1. Introduction
In illusory line motion (ILM), a dot is presented, followed by, after a short delay, a line or bar, abutting the spot at one of its ends. The spot remains throughout the presentation of the bar, and although the bar is presented as a complete unit, it appears to be growing outward and away from the dot [
1,
2,
3]. The reverse perceptual outcome is obtained when the bar is removed, as it appears as if it is contracting into the dot (i.e., Reverse ILM, RevILM). The effect was firstly described by Kanizsa [
1], Kanizsa and Legrenzi [
4] (who named it polarized gamma motion, PGM) and then re-discovered by Hikosaka, Miyauchi and Shimojo [
2,
3], who attributed it to an attentional gradient such that regions of the line closest to the attended spot are processed faster, and thereby activate higher-level motion detectors sooner. However, this sensory account has been questioned in subsequent studies, favoring instead an account in which the illusion is the result of an impletion process that fills in interpolated events after the cue and the line are linked as successive states of a single object in apparent motion [
5]. This latter explanation for ILM therefore emphasizes higher-level processes that ensure object continuity and coherence, rather than the firing of motion detectors resulting from an attention-induced asynchrony (as is suggested by the attentional gradient account). It is worth noticing that in both the explanations that have been suggested for ILM, exogenous attention plays a key role. Indeed, according to Hikosaka, Miyauchi and Shimojo [
2,
3], exogenous spatial attention has a facilitatory effect, while, according to Downing and Treisman [
5], it acts both as a mechanism for feature binding in multielement displays and as a bias, towards which sensory data are given precedence in initiating a representation of a single object.
Several variants of ILM have been proposed in attempting to understand the basic mechanisms underlying its occurrence [
6]: (i) if two dots are presented before the appearance of the line and the line is abutting the spots at both of its ends, then observers report a sensation of inward motion that meets at the center of the line [
5,
7]; (ii) if the bar is presented between two dots (or objects) that differ in some attribute, such as color or shape, then the bar matches one of the objects with respect to this attribute [
8,
9], in which case it is referred to as transformational apparent motion (TAM); (iii) if the bar appears between two dots, and one of the dots is flashing, then the bar appears to shoot out of the flashed box [
2,
3,
9,
10,
11,
12], while if the bar is removed following the flash of one dot, then the bar appears to contract into the non-flashed box. Given that in both these latter cases an illusory motion away from the flash is perceived, Han and Hamm [
13] named both cases as flash-ILM, suggesting to report as Reverse ILM (RevILM, which is in fact a RevFlashILM), the effect of which, if the bar is removed shortly after the onset of the flash, being that the illusion reverses direction and the bar appears to contract into the flashed box [
12,
14].
For all the variants reported above, different explanations have been suggested, all basically underlining the possibility of ILM being due to: (a) a low-level motion effect [
15], (b) a gradient of exogenous spatial attention [
16] or (c) both [
11].
Only recently has it been suggested that all these variants should be considered as different illusions [
13]. According to this view, the literature concerning ILM is possibly conflating a number of different illusions of line motion, some of which (such as PGM and TAM) could be accounted for by low-level mechanisms for motion detection, whilst others (such as flashILM) could be better explained by exogenous attention induced motion.
As discussed above, of the several variants of ILM presented in the literature, the first (i.e., the one in which the line is bilaterally cued) has consistently been reported as giving rise to an inward motion that meets at the center of the line. However, in a previous study performed in our laboratory [
17], we unexpectedly observed a tendency of participants to report a movement towards the left when the line was bilaterally cued, an effect that had never been reported before. This tendency to perceive the illusory motion in the same direction of the unilateral right cue condition suggests an asymmetrical attentional salience of the cues in the case of simultaneous double stimulation. This phenomenon is similar to that observed in patients with unilateral focal lesions that correctly detect unilateral stimuli but, in the case of a simultaneous double stimulation, report only the stimulus in the visual hemi-field contralateral to the lesion (i.e., extinction [
18,
19,
20]). Some studies have reported a phenomenon of the single detection of simultaneous double stimulation also in neurologically unimpaired individuals and named it pseudo-extinction [
21,
22,
23,
24,
25,
26,
27,
28]. Nevertheless, previous studies showed no spatial bias, given that participants missed equally often left and right stimuli.
The present study is aimed at verifying the existence of a leftward bias in bilaterally cued ILM, by using dot cues instead of faces. It is also aimed at understanding the mechanisms underlying the asymmetry.
In particular, Experiment 1 is aimed at replicating our previous results [
17] with a typical ILM display. In our previous study, which was aimed at testing the effect of attention-orientation on emotional faces, the classical dots were substituted with emotional faces, and both the faces and the bar were presented for 200 ms. In Experiment 1, we have instead used the classical paradigm developed by both Hikosaka, Miyauchi, and Shimojo [
2,
3], and Downing and Treisman [
5], with simple dots as cues and a presentation time (for both dots and bar) of 150 ms. Given that the attentional orientation asymmetry has been reported both for the left/right and the lower/higher visual hemifields [
29], Experiment 2 was aimed at testing whether a lower bias is also present when the bar is vertically oriented, while Experiment 3 was aimed at testing the radial orientation of 45° and 315° (thus presenting a diagonal bar cued at top-right/bottom-left or top-left/bottom-right respectively). Finally, in Experiment 4, we aimed at verifying a neuro-functional hypothesis, which is whether the superior colliculus (SC) is involved in the asymmetrical pseudo-extinction phenomenon of ILM. We chose this structure because it is involved in visuo-spatial attention and eye movements [
30,
31,
32]. In order to assess SC involvement behaviourally, we took advantage of the fact that this structure is ‘less sensitive’ to blue colors than to red ones. Indeed, blue colors are mainly detected by the S-cones, which do not project directly, or do so only in a sparse manner, to the SC [
33,
34,
35,
36,
37]. Therefore, if the SC is the locus of plays a role in the asymmetric ILM in the bilateral cue condition, the pseudo-extinction phenomenon should be attenuated by blue stimuli.
