feix, T.; Romero, J.; Schmiedmayer, H.B.; Dollar, A.M.; Kragic, D. The grasp taxonomy of human grasp types. IEEE Trans. Hum. Mach. Syst. 2015, 46, 66–77. [Google Scholar] [CrossRef]
- Venkataraman, V.V.; Kraft, T.S.; Dominy, N.J. Tree climbing and human evolution. Proc. Natl. Acad. Sci. USA 2013, 110, 1237–1242. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, B.; Kukla, M.; Warguła, Ł. The symmetric nature of the position distribution of the human body center of gravity during propelling manual wheelchairs with innovative propulsion systems. Symmetry 2021, 13, 154. [Google Scholar] [CrossRef]
- Annett, M. Left, Right, Hand and Brain: The Right Shift Theory; LEA Publishers: London, UK, 1985; 416p. [Google Scholar]
- Porac, C.; Coren, S. Lateral Preferences and Human Behavior; Springer: New York, NY, USA, 1981; 283p. [Google Scholar]
- McManus, I.C. The history and geography of human handedness. In Language Lateralization and Psychosis; Sommer, I.E.C., Kahn, R.S., Eds.; Cambridge University Press: Cambridge, UK, 2009; pp. 37–57. [Google Scholar]
- Llaurens, V.; Raymond, M.; Faurie, C. Why are some people left-handed? An evolutionary perspective. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 881–894. [Google Scholar] [CrossRef] [PubMed]
- Forrester, G.S.; Quaresmini, C.; Leavens, D.A.; Mareschal, D.; Thomas, M.S.C. Human handedness: An inherited evolutionary trait. Behav. Brain Res. 2013, 237, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Forrester, G.S. Hand, limb and other motor preferences: Methodological Considerations. In Lateralized Brain Functions; Vallortigara, G., Rogers, L., Eds.; Humana Press: New York, NY, USA, 2017; pp. 121–152. [Google Scholar]
- Hopkins, W.D.; Phillips, K.A.; Bania, A.; Calcutt, S.E.; Gardner, M.; Russell, J.; Schaeffer, J.; Lonsdorf, E.V.; Ross, S.R.; Schapiro, S.J. Hand preferences for coordinated bimanual actions in 777 great apes: Implications for the evolution of handedness in hominins. J. Hum. Evol. 2011, 60, 605–611. [Google Scholar] [CrossRef]
- Papademetriou, E.; Sheu, C.F.; Michel, G.F. A meta-analysis of primate hand preferences, particularly for reaching. J. Comp. Psychol. 2005, 119, 33–48. [Google Scholar] [CrossRef]
- Bardo, A.; Pouydebat, E.; Meunier, H. Do bimanual coordination, tool use, and body posture contribute equally to hand preferences in bonobos? J. Hum. Evol. 2015, 82, 159–169. [Google Scholar] [CrossRef]
- Hopkins, W.D.; Russell, J.L.; Cantalupo, C. Neuroanatomical correlates of handedness for tool use in chimpanzees (Pan troglodytes): Implication for theories on the evolution of language. Psychol. Sci. 2007, 18, 971–977. [Google Scholar] [CrossRef]
- Corballis, M.C. Cerebral asymmetry: Motoring on. Trends Cogn. Sci. 1998, 2, 152–157. [Google Scholar] [CrossRef]
- Gibson, K.R.; Ingold, T. Tools, Language and Cognition in Human Evolution; Cambridge University Press: Cambridge, UK, 1993; 496p. [Google Scholar]
- Uomini, N.T. The prehistory of handedness: Archaeological data and comparative ethology. J. Hum. Evol. 2009, 57, 411–419. [Google Scholar] [CrossRef]
- Corballis, M.C. From mouth to hand: Gesture, speech and the evolution of right- handedness. Behav. Brain Sci. 2003, 26, 199–260. [Google Scholar] [CrossRef]
- Meguerditchian, A.; Vauclair, J.; Hopkins, W.D. On the origins of human handedness and language: A comparative review of hand preferences for bimanual coordinated actions and gestural communication in nonhuman primates. Dev. Psychobiol. 2013, 55, 637–650. [Google Scholar] [CrossRef]
- Calvin, W.H. A stone’s throw and its launch window: Timing precision and its implication for language and hominids brain. J. Theor. Biol. 1983, 104, 121–135. [Google Scholar] [CrossRef]
