A Damage Model to Trabecular Bone and Similar Materials: Residual Resource, Effective Elasticity Modulus, and Effective Stress under Uniaxial Compression
Abstract
1. Introduction
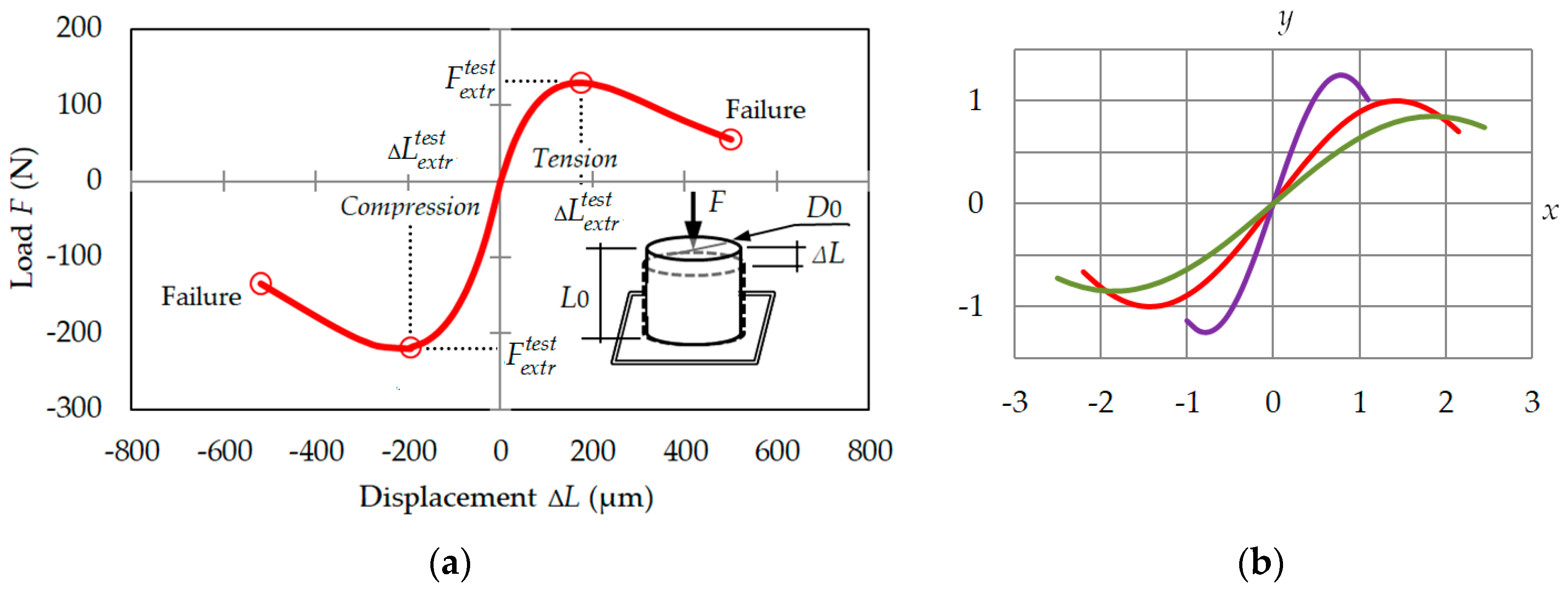
1.1. The Load–Displacement Interrelationships under Uniaxial Loading
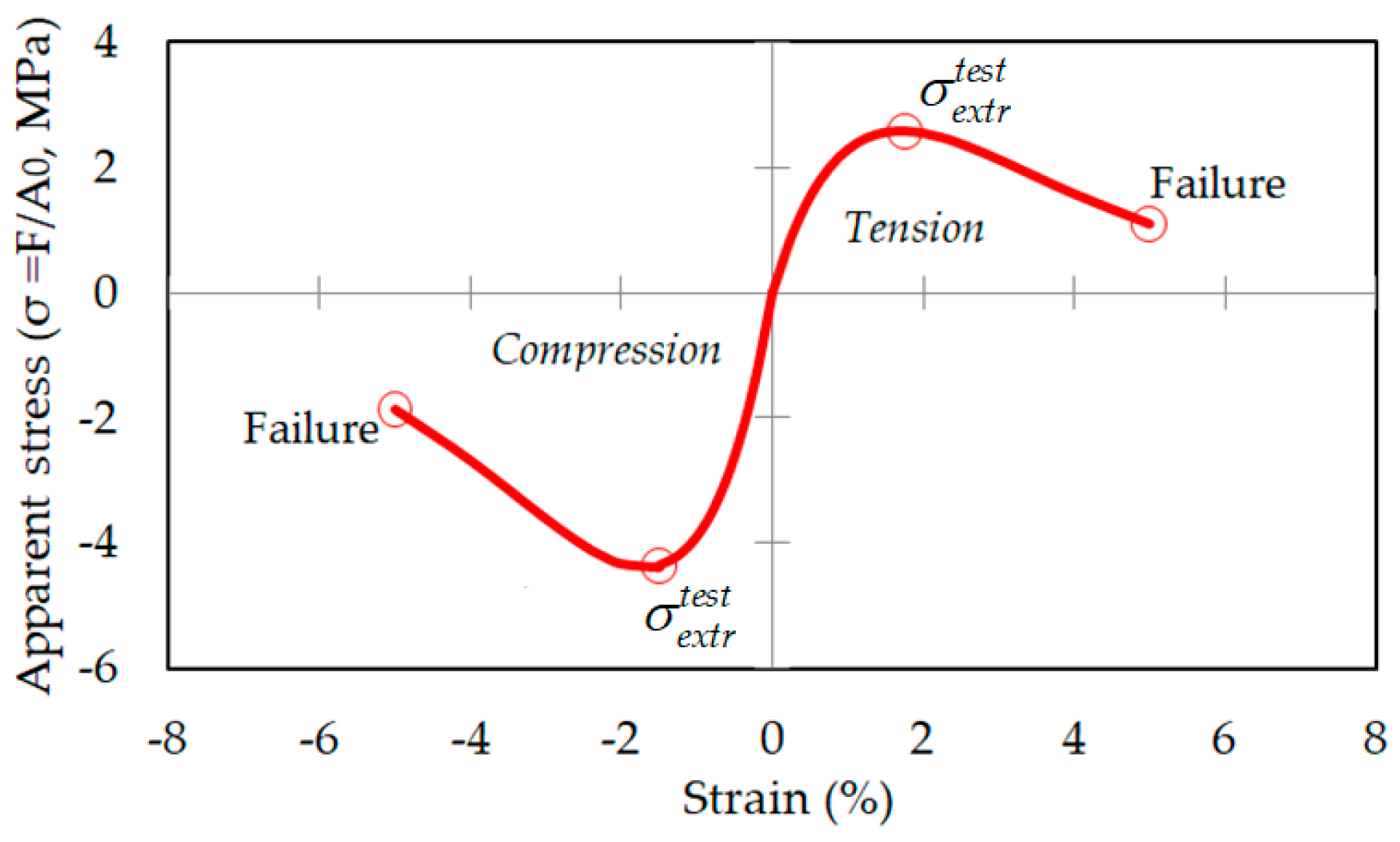
1.2. The Stress–Strain Ratio
1.3. The Problem of Consistency between Plots of Load–Displacement and Apparent Stress–Strain
2. Residual Resource Function, Damage Function, and Effective Elasticity Modulus
2.1. Bone as a Biomechanical System of Interacting Structural Units
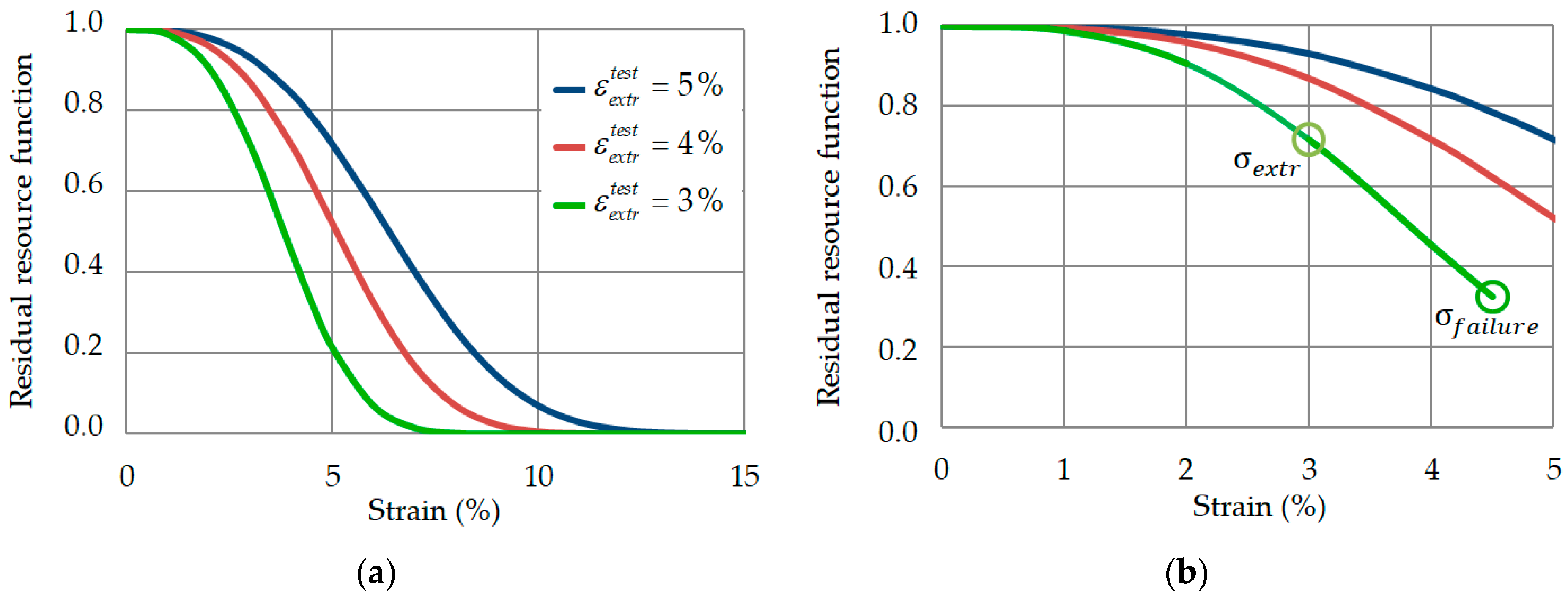
2.2. Effective Cross-Sectional Area and Residual Resource Function
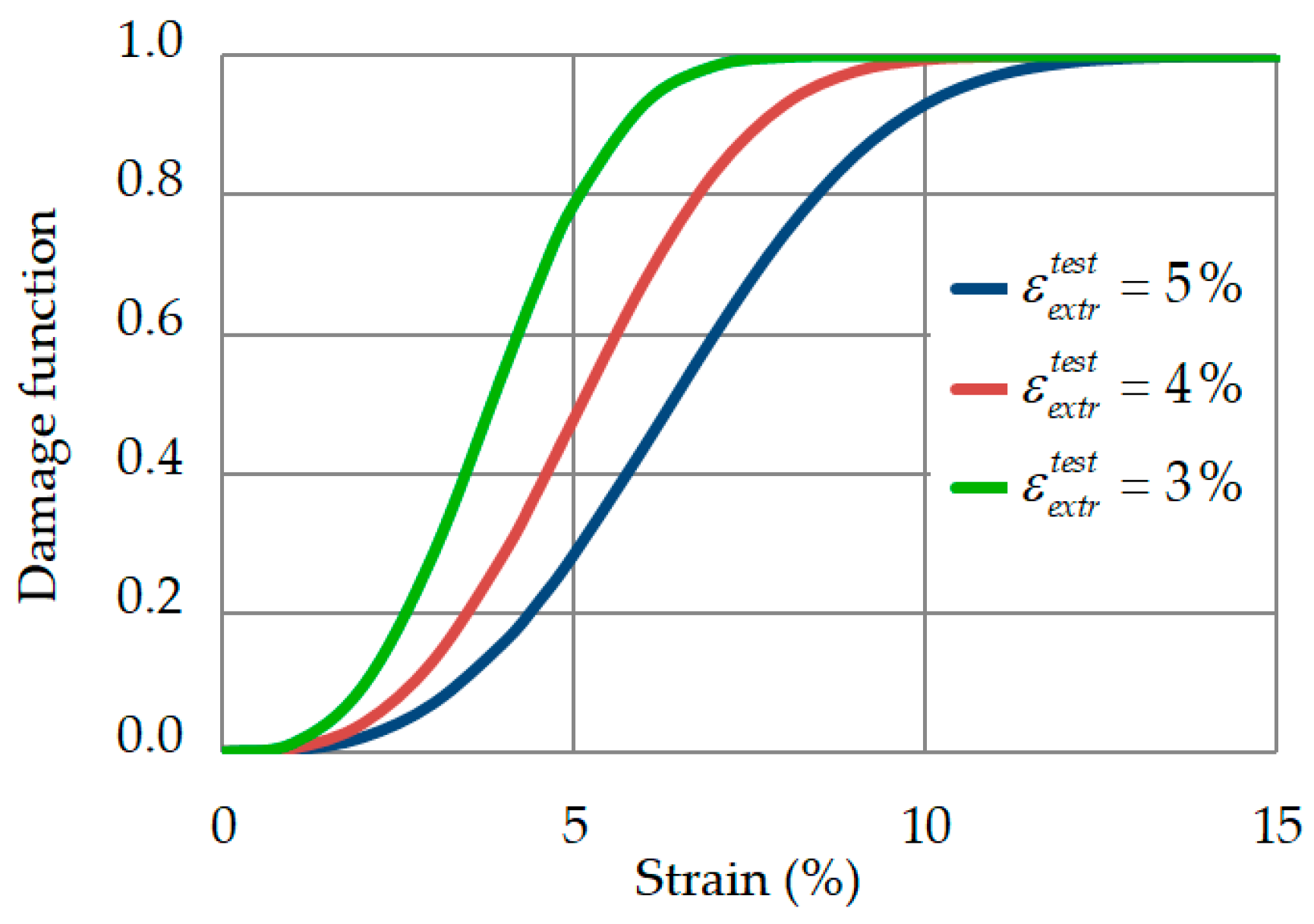
2.3. Damage Function
2.4. Effective Elasticity Modulus: Substantiation of Model Relationships
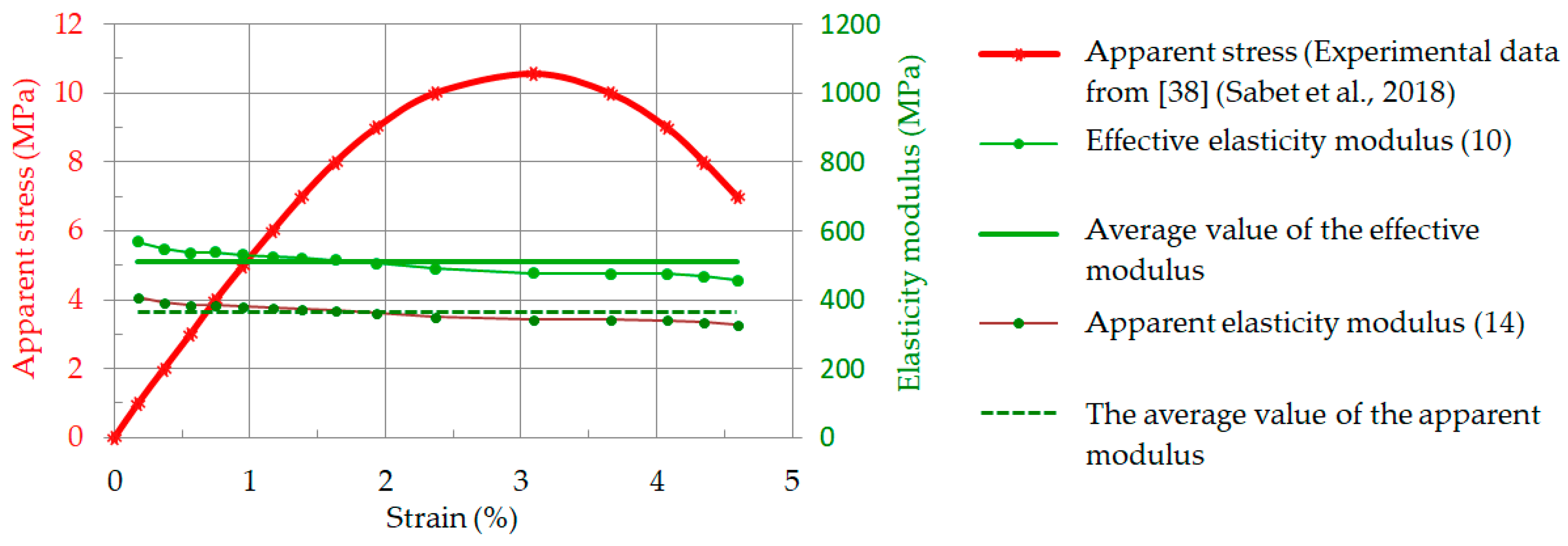
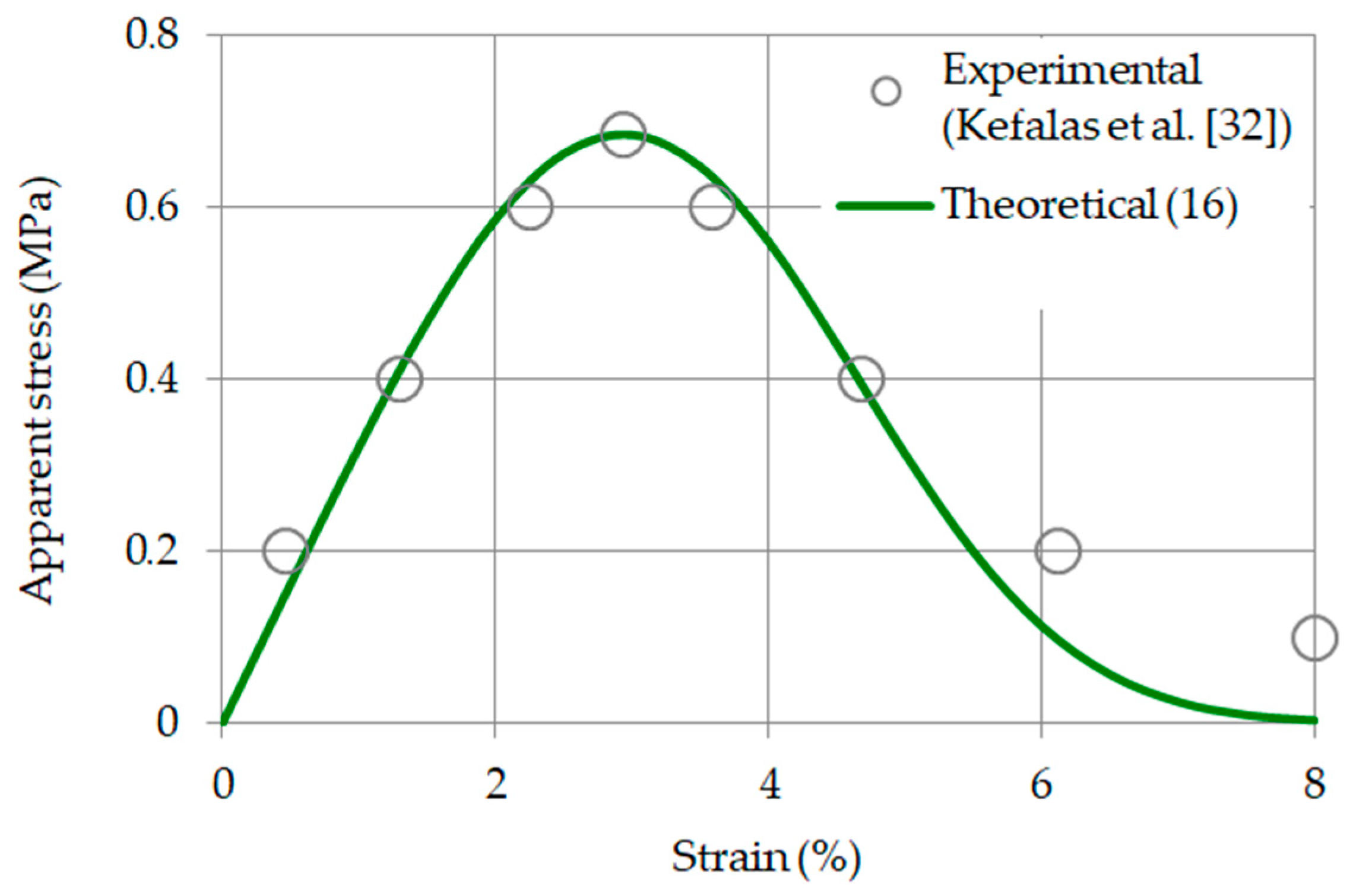
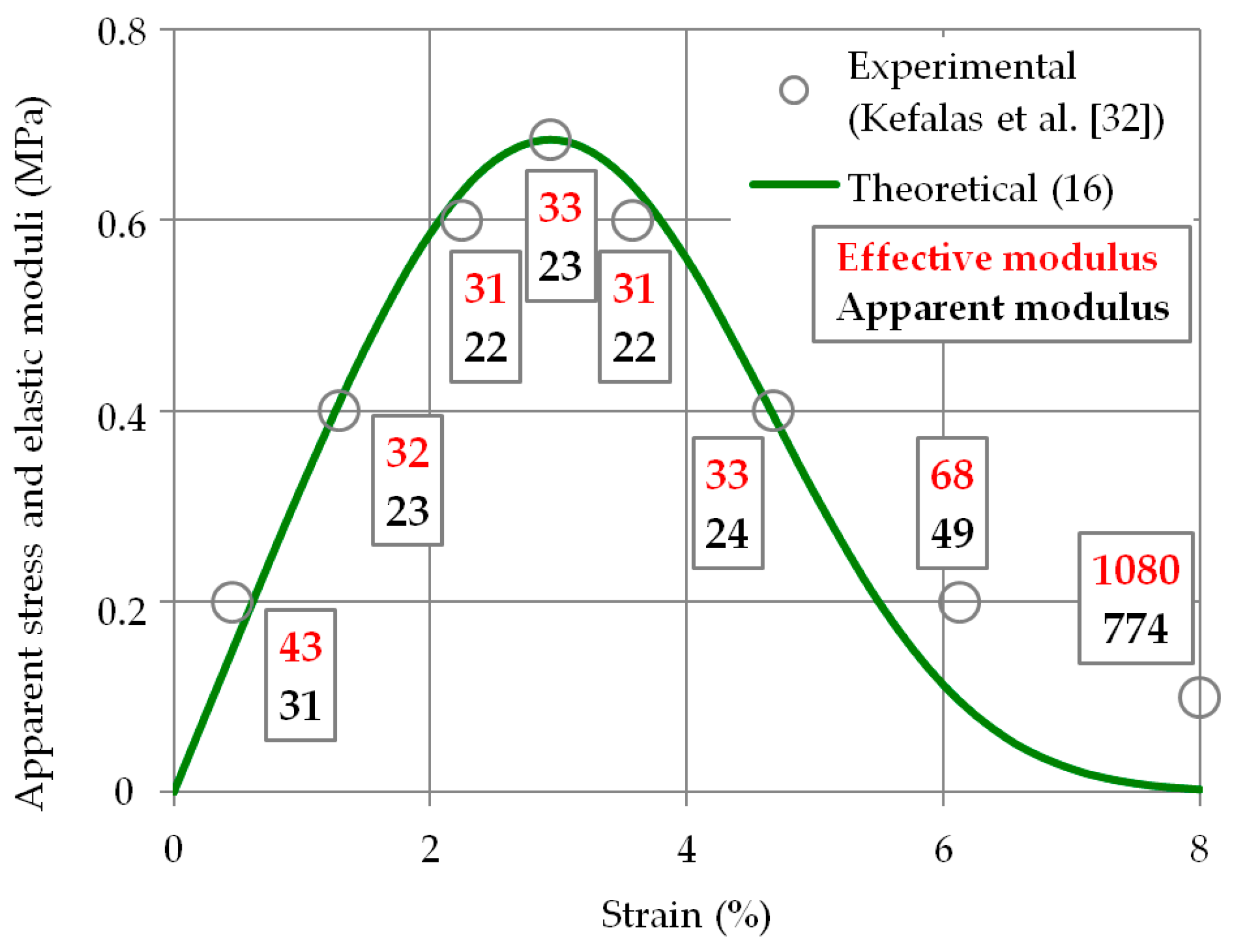
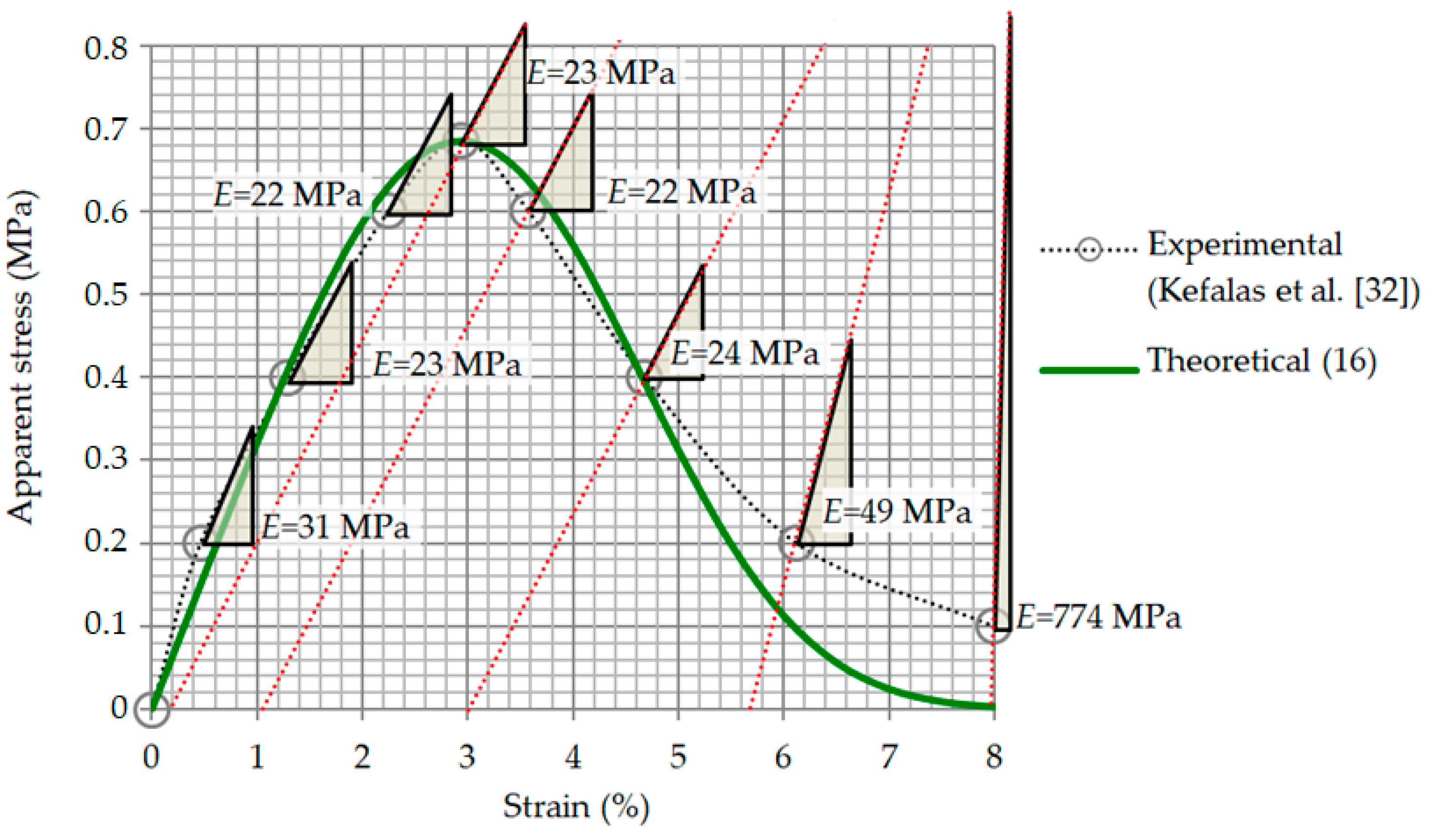
2.5. Effective Elasticity Modulus: Validation of Model Computations
3. Results
3.1. Load–Displacement Relationship
3.2. Relationship between Strain and Apparent Stress
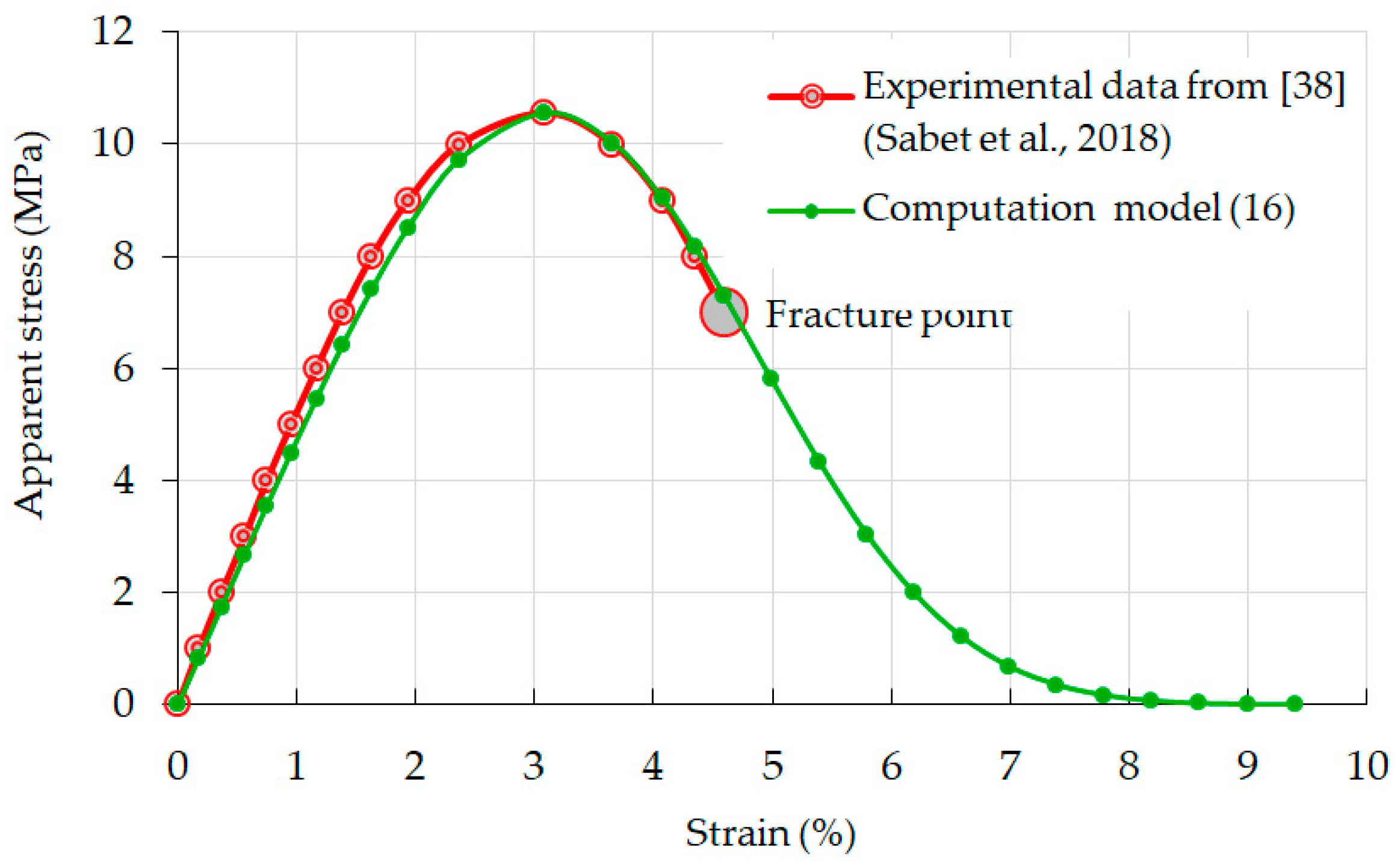
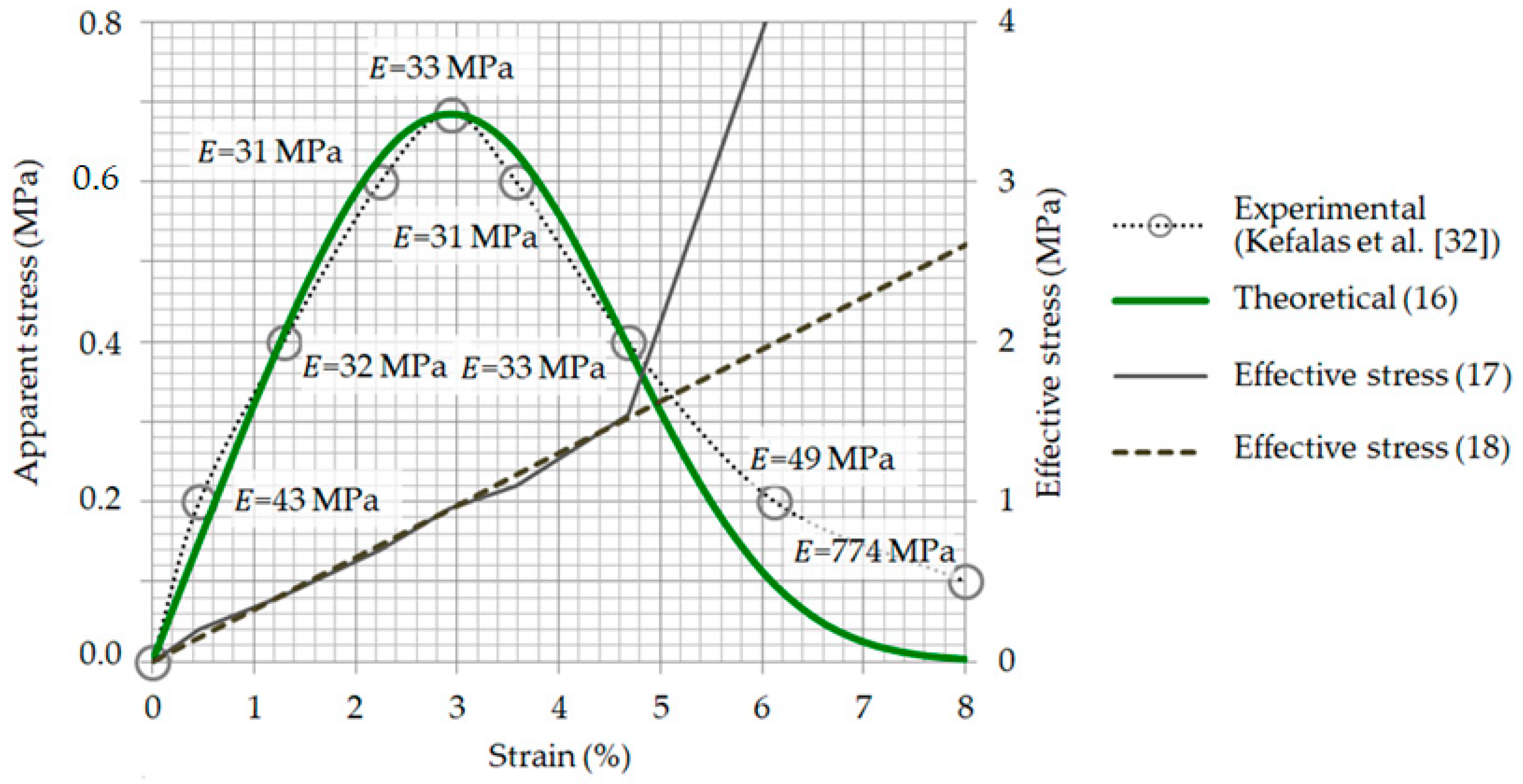
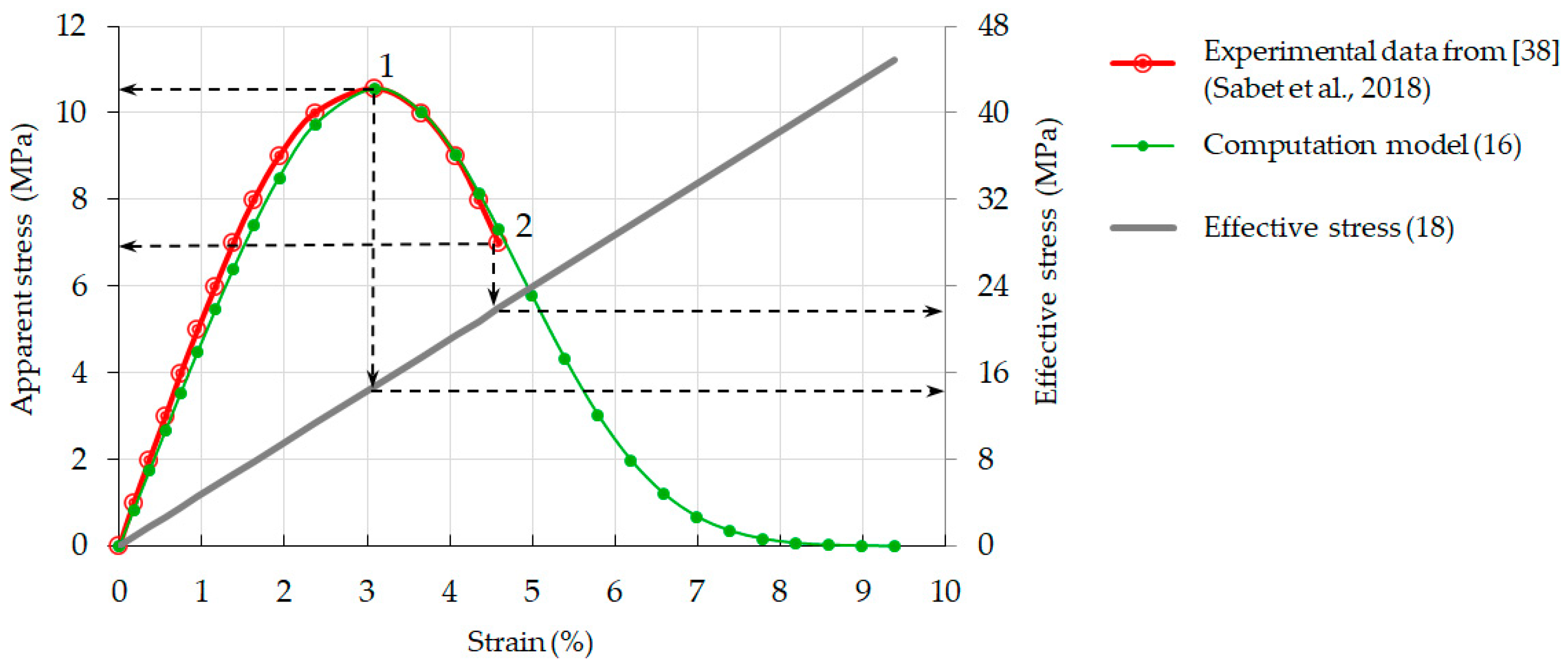
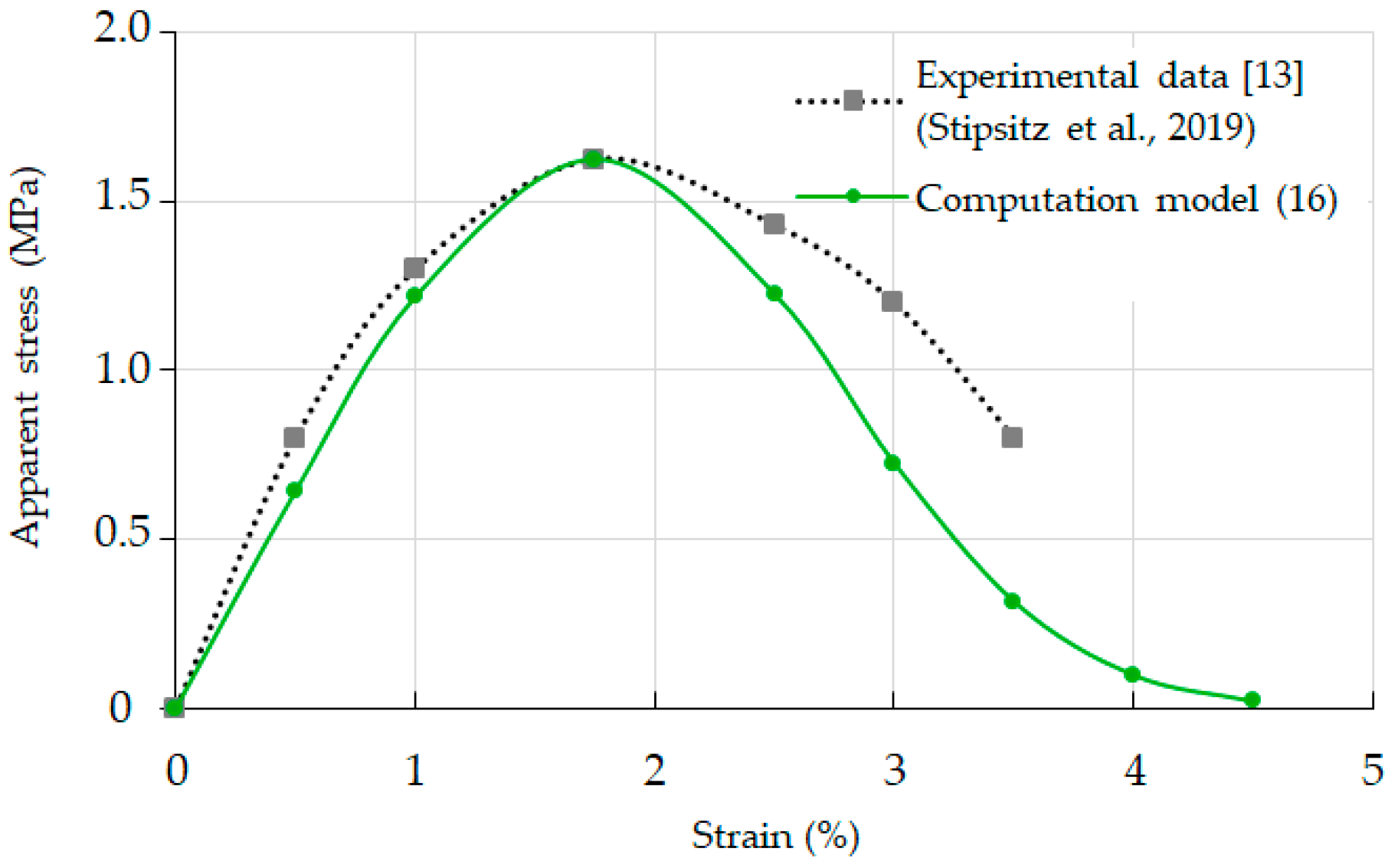
3.3. Relationship between Strain and Apparent Stress: Validation of Model Computations