In all four experiments, the presentation of the bar was preceded by either a single dot cueing one end of the line (for the two ends respectively) or two dots cueing both ends of the line. Surprisingly, to the best of our knowledge, no study on ILM has investigated the possible variation in the strength of the effect when the bar has different spatial orientations; the effect has typically been reported with horizontally orientated bars (although Downing and Treisman [
5] reported the ILM effect with diagonal bars). With our experiments, we should also be able to compare different spatial orientations of the bar directly within the same experimental paradigm.
2. Experiment 1
2.1. Materials and Methods
Participants: Twenty-six healthy, right-handed adult volunteers took part in the study (20 females, 6 males), aged between 20 and 29 (mean age: 22.76). All had normal or corrected-to-normal vision.
Apparatus and stimuli: All the stimuli were presented on a 17″ monitor, with a resolution of 1024 × 768 pixels and an 86 Hz refresh rate, connected to a Samsung SyncMaster 753S computer (Samsung, Seul, North Korea). The software used to present the stimuli was E-prime (Psychological Science Tools, Pittsburgh, USA) and the color space was sRGB.
A white line (15.53° × 0.43° of visual angle) was horizontally presented at the centre of a screen with a medium-gray background (RGB: 127). One or two red dots (2.21° × 2.21°) could appear, aligned with the line, at a distance of 2.6° from the ends.
Procedure: The experiment was conducted in a silent and dark room. Each participant sat in front of the monitor at a distance of 44 cm. In order to keep that distance and the gaze alignment with the centre of the screen constant, their heads were stabilized by a chin-rest.
The line could be presented in 4 different conditions: (1) line only (i.e., no red dots); (2) bilaterally cued line (i.e., two red dots at both ends of the line); unilaterally cued line (i.e., one red dot presented at the left (3) or at the right (4) end of the line, respectively).
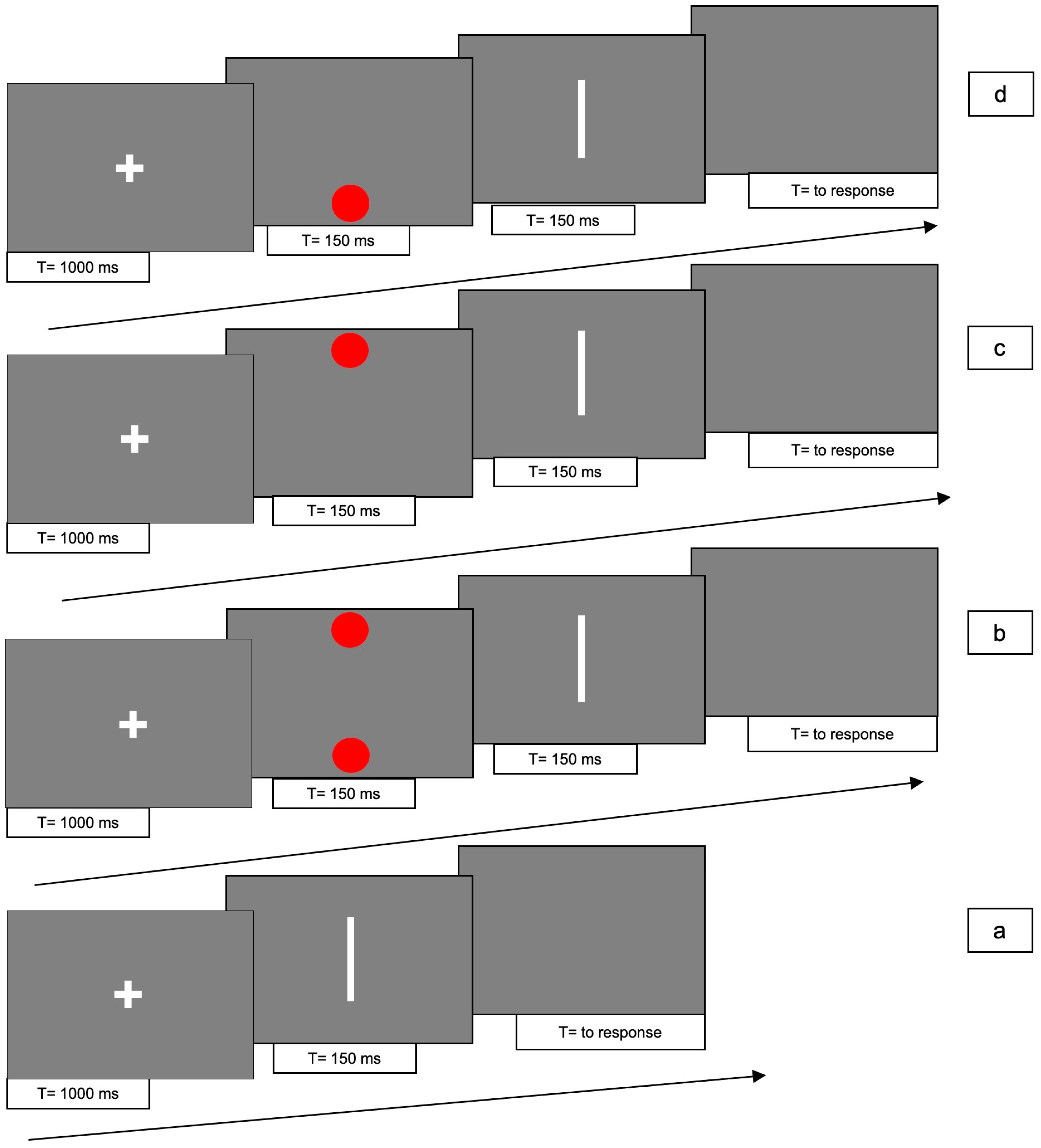
For conditions 2, 3 and 4, each trial consisted of the following 4 phases: (i) at the beginning of each trial a white fixation cross was presented at the centre of the screen for 1000 ms; (ii) immediately after the cross had disappeared, two (for condition 2) or one red dot(s) (for conditions 3 and 4) were presented for 150 ms; (iii) immediately after the disappearance of the dots, the white line was presented for 150 ms; (iv) after the disappearance of the bar, a gray screen appeared, which was visible until the participant responded.
