Abstract
The origin of life, based on the homochirality of biomolecules, is a persistent mystery. Did life begin by using both forms of chirality, and then one of the forms disappeared? Or did the choice of homochirality precede the formation of biomolecules that could ensure replication and information transfer? Is the natural choice of L-amino acids and D-sugars on which life is based deterministic or random? Is the handedness present in/of the Universe from its beginning? The whole biosystem on the Earth, all living creatures are chiral. Many theories try to explain the origin of life and chirality on the Earth: e.g., the panspermia hypothesis, the primordial soup hypothesis, theory of parity violation in weak interactions. Additionally, heavy neutrinos and the impact of the fact that only left-handed particles decay, and even dark matter, all have to be considered.
1. Introduction
There is no doubt that rotational and geometric isomers significantly influence the biological activities of the corresponding compounds. However, the greatest variety in these effects changes can be seen in molecules with chiral centers or that exhibit optical isomerism without chiral centers. There are many reasons for this, not only because most drugs and remedies whose biological activity depends on their stereochemistry are chiral. The utmost important reason is that at the molecular level, the living matter is composed of chiral macromolecules—proteins, glycolipids, polynucleotides—which are formed of homochiral (possessing the same sense of chirality) structural units of D-sugars and L-amino acids.
The origin of the single chirality of most biomolecules is still a great puzzle. The questions are: Did life begin by using both forms of chirality, and then one of the forms disappeared? Or did the choice of homochirality precede the formation of biomolecules that could ensure replication and information transfer? Is the natural choice of L-amino acids and D-sugars on which life is based deterministic or random? Is the handedness in/of the Universe present from the times of the Big Bang? These secrets posed tantalizing questions for generations of researchers [1].
2. Chirality and the Origin of Life
Chirality is a specific phenomenon. It pervades much of modern science, from the physics of elementary particles through astronomy to the chemistry of life. As we know it here on Earth, life is a chemical system capable of self-reproduction and evolution and is based on chiral molecules. Of course, there are many definitions of what life is. For instance, Pross in 2013 [2] proposed a new definition of life: “a self-sustaining kinetically stable dynamic reaction network derived from a replication reaction”. However, even according to the author, the term dynamic kinetic stability (DKS) is relatively difficult to quantify [3], and other readers may have a problem with this, e.g., [4,5]. Korthof states that “this is not an arbitrary definition of life, it is rather a theory of life that can be tested”. However, the question of why life is built on homochiral biomolecules or why it was not built upon their mirror images is still one of the greatest secrets concerning the origin of life. Another key question is: why the basis of our life is built upon L-amino acids and D-saccharides, although theoretically, nothing prevents from building up the biomolecules from opposite enantiomers? There are essentially three crucial questions, as set by Riehl in his excellent book [6], which require an answer:
- Does life have to be chiral, or can life arise, for example, using a mixture of racemic amino acids?
- Can life arise, as mentioned above, using D-amino acids and L-sugars?
- If the answer to question 2 is “yes”, why is terrestrial life based on L-amino acids and D-carbohydrates?
About 500 α-amino acids exist in nature, but surprisingly only 22 are proteogenic, i.e., constitute the building blocks of peptides/proteins. It has long been believed that mammalian life is based exclusively on L-amino acids, and their enantiomers, i.e., D-amino acids, are unsuitable for this purpose and are not a natural part of living systems. However, in the middle of the 20th century, the presence of D-amino acids in tissue derived from large bugs and in the cell wall of bacteria [7] was uncovered. Furthermore, in the 1970s, D-amino acids were found in plants, invertebrates, and vertebrates [8,9]. D-amino acids were also later found in human brain tissue, teeth, and the eye lens [10]. Over the past three decades, research focusing on D-amino acids in humans confirmed their abundance in the brain as well as in other tissues and body fluids, including blood plasma, urine, saliva, cerebrospinal fluid, amniotic fluid, arterial walls, skin, and bones [11]. Research has shown [12] that D-amino acids are significantly more widespread in biosystems than previously thought. The most abundant D-amino acids in vertebrates are D-aspartate and D-serine. In 2018, Noriko Fujii showed a very important feature for the first time (according to NF): the homochirality of amino acids in proteins is not guaranteed throughout life. The amounts of D-amino acids have been shown to increase in quantity as an organism grows older. When a D-amino acid appears in a peptide bond, the orientation of the side chain of the amino acid is reversed against the peptide plane; therefore, the peptide structure around the D-amino acid and peptide properties such as affinity for solvent and its interaction with other proteins are altered. For this reason, the presence of D-amino acids in a protein can lead to physiological changes and subsequently to age-related disorders such as cataracts, macular degeneration, arteriosclerosis, and Alzheimer’s disease [13,14].
Although most living systems are built up as mentioned, there are known examples, especially in the world of microorganisms; however, they are not just there, where such entities are built up from “unnatural” D-amino acids and some L-saccharides. For example, the D-alanyl-D-alanine sequence is a component of the peptidoglycan layer of the cell wall of many bacterial species [15], D-amino acid peptides are found in amphibia, and the nervous system of invertebrates such as cephalopods [16,17], and D-amino acids are present in many living tissues where they may have accumulated during aging [18,19,20,21,22].
Moreover, a significant amount of D-serine is found in mammals exclusively in the brain with a concentration of about one-third of L-serine with the highest levels in the forebrain [23,24,25]. D-aspartate is localized highly to neuroendocrine tissues with a selective concentration in the epinephrine-containing granules of the adrenal medulla, oxytocin/vasopressin neurons projecting from the hypothalamus to the posterior pituitary, the pineal gland, and particular neuronal populations in the brain [26]. Abundant recent evidence favors a neurotransmitter/neuromodulator role for D-serine. D-serine is synthesized from L-serine by serine racemase in astrocytic glia, which ensheath synapses, especially in brain regions that are enriched in NMDA (N-methyl-D-aspartate receptor), which is a glutamate receptor [27].
D-amino acids in the body may originate from multiple sources. First of all, the racemization of L-amino acids by racemase enzymes leads to the endogenous biosynthesis of D-amino acids. Currently, two racemase enzymes have been found in mammals: serine racemase and aspartate racemase [28]. Of these two enzymes, only serine racemase has been found in human tissue [29,30,31]. The presence of aspartate racemase, on the other hand, has been detected in mice, amphibians, mollusks, and bacterial species [32]. Human aspartate racemase has not been detected so far. Secondly, D-amino acids can enter the human body via the diet. Over the past decades, it has become clear that certain food processing techniques contribute to the racemization of L-amino acids into D-amino acids in several types of food [33]. Additionally, D-amino acids are found in fermented foods such as vinegar and dairy products [34,35]. Thirdly, at least one-third of the human D-amino acid pool is suggested to be derived from microbial synthesis [30]. According to recent evidence, the human gut microbiota might be a pivotal contributor to the systemic D-amino acid abundance in the host’s body [36,37].
There are also some drugs which structure bears a “unnatural” D-amino acid, for instance Figure 1 Goserelin (N-(21-((1H-indol-3-yl)methyl)-1,1-diamino-12-(tert-butoxymethyl)-6-(2-(2-carbamoylhydrazinecarbonyl) cyclopentanecarbonyl)-15-(4-hydroxybenzyl)-18-(hydroxymethyl)-25-(1H-imidazol-5-yl)-9-isobutyl-8,11,14,17,20,23-hexaoxo-2,7,10,13,16,19,22-heptaazapentacos-1-en-24-yl)-5-oxopyrrolidine-2-carboxamide), which is used in the form of acetate as an injectable gonadotropin releasing hormone super-agonist (GnRH agonist), also known as a luteinizing hormone-releasing hormone (LHRH) agonist containing D-serine within its molecule [38]. Goserelin acetate is used to suppress production of the sex hormones (testosterone and estrogen), particularly in the treatment of breast and prostate cancer [39].
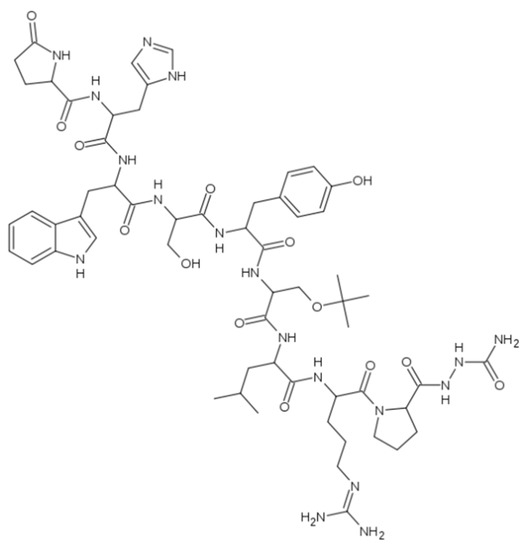
Figure 1.
Goserelin.
A large group of poisons isolated from the skin of South American frogs Phyllomedusa sauvag,i contains the dermorphin heptapeptide (Tyrosyl-D-alanyl-phenylalanyl-glycyl-tyrosyl-prolyl-serinamide; H-Tyr-D-Ala-Phe-Gly-Tyr-Pro-Ser-NH2) having the amino-terminal sequence of Tyr-D-Ala-Phe, wherein D-alanine is essential for the activity. As a result, dermorphin has high affinity and selectivity to μ-opioid receptors, is about a thousand times more potent than morphine, and causes long-lasting, deep analgesia [40].