3.4. Is There a Short-Term Aggregation of Separate Particles of a Destructible Sample at the Final Stage of Compression Tests?
3.5. Effective Stress
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Cristofolini, L. In vitro evidence of the structural optimization of the human skeletal bones. J. Biomech. 2015, 48, 787–796. [Google Scholar] [CrossRef]
- Yan, C.; Moshage, S.G.; Kersh, M.E. Play During Growth: The Effect of Sports on Bone Adaptation. Curr. Osteoporos. Rep. 2020, 18, 684–695. [Google Scholar] [CrossRef] [PubMed]
- Roesler, H. The history of some fundamental concepts in bone biomechanics. J. Biomech. 1987, 20, 1025–1034. [Google Scholar] [CrossRef]
- Pahr, D.H.; Reisinger, A.G. A Review on Recent Advances in the Constitutive Modeling of Bone Tissue. Curr. Osteoporos. Rep. 2020, 18, 696–704. [Google Scholar] [CrossRef]
- Roux, J.P.; Boutroy, S.; Bouxsein, M.L.; Chapurlat, R.; Wegrzyn, J. Local and global microarchitecture is associated with different features of bone biomechanics. Bone Rep. 2020, 13, 100716. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Nyman, J.S.; Dong, X.; Leng, H.; Reyes, M. Fundamental biomechanics in bone tissue engineering. Synth. Lect. Tissue Eng. 2010, 2, 1–225. [Google Scholar] [CrossRef]
