Asymmetries of Cerebellar Lobe in the Genus Homo
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
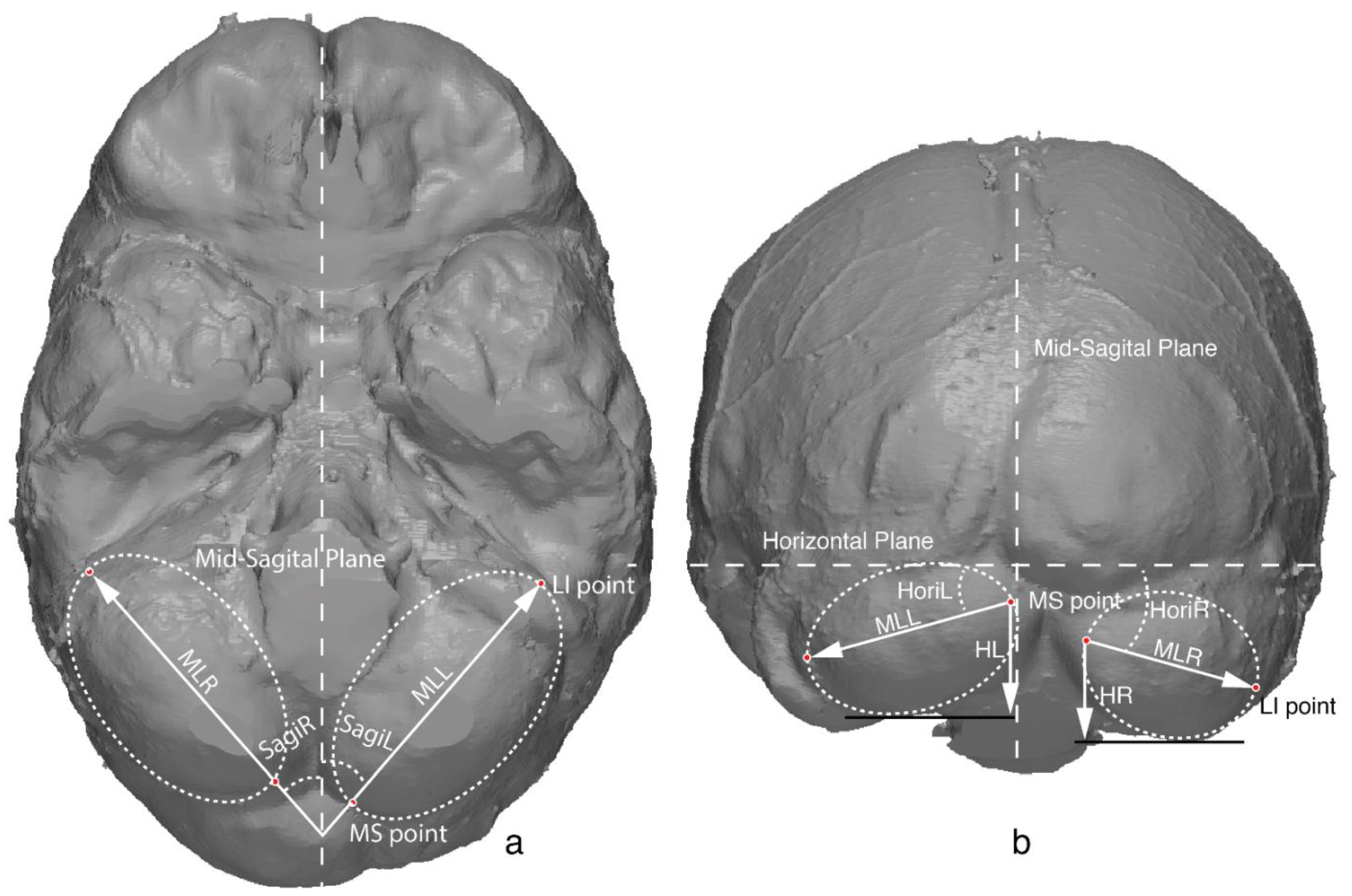
2.2. Cerebellar Metrics
2.3. Descriptive Statistics of the Asymmetries
2.4. Analysis of Covariance
3. Error Evaluation
4. Results
4.1. Description of Cerebellar Asymmetries
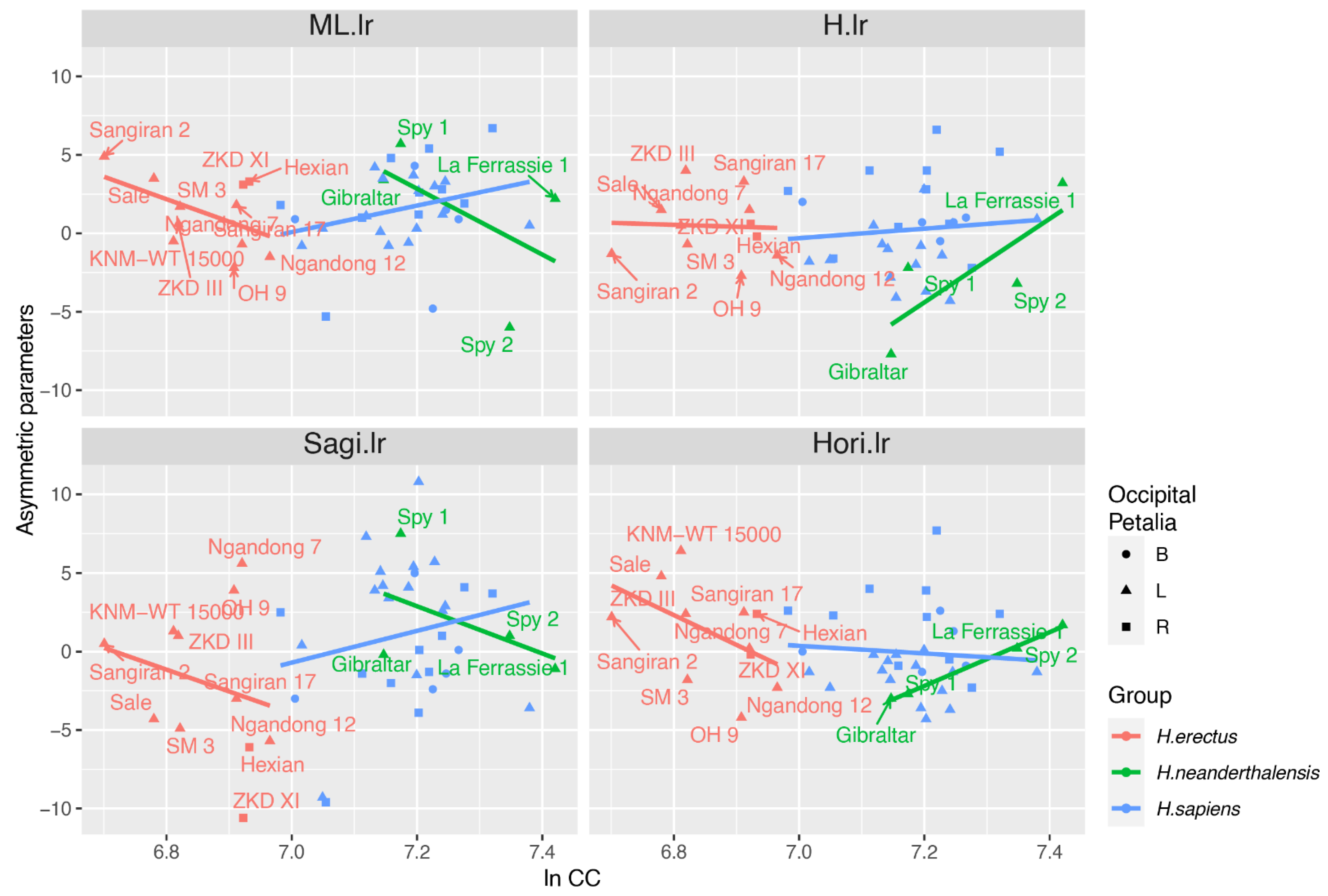
4.2. ANCOVA
5. Discussion
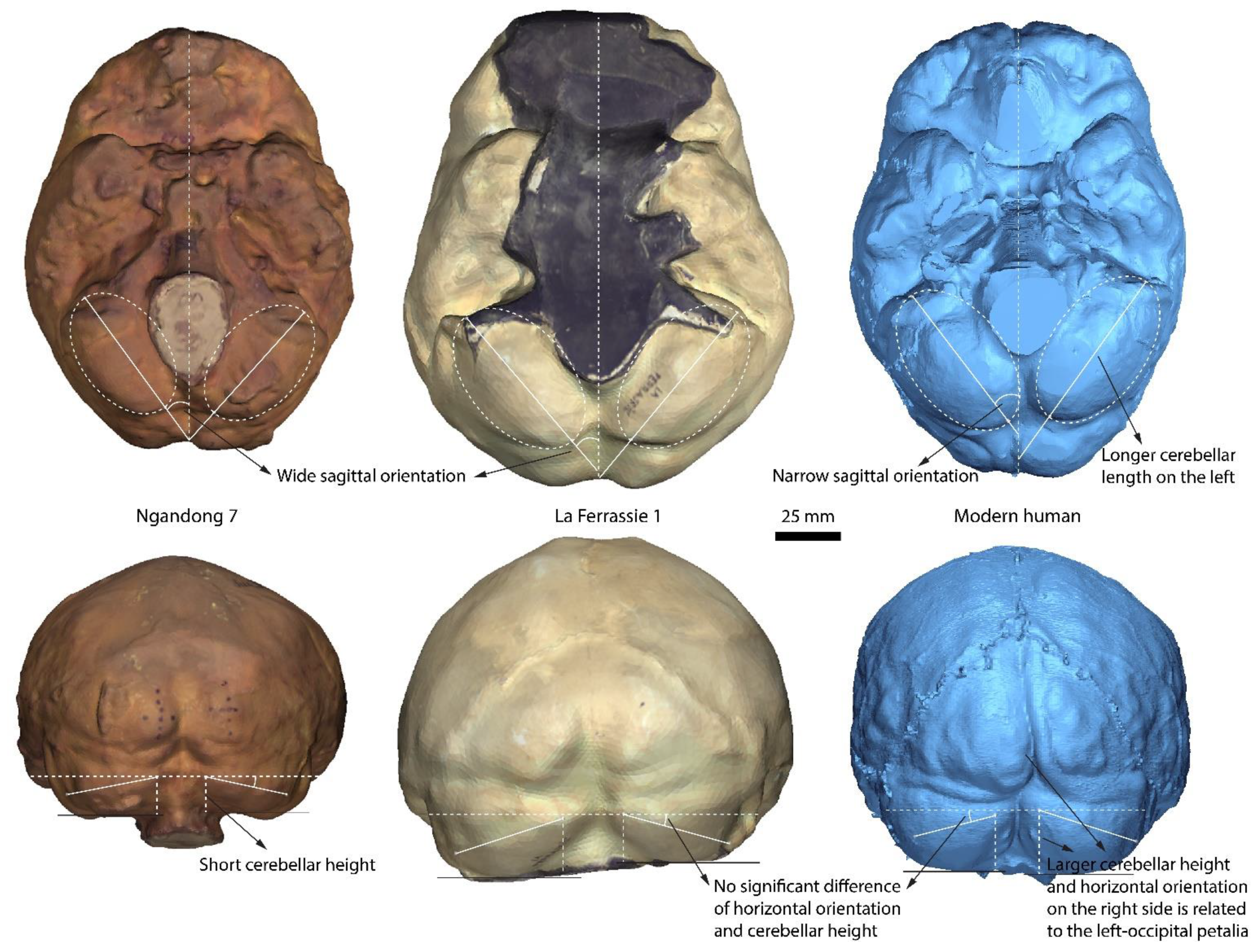
5.1. Cerebellar Asymmetric Pattern
5.2. Cerebellar Asymmetry in the Genus Homo
5.3. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holloway, R.L.; Broadfield, D.C.; Yuan, M.S.; Schwartz, J.H.; Tattersall, I. The Human Fossil Record, Brain Endocasts--The Paleoneurological Evidence, 1st ed.; Wiley-Liss: New York, NY, USA, 2004; p. 315. [Google Scholar]
- Holloway, R.L. Evolution of the Human Brain; Lock, A., Peters, C.R., Eds.; Clarendon Press: Oxford, UK, 1996; pp. 74–125. [Google Scholar]
- Holloway, R.L. Cranial capacity, neural reorganization, and hominid evolution: A search for more suitable parameters. Am. Anthrop. 1966, 68, 103–121. [Google Scholar]
- Holloway, R.L. New Endocranial Values for the Australopithecines. Nature 1970, 227, 199–200. [Google Scholar] [CrossRef]
- Holloway, R.L. New Endocranial Values for the East African Early Hominids. Nature 1973, 243, 97–99. [Google Scholar] [CrossRef]
- Conroy, G.C.; Vannier, M.W.; Tobias, P.V. Endocranial features of Australopithecus africanus revealed by 2- and 3-D computed tomography. Science 1990, 247, 838–841. [Google Scholar] [CrossRef]
- Conroy, G.C.; Weber, G.W.; Seidler, H.; Tobias, P.V.; Kane, A.; Brunsden, B. Endocranial capacity in an early hominid cranium from Sterkfontein, South Africa. Science 1998, 280, 1730–1731. [Google Scholar] [CrossRef] [PubMed]
- Falk, D.; Conroy, G.C. The cranial venous sinus system in Australopithecus afarensis. Nature 1983, 306, 779–781. [Google Scholar] [CrossRef]
- Falk, D. Meningeal arterial patterns in great apes: Implications for hominid vascular evolution. Am. J. Phys. Anthropol. 1993, 92, 81–97. [Google Scholar] [CrossRef] [PubMed]