In condition 1, phase (ii) was absent.
Figure 1 shows the experimental paradigm. Each trial lasted approximately 2 s.
A total of 10 trials were performed for each condition, with a total of 80 trials, randomly presented.
The task of each participant was to decide whether the direction of the movement of the bar was rightward or leftward, by pressing the left or right arrow key, respectively (2 alternative forced choice method, 2AFC) with the right hand. If the bar did not move, one of the two arrow keys still had to be pressed at random.
Before the experiment, each participant performed a practice trial.
2.2. Results
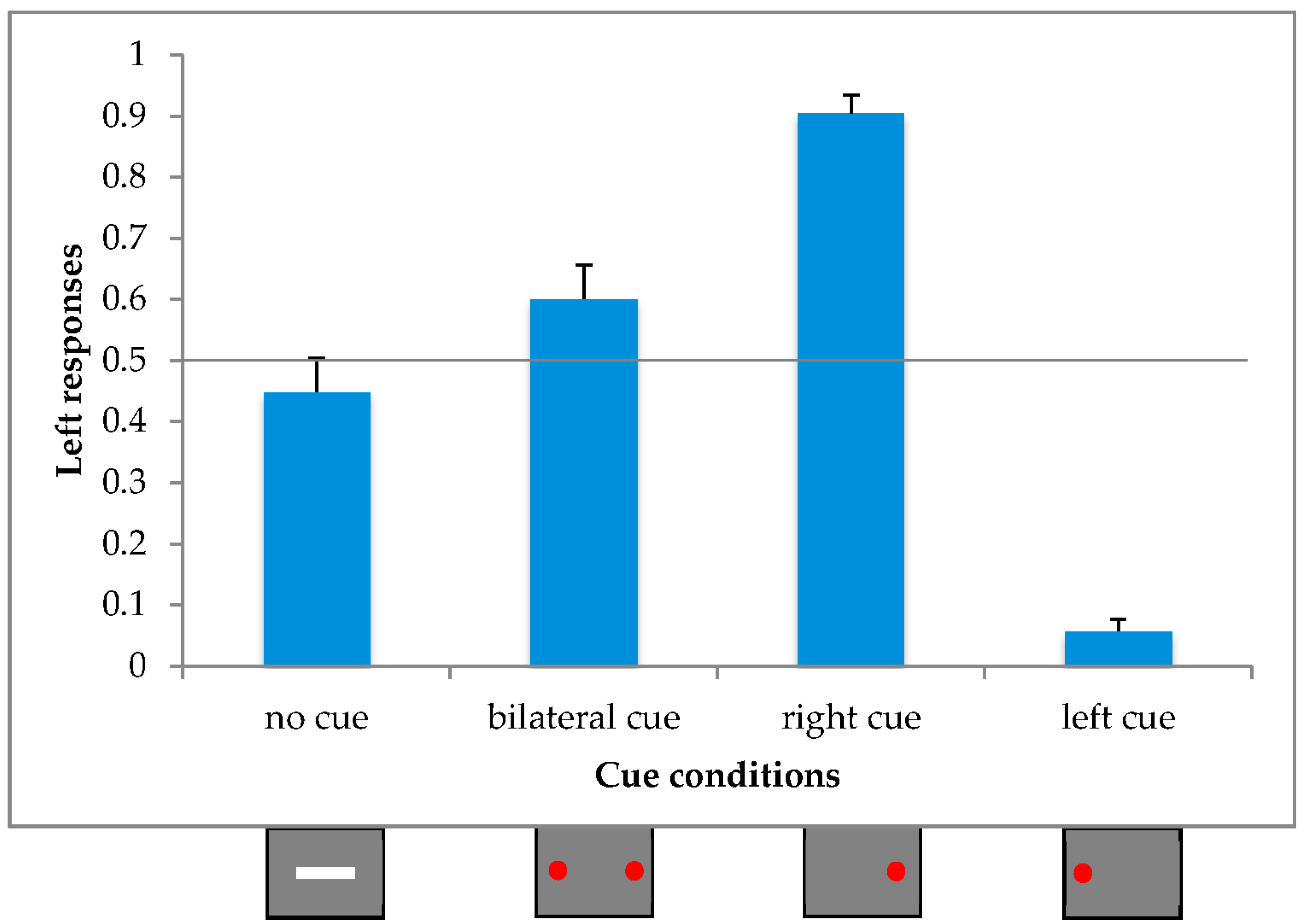
Since the only two conditions with an expected illusory effect, with a precise direction, were the unilaterally cued conditions, we excluded those participants who did not show one or both unilateral ILM. We calculated the mean proportion of leftward responses and the standard deviation for both unilaterally cued conditions and excluded those participants with a response greater than or equal to three standard deviations from the mean value, resulting in 5 out of the 26 participants being rejected.
In order to test whether an illusory effect was present not only under unilateral but also under bilateral conditions, we ran a within-subjects ANOVA with the proportions of leftward responses as the dependent variable and the Cue Condition as a repeated factor (4 levels; right unilateral cue, left unilateral cue, bilateral cue and no cue).
