Cytoskeletal Filaments Deep Inside a Neuron Are not Silent: They Regulate the Precise Timing of Nerve Spikes Using a Pair of Vortices
Abstract
1. Introduction
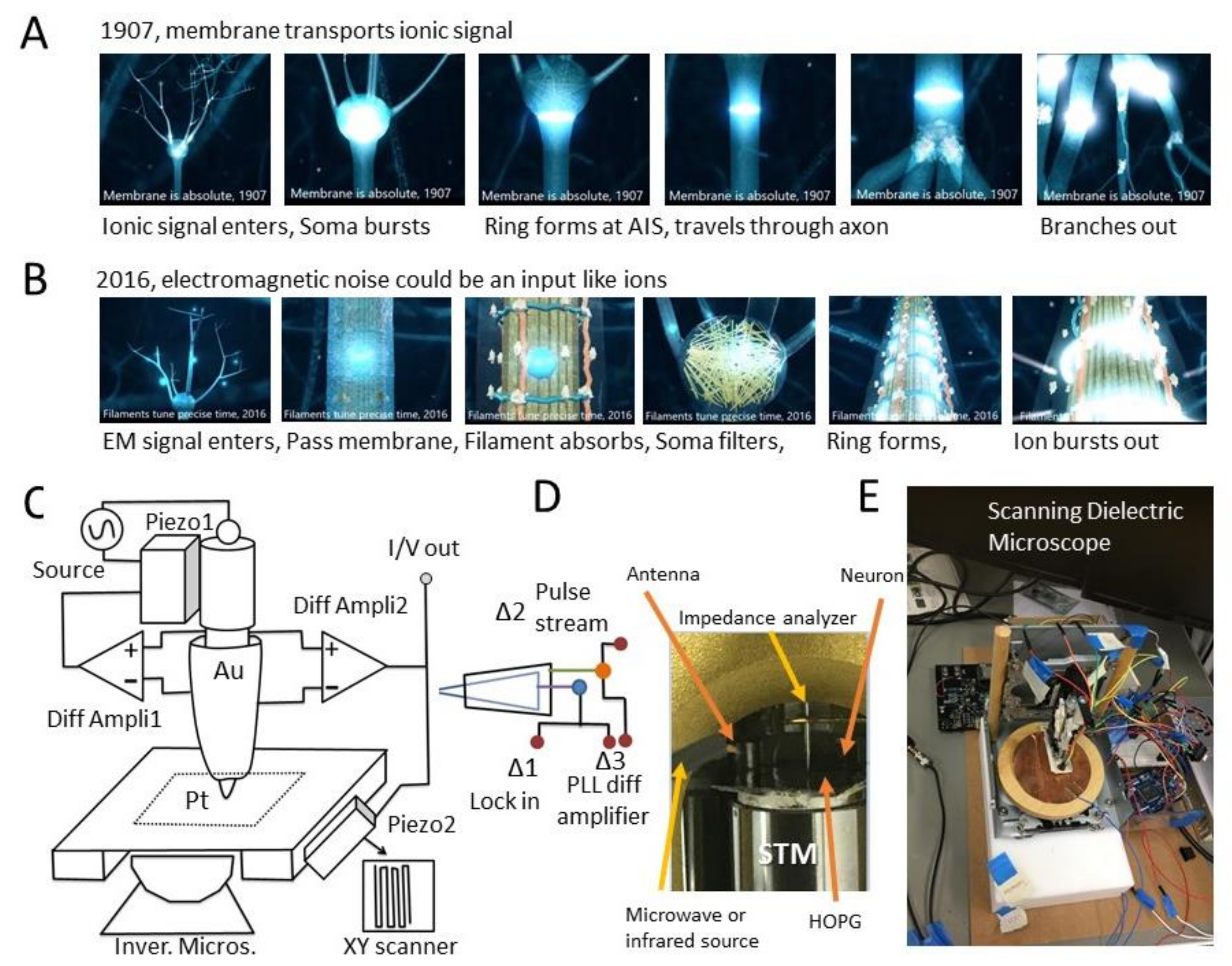
1.1. Can the Neuron’s Membrane Alone Generate and Transmit Nerve Spikes?
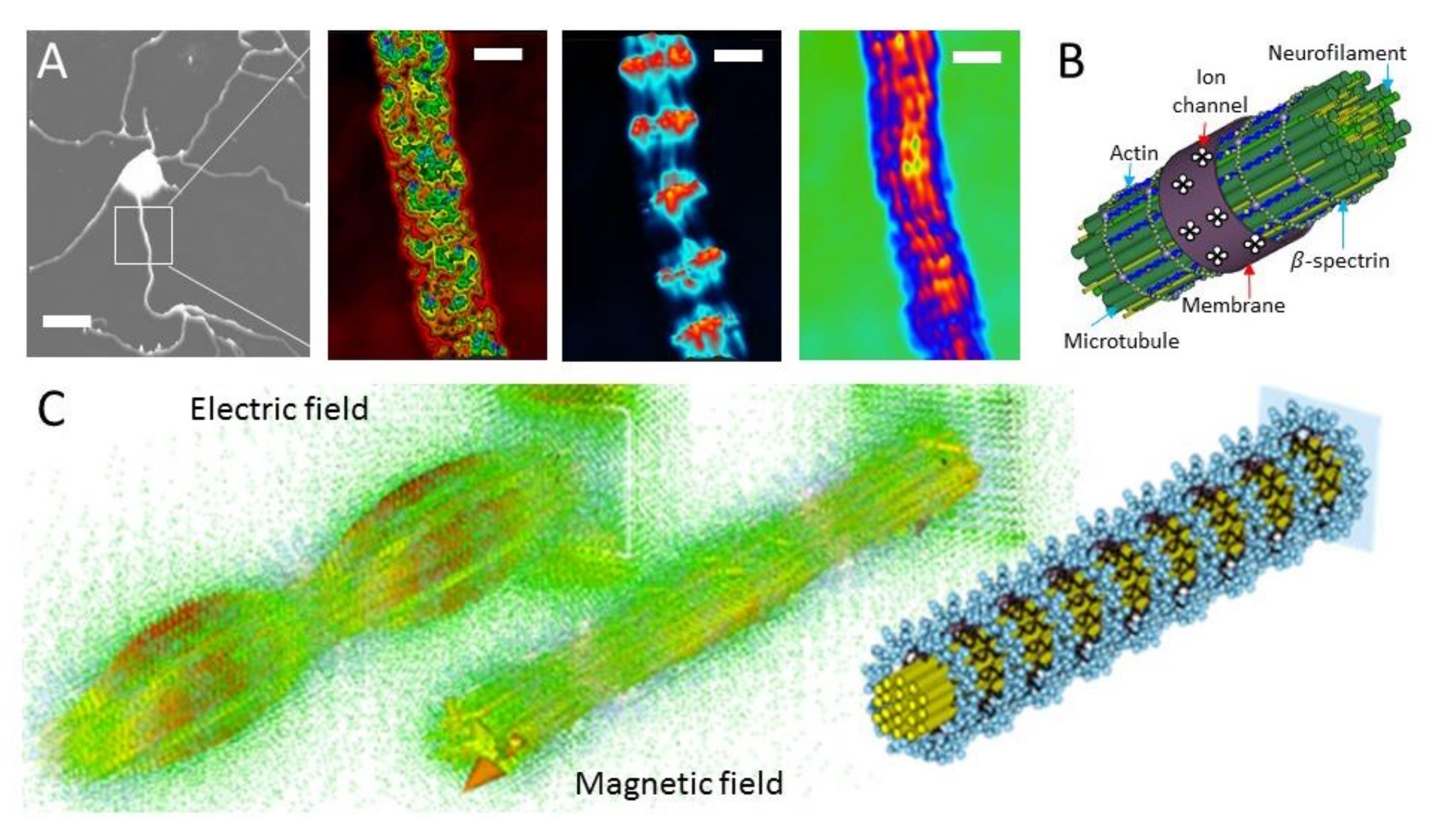
1.2. Communication of Electromagnetic Signals through the Filamentary Bundle
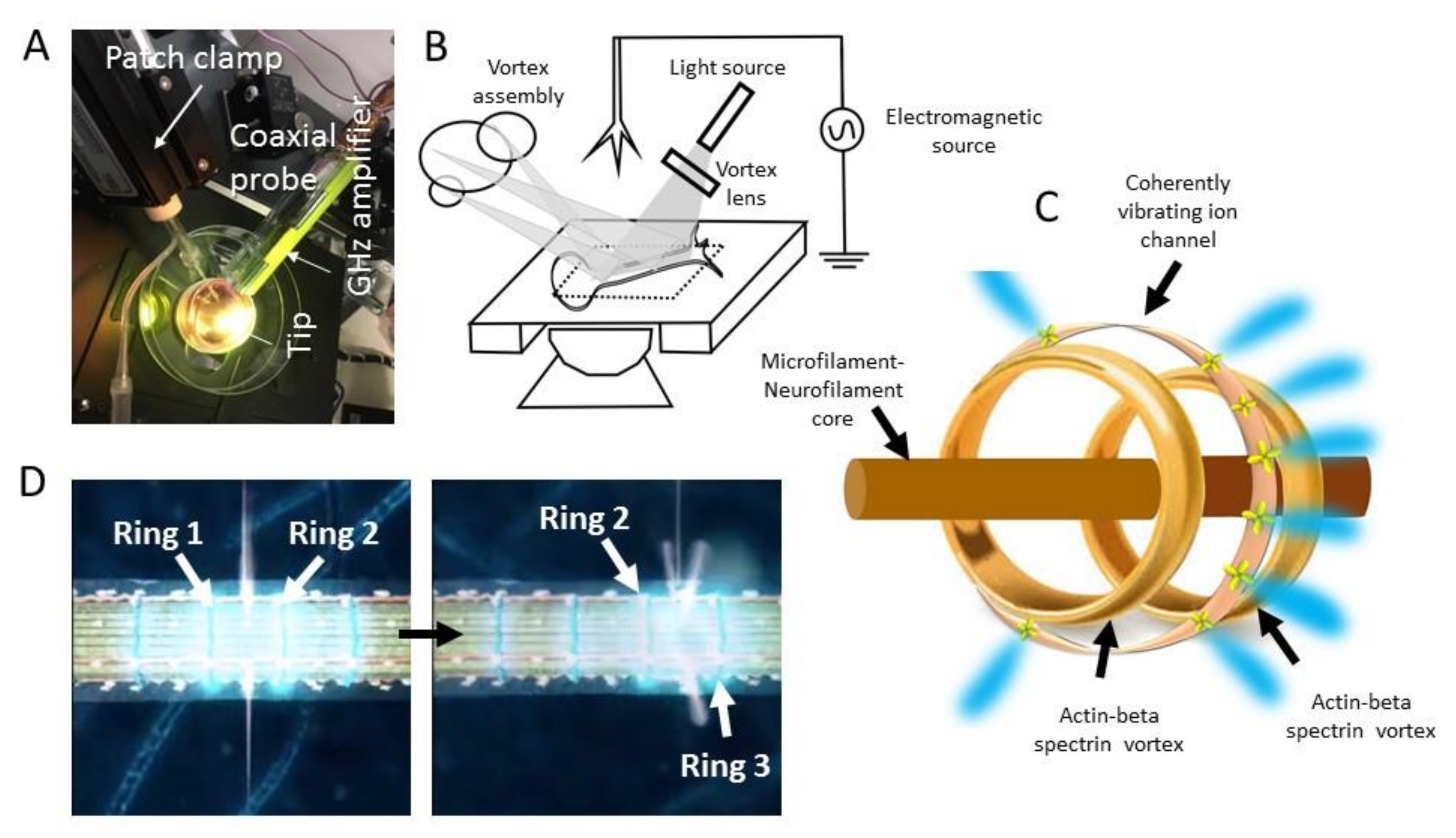
2. Experimental Details
2.1. Neuron Culture
2.2. Dielectric Resonance Imaging and Optical Vortex Study: Simultaneous Measurement of the Membrane, Beta-Spectrin, and Microtubule-Neurofilament Core
2.3. Microtubule Reconstitution from Tubulin for Deconvolution of Neuron’s Optical Vortex
2.4. Actin Filament Reconstitution from Actin and Tubulin Solution for Deconvolution of Neuron’s Optical Vortex
3. Results and Discussion
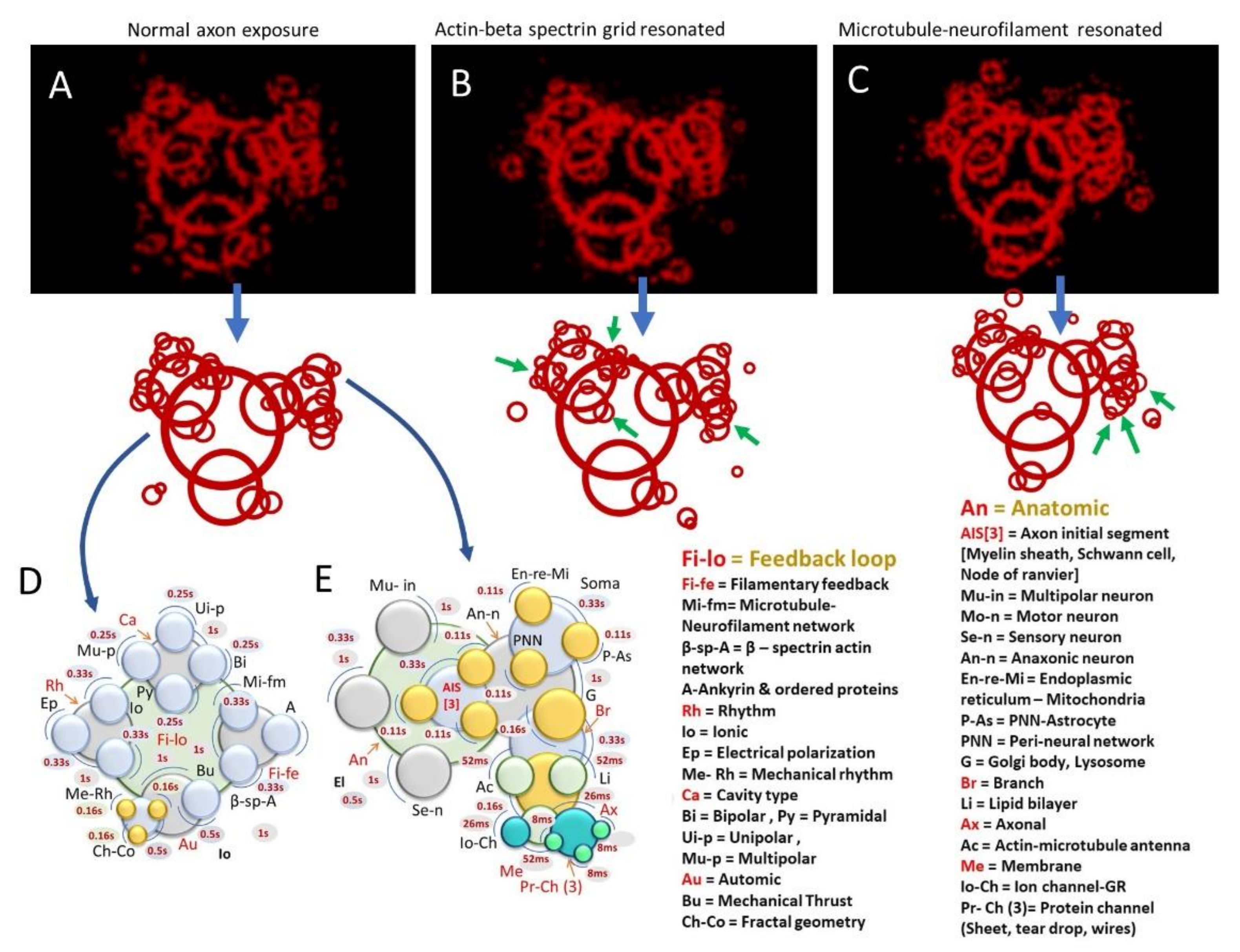
3.1. Ionic Nerve Spike Is Not a 1D Gaussian Packet but a 3D Ring of Electric Field
3.2. Wirelessly Imaging Internal Neural Components Using Scanning Dielectric Microscope Operating at Selected Frequencies
3.3. Optical Vortex: Angular Momentum Modulation, Composition and Superposition
3.4. Light–Matter Interaction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lapicque, L. Recherches quantitatives sur l’excitation électrique des nerfs traitée comme une polarisation. J. Physiol. Pathol. Gen. 1907, 9, 620–635. [Google Scholar]
- Brunel, N.; Van Rossum, M.C.W. Lapicque’s 1907 paper: From frogs to integrate-and-fire. Biol. Cybern. 2007, 97, 337–339. [Google Scholar] [CrossRef]
- Katz, B.; Schmitt, O.H. Electric Interaction Between Two Adjacent Nerve Fibers. J. Physiol. 1940, 97, 471–488. [Google Scholar] [CrossRef]
- Baker, P.F.; Hodgkin, A.L.; Shaw, T.I. Replacement of the Protoplasm of a Giant Nerve Fibre with Artificial Solutions. Nat. Cell Biol. 1961, 190, 885–887. [Google Scholar] [CrossRef]