- Greenfield, P.M. Language, tools and brain: The ontogeny and phylogeny of hierarchically organized sequential behavior. Behav. Brain Sci. 1991, 14, 531–595. [Google Scholar] [CrossRef]
- Bradshaw, J.L.; Nettleton, N.C. Language lateralization to the dominant hemisphere: Tool use, gesture and language in hominid evolution. Curr. Psychol. 1982, 2, 171–192. [Google Scholar] [CrossRef]
- Armstrong, D.F.; Stokoe, W.C.; Wilcox, S.E. Gesture and the Nature of Language; Cambridge University Press: Cambridge, UK, 1995; 270p. [Google Scholar]
- Uomini, N.T. Handedness in Neanderthals. In Neanderthal Lifeways, Subsistence and Technology; Conard, N.J., Richter, J., Eds.; Springer: Dordrecht, Netherlands, 2011; pp. 139–154. [Google Scholar]
- Estalrrich, A.; Rosas, A. Handedness in Neandertals from the El Sidrón (Asturias, Spain): Evidence from instrumental striations with ontogenetic inferences. PLoS ONE 2013, 8, e62797. [Google Scholar] [CrossRef] [PubMed]
- Courtney, A.J. Hand anthropometry of Hong Kong Chinese females compared to other ethnic groups. Ergonomics 1984, 27, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Okunribido, O.O. A survey of hand anthropometry of female rural farm workers in Ibadan, Western Nigeria. Ergonomics 2000, 43, 282–292. [Google Scholar] [CrossRef]
- Kar, S.K.; Ghosh, S.; Manna, I.; Banerjee, S.; Dhara, P. An investigation of hand anthropometry of agricultural workers. J. Hum. Ecol. 2003, 14, 57–62. [Google Scholar] [CrossRef]
- Imrhan, S.N.; Sarder, M.D.; Mandahawi, N. Hand anthropometry in Bangladeshis living in America and comparisons with other populations. Ergonomics 2009, 52, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Lazenby, R.; Smashnuk, A. Osteometric variation in the Inuit second metacarpal: A test of Allen’s Rule. Int. J. Osteoarchaeol. 1999, 9, 182–188. [Google Scholar] [CrossRef]
- Betti, L.; Lycett, S.J.; von Cramon-Taubadel, N.; Pearson, O.M. Are human hands and feet affected by climate? A test of Allen’s rule. Am. J. Phys. Anthropol. 2015, 158, 132–140. [Google Scholar] [CrossRef]
- Dianat, I.; Nedaei, M.; Nezami, M.A.M. The effects of tool handle shape on hand performance, usability and discomfort using masons’ trowels. Int. J. Ind. Ergon. 2015, 45, 13–20. [Google Scholar] [CrossRef]
- Kong, Y.K.; Lowe, B.D. Optimal cylindrical handle diameter for grip force tasks. Int. J. Ind. Ergon. 2005, 35, 495–507. [Google Scholar] [CrossRef]
- Rolian, C.; Lieberman, D.E.; Zermeno, J.P. Hand biomechanics during simulated stone tool use. J. Hum. Evol. 2011, 61, 26–41. [Google Scholar] [CrossRef]
- Key, A.J.; Lycett, S.J. Investigating interrelationships between Lower Palaeolithic stone tool effectiveness and tool user biometric variation: Implications for technological and evolutionary changes. Archaeol. Anthrop. Sci. 2018, 10, 989–1006. [Google Scholar] [CrossRef]
- Mathiowetz, V.; Kashman, N.; Volland, G.; Weber, K.; Dowe, M.; Rogers, S. Grip and pinch strength: Normative data for adults. Arch. Phys. Med. Rehabil. 1985, 66, 69–74. [Google Scholar]
- Bohannon, R.W. Dynamometer measurements of hand-grip strength predict multiple outcomes. Percept. Mot. Skills 2001, 93, 323–328. [Google Scholar] [CrossRef]
- Bohannon, R.W. Hand-grip dynamometry predicts future outcomes in aging adults. J. Geriat. Phys. Ther. 2008, 31, 3–10. [Google Scholar] [CrossRef]
- Cuesta-Vargas, A.; Hilgenkamp, T. Reference values of grip strength measured with a Jamar dynamometer in 1526 adults with intellectual disabilities and compared to adults without intellectual disability. PLoS ONE 2015, 10, e0129585. [Google Scholar] [CrossRef] [PubMed]