- Fischer, B.; Hofmann, A.; Kurz, S.; Edel, M.; Zajonz, D.; Roth, A.; Schleifenbaum, S. Influence of the fixation technique on the mechanical properties of human cancellous bone of the femoral head. Clin. Biomech. 2021, 82, 105280. [Google Scholar] [CrossRef] [PubMed]
- Henkel, J.; Woodruff, M.A.; Epari, D.R.; Steck, R.; Glatt, V.; Dickinson, I.C.; Choong, P.; Schuetz, M.A.; Hutmacher, D.W. Bone regeneration based on tissue engineering conceptions—A 21st century perspective. Bone Res. 2013, 1, 216–248. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, D.; Wang, T. Hierarchical structures of bone and bioinspired bone tissue engineering. Small 2016, 12, 4611–4632. [Google Scholar] [CrossRef] [PubMed]
- Razi, H.; Predan, J.; Fischer, F.D.; Kolednik, O.; Fratzl, P. Damage tolerance of lamellar bone. Bone 2020, 130, 115102. [Google Scholar] [CrossRef]
- Luo, Y. A biomechanical sorting of clinical risk factors affecting osteoporotic hip fracture. Osteoporos. Int. 2016, 27, 423–439. [Google Scholar] [CrossRef]
- Schwiedrzik, J.; Gross, T.; Bina, M.; Pretterklieber, M.; Zysset, P.; Pahr, D. Experimental validation of a nonlinear μFE model based on cohesive-frictional plasticity for trabecular bone. Int. J. Numer. Methods Biomed. Eng. 2016, 32, e02739. [Google Scholar] [CrossRef]
- Stipsitz, M.; Zysset, P.K.; Pahr, D.H. Efficient materially nonlinear μFE solver for simulations of trabecular bone failure. Biomech. Modeling Mechanobiol. 2019, 19, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Arnold, M.; Ma, S.; Abel, R.L.; Cobb, J.P.; Hansen, U.; Boughton, O. Standardizing compression testing for measuring the stiffness of human bone. Bone Jt. Res. 2018, 7, 524–538. [Google Scholar] [CrossRef]