- Baker, P.F.; Hodgkin, A.L.; Shaw, T.I. Replacement of the axoplasm of giant nerve fibres with artificial solutions. J. Physiol. 1962, 164, 330–354. [Google Scholar] [CrossRef] [PubMed]
- Arvanitaki, A. Effects Evoked in an Axon by the Activity of a Contigious One. J. Neurophysiol. 1942, 5, 89–108. [Google Scholar] [CrossRef]
- Ramon, F.; Moore, J.W. Ephaptic transmission in squid giant axons. Am. J. Physiol. Physiol. 1978, 234, C162–C169. [Google Scholar] [CrossRef]
- Radman, T.; Su, Y.; An, J.H.; Parra, L.C.; Bikson, M. Spike Timing Amplifies the Effect of Electric Fields on Neurons: Implications for Endogenous Field Effects. J. Neurosci. 2007, 27, 3030–3036. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Momose-Sato, Y.; Sato, K.; Greer, J.J. Rhythmic Neuronal Discharge in the Medulla and Spinal Cord of Fetal Rats in the Absence of Synaptic Transmission. J. Neurophysiol. 2006, 95, 527–534. [Google Scholar] [CrossRef]
- Dudek, F.E.; Yasumura, T.; Rash, J.E. ‘Non-Synaptic‘ mechanisms in seizures and epileptogenesis. Cell Biol. Int. 1998, 22, 793–805. [Google Scholar] [CrossRef]
- Alekseev, S.I.; Ziskin, M.C. Millimeter microwave effect on ion transport across lipid bilayer membranes. Bioelectromagnetics 1995, 16, 124–131. [Google Scholar] [CrossRef]
- Maskey, D.; Kim, M.; Aryal, B.; Pradhan, J.; Choi, I.-Y.; Park, K.-S.; Son, T.; Hong, S.-Y.; Kim, S.B.; Kim, H.G.; et al. Effect of 835 MHz radiofrequency radiation exposure on calcium binding proteins in the hippocampus of the mouse brain. Brain Res. 2010, 1313, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Fraser, A.; Frey, A.H. Electromagnetic Emission at Micron Wavelengths from Active Nerves. Biophys. J. 1968, 8, 731–734. [Google Scholar] [CrossRef]