Even in the case of some spider venom, the protein sequence contains D-amino acid, in this case D-serine, which is essential for activity. Although, interestingly, the insect organism, as well as some reptiles, produce the respective peptide exclusively from L-amino acids, and one or more L-amino acids are inverted to the D-isomers by the isomerase contained in the toxin [41].
D-amino acids are also found in many bacterial antibiotic products, e.g., in the macrocyclic antibiotics bacitracin, tyrocidin, gramicidin, polymyxins, etc., [42]. Martinez-Rodriguez [43] presented an interesting overview of how the D-amino acids could be used in the pharmaceutical industry and their applications, predominantly as several types of antibiotics.
Ciclosporin A, a well-known immunosuppressant, is another example of a molecule containing D-amino acid. Without this compound, a product of ascomycetes fungus Tolypocladium inflatum, most transplantations would not be possible.
(Ciclosporine A is the INN name of the molecule, sometimes written as Cyclosporine A) (Figure 2). This remedy is also taken for rheumatoid arthritis, psoriasis, Crohn’s disease, nephrotic syndrome, and organ transplants to prevent rejection. It is also used as eye drops for keratoconjunctivitis sicca (dry eyes) [44]. Ciclosporine A is a cyclic peptide containing 11 amino acids; out of them, one is a D-form of alanine. Unlike most peptides, ciclosporine is not synthesized by ribosomes. All its metabolites (ciclosporine B, C, D, E, H, and L) have less than 10% of ciclosporine’s immunosuppressant activity and are associated with higher kidney toxicity [45].
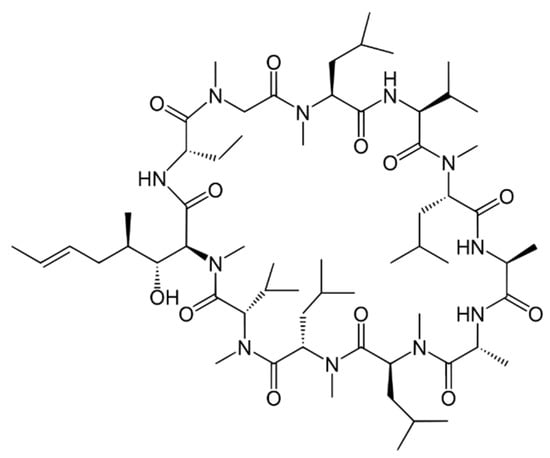
Figure 2.
Ciclosporine A.
Although the appearance of L-sugars in nature is extremely rare [46], some exceptions such as arabinose, fucose, and rhamnose are peculiar amongst the simple monosaccharides because they occur more commonly in the L- than in the D-configuration. Additionally, L-galactose (L-Gal) is a rare sugar that, in terrestrial organisms, has been found as a component of polysaccharides from some plants and in snail galactans. In the marine environment, L-Gal has been detected in red seaweeds, in several species of tunicates, and in sulfated glycoproteins from soft corals. In the case of the polysaccharides from red seaweeds, it has been shown that L-Gal may appear in place of or jointly with D-Gal, while in tunicates, the polysaccharides which present L-Gal are devoid of D-Gal [47]. Additionally, there is even the extreme case of rhamnose, which occurs in nature only in its L-form. It is commonly bound to other sugars found in plants and diatoms (microalgae), a component of the outer cell membranes.
It is worth to mention some references dealing with D-amino acids, especially some reviews on the topic, e.g., [48,49,50,51,52,53,54].
Many theories try to explain the phenomenon of homochirality concerning D-amino acids and L-sugars; however, no one has brought a satisfactory explanation, for example [55,56,57]. Nevertheless, homochirality is treated as a concept in terms of “yes” or “no”. Thus, homochirality either exists or does not. An excellent discussion on chirality indicators of extraterrestrial life, including homochirality, can be found in Avnir’s paper from 2021 [58].
First of all, we have to accept that in the whole biosystem on the Earth, all living creatures, as already said, are chiral.
An attempt to answer the question of why are, e.g., left-handed amino acids selected as building units of proteins is presented by Meierhenrich [59]. As we will show later, mammals, vertebrates, plants, the smallest microorganisms, and even the atoms and their particles and sub-particles are chiral. Second, we have to consider that some of the underlying principles of life are recognition, response, replication, and evolution. Recognition or discrimination at the level of sophistication required for life must be asymmetric. Furthermore, life must be energy efficient. It makes no sense for a living system to expend the energy to produce racemic mixtures that are inherently symmetric (if the enantiomeric excess (ee) ratio is 1:1) where there is used for only one enantiomer. Just as inefficient would be a living system that was functioning with two competing pathways. Life cannot originate in racemic mixtures [60,61]. Third, for the moment, it seems so, and we believe that the Universe we know is also governed by the 2nd Law of Thermodynamics, i.e., to obtain the maximum yield (or benefit) by minimum energy consumption. Therefore, it is in concert with the laws of nature that all life in all worlds should display chiral exclusivity. A fundamental mystery in physics, chemistry, and biology is how life abides by the second law of thermodynamics yet evolutionarily complexifies and maintains its intrinsic order [62].
2.1. Theories of Chirality of Molecules and the Origin of Life
The origin of life is broadly discussed, not only in today’s literature [63]. It is currently accepted that our planet, Earth, arose about 4.56 billion years ago from a protoplanetary disk and that life on Earth appeared about 3.5 billion years ago. The dating of fossils shows that life certainly existed on Earth 1 billion years ago (pp. 293–313 in [63]). When talking about life, we know only life on Earth and therefore the question: “what is life?” We can only try to answer from that point of view.
2.1.1. The “Sterile” and the Left-Handed Neutrino
We know that life on Earth is always based on the same type of biochemistry, carbon-containing biopolymers, amino acids, and nucleic acids. We assume that carbon-based extraterrestrial life is organized similarly (pp. 249–292 in [63]). The prove of the non-existence of “sterile neutrinos” (with right-handed spin) in 2016 and the discovery that only subatomic particles with left-handed spin decay, and thus the Universe has a left-handed bias or is inherently left-handed, supports this assumption [64,65,66,67,68]. “Sterile neutrinos” were intensively searched for but without any effect. It was assumed that neutrinos are the only particles in the Standard Model of particle physics that have only been observed with left-handed chirality to date [69]. If right-handed neutrinos exist, they could be responsible for several phenomena that have no explanation within the Standard Model, including neutrino oscillations [70], the baryon asymmetry of the universe, dark matter, and dark radiation. Neutrinos and their antimatter counterparts, antineutrinos, come in three “flavors”–muon, electron, and tau. They can spontaneously switch between these “flavors” in a process called neutrino oscillation [71]. All three known types of neutrinos have a left-handed spin. Thus it was argued there must be another type with right-handed spin called “sterile” neutrinos [72]. However, it was hypothesized that if “sterile” neutrinos had masses trillions of times greater than their normal left-handed counterparts, they would have decayed into these lighter neutrinos within the first seconds after the Big Bang, or at latest at the atom-forming processes. Therefore, only the left-handed neutrinos were left over. That may have influenced the further development of handedness of the Universe and later also on the primordial protoplanets. When we write about handedness and neutrinos, we discuss two different but related things: helicity and chirality. Helicity is a property similar to spin, energy, or momentum—it is a conserved quantity, but it depends on the reference frame. On the other hand, chirality is an intrinsic, fundamental property of the particle. It does not rely on reference points or perspective. Neutrinos can be left-chiral or right-chiral. Until now, we have seen only neutrinos born in a left-chiral state. Neutrinos turn out to be an anomaly. Other particles such as the quarks and the other three leptons (the electron, muon, and tau) have both left-handed and right-handed versions of the matter particle and their antimatter partner [73].
That leads to the question: Where are all the right-handed neutrinos and the left-handed antineutrinos? It remains a mystery, but scientists suspect that, because we have not seen them yet if these right-handed neutrinos exist, they will be very different from the left-handed neutrinos we know. Perhaps they are much heavier or do not interact via the weak force but instead interact only via gravity (these are the so-called “sterile neutrinos”). Right-handed neutrinos are a good candidate for the sterile neutrinos [73], which could be responsible for right-handed “dark matter” [74]. In other words: left-handedness is a phenomenon that appears to be here from the beginning of the Universe.
2.1.2. The Panspermia Hypothesis and the Origin of Water on Earth
There are many theories and hypotheses about how life has arisen on Earth. For instance, the panspermia hypothesis has many forms, some of which suggest that life started elsewhere in the universe and arrived on Earth by cometary, meteoric, or planetary delivery. We now know that some very complex organic molecules (also including proteogenic chiral L-amino acids) are delivered to the Earth on meteorites. Perhaps at the beginning of planet Earth, asteroids or comets were the building blocks of life. One estimate, for example, is that as much as 50% of the water on Earth is the result of comet impacts [75,76]. Certainly, this was not some distilled water. The amount of liquid water on Earth (~1.4 × 1018 Metric Tons), however, is only a fraction (0.02%) of the total Earth-mass [77].
