Temporal Dynamics of Event-Related Potentials during Inhibitory Control Characterize Age-Related Neural Compensation
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Materials
2.2.1. Stop-Signal Task
2.2.2. EEG Data Acquisition and ERPs
2.2.3. Procedure
3. Results
3.1. Descriptive Statistics and Excluded Data
3.2. Task Performance Analyses
3.3. ERP Analyses
3.3.1. N200 Component
3.3.2. P300 Component
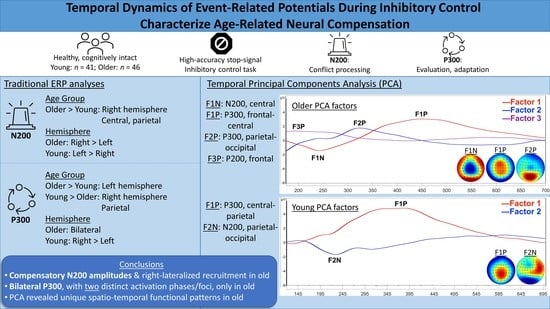
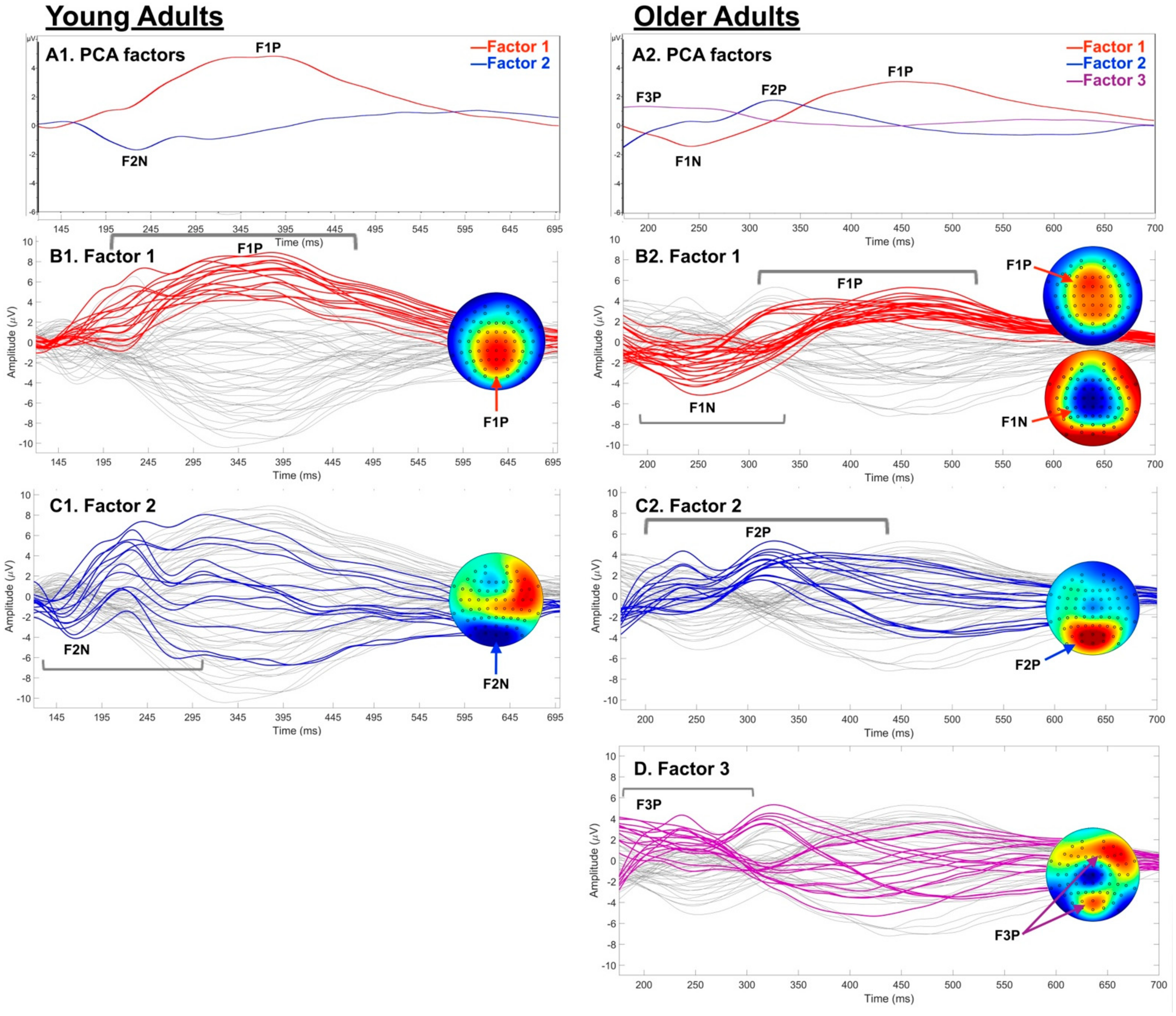
3.3.3. Post-Hoc Temporal PCA
4. Discussion
4.1. N200 Age Group Differences
4.2. P300 Age Group Differences
4.3. Spatio-Temporal Age Group Differences
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salthouse, T.A. When Does Age-Related Cognitive Decline Begin? Neurobiol. Aging 2009, 30, 507–514. [Google Scholar] [CrossRef]
- Verhaeghen, P.; Salthouse, T.A. Meta-Analyses of age–Cognition Relations in Adulthood: Estimates of Linear and Nonlinear Age Effects and Structural Models. Psychol. Bull. 1997, 122, 231–249. [Google Scholar] [CrossRef]
- Deary, I.J.; Corley, J.; Gow, A.J.; Harris, S.E.; Houlihan, L.M.; Marioni, R.E.; Penke, L.; Rafnsson, S.B.; Starr, J.M. Age-Associated Cognitive Decline. Br. Med. Bull. 2009, 92, 135–152. [Google Scholar] [CrossRef]
- Munakata, Y.; Herd, S.A.; Chatham, C.H.; Depue, B.E.; Banich, M.T.; O’Reilly, R.C. A Unified Framework for Inhibitory Control. Trends Cognit. Sci. 2011, 15, 453–459. [Google Scholar] [CrossRef]
- West, R.; Alain, C. Age-Related Decline in Inhibitory Control Contributes to the Increased Stroop Effect Observed in Older Adults. Psychophysiology 2000, 37, 179–189. [Google Scholar] [CrossRef]
- Sweeney, J.A.; Rosano, C.; Berman, R.A.; Luna, B. Inhibitory Control of Attention Declines More Than Working Memory During Normal Aging. Neurobiol. Aging 2001, 22, 39–47. [Google Scholar] [CrossRef]
- Pires, L.; Leitão, J.; Guerrini, C.; Simões, M.R. Event-Related Brain Potentials in the Study of Inhibition: Cognitive Control, Source Localization and Age-Related Modulations. Neuropsychol. Rev. 2014, 24, 461–490. [Google Scholar] [CrossRef]
- Votruba, K.L.; Rapport, L.J.; Vangel, S.J., Jr.; Hanks, R.A.; Lequerica, A.; Whitman, R.D.; Langenecker, S. Impulsivity and Traumatic Brain Injury: The Relations among Behavioral Observation, Performance Measures, and Rating Scales. J. Head Trauma Rehabilit. 2008, 23, 65–73. [Google Scholar] [CrossRef]
- Rubia, K.; Russell, T.; Overmeyer, S.; Brammer, M.J.; Bullmore, E.T.; Sharma, T.; Simmons, A.; Williams, S.C.; Giampietro, V.; Andrew, C.M. Mapping Motor Inhibition: Conjunctive Brain Activations across Different Versions of Go/No-Go and Stop Tasks. Neuroimage 2001, 13, 250–261. [Google Scholar] [CrossRef]