- Ratte, S.; Lankarany, M.; Rho, Y.A.; Patterson, A.; Prescott, S.A. Subthreshold membrane currents confer distinct tuning properties that enable neurons to encode the integral or derivative of their input. Front. Cell Neurosci. 2015, 8, 452. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Han, Z.; Platisa, J.; Wooltorton, J.R.; Cohen, L.B.; Pieribone, V.A. Single Action Potentials and Subthreshold Electrical Events Imaged in Neurons with a Fluorescent Protein Voltage Probe. Neuron 2012, 75, 779–785. [Google Scholar] [CrossRef]
- Duke, A.R.; Jenkins, M.W.; Lu, H.; McManus, J.M.; Chiel, H.J.; Jansen, E.D. Transient and selective suppression of neural activity with infrared light. Sci. Rep. 2013, 3, srep02600. [Google Scholar] [CrossRef]
- Howarth, J.V.; Keynes, R.D.; Ritchie, J.M.; Von Muralt, A. The heat production associated with the passage of a single impulse in pike olfactory nerve fibres. J. Physiol. 1975, 249, 349–368. [Google Scholar] [CrossRef]
- Westerfield, M.; Joyner, R.W.; Moore, J.W. Temperature-sensitive conduction failure at axon branch points. J. Neurophysiol. 1978, 41, 1–8. [Google Scholar] [CrossRef]
- Abbott, B.C.; Hill, A.V.; Howarth, J.V. The positive and negative heat production associated with a nerve impulse. Proc. R. Soc. London. Ser. B Boil. Sci. 1958, 148, 149–187. [Google Scholar] [CrossRef]
- Buzatu, S. The temperature-induced changes in membrane potential. Riv. Boil. 2010, 102, 199–217. [Google Scholar]
- Maheswari, U.; Tatsumi, H.; Katayama, Y.; Ohtsua, M. Observation of subcellular nanostructure of single neurons with an illumination mode photon scanning tunneling microscope. Opt. Commun. 1995, 120, 325–334. [Google Scholar] [CrossRef]
- Hirokawa, N. From electron microscopy to molecular cell biology, molecular genetics and structural biology: Intracellular transport and kinesin superfamily proteins, KIFs: Genes, structure, dynamics and functions. Microscopy 2011, 60, S63–S92. [Google Scholar] [CrossRef] [PubMed]