Nevertheless, similar to neutrinos and water on Earth, it is not as simple as it looks. In the Universe as a whole, water was not present in its very early phases. The origin of hydrogen and oxygen seems straightforward. Approximately 380,000 years after the start of the Universe’s expansion (commonly referred to as the Big Bang), the whole amount of hydrogen was already available, while the formation of oxygen required thermonuclear fusion [78]. Water in Universe is always imaged as a scarce and precious resource. As more and more has been learned about the Universe and our place in it, we become more aware that water is present everywhere. It has been detected in planetary systems [79], in the atmospheres of giant planets orbiting other stars, in the 3000 K hot sunspots, in cold molecular interstellar clouds, even in galaxies that are at a distance of several hundreds of million light-years from us.
The Endogenous theory originally stated that the origin of the water on Earth could have come directly from the solar nebula during the stage of the formation of the Earth [80]. Recent geological findings have added additional information in favor of the Endogenous theory. For example, the minerals olivine and ringwoodite ((Mg,Fe)2 SiO4) found in the Earth’s “transition zone” (between 410 and 660 km depth) could accommodate about 1% of water in weight trapped in minerals chemically bound as crystalline water. This amount of crystalline water could fill the Earth’s oceans more than three times (if in a liquid state) [81]. The question is: how do we obtain the water in liquid form from those crystals?
The Exogenous theory attributes the origin of water to the infall of celestial bodies. Water comes in a variety of isotopic compositions: H2O, HDO, and D2O; D = deuterium). If measuring the D/H ratio, we can estimate the cosmic contributors to the terrestrial water. According to the Vienna model in ocean water, D/H = ~1.5 × 10−4 [75,82]. A similar value is present in the carbonaceous chondrites and the eucrites. By contrast, the giant planets and protosolar nebula value is about one order of magnitude lower. The value of D/H in several comets is two to three times higher than in the terrestrial oceans (~3 × 10−4 [75]). For instance, high D/H ratios were found in Halley, Hale–Bopp, Hyakutake comets, and comet 67/P Churyumov–Gerasimenko [83]. At the moment, we know that the meteorites come from the (Kuiper) asteroid belt; in particular, carbonaceous chondrites and eucrites have the same D/H ratio as the Earth’s oceans, and the recently discovered Main Belt Comets represent ideal candidates as external sources of water in our planet during the Late Heavy Bombardment [78]. The D/H ratio in Earth’s oceans closely matches that of asteroids rather than comets [84].
2.1.3. The Primordial Soup Hypothesis and the Water Paradox
The primordial soup hypothesis, also known as the Oparin-Haldane model, proposes that during the Earth’s early evolution, a reducing atmosphere provided the correct environment for the formation of basic organic compounds [85,86]. When laboratory research into life’s origins started in the 1950s, many researchers assumed that life began in the sea, with a rich mix of carbon-based chemicals dubbed the primordial soup. This idea was independently proposed in the 1920s by biochemist Alexander Oparin and geneticist J.B.S. Haldane in the United Kingdom. Each imagined the young Earth as a huge chemical factory, with multitudes of carbon-based chemicals dissolved in the huge amount of shallow waters of the early oceans. Oparin reasoned that increasingly complex particles were formed, culminating in carbohydrates and proteins: what he called “the foundation of life”. However, there is a fundamental problem with this idea: life’s cornerstone molecules break down in the water. This is because proteins, and nucleic acids such as DNA and RNA, are vulnerable at their joints. Proteins are made of chains of amino acids, and nucleic acids are chains of nucleotides. If the chains are placed in water, it attacks the links and eventually breaks them due to hydrolysis. In carbon chemistry, “water is an enemy to be excluded as rigorously as possible” (Shapiro, R. in 1986), which critiqued the primordial ocean hypothesis [87]. This is called “the water paradox”. It is known that formamide is also a key prebiotic precursor for synthesis, e.g., chiral amino acids. Under certain conditions, formamide can be formed from HCNO. Theoretically, it could also serve as a micro-environment for prebiotic chemical reactions. This, in some way, could diminish or bypass the problem of “the water paradox”. Interestingly, formamide was also detected in a few comets, e.g., Churyumov-Gerasimenko [88,89].
Though the soup model has matured in recent decades, it has difficulty explaining the exact conditions of the early Earth atmosphere and the manner and order of emergence of polymeric systems. In the iron–sulfur world theory, primitive life is assumed to have started at deep-sea hydrothermal vents as a mineral base; redox reactions provided the chemical energy to drive the emergence of cellular life [90]. However, this model does not explain the origin of genetic information, membrane systems, or the complexification or diversity of cellular structure.
The “RNA world hypothesis” posits that ribonucleotide-based genetic systems evolved before to protein and deoxyribonucleic acid (DNA) [91,92].
A relatively new theory based on gyre (spiral) as a core model for understanding life [93] is based on spirals and spiraling. Perhaps one reason for their theoretical appeal is that gyres are detectable throughout the cosmic and tellurian spheres. One of Einstein’s “forgotten” theories already says that the universe is crooked and is twisting like a typhoon. Astronomically, galaxies, solar systems, comets, and lunar bodies gyrate. Atmospherically, tornadoes, hurricanes, eddies, and vortex streets are all gyres. Oceanographically, there are seven major gyres. Molecularly, numerous nucleic acid and protein structures—DNA double helix, RNA hairpins, pseudoknots, α-helices, coiled coils, and β-propellers—all gyrate. Cellularly and organismally, shells, horns, antennae, flagellae, and cochlea carry a spiral imprint. The ubiquity of rotation may be the reason why the gyration model could be a significant candidate for the principal model of natural systems [93].
2.1.4. Parity Violation in Weak Interactions—Energy Difference in Enantiomers
Living systems are predominantly based on CHNOPS atoms; thus, those atoms and their subatomic particles influence the properties of complex molecules of life. One of the theories which could strongly contribute to explaining the chirality of molecules of life is the theory of parity violation in weak interactions [94,95,96,97]. Until 1956, it was generally believed that all physical laws were the same for the image as for the mirror image; in other words, parity was conserved. The theoretical prediction (1956) [98] of the violation of parity in weak interactions and the experimental proof thereof some months later (1957) [99] was not only a surprise for the research community, but it was also a shock.
The first experimental works indicated that in processes linked to weak interactions (for example, the interaction of enantiomers with electrons, nucleons, and some nucleon components), a violation of parity occurs, and mirror images do not have the same energies. It was found that interactions of this kind cause one of the enantiomers to become somewhat more stable than the other. This was an extremely important and revolutionary finding, because it may explain, for example, the asymmetric composition of proteins (they consist mostly of L-amino acids) and saccharide biopolymers (consisting primarily of D-saccharides) and it may perhaps even help to explain the origin of life on this planet because of energy difference in enantiomers of organic molecules, e.g., amino acids.
Later, it was concluded that all atoms are inherently chiral, which is precisely the result of violations of the core parity and the chiral nature of electrons [100,101]. It has been shown that heavy neutrinos and even dark matter must be taken into account [102,103]. Various experiments have shown that even enantiomers of molecules cannot be completely energy equivalent [104,105]. The Universe as we know it is made of matter, not antimatter, and ”CP violation” in particle decays (C–charge conjugation, P–parity) could be the reason [106]. On the other hand, recently, it has been shown [107] that even when the parity violation energy differences in enantiomers could exist, they are very small at 10−4 eV (Jovian scenario) or 10−7 eV (terrestrial scenario; if taking into account the astrophysical-based experiments the energies are 10−25 eV and 10−28 eV, respectively), making them hard to observe.
It is assumed that in chemical reactions in which enantiomers are formed, molecules with an enantiomeric excess could emerge only under autocatalytic conditions. To date, only one such reaction is documented: the Soai reaction [108]. That model system (which was not present on Earth in prebiotic times) was used to explain the energy differences necessary for symmetry breaking and chiral amplification in molecular self-replication. In 2019, Hawbaker and Blackmond using this model reaction found out that the energy required to break symmetry with consistent chiral bias lies between 1.5 × 10−7 and 1.5 × 10−8 kJ/mol. This means that the biohomochirality is not based on simple and single reactions, and the energetics cannot account for one chirality to be favored over the other in prebiotic reactions, but it is instead a series of persistent chemical, physical, and biological synergistically acting processes [109]. Therefore, we may conclude that a tiny energy advantage in one isomer is likely not enough to push biological molecules all one way. According to J. Moran from the University of Strasbourg, “this work ([109]) effectively rules out one of the popular explanations (the parity violation) for how life became homochiral”.