- Zaggelidis, G. Maximal isometric handgrip strength (HGS) in Greek elite male judo and karate athletes. Sport Sci. Rev. 2016, 25, 320. [Google Scholar] [CrossRef]
- Josty, I.C.; Tyler, M.P.H.; Shewell, P.C.; Roberts, A.H.N. Grip and pinch strength variations in different types of workers. J. Hand Surg. 1997, 22, 266–269. [Google Scholar] [CrossRef]
- Nevill, A.M.; Holder, R.L. Modelling handgrip strength in the presence of confounding variables: Results from the Allied Dunbar National Fitness Survey. Ergonomics 2000, 43, 1547–1558. [Google Scholar] [CrossRef]
- Saremi, M.; Rostamzadeh, S. Hand Dimensions and Grip Strength: A Comparison of Manual and Non-manual Workers. In Proceedings of the 20th Congress of the International Ergonomics Association, Florence, Italy, 26–30 August 2018; Bagnara, S., Tartaglia, R., Albolino, S., Alexander, T., Fujita, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 520–529. [Google Scholar]
- Burdukiewicz, A.; Pietraszewska, J.; Andrzejewska, J.; Chromik, K.; Stachoń, A. Asymmetry of Musculature and Hand Grip Strength in Bodybuilders and Martial Artists. Int. J. Environ. Res. Public Health 2020, 17, 4695. [Google Scholar] [CrossRef]
- Desrosiers, J.; Bravo, G.; Hẻbert, R.; Dutil, E. Normative data forgrip strength of elderly men and women. Am. J. Occup. Ther. 1995, 49, 637–644. [Google Scholar] [CrossRef]
- Vaz, M.; Hunsberger, S.; Diffey, B. Prediction equations for hand grip strength in healthy Indian male and female subjects encompassing a wide age range. Ann. Hum. Biol. 2002, 29, 131–141. [Google Scholar] [CrossRef]
- Bohannon, R.W.; Bear-Lehman, J.; Desrosiers, J.; Massy-Westropp, N.; Mathiowetz, V. Average grip strength: A meta-analysis of data obtained with a Jamar dynamometer from individuals 75 years or more of age. J. Geriatr. Phys. Ther. 2007, 30, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Clerke, A.M.; Clerke, J.P.; Adams, R.D. Effects of hand shape on maximal isometric grip strength and its reliability in teenagers. J. Hand Ther. 2005, 18, 19–29. [Google Scholar] [CrossRef]
- Hone, L.S.; McCullough, M.E. 2D: 4D ratios predict hand grip strength (but not hand grip endurance) in men (but not in women). Evol. Hum. Behav. 2012, 33, 780–789. [Google Scholar] [CrossRef]
- Petersen, P.; Petrick, M.; Connor, H.; Conklin, D. Grip strength and hand dominance: Challenging the 10% rule. Am. J. Occup. Ther. 1989, 43, 444–447. [Google Scholar] [CrossRef] [PubMed]
- Leyk, D.; Gorges, W.; Ridder, D.; Wunderlich, M.; Rüther, T.; Sievert, A.; Essfeld, D. Hand-grip strength of young men, women and highly trained female athletes. Eur. J. Appl. Physiol. 2007, 99, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Angst, F.; Drerup, S.; Werle, S.; Herren, D.B.; Simmen, B.R.; Goldhahn, J. Prediction of grip and key pinch strength in 978 healthy subjects. BMC Musculoskelet. Disord. 2010, 11, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Massy-Westropp, N.M.; Gill, T.K.; Taylor, A.W.; Bohannon, R.W.; Hill, C.L. Hand Grip Strength: Age and gender stratified normative data in a population-based study. BMC Res. Notes 2011, 4, 1–5. [Google Scholar] [CrossRef]
- Hanten, W.P.; Chen, W.-Y.; Austin, A.A.; Brooks, R.E.; Carter, H.C.; Law, C.A.; Morgan, M.K.; Sanders, D.J.; Swan, C.A.; Vanderslice, A.L. Maximum grip strength in normal subjects from 20 to 64years of age. J. Hand. Ther. 1999, 12, 193–200. [Google Scholar] [CrossRef]
- Bohannon, R.W. Grip strength: A summary of studies comparing dominant and nondominant limb measurements. Percept. Mot. Ski. 2003, 96, 728–730. [Google Scholar] [CrossRef] [PubMed]