- Wright, T.M.; Hayes, W.C. Tensile testing of bone over a wide range of strain rates: Effects of strain rate, microstructure and density. Med. Biol. Eng. 1976, 14, 671–680. [Google Scholar] [CrossRef]
- Cole, J.H.; van der Meulen, M.C.H. Biomechanics of bone. In Osteoporosis, Contemporary Endocrinology; Leder, B.Z., Wein, M.N., Eds.; Springer: Cham, Switzerland, 2020; pp. 185–209. [Google Scholar] [CrossRef]
- Hollister, S.J.; Brennan, J.M.; Kikuchi, N. A homogenization sampling procedure for calculating trabecular bone effective stiffness and tissue level stress. J. Biomech. 1994, 27, 433–444. [Google Scholar] [CrossRef]
- Manzini, B.M.; Machado, L.M.R.; Noritomi, P.Y.; da Silva, J.V.L. Advances in Bone tissue engineering: A fundamental review. J. Biosci. 2021, 46, 1–18. [Google Scholar] [CrossRef]
- Giner, E.; Arango, C.; Vercher, A.; Fuenmayor, F.J. Numerical modelling of the mechanical behaviour of an osteon with microcracks. J. Mech. Behav. Biomed. Mater. 2014, 37, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Isaksson, P.; Ferguson, S.J.; Persson, C. Young’s modulus of trabecular bone at the tissue level: A review. Acta Biomater. 