2.1.5. Could Enantiomeric Excess Be an Indicator of Life?
Polymerization of amino acids in β-sheets (rather than α-helices) could conceivably have generated enantioenriched ensembles of chiral polymers [110]. Alternatively, the single chirality of RNA or DNA (with D-sugar backbones) on the one hand, and of proteins (comprising in the most general form both α-helix and β-sheet domains) on the other hand, stemmed from a chiral pool of already enantioenriched monomeric amino acids and sugars. We are faced with the question of which came first, i.e., whether the single chirality of amino acids might have caused the single chirality of sugars or vice versa. Pizzarello and Weber first suggested that gluconeogenesis might have proceeded stereoselectively in the presence of catalytically active amino acids [111,112]. The connection between the naturally mostly occurring L-amino acids and the predominantly naturally occurring D-sugars has been established for serine octamers [113]. It was shown that racemic amino acid solutions could be kinetically resolved by a small enantiomeric excess in the carbohydrate [114]. It might be rewarding to consider a further possibility: the homochirality of biomolecules could have originated endogenously from the carbohydrates rather than from their interaction with amino acids [115]. Life on planet Earth is not purely homochiral; its level is high, but it is not absolute. Neither amino acids, nor sugars, nor any of the biomolecules in general, comprise pure homochiral sets of molecules in our biosphere. However, for the recognition and selection of compatible substrates and receptors and to be a “biomarker of life”, the enantiomeric excess (ee) is crucial. Once the global enantiomeric excess of a certain molecule of biological origin in a biosphere is not zero, then non-zero values must be considered possible biomarkers of life. Furthermore, after the discovery of thousands of exoplanets (4438 [116]), exploration of comets, Mars, planets, and their moons, etc., and other new astronomical discoveries, the need for biomarkers for extraterrestrial life has become more than necessary. If the ee of the extraterrestrial subject is significantly high, it could be taken as a positive indicator. The first experiments to detect the ee on Mars, for example, have been carried out by NASA’s Curiosity Rover [117,118] and the Philae lander of Rosetta spacecraft [119] using GC methods.
Additionally, of interest is the view that amphiphilic molecules have played an important, though perhaps not key, role in the formation of biological homochirality [120]. However, there is a specific question about lipids: why is the chiral unit of the archaeis sn-glycerol-1-phosphate (G1P) while the chiral units of bacteria and eukaryotes are sn-glycerol-3-phosphate (G3P) [121,122,123]? Both of the phosphates are mirror images. There are even opinions that biopolymers such as, e.g., RNA, proteins, proteinoid microspheres, and the whole protein world itself [124], arose only after the formation of supramolecular structures (e.g., micelles, vesicles, coacervates, oil drops, etc.), which were here earlier and represented the lipid world hypothesis [125].
Some studies based on the analysis of Murchison meteorite but also other chondrites (e.g., Murray, Kentucky USA, impact in 1950 [126] or arctic chondrites) show that unusual α-methylamino acids (e.g., L-isovaline, i.e., α-amino-2-methylbutanoic acid, extremely rarely used in living systems and at the same time retaining its stereochemistry for billions of years [127,128]) with an excess of the L-form may produce standard amino acids which also prefer the L-isomer. It was shown that as little as 1% excess of the L-isomers could (for example, L-phenylalanine) be amplified up to a 95:5 ratio of L over D on simple evaporation of a solution, thus life could start with such a solution in which the dominant L-isomers would be selectively chosen. The same is true for the reaction of formaldehyde with glycolaldehyde forming glyceraldehyde catalyzed under prebiotic conditions to D:L ratios greater than 1, to as much as 60:40, by a representative group of L-amino acids (except for L-proline). The D:L glyceraldehyde ratio in water solution is amplified to 92:8 using the D and the D,L forms’ simple selective solubilities. This D center would then be carried into the prebiotic syntheses of larger sugars [129]. This theory is in accord with the paper of Morowitz, who described the theoretical background of such processes and some experimental exploration of them [130]. On the other hand, severe doubts exist about this theory, for example [131], questioning the central idea of transfer and amplification of enantiomeric excess to other molecules, thus the picture is far from clear. Such speculations are based upon laboratory results that definitively could not simulate the prebiotic conditions on Earth exactly and in full.
Nevertheless, we do have data on extraterrestrial ee, provided by carbonaceous meteorites, particularly on the Murchison meteorite [132], which are most important ones: whenever a measurable non-zero ee is detected for amino acids, there seems to be a consistent L-excess [133,134,135,136,137,138,139,140] similar to Earth. Moreover, data on sugar ee in such meteorites show a D-excess of some of them [141] as on planet Earth.
The calculated energy differences between the enantiomers of amino acids and carbohydrates are very small (ΔEPV ≈ 10−13 J × mol−1) [142] (for example, L-alanine energy is 10−12 J/mol lower than D-alanine energy). However, this energy difference is generally believed to be too small to account for the exclusive occurrence of L-amino acids in biomolecules) [143], and could hardly lead to one enantiomeric form predominating in the evolution of life due to parity violation [105,134,144]. For a homochiral molecule to play a key role in the chiral origin of life, there would have to be some previously unknown amplifying mechanism with a factor of up to 1017 [145]. On the other hand, it is also necessary to consider that, unlike man, nature has a virtually endless amount of time. In these theories, one must also remember that chirality is not an exclusive property of life on Earth. Certainly, chiral preferences exist throughout our Universe and even dominate it. Starting with masses of cosmic dimensions, such as spiral galaxies [146], (Figure 3) to subatomic particles.

Figure 3.
A model of “racemic” spiral galaxies.
Even our Earth is inherently chiral [147] Figure 4.

Figure 4.
The chiral Blue Marble.
This is also confirmed by the fact that, according to research, enantiomeric excesses (ee) of amino acids of up to 18.5% have been detected in different types of carbonaceous chondrites, which fell to Earth during the 20th century, all in favor of the L-enantiomer. The number of samples studied is not very large, but as the samples come from different parent bodies and all show an ee in favor of the same enantiomer, it is tempting to conclude that these ees have a deterministic origin [148]. Nevertheless, even the authors state that, e.g., the enantioselective photolysis by UV–CPL (circularly polarized light) radiation in the interstellar gas phase is perhaps not the deterministic cause they were looking for, even when some authors believe that UV-CP may play a key role in producing an enantiomeric excess in extra-terrestrial space [149]. Moreover, for terrestrial purposes, UV-CPL is not useful. UV-CPL is not detectable at Earth’s surface because of scattering in the atmosphere. (There was a device on the Hubble telescope capable of measuring polarization. This instrument was seldom used, and eventually was removed from the telescope during one space shuttle service mission [6], p. 67.) Hardly is the parity violation. Definitively not some cooperative nucleation and differential crystallization of enantiomers [49]. However, concerning the differential crystallization, it must not be omitted that under some specific conditions, deracemization could take place. These experiments are nothing new. Already in 1990 and 1991, Kondepudi [150] and McBride [151] have shown that there is a possibility of homochiral crystallization yielding mostly enantiomorphous crystals of single-handedness. In 2011, Viedma and Cintas published their results [152] on how a single-chirality solid phase can be obtained in boiling solutions containing a racemic mixture of left- and right-handed enantiomorphous crystals due to dissolution–crystallization cycles induced by a temperature gradient.
On the other hand, it is relatively easy to explain and also to prove in the laboratory why polymerization reactions produce stereospecific polymers (e.g., L-polypeptides or D-oligosaccharides) that are necessary for our chiral life (e.g., in recognition, self-replication, protein synthesis, regulation, and perfect gene expression) are not very effective in the presence of racemates. Namely, the addition of the wrong stereoisomer stops the reaction [105].
Last but not least, we should not forget the role of light and photochemistry in the origin of life and evolution. According to Giannetto [153], “life and intelligence are a phenomenon of “super-radiance” of light and evolution has mainly dependent on the ability to absorb electromagnetic bio-information from sunlight... A global information function governs the quantum cosmological evolution of the universe: light is living intelligence”.
3. Closing Remarks
The question of the origin of chirality in life is still open. The “happy coincidence” seems to be also very unlikely. Till now, no consensus model for life and homochirality has emerged. Therefore, there must also be other possibilities that other, still unknown, deterministic causes exist.
However, research in this area continues. In 2015, Ulmer et al. [154], conducting experiments on the CERN antiproton decelerator, showed that matter and antimatter are perfect mirror images of each other, but matter outweighs antimatter. In other words, one of the “pictures” predominates. It has also been shown that only subatomic particles with a left-handed spin decompose due to the consequence of weak internuclear forces, which are, as already mentioned earlier, one of the fundamental forces of nature, suggesting that the Universe has a left-handed preference [67].
At the end of this chapter, let us present the reaction of one of the most influential physicists of the time when the principle of parity violation was discovered. Not only for him but for many eminent physicists, the news came as a rather shocking surprise. Wolfgang Pauli, a pioneer of the modern quantum theory of matter, discoverer of the Exclusion Principle, comments on these developments critically. In a letter dated August 1957 to the famous psychologist C. G. Jung, Pauli remarks that “…So it is now certain that God is a weak left-hander…However, His reasons we do not know… In such a possibility I never would have believed before January of this year” [155].
Funding
This research was funded by KEGA grant No. 077UK-4/2017 (Cultural and Educational Agency of Ministry of Education, Science, Research and Sport of Slovak republic) and supported by APVV grant No. 17-0373 (Slovak Research and Development Agency).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data cited in the manuscript are available from the published papers.
Acknowledgments
I have benefited from discussions with Professor Ivan Lacko over the years, to whom I express my profound gratitude. I would also like to thank professors Dušan Mlynarčík and Pavol Balgavý for their helpful consultations and exchange of views.
Conflicts of Interest
The author declares no conflict of interest.