The main effect of Cue Condition [F(3,60) = 76.74, p < 0.001; η2p = 0.79] attained statistical significance.
Post-hoc comparisons (Duncan) revealed a significant difference between all four conditions (
p > 0.01). In particular, not only did the absence of any cue (0.45) differ from the left (0.06) and the right (0.90) unilateral cue condition, but it also differed from the bilateral (0.60) cue condition in the same direction as the right unilateral stimulus (see
Figure 2).
2.3. Discussion
This experiment confirms the presence of the illusory effect in our experimental procedure, as deduced from the unilateral right and left cue conditions.
In addition, as already observed in a previous study [
17], we found an asymmetrical effect in the symmetrical bilateral cue condition. In particular, the proportion of leftward responses was higher than for the simple line condition, denoted by a bias to the right.
As the effect of the inducing stimuli arose at a presentation time of 150 ms, which is below the time of endogenous attention [
38] and top-down saccadic movements (i.e., >250 ms; e.g., [
39]), these data suggest that the asymmetry was induced by exogenous mechanisms.
This could imply a bottom-up orientating of attention or, eventually, a bottom-up and top-down integration.
Whether it is either a simple orientation of attention (gradient) or a sensory activation facilitated by attention, attention certainly plays an important role.
The subsequent experiments (2 and 3) were devised to describe the leftward bias (right cue salience) better in terms of spatial orientation.
3. Experiment 2
Experiment 2 was designed to test whether the asymmetrical effect in the bilateral cue condition observed in the first experiment was also present in the vertical plane. Therefore, we presented a vertical line cued at the top and bottom ends.
3.1. Materials and Methods
Participants: Sixteen healthy, right-handed adult volunteers took part in the study (10 females, 6 males), aged between 20 and 25 (mean age: 22.53). Each had normal or corrected-to-normal vision.
Apparatus and stimuli: All the stimuli were presented on a 17″ monitor, with a resolution of 1024 × 768 pixels and an 86 Hz refresh rate, connected to a Samsung SyncMaster 753S computer (Samsung, Seul, North Korea). The software used to present the stimuli was E-prime (Psychological Science Tools, Pittsburgh, USA) and the color space was sRGB.
A white line (0.43° × 15.25°) was vertically presented at the centre of the screen on a medium-gray background (RGB: 127). At the top and bottom ends of the white line one or two red dots could appear (2.21° × 2.21°), at a distance of 0.65°.
Procedure: The experiment was conducted in a silent and dark room. Participants sat in front of the monitor at a distance of 44 cm. The head of each participant was stabilized with a chin-rest whose height was adjusted according to the requirements of each one so that the gaze was aligned with the center of the screen.
The line could be presented in 4 different conditions: (1) line only (i.e., no red dots); (2) bilaterally cued line (i.e., two red dots at both ends of the line); (3) unilaterally cued line at the top end of the line; (4) unilaterally cued line at the bottom end of the line.
For conditions 2, 3 and 4, each trial consisted of the following 4 phases: (i) t the beginning of each trial, a white fixation cross was presented at the centre of the screen for 1000 ms; (ii) immediately after the cross had disappeared, two (for condition 2) or one red dot(s) (for conditions 3 and 4) were presented for 150 ms; (iii) immediately after the disappearance of the dots, the white line was presented for 150 ms; (iv) after the disappearance of the bar, a gray screen appeared, which was visible until the participant responded.
In condition 1, phase (ii) was absent.
Figure 3 shows the experimental paradigm. Each trial lasted approximately 2 s.
There were 10 trials for each condition, with a total of 80 trials that were randomly presented.
The task of each participant was to determine the direction of the movement of the bar, be it upward or downward, and to press either the up- or down-arrow keys with the right hand, as per 2AFC. If the bar did not move, one of the two arrow keys still had to be pressed at random.
Before the experiment, each participant performed a practice trial.
3.2. Results
We calculated the mean proportion of downward responses and the standard deviation for both unilaterally cued conditions and excluded those participants with a response greater than or equal to three standard deviations from the mean value, resulting in 4 out of the 16 participants being rejected.
To test whether there were significant differences in the choice of either upward or downward, a within-subjects ANOVA was carried out with Condition as repeated factor (4 levels: bottom cue, top cue, bilateral cue and no cue).
The analysis demonstrated a main effect of the condition [F(3,33) = 13.29, p < 0.05; η2p = 0.55].
We performed post hoc comparisons (Duncan) from which it appeared that the bottom-unilateral condition differs significantly from all others (
p < 0.05). As can be seen from
Figure 4, when the inducing cue is below the apparent movement it is perceived to be upward, while in all the other conditions it is perceived to be downward.
3.3. Discussion
The results of this experiment confirm the presence of the illusory effect only for the bottom cue condition, in the vertical plane. The bilateral condition did not differ significantly from the bar condition and in both cases there is a downward asymmetry. There is a bias in the downward responses, but not an asymmetrical pseudo-extinction phenomenon.
4. Experiment 3
Experiments 3 was designed to test the line-motion illusion in the radial orientation of 45° clockwise and counter-clockwise, and the asymmetric effect in the bilateral cue condition observed in the first experiment. Therefore, we presented a diagonal line cued at top-right, bottom-left or top-left/bottom-right.
The two display orientations, clockwise and counter-clockwise, were initially run as two different experiments. Since the participants and the apparatus were the same, after the revision, we combined the description and the analyses in a single experiment.
4.1. Materials and Methods
Participants: Twenty healthy, right-handed adult volunteers took part in the study (15 females, 5 males), aged between 19 and 39 (mean age: 27.1). All had normal or corrected-to-normal vision.
Apparatus and stimuli: All the stimuli were presented on a 22″ monitor whose resolution was 1024 × 768 and an 86 Hz refresh rate, connected to a Samsung SyncMaster 1200NF computer (Samsung, Seul, North Korea).