- Aghazadeh, F.; Waikar, A.M.; Lee, K.S.; Backhouse, T.; Davis, P. Impact of anthropometric variables and sex on grip strength. In Advances in Industrial Ergonomics and Safety; Mital, A., Ed.; Taylor & Francis: London, UK, 1989; Volume I, pp. 501–505. [Google Scholar]
- Yakou, T.; Yamamoto, K.; Koyama, M.; Hyodo, K. Sensory evaluation of grip using cylindrical objects. JSME Int. J. Ser. C Mech. Syst. Mach. Elem. Manuf. 1997, 40, 730–735. [Google Scholar] [CrossRef][Green Version]
- Crawford, J.O.; Wabine, E.; Nayak, L. The interaction between lid diameter, height and shape on wrist torque exertion in younger and older adults. Ergonomics 2002, 45, 922–933. [Google Scholar] [CrossRef]
- Kong, Y.K.; Kim, D.M. The relationship between hand anthropometrics, total grip strength and individual finger force for various handle shapes. Int. J. Occup. Saf. Ergon. 2015, 21, 187–192. [Google Scholar] [CrossRef]
- Barut, Ç.; Demirel, P.; Kiran, S. Evaluation of hand anthropometric measurements and grip strength in basketball, volleyball and handball players. Anatomy 2008, 2, 55–59. [Google Scholar] [CrossRef]
- Carson, R.G. Get a grip: Individual variations in grip strength are a marker of brain health. Neurobiol. Aging 2018, 71, 189–222. [Google Scholar] [CrossRef] [PubMed]
- Praetorius Björk, M.; Johansson, B.; Hassing, L.B. I forgot when I lost my grip-strong associations between cognition and grip strength in level of performance and change across time in relation to impending death. Neurobiol. Aging 2016, 38, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Lau, V.W.-S.; Ip, W.-Y. Comparison of power grip and lateral pinch strengths between the dominant and non-dominant hands for normal Chinese male subjects of different occupational demand. Hong Kong Physiother. J. 2006, 24, 16–22. [Google Scholar] [CrossRef]
- Mitsionis, G.; Pakos, E.E.; Stafilas, K.S.; Paschos, N.; Papakostas, T.; Beris, A.E. Normative data on hand grip strength in a Greek adult population. Int. Orthop. 2009, 33, 713–717. [Google Scholar] [CrossRef]
- Hossain, M.G.; Zyroul, R.; Pereira, B.P.; Kamarul, T. Multiple regression analysis of factors influencing dominant hand grip strength in an adult Malaysian population. J. Hand Surg. 2012, 37, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W.; Peolsson, A.; Massy-Westropp, N.; Desrosiers, J.; Bear-Lehman, J. Reference values for adult grip strength measured with a Jamar dynamometer: A descriptive meta-analysis. Physiotherapy 2006, 92, 11–15. [Google Scholar] [CrossRef]
- Nicolay, C.W.; Walker, A.L. Grip strength and endurance: Influences of anthropometric variation, hand dominance, and gender. Int. J. Ind. Ergon. 2005, 35, 605–618. [Google Scholar] [CrossRef]
- Wang, Y.C.; Bohannon, R.W.; Li, X.; Sindhu, B.; Kapellusch, J. Hand-grip strength: Normative reference values and equations for individuals 18 to 85 years of age residing in the United States. J. Orthop. Sports Phys. Ther. 2018, 48, 685–693. [Google Scholar] [CrossRef]
- Cardoso, H.F.V.; Severino, R.S.S. The chronology of epiphyseal union in the hand and foot from dry bone observations. Int. J. Osteoarchaeol. 2010, 20, 737–746. [Google Scholar] [CrossRef]
- Cunningham, C.; Scheuer, L.; Black, S. Developmental Juvenile Osteology, 2nd ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 283–350. [Google Scholar]
- Fess, E.E. Grip strength. In Clinical Assessment Recommendations, 2nd ed.; Casanova, J.S., Ed.; American Society of Hand Therapists: Chicago, IL, USA, 1992; pp. 41–45. [Google Scholar]
- Rohlf, F.J. tpsDig2 Software; Version 2.31; The State University of New York at Stony Brook: Stony Brook, NY, USA, 2017; Available online: http://www.sbmorphometrics.org/soft-dataacq.html (accessed on 12 October 2020).