References
- Mauksch, M.; Shengwei, W.; Freund, M.; Zamfir, A.; Tsogoeva, S.B. Spontaneous Mirror Symmetry Breaking in the Aldol Reaction and its Potential Relevance in Prebiotic Chemistry. Orig. Life Evol. Biosph. 2010, 40, 79–91. [Google Scholar] [CrossRef]
- Pross, A. What Is Life? How Chemistry Becomes Biology; Oxford University Press: Oxford, UK, 2013; p. 164. ISBN 978-0-19-964101-7. [Google Scholar]
- Pascal, R.; Pross, A.; Sutherland, J.D. Towards an evolutionary theory of the origin of life based on kinetics and thermodynamics. Open Biol. 2013, 3, 130156. [Google Scholar] [CrossRef]
- Lehman, N. Kinetics to the rescue. Trends Evol. Biol. 2013, 5, br1. [Google Scholar] [CrossRef]
- Korthof, G. Available online: http://wasdarwinwrong.com/korthof99.htm (accessed on 27 September 2021).
- Riehl, J.P. Mirror-Image Asymmetry: An Introduction to the Origin and Consequences of Chirality; J. Wiley&Sons: Hoboken, NJ, USA, 2010; p. 59. ISBN 978-0-470-38759-7. [Google Scholar]
- Stevens, C.M.; Halpern, P.E.; Gigger, R.P. Occurrence of d-amino acids in some natural materials. J. Biol. Chem. 1951, 190, 705–710. [Google Scholar] [CrossRef]
- Robinson, T. D-amino acids in higher plants. Life Sci. 1976, 19, 1097–1102. [Google Scholar] [CrossRef]
- Corrigan, J.J. D-Amino Acids in Animals. Science 1969, 164, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Masters, P.M.; Bada, J.L.; Zigler, J.S., Jr. Aspartic acid racemization in heavy molecular weight crystallins and water insoluble protein from normal human lenses and cataracts. Proc. Natl. Acad. Sci. USA 1978, 75, 1204–1208. [Google Scholar] [CrossRef]
- Bastings, J.J.A.J.; van Eik, H.M.; Olde Damink, S.W.; Rensen, S.S. D-amino Acids in Health and Disease: A Focus on Cancer. Nutrients 2019, 11, 2205. [Google Scholar] [CrossRef]
- Brückner, H.; Fujii, N. (Eds.) D-Amino Acids in Chemistry, Life Sciences, and Biotechnology; Part 1: Transpeptidationa and isomerization; Part 5: Racemization mechanisms and chemistry of D-amino acids; J. Wiley-VCH, Verlag Helvetica Chimica Acta: Zürich, Switzerland, 2010; ISBN 978-3-906390-659. [Google Scholar]
- Fujii, N.; Takata, T.; Fujii, N.; Aki, K.; Sakaue, H. D-amino acids in protein: The mirror of life as a molecular index of aging. BBA-Proteins Proteom. 2018, 1866, 840–847. [Google Scholar] [CrossRef]
- Masters, P.M.; Bada, J.L.; Zigler, J.S., Jr. Aspartic acid racemisation in the human lens during ageing and in cataract formation. Nature 1977, 268, 71–73. [Google Scholar] [CrossRef]
- Sela, M.; Zisman, E. Different roles of D-amino acids in immune phenomena. FASEB J. 1997, 11, 449–456. [Google Scholar] [CrossRef]
- Avnir, G. Peptides containing a D-amino acid from frogs and molluscs. J. Biol. Chem. 1994, 269, 10967–10970. [Google Scholar]
- Mor, A.; Amiche, M.; Nicolas, P. Enter a new posttranslational modification: D-amino acids in gene-encoded peptides. Trends Biochem. Sci. 1992, 17, 481–485. [Google Scholar] [CrossRef]
- Fisher, G.H.; Petrucelli, L.; Gardner, C.; Emory, C.; Frey, W.H.; Amaducci, L.; Sorbi, S.; Sorrentino, G.; Borghi, M.; D’Aniello, A. Free D-amino acids in human cerebrospinal fluid of Alzheimer disease, multiple sclerosis, and healthy control subjects. Mol. Chem. Neuropathol. 1994, 23, 115–124. [Google Scholar] [CrossRef]
- Fisher, G.H.; Torres, D.; Bruna, J.; Cerwinski, S.; Martin, T.; Bergljung, C.; Gruneiro, A.; Chou, S.J.; Man, E.H.; Pappatheodorou, S. Presence of D-aspartate and D-glutamate in tumor protein. Cancer Biochem. Biophys. 1995, 15, 79–82. [Google Scholar]
- Nagata, Y.; Borghi, M.; Fisher, G.H. Free D-serine concentration in normal and Alzheimer human brain. Brains Res. Bull. 1995, 38, 181–183. [Google Scholar] [CrossRef]
- Schell, M.J.; Molliver, M.E.; Snyder, S.H. D-Serine, an endogenous synaptic modulator leocalization to astrocytes and glutamate-stimulated release. Proc. Natl. Acad. Sci. USA 1995, 92, 3948–3952. [Google Scholar] [CrossRef] [PubMed]
- Konno, R.; Brückner, H.; D’Aniello, A.; Fischer, G.; Fujii, N.; Homma, H. (Eds.) D-Amino Acids: A New Frontier in Amino Acids and Protein Research, Practical Methods and Protocols; Nova Science Publishers, Inc.: New York, NY, USA, 2007; ISBN 1-60021-075-9. [Google Scholar]
- Hashimoto, A.; Nishikawa, T.; Oka, T.; Takahashi, K. Endogenous D-serine in rat brain: N-methyl-D-aspartate receptor-related distribution and aging. J. Neurochem. 1993, 60, 783–786. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, A.; Nishikawa, T.; Konno, R.; Niwa, A.; Yasumura, Y.; Oka, T.; Takahashi, K. Free D-serine, D-aspartate and D-alanine in central nervous system and serum in mutant mice lacking D-amino acid oxidase. Neurosci. Lett. 1993, 152, 33–36. [Google Scholar] [CrossRef]
- Hashimoto, A.; Oka, T. Free D-aspartate and D-serine in the mammalian brain and periphery. Prog. Neurobiol. 1997, 52, 325–353. [Google Scholar] [CrossRef]
- Schell, M.J.; Cooper, O.B.; Snyder, S.H. D-aspartate localizations imply neuronal and neuroendocrine roles. Proc. Natl. Acad. Sci. USA 1997, 94, 2013–2018. [Google Scholar] [CrossRef]
- Mustafa, A.K.; Kim, P.M.; Snyder, S.H. D-Serine as a putative glial neurotransmitter. Neuron Glia Biol. 2004, 1, 275–281. [Google Scholar] [CrossRef][Green Version]
- Ohide, H.; Miyoshi, Y.; Maruyama, R.; Hamase, K.; Konno, R. D-Amino acid metabolism in mammals: Biosynthesis, degradation and analytical aspects of the metabolic study. J. Chrom. B Analyt. Technol. Biomed. Life Sci. 2011, 879, 3162–3168. [Google Scholar] [CrossRef] [PubMed]
- Wolosker, H.; Blackshaw, S.; Snyder, S.H. Serine racemase: A glial enzyme synthesizing D-serine to regulate glutamate-N-methyl-D-aspartate neurotransmission. Proc. Natl. Acad. Sci. USA 1999, 96, 13409. [Google Scholar] [CrossRef]
- Foltyn, V.N.; Bendikov, I.; De Miranda, J.; Panizzutti, R.; Dumin, E.; Shleper, M.; Li, P.; Toney, M.D.; Kartvelishvily, E.; Wolosker, H. Serine racemase modulates intracellular D-serine levels through an alpha,beta-elimination activity. J. Biol. Chem. 2005, 280, 1754–1763. [Google Scholar] [CrossRef] [PubMed]
- Raboni, S.; Marchetti, M.; Faggiano, S.; Campanini, B.; Bruno, S.; Marchesani, F.; Margiotta, M.; Mozzarelli, A. The Energy Landscape of Human Serine Racemase. Front. Mol. Biosci. 2019, 5, 112. [Google Scholar] [CrossRef] [PubMed]
- Uda, K.; Abe, K.; Dehara, Y.; Mizobata, K.; Sogawa, N.; Akagi, Y.; Saigan, M.; Radkov, A.D.; Moe, L.A. Distribution and evolution of the serine/aspartate racemase family in invertebrates. Amino Acids 2016, 48, 387–402. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Origin, microbiology, nutrition, and pharmacology of D-amino acids. Chem. Biodivers. 2010, 7, 1491–1530. [Google Scholar] [CrossRef]
- Mutaguchi, Y.; Ohmori, T.; Akano, H.; Doi, K.; Ohshima, T. Distribution of D-amino acids in vinegars and involvement of lactic acid bacteria in the production of d-amino acids. SpringerPlus 2013, 2, 691. [Google Scholar] [CrossRef]
- Jin, D.; Miyahara, T.; Oe, T.; Toyo’oka, T. Determination of D-amino acids labeled with fluorescent chiral reagents, R(-)- and S(+)-4-(3-isothiocyanatopyrrolidin-1-yl)-7-(N,N-dimethylami nosulfonyl)-2,1,3-benzoxadiazoles, in biological and food samples by liquid chromatography. Anal. Biochem. 1999, 269, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Aliashkevich, A.; Alvarez, L.; Cava, F. New Insights Into the Mechanisms and Biological Roles of D-Amino Acids in Complex Eco-Systems. Front. Microbiol. 2018, 9, 683. [Google Scholar] [CrossRef]
- Sasabe, J.; Miyoshi, Y.; Rakoff-Nahoum, S.; Zhang, T.; Mita, M.; Davis, B.M.; Hamase, K.; Waldor, M.K. Interplay between microbial D-amino acids and host D-amino acid oxidase modifies murine mucosal defence and gut microbiota. Nat. Microbiol. 2016, 1, 16125. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.W. Chiral Toxicology: It’s the Same Thing...Only Different. Toxicol. Sci. 2009, 110, 4–30. [Google Scholar] [CrossRef]
- Kotake, T.; Usami, M.; Akaza, H.; Koiso, K.; Homma, Y.; Kawabe, K.; Aso, Y.; Orikasa, S.; Shimazaki, J.; Isaka, S.; et al. Goserelin Acetate with or without Antiandrogen or Estrogen in the Treatment of Patients with Advanced Prostate Cancer: A Multicenter, Randomized, Controlled Trial in Japan. Jpn. J. Clin. Oncoi. 1999, 29, 562–570. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Melchiorri, P.; Negri, L. The dermorphin peptide family. Gen. Pharmacol. 1996, 27, 1099–1107. [Google Scholar] [CrossRef]
- Kreil, G. Conversion of L- to D-Amino Acids: A Posttranslational Reaction. Science 1994, 266, 996–997. [Google Scholar] [CrossRef]
- Jung, G. Proteine aus der D-chiralen Welt. Angew. Chem. 1992, 104, 1484–1486. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, S.; Martínez-Gómez, A.I.; Rodríguez-Vico, F.; Clemente-Jiménez, J.M.; Las Hera-Vázquez, F.J. Natural Occurrence and Industrial Applications of D-Amino Acids: An Overview. Chem. Biodivers. 2010, 4, 1531–1548. [Google Scholar] [CrossRef]
- Monograph for Professionals. Drugs.com. Available online: https://www.drugs.com/monograph/cyclosporine.html (accessed on 19 June 2019).