- Weidenreich, F. The ramification of the middle meningeal artery in fossil hominids: And its bearing upon phylogenetic problems. Palaeontol. Sin. 1938, 110, 1–16. [Google Scholar]
- Bruner, E.; Sherkat, S. The middle meningeal artery: From clinics to fossils. Child’s Nerv. Syst. 2008, 24, 1289–1298. [Google Scholar] [CrossRef]
- Bruner, E. Geometric morphometrics and paleoneurology: Brain shape evolution in the genus Homo. J. Hum. Evol. 2004, 47, 279–303. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Pedro, A.S.; Bruner, E.; Gunz, P.; Neubauer, S. A morphometric comparison of the parietal lobe in modern humans and Neanderthals. J. Hum. Evol. 2020, 142, 102770. [Google Scholar] [CrossRef]
- Wu, X.-j.; Bruner, E. The endocranial anatomy of maba 1. Am. J. Phys. Anthropol. 2016, 160, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Balzeau, A.; Holloway, R.L.; Grimaud-Hervé, D. Variations and asymmetries in regional brain surface in the genus Homo. J. Hum. Evol. 2012, 62, 696–706. [Google Scholar] [CrossRef]
- Balzeau, A.; Gilissen, E.; Holloway, R.L.; Prima, S.; Grimaud-Hervé, D. Variations in size, shape and asymmetries of the third frontal convolution in hominids: Paleoneurological implications for hominin evolution and the origin of language. J. Hum. Evol. 2014, 76, 116–128. [Google Scholar] [CrossRef]
- Sherwood, C.C.; Broadfield, D.C.; Holloway, R.L.; Gannon, P.J.; Hof, P.R. Variability of Broca’s area homologue in African great apes: Implications for language evolution. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. 2003, 271, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Bruner, E.; Holloway, R.L. A bivariate approach to the widening of the frontal lobes in the genus Homo. J. Hum. Evol. 2010, 58, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Xiang, L.; Crow, T.J.; Leroy, F.; Rivière, D.; Mangin, J.-F.; Roberts, N. Measurement of Sylvian Fissure asymmetry and occipital bending in humans and Pan troglodytes. Neuroimage 2019, 184, 855–870. [Google Scholar] [CrossRef]
- LeMay, M. Morphological Cerebral Asymmetries of Modern Man, Fossil Man, and Nonhuman Primate. Ann. N. Y. Acad. Sci. 1976, 280, 349–366. [Google Scholar] [CrossRef] [PubMed]