- Gamer, M.; Lemon, J.; Fellows, I.; Singh, P. irr: Various Coefficients of Interrater Reliability and Agreement; R Package Version 0.84.1.; The R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://CRAN.R-project.org/package=irr (accessed on 3 March 2021).
- Fox, J.; Weisberg, S. Visualizing Fit and Lack of Fit in Complex Regression Models with Predictor Effect Plots and Partial Residuals. J. Stat. Softw. 2018, 87, 1–27. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 6 June 2020).
- Golden, C.J.; Espe-Pfeifer, P.; Wachsler-Felder, J. Neuropsychological Interpretation of Objective Psychological Tests. Motor and Sensory Tests; Springer: New York, NY, USA, 2002; 243p. [Google Scholar]
- Porac, C. Are age trends in adult hand preference best explained by developmental shifts or generational differences? Can. J. Exp. Psychol. 1993, 47, 697–713. [Google Scholar] [CrossRef]
- Armstrong, C.A.; Oldham, J.A. A comparison of dominant and non-dominant hand strengths. J. Hand Surg. Eur. Vol. 1999, 24, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Crosby, C.A.; Wehbé, M.A. Hand strength: Normative values. J. Hand Surg. 1994, 19, 665–670. [Google Scholar] [CrossRef]
- Incel, N.A.; Ceceli, E.; Durukan, P.B.; Erdem, H.R.; Yorgancioglu, Z.R. Grip strength: Effect of hand dominance. Singap. Med. J. 2002, 43, 234–237. [Google Scholar]
- Lewandowski, L.; Kobus, D.A.; Church, K.L.; Van Orden, K. Neuropsychological implications of hand preference versus hand grip performance. Percep. Mot. Skills 1982, 55, 311–314. [Google Scholar] [CrossRef]
- Ziyagil, M.A.; Gürsoy, R.; Dane, Ş.; Türkmen, M.; Çebi, M. Effects of handedness on the hand grip strength asymmetry in Turkish athletes. Compr. Psychol. 2015, 4, 20. [Google Scholar] [CrossRef]
- Bagis, S.; Sahin, G.; Yapici, Y.; Cimen, Ö.B.; Erdogan, C. The effect of hand osteoarthritis on grip and pinch strength and hand function in postmenopausal women. Clin. Rheumatol. 2003, 22, 420–424. [Google Scholar] [CrossRef]
- Fontana, L.; Neel, S.; Claise, J.M.; Uqhetto, S.; Catilina, P. Osteoarthritis of the thumb carpometacarpal joint in women and occupational risk factors: A case-control study. J. Hand. Surg. Am. 2007, 32, 459–465. [Google Scholar] [CrossRef]
- Ceceli, E.; Gül, S.; Borman, P.N.; Uysal, S.R.; Okumuş, M. Hand function in female patients with hand osteoarthritis: Relation with radiological progression. Hand 2012, 7, 335–340. [Google Scholar] [CrossRef]
- Marzke, M.W.; Toth, N.; Schick, K.; Reece, S.; Steinberg, B.; Hunt, K.; Linscheid, R.L.; An, K.N. EMG study of hand muscle recruitment during hard hammer percussion manufacture of Oldowan tools. Am. J. Phys. Anthropol. 1998, 105, 315–332. [Google Scholar] [CrossRef]
- Key, A.J.; Dunmore, C.J. The evolution of the hominin thumb and the influence exerted by the non-dominant hand during stone tool production. J. Hum. Evol. 2015, 78, 60–69. [Google Scholar] [CrossRef]
- Shaw, C.N.; Hofmann, C.L.; Petraglia, M.D.; Stock, J.T.; Gottschall, J.S. Neandertal humeri may reflect adaptation to scraping tasks, but not spear thrusting. PLoS ONE 2012, 7, e40349. [Google Scholar] [CrossRef]
- Key, A.J. Manual loading distribution during carrying behaviors: Implications for the evolution of the hominin hand. PLoS ONE 2016, 11, e0163801. [Google Scholar] [CrossRef]