- Elverman, K.H.; Paitel, E.R.; Figueroa, C.M.; McKindles, R.J.; Nielson, K.A. Event-Related Potentials, Inhibition and Risk for Alzheimer’s Disease among Cognitively Intact Elders. J. Alzheimer’s Dis. 2021, 80, 1413–1428. [Google Scholar] [CrossRef]
- Zhang, R.; Geng, X.; Lee, T.M. Large-Scale Functional Neural Network Correlates of Response Inhibition: An fMRI Meta-Analysis. Brain Struct. Funct. 2017, 222, 3973–3990. [Google Scholar] [CrossRef]
- Slotnick, S.D. fMRI Versus ERPs. In Cognitive Neuroscience of Memory; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar]
- Kok, A.; Ramautar, J.R.; De Ruiter, M.B.; Band, G.P.; Ridderinkhof, K.R. ERP Components Associated with Successful and Unsuccessful Stopping in a Stop-Signal Task. Psychophysiology 2004, 41, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Chevrier, A.D.; Noseworthy, M.D.; Schachar, R. Dissociation of Response Inhibition and Performance Monitoring in the Stop Signal Task Using Event-Related fMRI. Hum. Brain Mapp. 2007, 28, 1347–1358. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.; Lin, Y.-C. Stopping Ability in Younger and Older Adults: Behavioral and Event-Related Potential. Cognit. Affect. Behav. Neurosci. 2017, 17, 348–363. [Google Scholar] [CrossRef] [PubMed]
- Huster, R.; Westerhausen, R.; Pantev, C.; Konrad, C. The Role of the Cingulate Cortex as Neural Generator of the N200 and P300 in a Tactile Response Inhibition Task. Hum. Brain Mapp. 2010, 31, 1260–1271. [Google Scholar] [CrossRef] [PubMed]
- Enriquez-Geppert, S.; Konrad, C.; Pantev, C.; Huster, R.J. Conflict and Inhibition Differentially Affect the N200/P300 Complex in a Combined Go/Nogo and Stop-Signal Task. Neuroimage 2010, 51, 877–887. [Google Scholar] [CrossRef]
- Huster, R.J.; Enriquez-Geppert, S.; Lavallee, C.F.; Falkenstein, M.; Herrmann, C.S. Electroencephalography of Response Inhibition Tasks: Functional Networks and Cognitive Contributions. Int. J. Psychophysiol. 2013, 87, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, M.; Marzinzik, F.; Weisbrod, M.; Scherg, M.; Spitzer, M. The Time Course of Brain Activations During Response Inhibition: Evidence from Event-Related Potentials in a Go/No Go task. Neuroreport 1998, 9, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Falkenstein, M.; Hoormann, J.; Hohnsbein, J. Inhibition-Related ERP Components: Variation with Modality, Age, and Time-on-Task. J. Psychophysiol. 2002, 16, 167. [Google Scholar] [CrossRef]
- Falkenstein, M.; Hoormann, J.; Hohnsbein, J. ERP Components in Go/No-Go Tasks and Their Relation to Inhibition. Acta Psychol. 1999, 101, 267–291. [Google Scholar] [CrossRef]