- Hirokawa, N.; Pfister, K.; Yorifuji, H.; Wagner, M.C.; Brady, S.T.; Bloom, G.S. Submolecular domains of bovine brain kinesin identified by electron microscopy and monoclonal antibody decoration. Cell 1989, 56, 867–878. [Google Scholar] [CrossRef]
- Hirokawa, N.; Shiomura, Y.; Okabe, S. Tau proteins: The molecular structure and mode of binding on microtubules. J. Cell Biol. 1988, 107, 1449–1459. [Google Scholar] [CrossRef]
- Chen, J.; Kanai, Y.; Cowan, N.J.; Hirokawa, N. Projection domains of MAP2 and tau determine spacings between microtubules in dendrites and axons. Nat. Cell Biol. 1992, 360, 674–677. [Google Scholar] [CrossRef] [PubMed]
- Stone, M.C.; Roegiers, F.; Rolls, M.M. Microtubules Have Opposite Orientation in Axons and Dendrites of Drosophila Neurons. Mol. Biol. Cell 2008, 19, 4122–4129. [Google Scholar] [CrossRef] [PubMed]
- Heidemann, S.R.; Landers, J.M.; A Hamborg, M. Polarity orientation of axonal microtubules. J. Cell Biol. 1981, 91, 661–665. [Google Scholar] [CrossRef]
- Heidemann, S.R.; A Hamborg, M.; Thomas, S.J.; Song, B.; Lindley, S.; Chu, D. Spatial organization of axonal microtubules. J. Cell Biol. 1984, 99, 1289–1295. [Google Scholar] [CrossRef] [PubMed]
- Baas, P.W.; Deitch, J.S.; Black, M.M.; Banker, G.A. Polarity orientation of microtubules in hippocampal neurons: Uniformity in the axon and nonuniformity in the dendrite. Proc. Natl. Acad. Sci. USA 1988, 85, 8335–8339. [Google Scholar] [CrossRef]
- Xu, K.; Zhong, G.; Zhuang, X. Actin, spectrin and associated proteins form a periodic cytoskeleton structure in axons. Science 2013, 339, 452–456. [Google Scholar] [CrossRef]
- He, J.; Zhou, R.; Heller, E.; Freeman, M.R.; Shen, K.; Maniatis, T.; Tessier-Lavigne, M.; Zhuang, X.; Wu, Z.; Carrasco, M.A.; et al. Prevalent presence of periodic actin–spectrin-based membrane skeleton in a broad range of neuronal cell types and animal species. Proc. Natl. Acad. Sci. USA 2016, 113, 6029–6034. [Google Scholar] [CrossRef] [PubMed]