2018, 78, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ferregut, C.; Nazarian, S.; Vennalaganti, K.; Chang, C.C.; Kreinovich, V. Fast error estimates for indirect measurements: Applications to pavement engineering. Reliab. Comput. 1996, 2, 219–228. [Google Scholar] [CrossRef][Green Version]
- Turunen, M.J.; Le Cann, S.; Tudisco, E.; Lovric, G.; Patera, A.; Hall, S.A.; Isaksson, H. Sub-trabecular strain evolution in human trabecular bone. Sci. Rep. 2020, 10, 13788. [Google Scholar] [CrossRef]
- Pugno, N.M.; Ruoff, R.S. Quantized fracture mechanics. Philos. Mag. 2004, 84, 2829–2845. [Google Scholar] [CrossRef]
- Pugno, N.M. Dynamic quantized fracture mechanics. Int. J. Fract. 2006, 140, 159–168. [Google Scholar] [CrossRef]
- Novozhilov, V.V. On a necessary and sufficient criterion for brittle strength. Prik. Mat. Mek. 1969, 33, 212–222. [Google Scholar] [CrossRef]
- Cornetti, P.; Pugno, N.; Carpinteri, A.; Taylor, D. A Coupled Stress and Energy Criterion within Finite Fracture Mechanics. In Fracture of Nano and Engineering Materials and Structures; Gdoutos, E.E., Ed.; Springer: Dordrecht, The Netherlands, 2006; pp. 1107–1108. [Google Scholar] [CrossRef]
- Kolesnikov, G. Analysis of Concrete Failure on the Descending Branch of the Load-Displacement Curve. Crystals 2020, 10, 921. [Google Scholar] [CrossRef]
- Lv, J.; Zhou, T.; Du, Q.; Li, K.; Sun, K. Evaluation of Stress–Strain Behavior of Self-Compacting Rubber Lightweight Aggregate Concrete under Uniaxial Compression Loading. Materials 2019, 12, 4064. [Google Scholar] [CrossRef]