- Smith, J.L.; Johnstone, S.J.; Barry, R.J. Movement-Related Potentials in the Go/No-Go Task: The P3 Reflects Both Cognitive and Motor Inhibition. Clin. Neurophysiol. 2008, 119, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Groom, M.J.; Cragg, L. Differential Modulation of the N2 and P3 Event-Related Potentials by Response Conflict and Inhibition. Brain Cognit. 2015, 97, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Huster, R.J.; Messel, M.S.; Thunberg, C.; Raud, L. The P300 as Marker of Inhibitory Control–Fact or Fiction? Cortex 2020, 132, 334–348. [Google Scholar] [CrossRef] [PubMed]
- Fallgatter, A.J.; Strik, W.K. The NoGo-Anteriorization as a Neurophysiological Standard-Index for Cognitive Response Control. Int. J. Psychophysiol. 1999, 32, 233–238. [Google Scholar] [CrossRef]
- Salisbury, D.F.; Griggs, C.B.; Shenton, M.E.; McCarley, R.W. The NoGo P300 Anteriorization Effect and Response Inhibition. Clin. Neurophysiol. 2004, 115, 1550–1558. [Google Scholar] [CrossRef][Green Version]
- González-Villar, A.J.; Bonilla, F.M.; Carrillo-de-la-Peña, M.T. When the Brain Simulates Stopping: Neural Activity Recorded During Real and Imagined Stop-Signal Tasks. Cognit. Affect. Behav. Neurosci. 2016, 16, 825–835. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vallesi, A. Targets and Non-Targets in the Aging Brain: A Go/Nogo Event-Related Potential Study. Neurosci. Lett. 2011, 487, 313–317. [Google Scholar] [CrossRef]
- Vallesi, A.; Stuss, D.T.; McIntosh, A.R.; Picton, T.W. Age-Related Differences in Processing Irrelevant Information: Evidence from Event-Related Potentials. Neuropsychology 2009, 47, 577–586. [Google Scholar] [CrossRef]
- Hong, X.; Sun, J.; Bengson, J.J.; Tong, S. Age-Related Spatiotemporal Reorganization During Response Inhibition. Int. J. Psychophysiol. 2014, 93, 371–380. [Google Scholar] [CrossRef]
- Pfefferbaum, A.; Ford, J.M. ERPs to Stimuli Requiring Response Production and Inhibition: Effects of Age, Probability and Visual Noise. Electroencephalogr. Clin. Neurophysiol. 1988, 71, 55–63. [Google Scholar] [CrossRef]
- Reuter-Lorenz, P.A.; Park, D.C. How Does It STAC up? Revisiting the Scaffolding Theory of Aging and Cognition. Neuropsychol. Rev. 2014, 24, 355–370. [Google Scholar] [CrossRef] [PubMed]
- Cabeza, R.; Anderson, N.D.; Locantore, J.K.; McIntosh, A.R. Aging Gracefully: Compensatory Brain Activity in High-Performing Older Adults. Neuroimage 2002, 17, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.W.; Dennis, N.A.; Daselaar, S.M.; Fleck, M.S.; Cabeza, R. Que PASA? The Posterior–Anterior Shift in Aging. Cereb. Cortex 2008, 18, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Nielson, K.A.; Langenecker, S.A.; Garavan, H. Differences in the Functional Neuroanatomy of Inhibitory Control across the Adult Life Span. Psychol. Aging 2002, 17, 56. [Google Scholar] [CrossRef]