- D’Este, E.; Kamin, D.; Velte, C.; Göttfert, F.; Simons, M.; Hell, S.W. Subcortical cytoskeleton periodicity throughout the nervous system. Sci. Rep. 2016, 6, 22741. [Google Scholar] [CrossRef] [PubMed]
- Gervasi, M.G.; Xu, X.; Carbajal-Gonzalez, B.; Buffone, M.G.; Visconti, P.E.; Krapf, D. The actin cytoskeleton of the mouse sperm flagellum is organizedin a helical structure. J. Cell Sci. 2018, 131, jcs215897. [Google Scholar] [CrossRef]
- D’Este, E.; Kamin, D.; Göttfert, F.; El-Hady, A.; Hell, S.W. STED Nanoscopy Reveals the Ubiquity of Subcortical Cytoskeleton Periodicity in Living Neurons. Cell Rep. 2015, 10, 1246–1251. [Google Scholar] [CrossRef] [PubMed]
- Seidl, A.H.; Rubel, E.W.; Harris, D.M. Mechanisms for Adjusting Interaural Time Differences to Achieve Binaural Coincidence Detection. J. Neurosci. 2010, 30, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Lundstrom, B.N.; Famulare, M.; Sorensen, L.B.; Spain, W.J.; Fairhall, A.L. Sensitivity of firing rate to input fluctuations depends on time scale separation between fast and slow variables in single neurons. J. Comput. Neurosci. 2009, 27, 277–290. [Google Scholar] [CrossRef]
- Usher, M.; Stemmler, M.; Koch, C.; Olami, Z. Network Amplification of Local Fluctuations Causes High Spike Rate Variability, Fractal Firing Patterns and Oscillatory Local Field Potentials. Neural Comput. 1994, 6, 795–836. [Google Scholar] [CrossRef]
- Ghosh, S.; Sahu, S.; Agrawal, L.; Shiga, T.; Bandyopadhyay, A. Inventing a co-axial atomic resolution patch clamp to study a single resonating protein complex and ultra-low power communication deep inside a living neuron cell. J. Integr. Neurosci. 2016, 15, 1–31. [Google Scholar] [CrossRef]