The software used to present the stimuli was E-prime (Psychological Science Tools, Pittsburgh, USA) and the color space was sRGB.
A white line, 0.33° × 12°, was diagonally oriented (45° clockwise or counterclockwise) and was presented at the centre of the screen on a medium-gray background (RGB: 127). In a position corresponding to the extremes of the white line, one or two red dots (1.7° × 0.17) could appear at a distance of 1° from the end of the line.
Procedure: The experiment was conducted in a silent and dark room. Each participant sat in front of the monitor at a distance of 57 cm and the head was stabilized by a chin-rest whose height was adjusted according to the requirements of each participant so that the gaze was aligned with the centre of the screen.
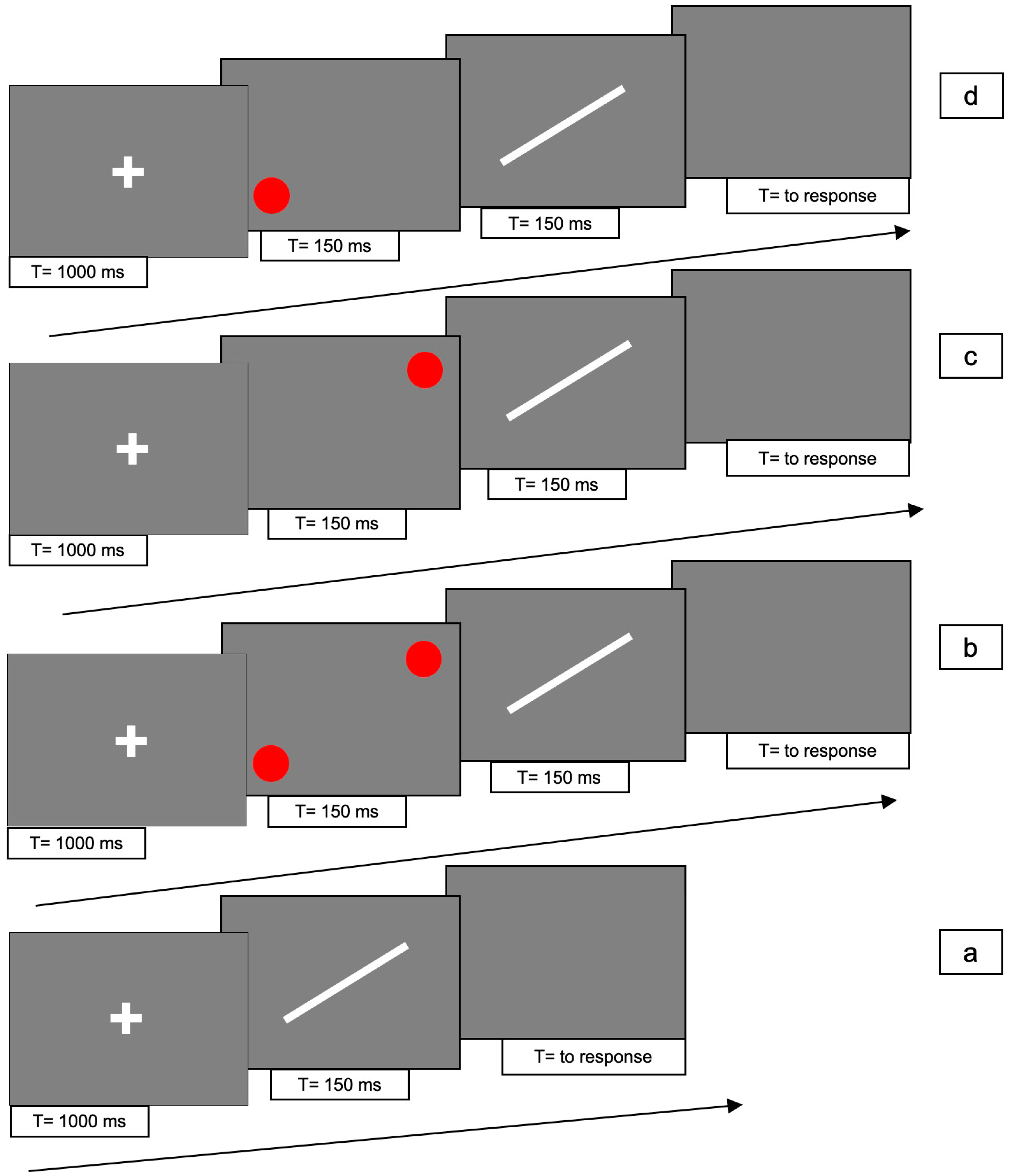
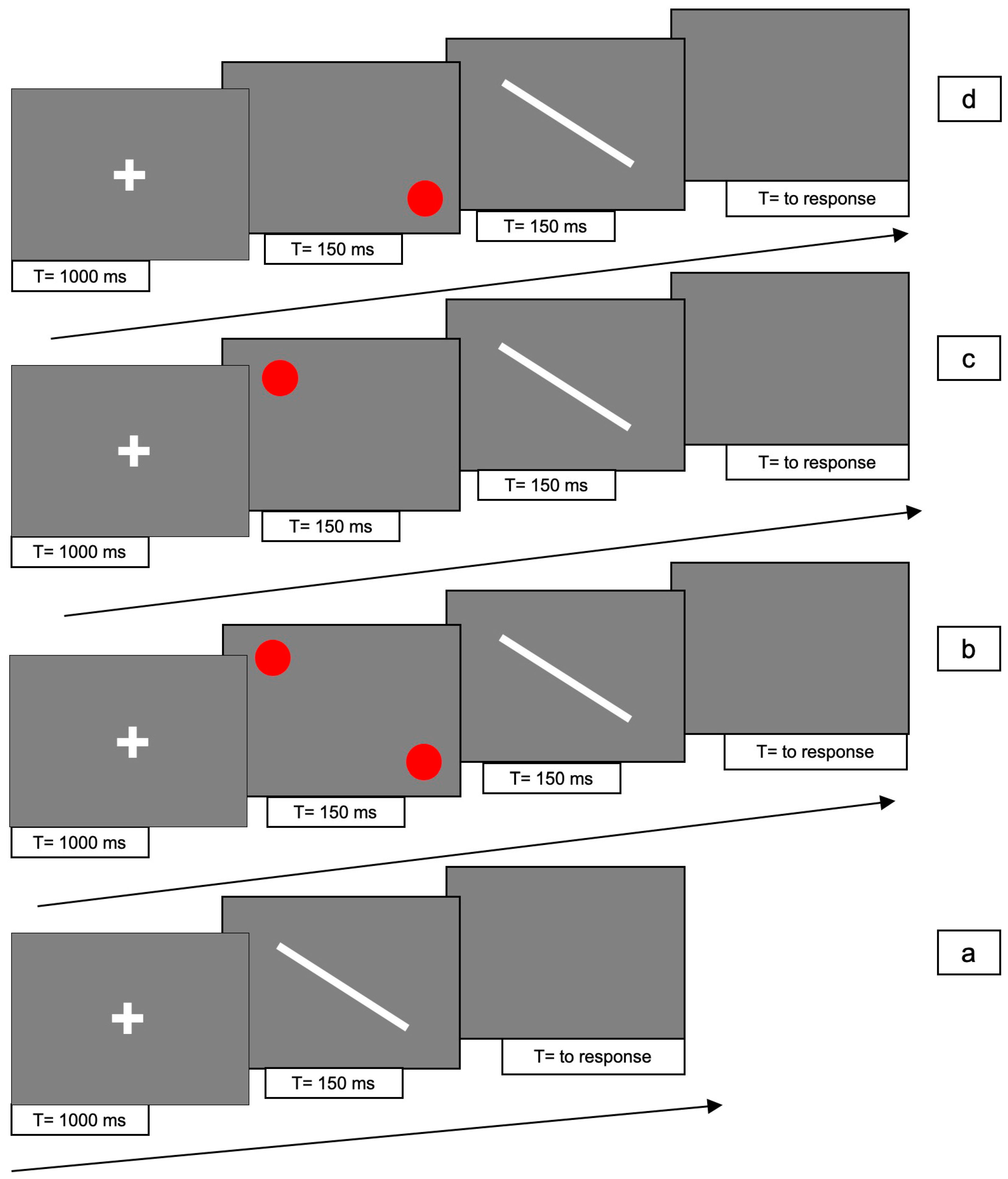
The line could be presented in 4 different conditions for each one of two line orientations, 45° clockwise and counterclockwise: (1) line only (i.e., no red dots); (2) bilaterally cued line (i.e., two red dots at both ends of the line); (3) unilaterally cued line at the top-right; (4) unilaterally cued line at the bottom-left.
For conditions 2, 3 and 4, each trial consisted of the following 4 phases: (i) at the beginning of each trial a white fixation cross was presented at the centre of the screen for 1000 ms; (ii) Immediately after the cross had disappeared, one or two red dots were presented for 150 ms; (iii) immediately after disappearance of the dots, the white line was presented for 150 ms; (iv) after the disappearance of the line, a gray screen appeared which lasted until participant responded.
In condition 1, phase (ii) was absent.
Figure 5 and
Figure 6 shows the experimental paradigm. Each trial lasted approximately 2 s.
There were 10 trials for each condition, with a total of 80 trials presented randomly for each of the two orientations.