- Langenecker, S.A.; Nielson, K.A. Frontal Recruitment During Response Inhibition in Older Adults Replicated with fMRI. Neuroimage 2003, 20, 1384–1392. [Google Scholar] [CrossRef]
- Langenecker, S.A.; Nielson, K.A.; Rao, S.M. fMRI of Healthy Older Adults During Stroop Interference. Neuroimage 2004, 21, 192–200. [Google Scholar] [CrossRef]
- Kleerekooper, I.; van Rooij, S.J.; van den Wildenberg, W.P.; de Leeuw, M.; Kahn, R.S.; Vink, M. The Effect of Aging on Fronto-striatal Reactive and Proactive Inhibitory Control. Neuroimage 2016, 132, 51–58. [Google Scholar] [CrossRef]
- Sebastian, A.; Baldermann, C.; Feige, B.; Katzev, M.; Scheller, E.; Hellwig, B.; Lieb, K.; Weiller, C.; Tüscher, O.; Klöppel, S. Differential Effects of Age on Subcomponents of Response Inhibition. Neurobiol. Aging 2013, 34, 2183–2193. [Google Scholar] [CrossRef]
- Cabeza, R. Hemispheric Asymmetry Reduction in Older Adults: The HAROLD Model. Psychol. Aging 2002, 17, 85–100. [Google Scholar] [CrossRef]
- Luck, S.J. An Introduction to the Event-Related Potential Technique; Massachusetts Institute of Technology Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Patel, S.H.; Azzam, P.N. Characterization of N200 and P300: Selected Studies of the Event-Related Potential. Int. J. Med. Sci. 2005, 2, 147–154. [Google Scholar] [CrossRef]
- Koen, J.D.; Rugg, M.D. Neural Dedifferentiation in the Aging Brain. Trends Cognit. Sci. 2019, 23, 547–559. [Google Scholar] [CrossRef]
- Jurica, P.J.; Leitten, C.L.; Mattis, S. Dementia Rating Scale-2: DRS-2: Professional Manual; Psychological Assessment Resources, Inc.: Lutz, FL, USA, 2001. [Google Scholar]
- Mattis, S. Dementia Rating Scale: DRS: Professional Manual; Psychological Assessment Resources, Inc.: Lutz, FL, USA, 1988. [Google Scholar]
- Monsch, A.U.; Bondi, M.W.; Salmon, D.P.; Butters, N.; Thal, L.J.; Hansen, L.A.; Wiederholt, W.C.; Cahn, D.A.; Klauber, M.R. Clinical Validity of the Mattis Dementia Rating Scale in Detecting Dementia of the Alzheimer type: A Double Cross-Validation and Application to a Community-Dwelling Sample. Arch. Neurol. 1995, 52, 899–904. [Google Scholar] [CrossRef]
- Logan, G.D. On the Ability to Inhibit Thought and Action: A Users’ Guide to the Stop Signal Paradigm. In Inhibitory Processes in Attention, Memory, and Language; Dagenbach, D., Carr, T.H., Eds.; Academic Press: Cambridge, MA, USA, 1994; pp. 189–239. [Google Scholar]