- Agrawal, L.; Sahu, S.; Ghosh, S.; Shiga, T.; Fujita, D.; Bandyopadhyay, A. Inventing atomic resolution scanning dielectric microscopy to see a single protein complex operation live at resonance in a neuron without touching or adulterating the cell. J. Integr. Neurosci. 2016, 15, 435–462. [Google Scholar] [CrossRef]
- Singh, P.; Ghosh, S.; Sahoo, P.; Ray, K.; Fujita, D.; Bandyopadhyay, A. Reducing the Dimension of a Patch-Clamp to the Smallest Phyisical Limit using a Coaxial Atom Probe. Prog. Electromagn. Res. B 2020, 89, 29–44. [Google Scholar] [CrossRef]
- Saxena, K.; Singh, P.; Sahoo, P.; Sahu, S.; Ghosh, S.; Ray, K.; Fujita, D.; Bandyopadhyay, A. Fractal, Scale Free Electromagnetic Resonance of a Single Brain Extracted Microtubule Nanowire, a Single Tubulin Protein and a Single Neuron. Fractal Fract. 2020, 4, 11. [Google Scholar] [CrossRef]
- Amari, S. Topographic organization of nerve fields. Bull. Math. Biol. 1980, 42, 339–364. [Google Scholar] [CrossRef]
- Giustia, C.; Pastalkovac, E.; Curto, C.; Itskov, V. Clique topology reveals intrinsic geometric structure in neural correlations. Proc. Natl. Acad. Sci. USA 2015, 112, 13455–13460. [Google Scholar] [CrossRef] [PubMed]
- Best, E.N. Null Space and Phrase Resetting Curves for the Hodgkin-Huxley Equations. Ph.D. Thesis, Purdue University, West Lafayette, IN, USA, 1976. [Google Scholar]
- Guckenheimer, J.; Labouriau, J.S.; Labouriau, I. Bifurcation of the Hodgkin and Huxley equations: A new twist. Bull. Math. Biol. 1993, 55, 937–952. [Google Scholar] [CrossRef]
- Sardi, S.; Vardi, R.; Sheinin, A.; Goldental, A.; Kanter, I. New Types of Experiments Reveal that a Neuron Functions as Multiple Independent Threshold Units. Sci. Rep. 2017, 7, 1–17. [Google Scholar] [CrossRef]
- McCormick, D.A.; Shu, Y.; Yu, Y. Neurophysiology: Hodgkin and Huxley model–still standing? Nature 2007, 445, E1–E2. [Google Scholar] [CrossRef] [PubMed]