The task of each participant was to decide the direction of the movement of the line, be it upward or downward, by pressing either the up- or down-arrow key (2AFC) with the right hand. If the bar did not move, one of the two arrow keys still had to be pressed at random.
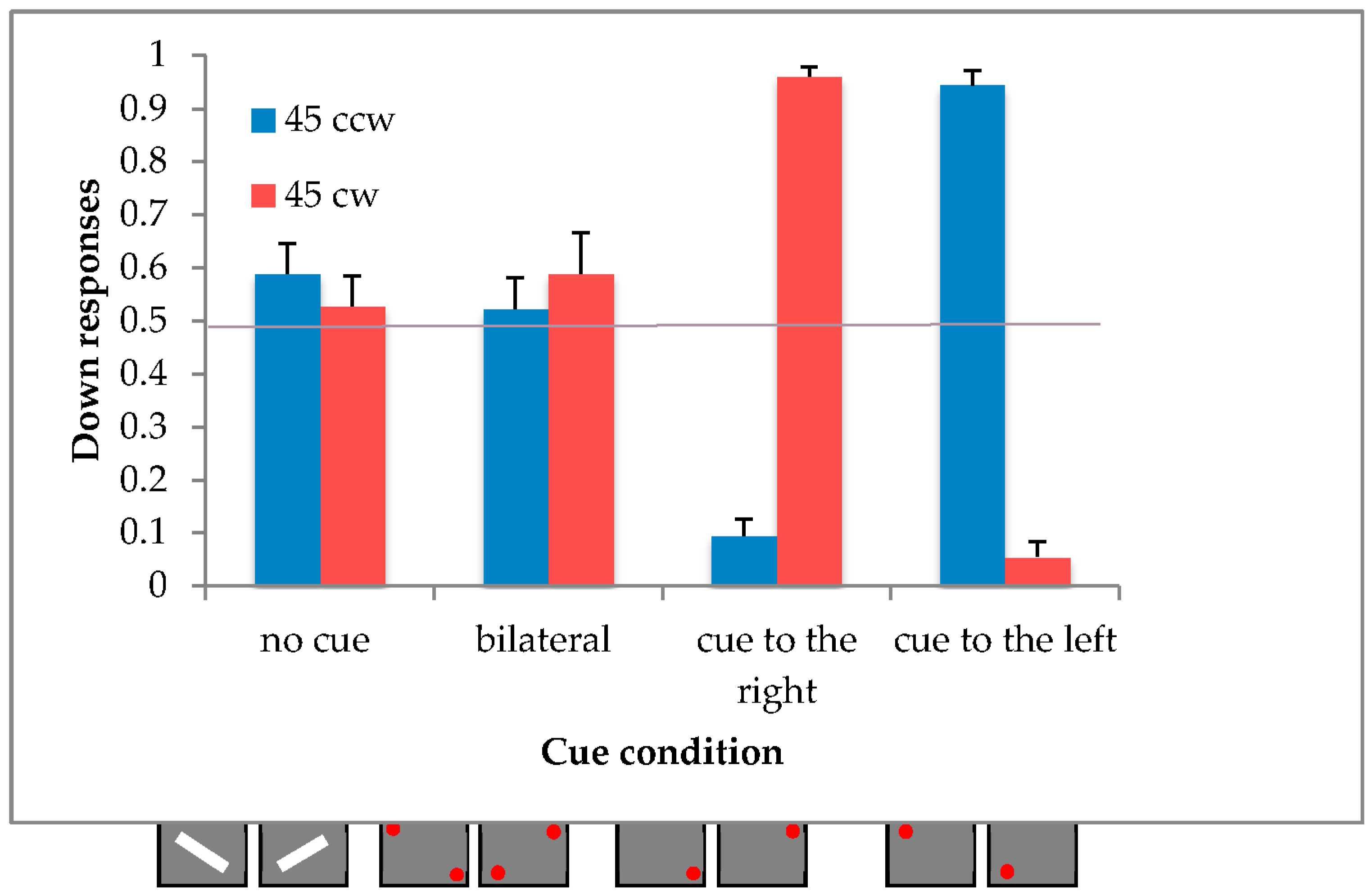
4.2. Results
We calculated the mean proportion of downward responses and the standard deviation for both unilaterally cued conditions and excluded those participants with a response greater than or equal to three standard deviations from the mean value, resulting in 2 out of the 20 participants being rejected.
In order to verify the illusory effect of motion, a within-subjects ANOVA was carried out with two repeated factors: direction (2 levels: 45° clockwise and 45° counterclockwise) and condition (4 levels: top-right unilateral, bottom-left unilateral, bilateral cue and no cue).
The two main effects of direction [F(1,17) = 0.01, p = 0.9137; η2p = 0.00] and condition [F(3,51) = 0.69, p = 0.5635; η2p = 0.04] were not significant, and only the interaction direction x condition reached the significance [F(3,51) = 128.68, p < 0.0001; η2p = 0.88].
A post-hoc comparison (Duncan) revealed a significant difference between the no-cue condition and the unilateral cue conditions (all
p < 0.0001), while the bilateral cue conditions did not differ from the bar (see
Figure 7).
4.3. Discussion
The results of the actual experiment confirm the presence of the illusory effect in the unilateral conditions, but not the asymmetrical effect of the bilateral cue conditions.
These results suggest that the bilateral cue condition is associated with the horizontal plane.
5. Experiment 4
The aim of this experiment was to test the involvement of the collicular pathway in the bilateral asymmetric effect obtained in Experiment 1. We used the blue stimuli because they stimulate the M- and L-cones “less” and activated the Superior Colliculus (SC) “less” than the red stimuli do.