- Logan, G.D.; Cowan, W.B. On the Ability to Inhibit Thought and Action: A Theory of an Act of Control. Psychol. Rev. 1984, 91, 295. [Google Scholar] [CrossRef]
- Palmer, J.A.; Makeig, S.; Kreutz-Delgado, K.; Rao, B.D. Newton Method for the ICA Mixture Model. 2008 In Proceeding of the IEEE International Conference on Acoustics, Speech and Signal Processing, Las Vegas, NV, USA, 31 March–4 April 2008; IEEE: Piscataway, NJ, USA, 2008; pp. 1805–1808. [Google Scholar] [CrossRef]
- Tadel, F.; Baillet, S.; Mosher, J.C.; Pantazis, D.; Leahy, R.M. Brainstorm: A User-Friendly Application for MEG/EEG Analysis. Comput. Intell. Neurosci. 2011. [Google Scholar] [CrossRef] [PubMed]
- Dien, J.; Frishkoff, G.A. Principal Components Analysis of Event-Related Potential Datasets. In Event-Related Potentials: A Methods Handbook; A Bradford Book: Cambridge, MA, USA, 2005; pp. 189–208. [Google Scholar]
- Dien, J.; Beal, D.J.; Berg, P. Optimizing Principal Components Analysis of Event-Related Potentials: Matrix Type, Factor Loading Weighting, Extraction, and Rotations. Clin. Neurophysiol. 2005, 116, 1808–1825. [Google Scholar] [CrossRef]
- Makeig, S.; Debener, S.; Onton, J.; Delorme, A. Mining Event-Related Brain Dynamics. Trends Cognit. Sci. 2004, 8, 204–210. [Google Scholar] [CrossRef]
- Richards, J.E. Recovering Dipole Sources from Scalp-Recorded Event-Related-Potentials Using Component Analysis: Principal Component Analysis and Independent Component Analysis. Int. J. Psychophysiol. 2004, 54, 201–220. [Google Scholar] [CrossRef] [PubMed]
- Debener, S.; Makeig, S.; Delorme, A.; Engel, A.K. What is Novel in the Novelty Oddball Paradigm? Functional Significance of the Novelty P3 Event-Related Potential as Revealed by Independent Component Analysis. Cognit. Brain Res. 2005, 22, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Dien, J. Applying Principal Components Analysis to Event-Related Potentials: A Tutorial. Dev. Neuropsychol. 2012, 37, 497–517. [Google Scholar] [CrossRef]
- Dien, J. The ERP PCA Toolkit: An Open Source Program for Advanced Statistical Analysis of Event-Related Potential Data. J. Neurosci. Methods 2010, 187, 138–145. [Google Scholar] [CrossRef]
- Aron, A.R.; Robbins, T.W.; Poldrack, R.A. Inhibition and the Right Inferior Frontal Cortex: One Decade on. Trends Cognit. Sci. 2014, 18, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Overbye, K.; Walhovd, K.B.; Fjell, A.M.; Tamnes, C.K.; Huster, R. Electrophysiological and Behavioral Indices of Cognitive Conflict Processing across Adolescence. Dev. Cognit. Neurosci. 2021, 48. [Google Scholar] [CrossRef]