- Pethig, R. Dielectric and Electronic Properties of Biological Materials; Wiley: New York, NY, USA, 1979; p. 139. [Google Scholar]
- Asami, K. The scanning dielectric microscope. Meas. Sci. Technol. 1994, 5, 589–592. [Google Scholar] [CrossRef]
- Nixon, R.A.; Shea, T.B. Dynamics of neuronal intermediate filaments: A developmental perspective. Cell Motil. Cytoskelet. 1992, 22, 81–91. [Google Scholar] [CrossRef]
- Zhong, G.; He, J.; Zhou, R.; Lorenzo, D.; Babcock, H.P.; Bennett, V.; Zhuang, X. Developmental mechanism of the periodic membrane skeleton in axons. eLife 2014, 3, 04581. [Google Scholar] [CrossRef]
- Qiu, C.; Shivacharan, R.S.; Zhang, M.; Durand, D.M. Can Neural Activity Propagate by Endogenous Electrical Field? J. Neurosci. 2015, 35, 15800–15811. [Google Scholar] [CrossRef] [PubMed]
- Zaromytidou, A.-I. Synchronizing actin and microtubules for axonal branching. Nat. Cell Biol. 2012, 14, 792. [Google Scholar] [CrossRef]
- Schubert, T.; Akopian, A. Actin filaments regulate voltage-gated ion channels in salamander retinal ganglion cells. Neuroscience 2004, 125, 583–590. [Google Scholar] [CrossRef]
- Vollmer, F.; Braun, D.; Libchaber, A.J.; Khoshsima, M.; Teraoka, I.; Arnold, S. Protein detection by optical shift of a resonant microcavity. Appl. Phys. Lett. 2002, 80, 4057–4059. [Google Scholar] [CrossRef]
- Singh, P.; Saxena, K.; Singhania, A.; Sahoo, P.; Ghosh, S.; Chhajed, R.; Ray, K.; Fujita, D.; Bandyopadhyay, A. A Self-Operating Time Crystal Model of the Human Brain: Can We Replace Entire Brain Hardware with a 3D Fractal Architecture of Clocks Alone? Information 2020, 11, 238. [Google Scholar] [CrossRef]
- Bandyopadhyay, A. Nanobrain: The Making of an Artificial Brain from a Time Crystal; Taylor & Francis Inc. Imprint CRC Press Inc.: Boca Raton, FL, USA, 2020; pp. 1–336. [Google Scholar]