Thus, we should not obtain a reliable ILM with blue cues, as compared to red ones, if the SC plays a role in the present effects.
5.1. Materials and Methods
Participants: These were the same as already described for Experiment 3.
Apparatus and stimuli: All the stimuli were presented on a 22″ monitor with a resolution of 1024 × 768 and an 85 Hz refresh rate, connected to a Samsung SyncMaster 1200NF computer (Samsung, Seul, North Korea).
The software that was used to present the stimuli was E-prime (Psychological Science Tools, Pittsburgh, USA) and the color space was sRGB.
A white line, 12° × 0.33°, was horizontally presented at the centre of the screen. To mask any luminance changes produced by the sudden appearance of the stimuli, the background was a gray flickering surface, changing luminance every 67 ms, which produced random luminance masking that counteracted the colliculus sensitivity incoming effects to the luminance contrast [
30,
36,
37].
One or two red (RGB = 95,0,0; colorimetric values: x = 0.644, y = 0.328) or blue squares (RGB = 0,0,100; x = 0.147, y = 0.067) could appear (1.7° × 1.7°) at a distance of 1° from the position of the line ends. The luminance of the cues was set at 0.4 cd/m2; this value was equated to the average luminance of gray flickering background (RGB = 77,77,77; luminance range = 0.7–0.12 cd/m2). Luminance and colorimetric parameters were measured by a photometer (Konica Minolta CS-100 Spot Chroma Meter; Konica-Minolta, Tokyo, Japan).
Procedure: The experiment was conducted in a silent and dark room. Each participant sat in front of the monitor at a distance of 57 cm with a chin-rest whose height was adjusted according to specific requirements so that the gaze was aligned with the centre of the screen.