- Rentería, M.E. Cerebral asymmetry: A quantitative, multifactorial, and plastic brain phenotype. Twin Res. Hum. Genet. 2012, 15, 401–413. [Google Scholar] [CrossRef]
- Holloway, R.L.; De La Costelareymondie, M.C. Brain endocast asymmetry in pongids and hominids: Some preliminary findings on the paleontology of cerebral dominance. Am. J. Phys. Anthropol. 1982, 58, 101–110. [Google Scholar] [CrossRef]
- Galaburda, A.M.; LeMay, M.; Kemper, T.L.; Geschwind, N. Right-left asymmetrics in the brain. Science 1978, 199, 852–856. [Google Scholar] [CrossRef]
- Hervé, P.-Y.; Crivello, F.; Perchey, G.; Mazoyer, B.; Tzourio-Mazoyer, N. Handedness and cerebral anatomical asymmetries in young adult males. Neuroimage 2006, 29, 1066–1079. [Google Scholar] [CrossRef]
- Li, X.; Crow, T.J.; Hopkins, W.D.; Gong, Q.; Roberts, N. Human torque is not present in chimpanzee brain. Neuroimaging 2018, 165, 285–293. [Google Scholar] [CrossRef]
- Phillips, K.A.; Sherwood, C.C. Cerebral petalias and their relationship to handedness in capuchin monkeys (Cebus apella). Neuropsychologia 2007, 45, 2398–2401. [Google Scholar] [CrossRef] [PubMed]
- Falk, D.; Hildebolt, C.; Cheverud, J.; Vannier, M.; Helmkamp, R.C.; Konigsberg, L. Cortical asymmetries in frontal lobes of rhesus monkeys (Macaca mulatta). J. Brain Res. 1990, 512, 40–45. [Google Scholar] [CrossRef]
- MacLeod, C. Chapter 8—The missing link: Evolution of the primate cerebellum. In Progress in Brain Research; Hofman, M.A., Falk, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 195, pp. 165–187. [Google Scholar]
- Sereno, M.I.; Diedrichsen, J.; Tachrount, M.; Testa-Silva, G.; d’Arceuil, H.; De Zeeuw, C. The human cerebellum has almost 80% of the surface area of the neocortex. Proc. Natl. Acad. Sci. USA 2020, 117, 19538–19543. [Google Scholar] [CrossRef]
- Tanabe, H.C.; Kubo, D.; Hasegawa, K.; Kochiyama, T.; Kondo, O. Cerebellum: Anatomy, Physiology, Function, and Evolution. In Digital Endocasts: From Skulls to Brains; Bruner, E., Ogihara, N., Tanabe, H.C., Eds.; Springer: Tokyo, Japan, 2018; pp. 275–289. [Google Scholar]