- Mudunuru, M.K.; Panda, N.; Karra, S.; Srinivasan, G.; Chau, V.T.; Rougier, E.; Hunter, A.; Viswanathan, H.S. Surrogate Models for Estimating Failure in Brittle and Quasi-Brittle Materials. Appl. Sci. 2019, 9, 2706. [Google Scholar] [CrossRef]
- Baldonedo, J.; Fernández, J.R.; López-Campos, J.A.; Segade, A. Analysis of Damage Models for Cortical Bone. Appl. Sci. 2019, 9, 2710. [Google Scholar] [CrossRef]
- Gao, H. Application of fracture mechanics concepts to hierarchical biomechanics of bone and bone-like materials. Int. J. Fract. 2006, 138, 101–137. [Google Scholar] [CrossRef]
- Kefalas, V.; Eftaxiopoulos, D.A. Experimental study of cancellous bone under large strains and a constitutive probabilistic model. J. Mech. Behav. Biomed. Mater. 2012, 6, 41–52. [Google Scholar] [CrossRef]
- Ural, A. Advanced Modeling Methods—Applications to Bone Fracture Mechanics. Curr. Osteoporos. Rep. 2020, 18, 568–576. [Google Scholar] [CrossRef]
- Menon, A.; Mehrotra, K.; Mohan, C.K.; Ranka, S. Characterization of a class of sigmoid functions with applications to neural networks. Neural Netw. 1996, 9, 819–835. [Google Scholar] [CrossRef]
- Liu, D.; He, M.; Cai, M. A damage model for modeling the complete stress–strain relations of brittle rocks under uniaxial compression. Int. J. Damage Mech. 2018, 27, 1000–1019. [Google Scholar] [CrossRef]