- Larson, M.J.; Clayson, P.E.; Clawson, A. Making Sense of all the Conflict: A Theoretical Review and Critique of Conflict-Related ERPs. Int. J. Psychophysiol. 2014, 93, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Botvinick, M.M.; Braver, T.S.; Barch, D.M.; Carter, C.S.; Cohen, J.D. Conflict Monitoring and Cognitive Control. Psychol. Rev. 2001, 108, 624. [Google Scholar] [CrossRef] [PubMed]
- Park, D.C.; Reuter-Lorenz, P. The Adaptive Brain: Aging and Neurocognitive Scaffolding. Annu. Rev. Psychol. 2009, 60, 173–196. [Google Scholar] [CrossRef]
- Reuter-Lorenz, P.A.; Cappell, K.A. Neurocognitive Aging and the Compensation Hypothesis. Curr. Dir. Psychol. Sci. 2008, 17, 177–182. [Google Scholar] [CrossRef]
- Cheng, C.-H.; Tsai, H.-Y.; Cheng, H.-N. The Effect of Age on N2 and P3 Components: A Meta-Analysis of Go/Nogo Tasks. Brain Cognit. 2019, 135, 103574. [Google Scholar] [CrossRef]
- Schmajuk, M.; Liotti, M.; Busse, L.; Woldorff, M.G. Electrophysiological Activity Underlying Inhibitory Control Processes in Normal Adults. Neuropsychologia 2006, 44, 384–395. [Google Scholar] [CrossRef]
- Paitel, E.R.; Samii, M.R.; Nielson, K.A. A Systematic Review of Cognitive Event-Related Potentials in Mild Cognitive Impairment and Alzheimer’s Disease. Behav. Brain Res. 2021. [Google Scholar] [CrossRef]
- Van Dinteren, R.; Huster, R.; Jongsma, M.; Kessels, R.; Arns, M. Differences in Cortical Sources of the Event-Related P3 Potential between Young and Old Participants Indicate Frontal Compensation. Brain Topogr. 2018, 31, 35–46. [Google Scholar] [CrossRef]
- Salthouse, T.A.; Atkinson, T.M.; Berish, D.E. Executive Functioning as a Potential Mediator of Age-Related Cognitive Decline in Normal Adults. J. Exp. Psychol. Gen. 2003, 132, 566. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, M.K.; Boyle, P.A. Executive Dysfunction in Alzheimer’s Disease. In Research Progress in Alzheimer’s Disease and Dementia; Sun, M.-K., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2007; Volume 1, pp. 25–38. [Google Scholar]
- Reuter, E.-M.; Voelcker-Rehage, C.; Vieluf, S.; Lesemann, F.P.; Godde, B. The P3 Parietal-to-Frontal Shift Relates to Age-Related Slowing in a Selective Attention Task. J. Psychophysiol. 2016. [Google Scholar] [CrossRef]

| Older Adults (n = 46) | Young Adults (n = 41) | |
|---|---|---|
| Age (years) | 79.63 (4.68) a | 19.95 (2.74) a |
| Education (years) | 14.80 (2.65) a | 13.77 (1.16) a |
| Sex (% female) | 73.91% | 73.17% |
| Dementia Rating Scale-2nd Edition | 138.26 (2.88) | -- |
| Brief Symptom Inventory-Depression | 0.39 (0.53) | 0.56 (0.61) |
| Older Adults (n = 46) | Young Adults (n = 41) | |
|---|---|---|
| Go Task (prepotency): | ||
| % Correct Target Trials (PCTT) | 99.52 (0.83) | 99.50 (1.51) |