- MacLeod, C.E.; Zilles, K.; Schleicher, A.; Rilling, J.K.; Gibson, K.R. Expansion of the neocerebellum in Hominoidea. J. Hum. Evol. 2003, 44, 401–429. [Google Scholar] [CrossRef]
- Kim, S.; Ugurbil, K.; Strick, P. Activation of a cerebellar output nucleus during cognitive processing. Science 1994, 265, 949–951. [Google Scholar] [CrossRef] [PubMed]
- Raichle, M.E.; Fiez, J.A.; Videen, T.O.; MacLeod, A.-M.K.; Pardo, J.V.; Fox, P.T.; Petersen, S.E. Practice-related changes in human brain functional anatomy during nonmotor learning. Cereb. Cortex 1994, 4, 8–26. [Google Scholar] [CrossRef]
- Rae, C.; Harasty, J.A.; Dzendrowskyj, T.E.; Talcott, J.B.; Simpson, J.M.; Blamire, A.M.; Dixon, R.M.; Lee, M.A.; Thompson, C.H.; Styles, P. Cerebellar morphology in developmental dyslexia. Neuropsychologia 2002, 40, 1285–1292. [Google Scholar] [CrossRef]
- Eckert, M.A.; Leonard, C.M.; Richards, T.L.; Aylward, E.H.; Thomson, J.; Berninger, V.W. Anatomical correlates of dyslexia: Frontal and cerebellar findings. Brain 2003, 126, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Diedrichsen, J.; Balsters, J.H.; Flavell, J.; Cussans, E.; Ramnani, N. A probabilistic MR atlas of the human cerebellum. Neuroimage 2009, 46, 39–46. [Google Scholar] [CrossRef]
- Snyder, P.J.; Bilder, R.M.; Wu, H.; Bogerts, B.; Lieberman, J.A. Cerebellar volume asymmetries are related to handedness: A quantitative MRI study. Neuropsychologia 1995, 33, 407–419. [Google Scholar] [CrossRef]
- Weaver, A.H. Reciprocal evolution of the cerebellum and neocortex in fossil humans. Proc. Natl. Acad. Sci. USA 2005, 102, 3576–3580. [Google Scholar] [CrossRef]
- Barton, R.A. Embodied cognitive evolution and the cerebellum. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 2097–2107. [Google Scholar] [CrossRef]
- Barton, R.A.; Venditti, C. Rapid Evolution of the Cerebellum in Humans and Other Great Apes. Curr. Biol. 2014, 24, 2440–2444. [Google Scholar] [CrossRef]
- Naidich, T.P.; Duvernoy, H.M.; Delman, B.N.; Sorensen, A.G.; Kollias, S.S.; Haacke, E.M. Duvernoy’s Atlas of the Human Brain Stem and Cerebellum; Springer: Vienna, Austria, 2009. [Google Scholar]