- Baldwin, R.; North, M.A. Stress-strain curves of concrete at high temperature—A review. Fire Saf. Sci. 1969, 785, 1. Available online: http://www.iafss.org/publications/frn/785/-1/view/frn_785.pdf (accessed on 11 March 2021).
- Yu, M.H. Advances in strength theories for materials under complex stress state in the 20th century. Appl. Mech. Rev. 2002, 55, 169–218. [Google Scholar] [CrossRef]
- Sabet, F.; Jin, O.; Koric, S.; Jasiuk, I. Nonlinear micro-CT based FE modeling of trabecular bone—Sensitivity of apparent response to tissue constitutive law and bone volume fraction. Int. J. Numer. Methods Biomed. Eng. 2018, 34, e2941. [Google Scholar] [CrossRef] [PubMed]
- Aerens, R.; Vorkov, V.; Duflou, J.R. Springback prediction and elasticity modulus variation. Procedia Manuf. 2019, 29, 185–192. [Google Scholar] [CrossRef]
- Buccino, F.; Colombo, C.; Vergani, L.M. A Review on Multiscale Bone Damage: From the Clinical to the Research Perspective. Materials 2021, 14, 1240. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-H.; Lee, D.-Y.; Jung, S.-K. Comparison of Trabecular Bone Mineral Density Measurement Using Hounsfield Unit and Trabecular Microstructure in Orthodontic Patients Using Cone-Beam Computed Tomography. Appl. Sci. 2021, 11, 1028. [Google Scholar] [CrossRef]