- Copeland, K.R.; Yatscoff, R.W.; McKenna, R.M. Immunosuppressive activity of cyclosporine metabolites compared and characterized by mass spectroscopy and nuclear magnetic resonance. Clin. Chem. 1990, 36, 225–229. [Google Scholar] [CrossRef]
- Pikuta, E.V.; Hoover, R.B.; Klyce, B.; Davies, P.-C.W.; Davies, P. Bacterial utilization of L-sugars and D-amino acids. Proceedings 2006, 6309, 63090A. [Google Scholar] [CrossRef]
- Gutiérrez, M.; Capson, T.; Guzmán, H.M.; Quiňoá, E.; Riguera, R. L-Galactose as a natural product: Isolation from a marine octocoral of the first α-L-galactosyl saponin. Tetrahedron Lett. 2004, 45, 7833–7836. [Google Scholar] [CrossRef]
- Fujii, N. D-amino acids in living higher organisms. Orig. Life Evol. Biosph. 2002, 32, 103–127. [Google Scholar] [CrossRef] [PubMed]
- Deamer, D.; Dick, R.; Thiemann, W.; Shinitzky, M. Intrinsic Asymmetries of Amino Acid Enantiomers and Their Peptides: A Possible Role in the Origin of Biochirality. Chirality 2007, 19, 751–763. [Google Scholar] [CrossRef]
- Brückner, H.; Fujii, N. (Eds.) D-Amino Acids in Chemistry, Life Sciences, and Biotechnology; Wiley-VCH, Verlag Helvetica Chimica Acta: Zürich, Switzerland, 2011; p. 392. ISBN 978-3-906-39065-9. [Google Scholar]
- Gaspar, D.; Veiga, A.S.; Castanho, M.A.R.B. From antimicrobial to anticancer peptides. A review. Front. Microbiol. 2013, 4, 294. [Google Scholar] [CrossRef]
- Genchi, G. An overview on D-amino acids. Amino Acids 2017, 49, 1521–1533. [Google Scholar] [CrossRef]
- Pundir, C.S.; Lata, S.; Narval, V. Biosensors for determination of D- and L-amino acids: A Review. Biosens. Bioelectron. 2018, 117, 373–384. [Google Scholar] [CrossRef]
- Grishin, D.V.; Zhdanov, D.D.; Pokrovskaya, M.V.; Sokolov, N.N. D-amino acids in nature, agriculture and biomedicine. Front. Life Sci. 2020, 13, 11–22. [Google Scholar] [CrossRef]
- Podlech, J. Origin of organic molecules and biomolecular homochirality. Cell. Moll. Life Sci. 2001, 58, 44–60. [Google Scholar] [CrossRef] [PubMed]
- Gusev, V.A. Living Universe. Fundamental of Life; Chap-IV-06; Elsevier: Paris, France, 2001; p. 41. [Google Scholar]
- Plasson, R.; Kondepudi, D.K.; Bersini, H.; Commeyras, A.; Asakura, K. Emergence of homochirality in far-from-equilibrium systems: Mechanisms and role in prebiotic chemistry. Chirality 2007, 19, 589–600. [Google Scholar] [CrossRef]
- Avnir, D. Critical review of chirality indicators of extraterrestrial life. New Astron. Rev. 2021, 92, 101596. [Google Scholar] [CrossRef]
- Meierhenrich, U.J. Amino acids and the asymmetry of life. Eur. Rev. 2013, 21, 190–199. [Google Scholar] [CrossRef]
- Avetisov, V.; Goldanskii, V. Mirror symmetry breaking at the molecular level. Proc. Natl. Acad. Sci. USA 1996, 93, 11435–11442. [Google Scholar] [CrossRef] [PubMed]
- Cline, D.B. On the physical origin of the homochirality of life. Eur. Rev. 2005, 13, 49–59. [Google Scholar] [CrossRef]
- Schrödinger, E. What is Life? The Physical Aspect of the Living Cell; Mind and Matter; & Autobiographical Sketches; Cambridge University Press: Cambridge, UK; New York, NY, USA, 1992; p. 184. [Google Scholar]
- Dinner, A.R.; Rice, S.A. (Eds.) Proceedings of the 240 Conference Science’s Great Challenges; J. Wiley&Sons: Hoboken, NJ, USA, 2015; ISBN 978-1-118-95959-6. [Google Scholar]
- Aartsen, M.G. Searches for Sterile Neutrinos with the IceCube Detector. Phys. Rev. Lett. 2016, 117, 071801. [Google Scholar] [CrossRef]
- Angle, J.; The XENON10 Collaboration. Limits on spin-dependent WIMP-nucleon cross-sections from the XENON10 experiment. Phys. Rev. Lett. 2008, 101, 091301. [Google Scholar] [CrossRef]
- Only left-handed particles decay. Nature 2015, 524, 8. [CrossRef]
- The LHCb collaboration; Aaij, R.; Raven, G. Determination of the quark coupling strength |Vub| using baryonic decays. Nat. Phys. 2015, 11, 743–747. [Google Scholar] [CrossRef]
- Drewes, M. The Phenomenology of Right Handed Neutrinos. Int. J. Mod. Phys. E 2013, 22, 1330019. [Google Scholar] [CrossRef]
- Ge, S.-F.; Pasquini, P. Parity violation and chiral oscillation of cosmologic relic neutrinos. Phys. Lett. B 2020, 135961. [Google Scholar] [CrossRef]
- The T2K Collaboration. Constraint on the matter–antimatter symmetry-violating phase in neutrino oscillations. Nature 2020, 580, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Patrignani, C. Review of Particle Physics. Chin. Phys. C 2016, 40, 100001. [Google Scholar] [CrossRef]
- Dasgupta, B.; Kopp, J. Sterile neutrinos. Phys.Rep. 2021, 928, 1–63. [Google Scholar] [CrossRef]
- All Things Neutrino. Fermilab. Available online: https://neutrinos.fnal.gov/ (accessed on 8 August 2021).