- Eshed, V.; Gopher, A.; Galili, E.; Hershkovitz, I. Musculoskeletal stress markers in Natufian hunter-gatherers and Neolithic farmers in the Levant: The upper limb. Amer. J. Phys. Anth. 2004, 123, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Ward, C.V. Interpreting the posture and locomotion of Australopithecus afarensis: Where do we stand? Am. J. Phys. Anthropol. 2002, 119 (Suppl. 35), 185–215. [Google Scholar] [CrossRef]
- Kivell, T.L. Evidence in hand: Recent discoveries and the early evolution of human manual manipulation. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2015, 370, 20150105. [Google Scholar] [CrossRef] [PubMed]
- Carlson, K.J.; Green, D.J.; Jashashvili, T.; Pickering, T.R.; Heaton, J.L.; Beaudet, A.; Stratford, D.; Crompton, R.; Kuman, K.; Bruxelles, L.; et al. The pectoral girdle of StW 573 (‘Little Foot’) and its implications for shoulder evolution in the Hominina. J. Hum. Evol. 2021, 102983. [Google Scholar] [CrossRef]
- Cartmill, M. Climbing. In Functional Vertebrate Morphology; Hildebrand, M., Bramble, D.M., Liem, K.F., Wake, D.B., Eds.; Harvard University Press: Cambridge, MA, USA, 1985; pp. 73–88. [Google Scholar]
- Preuschoft, H. What does “arboreal locomotion” mean exactly and what are the relationships between “climbing”, environment and morphology? Z. Morphol. Anthropol. 2002, 83, 171–188. [Google Scholar]
- Kappelman, J.; Ketcham, R.A.; Pearce, S.; Todd, L.; Akins, W.; Colbert, M.W.; Feseha, M.; Maisano, J.A.; Witzel, A. Perimortem fractures in Lucy suggest mortality from fall out of tall tree. Nature 2016, 537, 503–507. [Google Scholar] [CrossRef]
- Rolian, C.; Gordon, A.D. Reassessing manual proportions in Australopithecus afarensis. Am. J. Phys. Anthropol. 2013, 152, 393–406. [Google Scholar] [CrossRef]
- Niewoehner, W.A.; Bergstrom, A.; Eichele, D.; Zuroff, M.; Clark, J.T. Manual dexterity in Neanderthals. Nature 2003, 422, 395. [Google Scholar] [CrossRef]
- Feix, T.; Kivell, T.L.; Pouydebat, E.; Dollar, A.M. Estimating thumb–index finger precision grip and manipulation potential in extant and fossil primates. J. R. Soc. Interface 2015, 12, 20150176. [Google Scholar] [CrossRef]
- Almécija, S.; Alba, D.M. On manual proportions and pad-to-pad precision grasping in Australopithecus afarensis. J. Hum. Evol. 2014, 73, 88–92. [Google Scholar] [CrossRef]
- Niewoehner, W.A. Neanderthal hands in their proper perspective. In Neanderthals Revisited: New Approaches and Perspectives; Harvati, K., Harrison, T., Eds.; Springer: Berlin, Germany, 2008; pp. 157–190. [Google Scholar]
- Bardo, A.; Moncel, M.H.; Dunmore, C.J.; Kivell, T.L.; Pouydebat, E.; Cornette, R. The implications of thumb movements for Neanderthal and modern human manipulation. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Misiak, M.; Butovskaya, M.; Oleszkiewicz, A.; Sorokowski, P. Digit ratio and hand grip strength are associated with male competition outcomes: A study among traditional populations of the Yali and Hadza. Am. J. Hum. Biol. 2020, 32, e23321. [Google Scholar] [CrossRef]
- Ruff, C.B.; Larsen, C.S. Long bone structural analyses and the reconstruction of past mobility: A historical review. In Reconstructing Mobility; Carlson, K.J., Marchi, D., Eds.; Springer: Boston, MA, USA, 2014; pp. 13–29. [Google Scholar]