- Stojković, N.; Perić, D.; Stojić, D.; Marković, N. New stress-strain model for concrete at high temperatures. Teh. Vjesn. 2017, 24, 863–868. [Google Scholar] [CrossRef][Green Version]
- Khoroshilov, K.V.; Katarov, V.K.; Gavrilov, T.A.; Kolesnikov, G.N. Strength and Elastic Modulus of Frozen Sandy Soil as a Material for a Logging Road. Resour. Technol. 2019, 16, 33–43. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolesnikov, G.; Meltser, R. A Damage Model to Trabecular Bone and Similar Materials: Residual Resource, Effective Elasticity Modulus, and Effective Stress under Uniaxial Compression. Symmetry 2021, 13, 1051. https://doi.org/10.3390/sym13061051
Kolesnikov G, Meltser R. A Damage Model to Trabecular Bone and Similar Materials: Residual Resource, Effective Elasticity Modulus, and Effective Stress under Uniaxial Compression. Symmetry. 2021; 13(6):1051. https://doi.org/10.3390/sym13061051
Chicago/Turabian StyleKolesnikov, Gennady, and Rudolf Meltser. 2021. "A Damage Model to Trabecular Bone and Similar Materials: Residual Resource, Effective Elasticity Modulus, and Effective Stress under Uniaxial Compression" Symmetry 13, no. 6: 1051. https://doi.org/10.3390/sym13061051
APA StyleKolesnikov, G., & Meltser, R. (2021). A Damage Model to Trabecular Bone and Similar Materials: Residual Resource, Effective Elasticity Modulus, and Effective Stress under Uniaxial Compression. Symmetry, 13(6), 1051. https://doi.org/10.3390/sym13061051