- Boyarsky, A.; Drewes, M.; Lasserre, T.; Mertens, S.; Ruchayskyi, O. Sterile neutrino dark matter. Prog. Part. Nucl. Phys. 2019, 104, 1–45. [Google Scholar] [CrossRef]
- Javoy, M. Where do oceans come from? Compt. Rend. Geosci. 2005, 337, 139–158. [Google Scholar] [CrossRef]
- From Suns to Life: A Chronological Approach to the History of Life on Earth; Gargaud, M., Clayes, P., López-Gárcia, P., Martin, H., Montmerle, T., Pascal, R., Reisse, J., Eds.; Springer: Dordrecht, The Netherlands, 2006; reprinted in Earth Moon Planets 2006, 98, 371; ISBN 978-0-387-45082-7. [Google Scholar] [CrossRef]
- Nath, B. Water on Earth: Where did it come from? Reson. -J. Sci. Educ. 2019, 24, 575–582. [Google Scholar] [CrossRef]
- Spiga, R.; Barbieri, C.; Bertini, I.; Lazzarini, M.; Nestola, F. The origin of water on Earth: Stars or diamonds? Rend. Fiss. Acc. Lincei 2019, 30, 261–268. [Google Scholar] [CrossRef]
- Wakeford, H.R.; Sing, D.K.; Deming, D.; Lewis, N.K.; Goyal, J.; Wilson, T.J.; Barstow, J.; Kataria, T.; Drummond, B.; Evans, T.M.; et al. The complete transmission spectrum of WASP-39b with a precise water constraint. Astronom. J. 2018, 155, 14. [Google Scholar] [CrossRef]
- Izidoro, A.; De Souza Torres, K.; Winter, O.C.; Haghighipour, N. A compound model for the origin of Earth’s water. Earth Planet Astrophys. 2013, 767, 54. [Google Scholar] [CrossRef]
- Nestola, F.; Smyth, J.R. Diamonds and water in the deep Earth: A new scenario. Int. Geol. Rev. 2016, 58, 263–276. [Google Scholar] [CrossRef]
- Hallis, L.J. D/H ratios of the inner solar system. Philos. Trans. R. Soc. A 2017, 375, 20150390. [Google Scholar] [CrossRef] [PubMed]
- Altwegg, K.; Balsiger, H.; Bar-Nun, A.; Berthelier, J.J.; Bieler, A.; Bochsler, P.; Briois, C.; Calmonte, U.; Combi, M.; De Keyser, J.; et al. 67P/Churyumov-Gerasimenko, a Jupiter family comet with a high D/H ratio. Science 2015, 347, 1261952. [Google Scholar] [CrossRef]
- Choi, C.Q. Most of Earth’s Water Came from Asteroids, Not Comets. Space.com. 10 December 2014. Available online: http://www.space.com/27969-earth-water-from-asteroids-not-comets.htm (accessed on 27 July 2021).
- Oparin, A.I.; Morgulis, S. The Origin of Life, 2nd ed.; Dover Publications: Mineola, NY, USA, 2003; p. 270. ISBN 10:0486602133. [Google Scholar]
- Bernal, J.D. The Origin of Life; World Publishing: Cleveland, OH, USA, 1967; p. 345. ISBN 13:978-0297170358. [Google Scholar]
- Shapiro, R. Origins: A Skeptic’s Guide to the Creation of Life on Earth; Bantam New Age: New York, NY, USA, 1986; p. 0553343556. ISBN 13: 9780553343557. [Google Scholar]
- Ferus, M.; Laitl, V.; Knizek, A.; Kubelík, P.; Sponer, J.; Kára, J.; Lefloch, B.; Cassone, G.; Civiš, S. HNCO-based synthesis of formamide in planetary atmospheres. Astron. Astrophys. 2018, 616, A150. [Google Scholar] [CrossRef]
- Adam, Z.R.; Hongo, Y.; Ii, H.J.C.; Yi, R.; Fahrenbach, A.C.; Yoda, I.; Aono, M. Estimating the capacity for production of formamide by radioactive minerals on the prebiotic Earth. Sci. Rep. 2018, 8, 265. [Google Scholar] [CrossRef] [PubMed]
- Wächtershäuser, G. On the chemistry and evolution of the pioneer organism. Chem. Biodivers. 2007, 4, 584–602. [Google Scholar] [CrossRef] [PubMed]
- Gesteland, R.F.; Cech, T.; Atkins, J.F. The RNA World: The Nature of Modern RNA Suggests a Prebiotic RNA World, 3rd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2006; p. 768. ISBN 0-87969-739-3. [Google Scholar]
- Jia, T.Z.; Kuruma, Y. Recent Advances in Origins of Life Research by Biophysicists in Japa: A Review. Challenges 2019, 10, 28. [Google Scholar] [CrossRef]
- Andrulis, E.D. Theory of the Origin, Evolution, and Nature of Life. Life 2012, 2, 1–105. [Google Scholar] [CrossRef]
- Lee, T.D. Obituary: Chien-Shiung Wu (1912-97), Experimental physicist, co-discoverer of parity violation. Nature 1997, 386, 334. [Google Scholar] [CrossRef]
- Bonner, W. Parity violation and the evolution of biomolecular homochirality. Chirality 2000, 12, 114–126. [Google Scholar] [CrossRef]
- Wagniére, G.H. On Chirality and the Universal Asymmetry: Reflections on Image and Mirror Image; Wiley-VCH: Zürich, Switzerland, 2007; ISBN 978-3-906-39038-3. [Google Scholar]
- Bernabeu, J. Symmetries and Their Breaking in the Fundamental Laws of Physics. Symmetry 2020, 12, 1316. [Google Scholar] [CrossRef]
- Lee, T.D.; Yang, C.N. Question of parity conservation in weak interactions. Phys. Rev. 1956, 104, 254–258. [Google Scholar] [CrossRef]
- Wu, C.S.; Ambler, E.; Hayward, R.W.; Hoppes, D.D.; Hudson, R.P. Experimental Test of Parity Conservation in Beta Decay. Phys. Rev. 1957, 105, 1413–1415. [Google Scholar] [CrossRef]
- Rubbia, C. Experimental observation of the intermediate vector bosons W+,W− and Z0. Rev. Mod. Phys. 1985, 57, 699–722. [Google Scholar] [CrossRef]
- Quack, M. How important is parity violation for molecular and biomolecular chirality? Angew. Chem. Int. Ed. Engl. 2002, 41, 4618–4630. [Google Scholar] [CrossRef]
- Quack, M. Molecular Parity Violation and Chirality: The Asymmetry of Life and the Symmetry Violation in Physics. In Quantum Systems in Chemistry and Physics: Progress in Methods and Applications; Chapter 3; Theoretical Chemistry and Physics; Nishikawa, K., Maruani, J., Brändas, E.J., Delgado-Barrio, G., Piecuch, P., Eds.; Springer Science: Dordrecht, The Netherlands, 2012; Volume 26, pp. 47–76. ISBN 13:9789400752979. [Google Scholar]
- Plankensteiner, K.; Righi, A.; Rode, B.M.; Gargallo, R.; Jaumot, J.; Tauler, R. Indications toward a stereoselectivity of the salt-induced peptide formation reaction. Inorg. Chim. Acta 2004, 357, 649–656. [Google Scholar] [CrossRef]
- Berger, R.; Quack, M. Electroweak quantum chemistry of alanine: Parity violation in gas and condensed phases. Chem. Phys. Chem. 2000, 1, 57–60. [Google Scholar] [CrossRef]
- Plankensteiner, K.; Reiner, H.; Rode, B.M. Stereoselective differentiation in the salt-induced formation reaction and its relevance for the origin of life. Peptides 2005, 26, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Peskin, R. The matter with antimatter. Nature 2002, 419, 25–27. [Google Scholar] [CrossRef]
- Santos, A.C.L.; Muniz, C.R.; Oliveira, L.T.; Souza, J.T. Contribution of a modified electrodynamics to the molecular biochirality. Chirality 2020, 32, 1186–1190. [Google Scholar] [CrossRef]
- Soai, K.; Shibata, T.; Morioka, H.; Choji, K. Asymmetric autocatalysis and amplification of enantiomeric excess of a chiral molecule. Nature 1995, 378, 767–768. [Google Scholar] [CrossRef]
- Hawbaker, N.A.; Blackmond, D.G. Energy threshold for chiral symmetry breaking in molecular self-replication. Nat. Chem. 2019, 11, 957–962. [Google Scholar] [CrossRef]
- Rubinstein, I.; Clodic, G.; Bolbach, G.; Weissbuch, I.; Lahav, M. Racemic beta-sheets as templates for the generation of homochiral (isotactic) peptides from aqueous solutions of (RS)-valin or -leucin N-carboxyanhydrides: Relevance to biochirogenesis. Chem. Eur. J. 2008, 14, 10999–11009. [Google Scholar] [CrossRef] [PubMed]
- Pizzarello, S.; Weber, A.L. Prebiotic amino acids as asymmetric catalysts. Science 2004, 303, 1151. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.L.; Pizzarello, S. The peptide-catalyzed stereospecific synthesis of tetroses: A possible model for prebiotic molecular evolution. Proc. Natl. Acad. Sci. USA 2006, 103, 12713–12717. [Google Scholar] [CrossRef]
- Nanita, S.C.; Cooks, R.G. Serine octamers: Clusters formation. Reaction, and implication for biomolecule homochirality. Angew. Chem. Int. Ed. Engl. 2006, 45, 554–569. [Google Scholar] [CrossRef] [PubMed]
- Cordova, A.; Sunden, H.; Xu, Y.; Ibrahem, I.; Zou, W.; Engquist, M. Sugar-assisted kinetic resolution of amino acids and amplification of enantiomeric excess of organic molecules. Chem. Eur. J. 2006, 12, 5446–5451. [Google Scholar] [CrossRef]
- Toxvaerd, S. Homochirality in bio-organic systems and glyceraldehyde in the formose reaction. J. Biol. Phys. 2005, 31, 599–606. [Google Scholar] [CrossRef]
- NASA Exoplanet Erchive. Available online: https://exoplanetarchive.ipac.caltech.edu (accessed on 27 July 2021).
- The Mars Curiosity Rover Can Detect Chiral Compounds. Available online: https://www.dailykos.com/stories/2012/8/25/11;24242/-The-Mars-Curiosity-Rover-Can-Detect-Chiral-Compounds (accessed on 27 July 2021).