- Chirchir, H.; Kivell, T.L.; Ruff, C.B.; Hublin, J.J.; Carlson, K.J.; Zipfel, B.; Richmond, B.G. Recent origin of low trabecular bone density in modern humans. Proc. Natl. Acad. Sci. USA 2015, 112, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Chirchir, H.; Ruff, C.B.; Junno, J.A.; Potts, R. Low trabecular bone density in recent sedentary modern humans. Am. J. Phys. Anthropol. 2017, 162, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Ryan, T.M.; Shaw, C.N. Gracility of the modern Homo sapiens skeleton is the result of decreased biomechanical loading. Proc. Natl. Acad. Sci. USA 2015, 112, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Cashmore, L.; Uomini, N.; Chapelain, A. The evolution of handedness in humans and great apes: A review and current issues. J. Anthropol. Sci. 2008, 86, 7–35. [Google Scholar]
- Provins, K.A. The specificity of motor skill and manual asymmetry: A review of the evidence and its implications. J. Mot. Behav. 1997, 29, 183–192. [Google Scholar] [CrossRef]
- Fagot, J.; Vauclair, J. Manual laterality in nonhuman primates: A distinction between handedness and manual specialization. Psychol. Bull. 1991, 109, 76. [Google Scholar] [CrossRef]
- Steele, J.; Uomini, N. Can the archaeology of manual specialization tell us anything about language evolution? A survey of the state of play. Cambr. Arch. J. 2009, 19, 97–110. [Google Scholar] [CrossRef][Green Version]
- Lazenby, R.A. Identification of sex from metacarpals: Effect of side asymmetry. J. Forensic Sci. 1994, 39, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Lazenby, R.A.; Cooper, D.M.L.; Angus, S.; Hallgrmsson, B. Articular constraint, handedness, and directional asymmetry in the human second metacarpal. J. Hum. Evol. 2008, 54, 857–885. [Google Scholar] [CrossRef] [PubMed]
- Stephens, N.B.; Kivell, T.L.; Gross, T.; Pahr, D.H.; Lazenby, R.A.; Hublin, J.J.; Hershkovitz, I.; Skinner, M.M. Trabecular architecture in the thumb of Pan and Homo: Implications for investigating hand use, loading, and hand preference in the fossil record. Am. J. Phys. Anthropol. 2016, 161, 603–619. [Google Scholar] [CrossRef] [PubMed]
- Boonsaner, K.; Louthrenoo, W.; Meyer, S.; Schumacher, H.R., Jr. Effect of dominancy on severity in rheumatoid arthritis. Br. J. Rheumatol. 1992, 31, 77–80. [Google Scholar] [CrossRef]
- Sarringhaus, L.A.; Stock, J.T.; Marchant, L.F.; McGrew, W.C. Bilateral asymmetry in the limb bones of the chimpanzee (Pan troglodytes). Am. J. Phys. Anthropol. 2005, 128, 840–845. [Google Scholar] [CrossRef]
- Warguła, Ł.; Kukla, M.; Krawiec, P.; Wieczorek, B. Impact of Number of Operators and Distance to Branch Piles on Woodchipper Operation. Forests 2020, 11, 598. [Google Scholar] [CrossRef]
- Wieczorek, B.; Kukla, M. Biomechanical Relationships Between Manual Wheelchair Steering and the Position of the Human Body’s Center of Gravity. J. Biomech. Eng. 2020, 142. [Google Scholar] [CrossRef]

| Age (Years) | Dominance to Write | Total Participants | Forceful Manual Labour | Office Job/Work | Precision Manual Work | Playing a Musical Instrument | Practicing Sport | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | F | M | F | M | F | M | F | M | F | M | F | ||
| 17–19 | R | 25 | 42 | - | - | 25 | 42 | - | - | 12 | 19 | 20 | 29 |