| Target Reaction Time (ms) | 678.71 (47.72) a | 596.26 (39.51) a |
| Stop-Signal Task: | ||
| % Correct Target Trials (PCTT) | 98.58 (2.63) | 98.16 (2.52) |
| % Correct Inhibitory Trials (PCIT) | 75.00 (11.92) | 77.64 (12.79) |
| Target Reaction Time (ms) | 769.72 (63.36) a | 684.01 (39.31) a |
| Stop-Signal Reaction Time (SSRT) (ms) | 541.47 (36.89) a | 450.59 (44.94) a |
| . | Group Contrasts | Hemisphere Contrasts | ||||||
|---|---|---|---|---|---|---|---|---|
| Effect | F | Effect | F | |||||
| N200 | Left | -- | Young | L > R ** | 8.95 | 0.10 | ||
| Right | O > Y *** | 25.17 | 0.23 | Older | R > L ** | 7.42 | 0.08 | |
| Frontal | -- | -- | ||||||
| Fronto-central | -- | -- | ||||||
| Central | O > Y *** | 31.79 | 0.28 | -- | ||||
| Parietal | -- | -- | ||||||
| P300 | Left | O > Y * | 5.61 | 0.06 | Young | R > L *** | 47.93 | 0.37 |
| Right | Y > O *** | 20.00 | 0.19 | Older | -- | |||
| Frontal | -- | -- | ||||||
| Fronto-central | -- | -- | ||||||
| Central | -- | -- | ||||||
| Parietal | Y > O ** | 10.40 | 0.11 | -- | ||||
| Young adults | Older adults | |||||||
|---|---|---|---|---|---|---|---|---|
| N200 | P300 | N200 | P300 | |||||
| Site | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM |
| F3 | −3.12 | 0.40 | 2.54 | 0.41 | −2.30 | 0.38 | 4.19 | 0.38 |
| F4 | −1.90 | 0.32 | 4.66 | 0.31 | −3.64 | 0.30 | 3.74 | 0.29 |
| FC3 | −3.69 | 0.42 | 3.22 | 0.34 | −2.07 | 0.40 | 4.51 | 0.32 |
| FC4 | −2.26 | 0.39 | 5.96 | 0.41 | −3.21 | 0.36 | 4.05 | 0.39 |
| C3 | −2.26 | 0.32 | 3.37 | 0.33 | −2.89 | 0.30 | 4.41 | 0.31 |
| C4 | −1.43 | 0.30 | 6.36 | 0.45 | −3.94 | 0.29 | 4.31 | 0.43 |
| P3 | −2.56 | 0.40 | 4.44 | 0.38 | −2.69 | 0.38 | 4.13 | 0.36 |
| P4 | −1.64 | 0.38 | 7.27 | 0.48 | −2.94 | 0.36 | 4.78 | 0.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paitel, E.R.; Nielson, K.A. Temporal Dynamics of Event-Related Potentials during Inhibitory Control Characterize Age-Related Neural Compensation. Symmetry 2021, 13, 2323. https://doi.org/10.3390/sym13122323
Paitel ER, Nielson KA. Temporal Dynamics of Event-Related Potentials during Inhibitory Control Characterize Age-Related Neural Compensation. Symmetry. 2021; 13(12):2323. https://doi.org/10.3390/sym13122323
Chicago/Turabian StylePaitel, Elizabeth R., and Kristy A. Nielson. 2021. "Temporal Dynamics of Event-Related Potentials during Inhibitory Control Characterize Age-Related Neural Compensation" Symmetry 13, no. 12: 2323. https://doi.org/10.3390/sym13122323
APA StylePaitel, E. R., & Nielson, K. A. (2021). Temporal Dynamics of Event-Related Potentials during Inhibitory Control Characterize Age-Related Neural Compensation. Symmetry, 13(12), 2323. https://doi.org/10.3390/sym13122323