- Goesmann, F.; The MOMA Science team. The Mars Organic Molecule Analyzer (MOMA) Instrument: Characterization of Organic Material in Martian Sediments. Astrobiology 2017, 17, 655–685. [Google Scholar] [CrossRef] [PubMed]
- Meierhenrich, U.J.; Cason, J.R.L.; Szopa, C.; Sternberg, R.; Raulin, F.; Thiemann, W.H.-P.; Goesmann, F. Evaluating the robustness of the enantioselective stationary phases on the Rosetta mission against space vacuum vaporization. Adv. Space Res. 2013, 52, 2080–2084. [Google Scholar] [CrossRef]
- Suzuki, N.; Itabashi, Y. Possible roles of amphiphilic molecules in the origin of biological homochirality. Symmetry 2019, 11, 966. [Google Scholar] [CrossRef]
- Lombard, J.; López-García, P.; Moreira, D. The early evolution of lipid membranes and the three domains of life. Nat. Rev. Microbiol. 2012, 10, 507–515. [Google Scholar] [CrossRef]
- Koga, Y. Early evolution of membrane lipids: How did the lipid divide occur? J. Mol. Evol. 2011, 72, 274–282. [Google Scholar] [CrossRef]
- Caforio, A.; Siliakus, M.E.; Exterkate, M.; Jain, S.; Jumde, V.R.; Andringa, R.L.H.; Kengen, S.W.M.; Minnaard, A.J.; Driessen, A.J.M.; van der Oost, J. Converting Escherichia coli into an archaebacterium with hybrid heterochiral membrane. Proc. Natl. Acad. Sci. USA 2018, 115, 3704–3709. [Google Scholar] [CrossRef]
- Ikehara, K. Possible steps to the emergence of life: The [GADV]-protein world hypothesis. Chem. Rec. 2005, 5, 107–118. [Google Scholar] [CrossRef]
- Deamer, D. The role of lipid membranes in life´s origin. Life 2017, 7, 5. [Google Scholar] [CrossRef]
- Cooper, G.; Kimmich, N.; Belisle, W.; Sarinana, J.; Brabham, K.; Garrel, L. Carbonaceous meteorites as a source of sugar-related organic compounds for the early Earth. Nature 2001, 414, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Glavin, D.P.; Dworkin, J.P. Enrichment of the amino acid L-isovaline by aqueous alteration on CI and CM meteorite parent bodies. Proc. Natl. Acad. Sci. USA 2009, 106, 5487–5492. [Google Scholar] [CrossRef]
- Glavin, D.P.; Callahan, M.P.; Dworkin, J.P.; Elsila, J.E. The effects of parent body processes on amino acids in carbonaceous chondrites. Meteorit. Planet. Sci. 2010, 45, 1948–1972. [Google Scholar] [CrossRef]
- Breslow, R.; Cheng, Z.-L. L-amino acids catalyze the formation of an excess of D-glyceraldehyde, and thus of other D sugars, under credible prebiotic conditions. Proc. Natl. Acad. Sci. USA 2010, 107, 5723–5725. [Google Scholar] [CrossRef]
- Morowitz, H. Mechanism for the amplification of fluctuations in racemic mixtures. J. Theor. Biol. 1969, 25, 491–494. [Google Scholar] [CrossRef]
- Bada, J.L. Enantiomeric excesses in the Murchison meteorite and the origin of homochirality in terrestrial biology. Proc. Natl. Acad. Sci. USA 2009, 106, E85. [Google Scholar] [CrossRef] [PubMed]
- Pizzarello, S.; Shock, E. Carbonaceous chondrite meteorites: The chronicle of a potential evolutionary path between stars and life. Orig. Life Evol. Biosph. 2017, 47, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Pizzarello, S.; Groy, T.L. Molecular asymmetry in extraterrestrial organic chemistry: An analytical perspective. Geochim. Cosmochim. Acta 2011, 75, 645–656. [Google Scholar] [CrossRef]
- Cronin, J.R.; Pizzarello, S. Enantiomeric excesses in meteoritic amino acids. Science 1997, 275, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Koga, T.; Naraoka, H. A new family of extraterrestrial amino acids in the Murchison Meteorite. Sci. Rep. 2017, 7, 636. [Google Scholar] [CrossRef]
- Pizzarello, S.; Yarnes, C.T. Chiral molecules in space and their possible passage to planetary bodies recorded by meteorites. Earth Planet. Sci. Lett. 2018, 496, 198–205. [Google Scholar] [CrossRef]
- Pizzarello, S.; Yarnes, C.T. The soluble organic compounds of the Mukundpura meteorite: A new CM chondrite fall. Planet. Space Sci. 2018, 164, 127–131. [Google Scholar] [CrossRef]
- Burton, A.S.; Berger, E. L Insights into abiotically-generated amino acid enantiomeric excesses in meteorites. Life 2018, 8, 14. [Google Scholar] [CrossRef]
- Elsila, J.E.; Aponte, J.C.; Blackmond, D.G.; Burton, A.S.; Dworkin, J.P.; Glavin, J.P. Meteoritic amino acids: Diversity in compositions reflects parent body histories. ACS Cent. Sci. 2016, 2, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Glavin, D.P.; Dworkin, J.P.; Aubrey, A.; Botta, O.; Doty III, J.H.; Martins, Z.; Bada, J.L. Amino acid analyses of Antarctic CM2 meteorites using liquid chromatography–time of flight–mass spectrometry. Meteorit. Planet. Sci. 2010, 41, 889–902. [Google Scholar] [CrossRef]
- Cooper, G.; Rios, A.C. Enantiomer excesses of rare and common sugar derivatives in carbonaceous meteorites. Proc. Natl. Acad. Sci. USA 2016, 113, E3322–E3331. [Google Scholar] [CrossRef]
- Lente, G. Stochastic analysis of the parity-violating energy differences between enantiomers and its implications for the origin of biological chirality. J. Phys. Chem. A 2006, 110, 12711–12713. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, R.; Pyda, M.; Pak, J.; Wunderlich, B.; Thompson, J.R.; Pagni, R.; Pan, H.; Barnes, C.; Schwerdtfeger, P.; Compton, R. Search for electroweak interactions in amino acid crystals. II. The Salam hypothesis. J. Phys. Chem. A 2003, 107, 6674–6680. [Google Scholar] [CrossRef]
- Pizzarello, S.; Cronin, J.R. Non-racemic amino acids in the Murray and Murchison meteorites. Geochim. Cosmochim. Acta. 2000, 64, 329–338. [Google Scholar] [CrossRef]
- Avalos, M.; Babiano, R.; Cintas, P.; Jiménez, J.L.; Palacios, J.C. Chiral autocatalysis: Where stereochemistry meets the origin of life. Chem. Commun. 2000, 887–892. [Google Scholar] [CrossRef]
- NASA Space Science. Available online: https://cdn.images.express.co.uk/img/dynamic/151/590x/secondary/NASA-space-science-1185919.jpg (accessed on 8 August 2021).
- Image Created by R. Stockli with the Help of A. Nelson, under the Leadership of F. Hasler. 26 April 2000. Available online: https://earthobservatory.nasa.gov/images/565/earth-the-blue-marble (accessed on 8 August 2021).
- Vandenbussche, S.; Reisse, J.; Bartik, K.; Lievin, J. The Search for a Deterministic Origin for the Presence of Nonracemic Amino-Acids in Meteorites: A computational Approach. Chirality 2011, 23, 367–373. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lucas, P.W.; Hough, J.H.; Bailey, J.; Chrysostomou, A.; Gledhill, T.M.; McCall, A. UV Circular polarisation in star formation regions: The origin of homochirality ? Orig. Life Evol. Biosph. 2005, 35, 29–60. [Google Scholar] [CrossRef]
- Kondepudi, D.K.; Kaufman, R.J.; Singh, N. Chiral Symmetry Breaking in Sodium Chlorate Crystallizaton. Science 1990, 250, 975–976. [Google Scholar] [CrossRef]
- McBride, J.M.; Carter, L.R. Spontaneous Resolution by Stirred Crystallization. Angew. Chem. Int. Ed. Engl. 1991, 30, 293–295. [Google Scholar] [CrossRef]
- Viedma, C.; Cintas, P. Homochirality beyond grinding: Deracemizing chiral crystals by temperature gradient under boiling. Chem. Commun. 2011, 47, 12786–12788. [Google Scholar] [CrossRef]
- Giannetto, E. (R.A.C.). The light of photophysics and photochemistry. In Prebiotic Photochemistry: From Urey-Miller-Like Experiments to Recent Findings; Saija, F., Cassone, G., Eds.; European Society for Photobiology, Rozal Society of Chemistry: London, UK, 2021; Chapter 1; pp. 1–8. ISBN 978-1-83916-177-3. [Google Scholar] [CrossRef]
- Ulmer, S.; Smora, C.; Mooser, A.; Franke, K.; Nagahama, H.; Schneider, G.; Higuchi, T.; VanGroup, S.; Blaum, K.; Matsuda, Y.; et al. High-precision comparison of the antiproton-to-proton charge-to-mass ratio. Nature 2015, 524, 196–199. [Google Scholar] [CrossRef]
- Meier, C.A. (Ed.) Der Briefwechsel. In Wolfgang Pauli und C. G. Jung; Springer: Berlin/Heidelberg, Germany, 1992. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).