- Falk, D. Apples, oranges, and the lunate sulcus. Am. J. Phys. Anthropol. 1985, 67, 313–315. [Google Scholar] [CrossRef] [PubMed]
- Falk, D. A reanalysis of the South African australopithecine natural endocasts. Am. J. Phys. Anthropol. 1980, 53, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Falk, D. Ape-like endocast of “ape-man” Taung. Am. J. Phys. Anthropol. 1989, 80, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Falk, D. The Taung endocast: A reply to Holloway. Am. J. Phys. Anthropol. 1983, 60, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Holloway, R.L. Revisiting the South African Taung australopithecine endocast: The position of the lunate sulcus as determined by the stereoplotting technique. Am. J. Phys. Anthropol. 1981, 56, 43–58. [Google Scholar] [CrossRef]
- Holloway, R.L. The taung endocast and the lunate sulcus: A rejection of the hypothesis of its anterior position. Am. J. Phys. Anthropol. 1984, 64, 285–287. [Google Scholar] [CrossRef]
- Holloway, R.L.; Clarke, R.J.; Tobias, P.V. Posterior lunate sulcus in Australopithecus africanus: Was Dart right? Comptes Rendus Palevol 2004, 3, 287–293. [Google Scholar] [CrossRef]
- Rightmire, G.P. Homo erectus and middle pleistocene hominins: Brain size, skull form, and species recognition. J. Hum. Evol. 2013, 65, 223–252. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2018. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: Dordrecht, The Netherlands, 2016. [Google Scholar]
- Enlow, D.H.; Hans, M. Essentials of Facial Growth; W.B. Saunders Company: Philadelphia, PA, USA, 1996. [Google Scholar]
- Enlow, D.H.; Kuroda, T.; Lewis, A.B. The morphological and morphogenetic basis for craniofacial form and pattern. Angle Orthod. 1971, 41, 161–188. [Google Scholar] [PubMed]
- Arsava, E.Y.; Arsava, E.M.; Oguz, K.K.; Topcuoglu, M.A. Occipital petalia as a predictive imaging sign for transverse sinus dominance. Neurol. Res. 2019, 41, 306–311. [Google Scholar] [CrossRef]