| L | 3 | 5 | - | - | 3 | 5 | - | - | 1 | 3 | 2 | 5 | |
| 20–24 | R | 43 | 56 | 6 | 4 | 32 | 46 | 5 | 6 | 15 | 27 | 36 | 33 |
| L | 3 | 2 | - | 1 | 3 | 1 | - | - | 2 | 1 | 2 | 1 | |
| 25–29 | R | 21 | 33 | 6 | 5 | 17 | 22 | 1 | 6 | 7 | 9 | 20 | 16 |
| L | 3 | 4 | - | - | 1 | 3 | 2 | 1 | 2 | 1 | 1 | - | |
| 30–34 | R | 21 | 34 | 2 | - | 17 | 28 | 2 | 6 | 7 | 10 | 17 | 16 |
| L | 3 | 4 | 1 | 1 | 2 | 3 | - | - | 1 | - | 2 | 2 | |
| 35–39 | R | 22 | 33 | 5 | 5 | 17 | 24 | - | 4 | 8 | 8 | 18 | 17 |
| L | 4 | 4 | - | - | 4 | 3 | - | 1 | 1 | - | 1 | 1 | |
| 40–44 | R | 28 | 47 | 5 | 2 | 21 | 42 | 2 | 3 | 9 | 13 | 17 | 21 |
| L | 3 | 4 | 1 | 1 | 2 | 2 | - | 1 | 1 | 2 | 2 | 1 | |
| 45–49 | R | 30 | 51 | 5 | 3 | 22 | 42 | 4 | 6 | 4 | 12 | 20 | 24 |
| L | - | 6 | 1 | - | - | 6 | - | - | - | 1 | - | 2 | |
| 50–54 | R | 23 | 31 | 2 | - | 19 | 29 | 2 | 2 | 6 | 11 | 15 | 19 |
| L | 4 | 4 | 1 | - | 3 | 4 | - | - | 2 | 1 | - | 2 | |
| 55–59 | R | 2 | 12 | - | - | 2 | 11 | - | 1 | 2 | 3 | 2 | 8 |
| L | 1 | 1 | - | - | 1 | 1 | - | - | - | 1 | 1 | - | |
| 60 and + | R | 16 | 23 | 1 | - | 14 | 20 | 1 | 3 | 4 | 4 | 6 | 10 |
| L | 2 | - | - | - | 2 | - | - | - | 2 | - | 2 | - | |
| Total | 257 | 396 | 36 | 22 | 207 | 334 | 19 | 40 | 86 | 126 | 184 | 207 | |
| Age (Years) | Sex | Dominant Hand | Non-Dominant Hand | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| 17–19 | M | 87.9 | 17.7 | 81.1 | 17.8 |
| F | 55.5 | 13 | 51.8 | 12.5 | |
| 20–24 | M | 93.1 | 18.9 | 88.9 | 18.1 |
| F | 56.3 | 11.7 | 54.2 | 11.7 | |
| 25–29 | M | 101 | 22.3 | 96 | 19.2 |
| F | 59.4 | 11.6 | 55.6 | 11.6 | |
| 30–34 | M | 106 | 19 | 96.7 | 20.5 |
| F | 56.8 | 15.3 | 54.2 | 13.6 | |
| 35–39 | M | 99.8 | 18.1 | 97.9 | 17.9 |
| F | 59.8 | 9.36 | 57.7 | 10.3 | |
| 40–44 | M | 102 | 21.1 | 96.7 | 19.1 |
| F | 57.7 | 11.5 | 57.5 | 12.1 | |
| 45–49 | M | 93.1 | 21 | 87.9 | 21.1 |
| F | 54.7 | 12.1 | 52.3 | 12.4 | |
| 50–54 | M | 92.3 | 17.7 | 87.8 | 17 |
| F | 55.4 | 12.3 | 54.2 | 11.2 | |
| 55–59 | M | 102 | 25 | 90.8 | 22.7 |
| F | 56.9 | 10.6 | 54.5 | 8.95 | |
| 60 and + | M | 81.4 | 22.2 | 75.1 | 17.1 |
| F | 48.4 | 9.31 | 44.1 | 11 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bardo, A.; Kivell, T.L.; Town, K.; Donati, G.; Ballieux, H.; Stamate, C.; Edginton, T.; Forrester, G.S. Get a Grip: Variation in Human Hand Grip Strength and Implications for Human Evolution. Symmetry 2021, 13, 1142. https://doi.org/10.3390/sym13071142
Bardo A, Kivell TL, Town K, Donati G, Ballieux H, Stamate C, Edginton T, Forrester GS. Get a Grip: Variation in Human Hand Grip Strength and Implications for Human Evolution. Symmetry. 2021; 13(7):1142. https://doi.org/10.3390/sym13071142
Chicago/Turabian StyleBardo, Ameline, Tracy L. Kivell, Katie Town, Georgina Donati, Haiko Ballieux, Cosmin Stamate, Trudi Edginton, and Gillian S. Forrester. 2021. "Get a Grip: Variation in Human Hand Grip Strength and Implications for Human Evolution" Symmetry 13, no. 7: 1142. https://doi.org/10.3390/sym13071142
APA StyleBardo, A., Kivell, T. L., Town, K., Donati, G., Ballieux, H., Stamate, C., Edginton, T., & Forrester, G. S. (2021). Get a Grip: Variation in Human Hand Grip Strength and Implications for Human Evolution. Symmetry, 13(7), 1142. https://doi.org/10.3390/sym13071142