- Andersen, S.S.L.; Jackson, A.D.; Heimburg, T. Towards a thermodynamic theory of nerve pulse propagation. Prog. Neurobiol. 2009, 88, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Hasenstaub, A.; Otte, S.; Callaway, E.; Sejnowski, T.J. Metabolic cost as a unifying principle governing neuronal biophysics. Proc. Natl. Acad. Sci. USA 2010, 107, 12329–12334. [Google Scholar] [CrossRef]
- Singh, P.; Saxena, K.; Sahoo, P.; Ghosh, S.; Bandyopadhyay, A. Electrophysiology using coaxial atom probe array: Live imaging reveals hidden circuits of a hippocampal neural network. J. Neurophysiol. 2021. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, P.; Sahoo, P.; Saxena, K.; Manna, J.S.; Ray, K.; Ghosh, S.; Bandyopadhyay, A. Cytoskeletal Filaments Deep Inside a Neuron Are not Silent: They Regulate the Precise Timing of Nerve Spikes Using a Pair of Vortices. Symmetry 2021, 13, 821. https://doi.org/10.3390/sym13050821
Singh P, Sahoo P, Saxena K, Manna JS, Ray K, Ghosh S, Bandyopadhyay A. Cytoskeletal Filaments Deep Inside a Neuron Are not Silent: They Regulate the Precise Timing of Nerve Spikes Using a Pair of Vortices. Symmetry. 2021; 13(5):821. https://doi.org/10.3390/sym13050821
Chicago/Turabian StyleSingh, Pushpendra, Pathik Sahoo, Komal Saxena, Jhimli Sarkar Manna, Kanad Ray, Subrata Ghosh, and Anirban Bandyopadhyay. 2021. "Cytoskeletal Filaments Deep Inside a Neuron Are not Silent: They Regulate the Precise Timing of Nerve Spikes Using a Pair of Vortices" Symmetry 13, no. 5: 821. https://doi.org/10.3390/sym13050821
APA StyleSingh, P., Sahoo, P., Saxena, K., Manna, J. S., Ray, K., Ghosh, S., & Bandyopadhyay, A. (2021). Cytoskeletal Filaments Deep Inside a Neuron Are not Silent: They Regulate the Precise Timing of Nerve Spikes Using a Pair of Vortices. Symmetry, 13(5), 821. https://doi.org/10.3390/sym13050821