- Gunz, P.; Neubauer, S.; Golovanova, L.; Doronichev, V.; Maureille, B.; Hublin, J.-J. A uniquely modern human pattern of endocranial development. Insights from a new cranial reconstruction of the Neandertal newborn from Mezmaiskaya. J. Hum. Evol. 2012, 62, 300–313. [Google Scholar] [CrossRef]
- Burke, A. Spatial abilities, cognition and the pattern of Neanderthal and modern human dispersals. Quat. Int. 2012, 247, 230–235. [Google Scholar] [CrossRef]
- Lieberman, P. The evolution of human speech-Its anatomical and neural bases. Curr. Anthropol. 2007, 48, 39–66. [Google Scholar] [CrossRef]
- Kochiyama, T.; Ogihara, N.; Tanabe, H.C.; Kondo, O.; Amano, H.; Hasegawa, K.; Suzuki, H.; Ponce de León, M.S.; Zollikofer, C.P.E.; Bastir, M.; et al. Reconstructing the Neanderthal brain using computational anatomy. Sci. Rep. 2018, 8, 6296. [Google Scholar] [CrossRef]

| Populations | Number | Specimens and Source |
|---|---|---|
| Homo erectus | 11 | ZKD III, ZKD XI, Hexian (IVPP); OH 9, WT 15000, Sale, Sangiran 2, Sangiran 17, Ngandong 7, Ngandong 12, Sambungmacan 3 (AMNH) |
| Homo neanderthalensis | 4 | La Ferrassie 1, Gibraltar, Spy 1, Spy 2 (AMNH) |
| Homo sapiens | 30 | Modern Chinese (IVPP) |
| Measurements | Abbreviation (Right/Left) | Definition |
|---|---|---|
| Cerebellar length | MLR/MLL | Length of the cerebellar major axis |
| Cerebellar height | HR/HL | Height of the cerebellum, measured from the MS point to the lowest margin of the cerebellum |
| Sagittal orientation | SagiR/SagiL | Orientation of the major axis relative to the sagittal plane, depicting how the cerebellar lobe orientated medial-laterally |
| Horizontal orientation | HoriR/HoriL | Orientation of the major axis relative to the horizontal plane, depicting how the cerebellar lobe orientated superior-inferiorly |
| Asymmetric parameters | ML.lr, H.lr, Sagi.lr, Hori.lr | Difference between the left and right side of the same measurement, calculated as (L–R) |
| Specimen | Side | ML | H | Sagi | Hori |
|---|---|---|---|---|---|
| Ngandong 7 | right | 2.42 | 7.95 | 8.88 | 16.33 |
| Ngandong 7 | left | 0.73 | 2.44 | 2.01 | 22.46 |
| Gibraltar | right | 1.75 | 8.34 | 3.70 | 40.03 |
| Gibraltar | left | 3.52 | 4.15 | 4.36 | 33.63 |
| yno4f | right | 0.86 | 4.12 | 3.29 | 24.41 |
| yno4f | left | 1.03 | 1.33 | 5.49 | 19.23 |
| Population | Side | Metrics | ML | H | Sagi | Hori |
|---|---|---|---|---|---|---|
| H. erectus (n = 12) | right | mean | 48.68 | 15.18 | 40.47 | 7.68 |
| sd | 4.21 | 1.83 | 5.49 | 2.18 | ||
| cv | 8.65 | 12.05 | 13.56 | 28.42 | ||
| left | mean | 49.95 | 15.65 | 38.45 | 8.81 | |
| sd | 3.56 | 1.30 | 5.11 | 3.23 | ||
| cv | 7.13 | 8.30 | 13.29 | 36.66 | ||
| p | 0.10 | 0.45 | 0.19 | 0.26 | ||
| H. neanderthalensis (n = 4) | right | mean | 60.33 | 22.85 | 41.35 | 11.20 |
| sd | 4.28 | 3.39 | 3.36 | 4.53 | ||
| cv | 7.09 | 14.84 | 8.13 | 40.47 | ||
| left | mean | 61.65 | 20.38 | 43.15 | 10.25 | |
| sd | 4.70 | 4.41 | 4.66 | 5.76 | ||
| cv | 7.63 | 21.64 | 10.79 | 56.19 | ||
| p | 0.64 | 0.43 | 0.25 | 0.37 | ||
| H. sapiens (n = 30) | right | mean | 58.17 | 21.78 | 35.40 | 9.90 |
| sd | 2.65 | 3.02 | 3.27 | 2.82 | ||
| cv | 4.55 | 13.86 | 9.24 | 28.52 | ||
| left | mean | 59.75 | 22.01 | 36.50 | 9.83 | |
| sd | 3.33 | 3.05 | 3.46 | 2.77 | ||
| cv | 5.57 | 13.85 | 9.48 | 28.14 | ||
| p | 0.01 * | 0.64 | 0.21 | 0.91 |
| Asymmetric Parameter | Term | H. erectus | H. sapiens | H. neanderthalensis |
|---|---|---|---|---|
| ML.lr | ln CC | 0.03 * | 0.11 | 0.45 |
| Occipital Petalia | 0.01 * | 0.42 | ||
| H.lr | ln CC | 0.89 | 0.51 | 0.21 |
| Occipital Petalia | 0.89 | 0.01 * | ||
| Sagi.lr | ln CC | 0.42 | 0.26 | 0.49 |
| Occipital Petalia | 0.06 | 0.12 | ||
| Hori.lr | ln CC | 0.14 | 0.56 | 0.00 * |
| Occipital Petalia | 0.51 | 0.01 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wu, X. Asymmetries of Cerebellar Lobe in the Genus Homo. Symmetry 2021, 13, 988. https://doi.org/10.3390/sym13060988
Zhang Y, Wu X. Asymmetries of Cerebellar Lobe in the Genus Homo. Symmetry. 2021; 13(6):988. https://doi.org/10.3390/sym13060988
Chicago/Turabian StyleZhang, Yameng, and Xiujie Wu. 2021. "Asymmetries of Cerebellar Lobe in the Genus Homo" Symmetry 13, no. 6: 988. https://doi.org/10.3390/sym13060988
APA StyleZhang, Y., & Wu, X. (2021). Asymmetries of Cerebellar Lobe in the Genus Homo. Symmetry, 13(6), 988. https://doi.org/10.3390/sym13060988