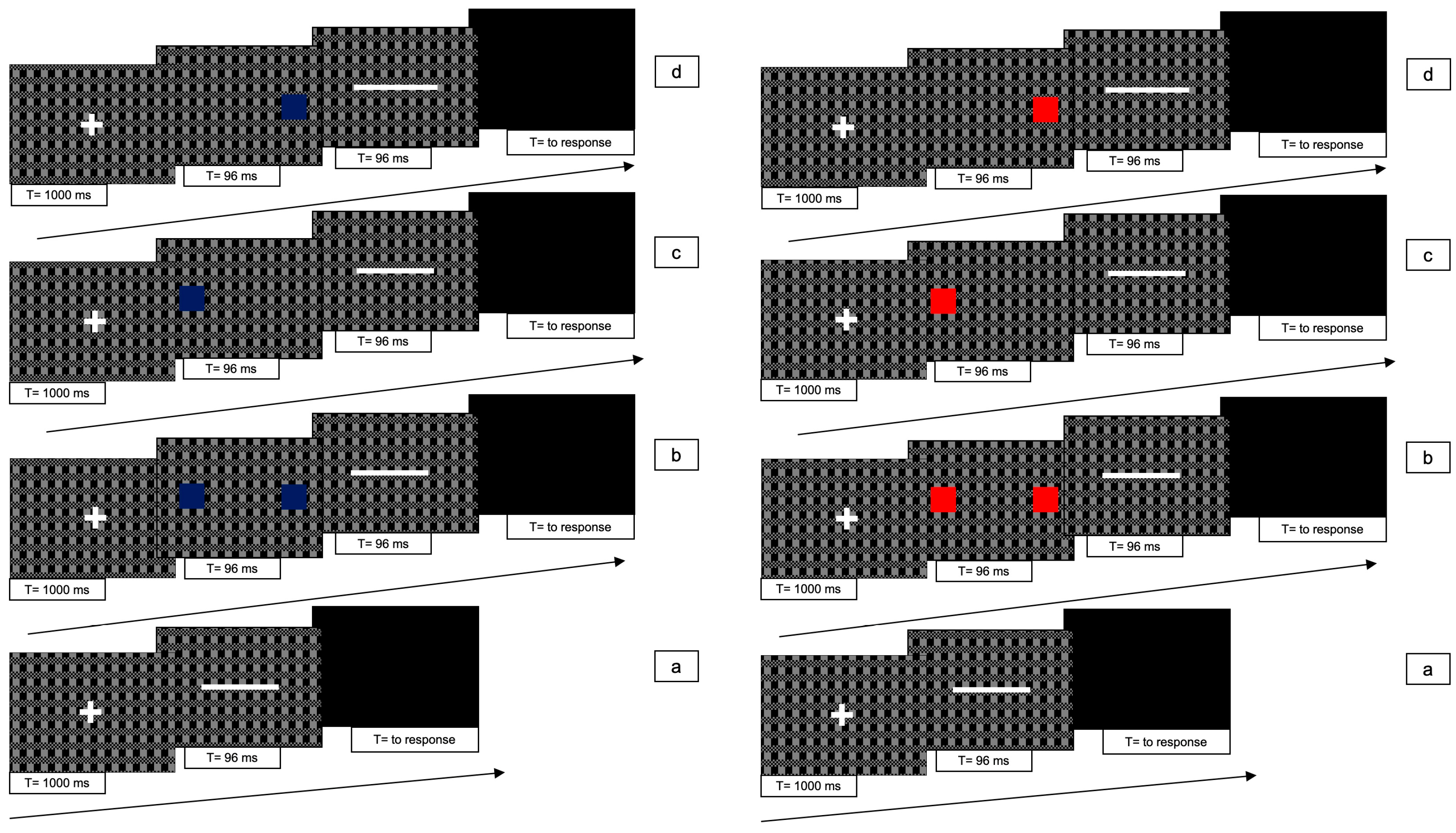
The line could be presented in 7 different conditions (see
Figure 8): (1) line only (i.e., no cues); (2) blue bilateral cues; (3) blue unilateral cue to the left; (4) blue unilateral cue to the right; (5) red bilateral cues; (6) red unilateral cue to the left; (7) red unilateral cue to the right. Each stimulus lasted 96 ms in order to accommodate the flicker alternation cycle.
For conditions 2 to 7, each trial consisted of the following 4 phases: (i) at the beginning of each trial, a white fixation cross was presented at the centre of the screen for 1000 ms; (ii) immediately after the cross had disappeared, one or two (red or blue squares were presented for 96 ms; (iii) immediately after the disappearance of the squares the white line was presented for 96 ms; (iv) after the disappearance of the bar a gray screen appeared which remained visible until participant responded.
In condition 1, phase (ii) was absent.
Figure 8 shows the experimental paradigm. Each trial lasted approximately 2 s.
There were 10 trials for each condition, with a total of 70 trials presented randomly.
The task of each participant was to decide on the direction of the movement of the bar, whether rightward or leftward, and to press the right- or left-arrow keys with the right hand, as per 2AFC. If the bar did not move, they still had to press one of the two arrow keys at random.
5.2. Results
Firstly, in order to confirm the presence of the pseudo-extinction effect, the no-cue condition was compared with both bilateral cue conditions. A within-subjects ANOVA was carried out with the 3-levels factor condition (no-cue condition, blue bilateral cues, red bilateral cues). The dependent variable was always the proportion of leftward responses.
Condition was statistically significant [F(2,38) = 6.92,
p < 0.005; η
2p = 0.27]. A post-hoc comparison (Duncan) revealed a difference between the no-cue condition and each of the bilateral cue conditions (blue:
p < 0.01; red:
p = 0.01), while the two bilateral cue conditions did not differ from each other.
Figure 9 shows an illusory effect of the bilateral cue conditions shifted leftward in comparison to the no-cue condition.
In order to compare the illusory effect with unilateral and bilateral SC-sensitive and insensitive cue colors, a within-subjects ANOVA was carried out with 2 repeated factors: color (2 levels: red and blue) and cue condition (unilateral left, unilateral right, bilateral).
Only the expected main effect of cue condition [F(2,38) = 92.22, p < 0.001; η2p = 0.83] was found but there was no effect of color [F(1,19) = 0.63, p = 0.4368; η2p = 0.03] or interaction [F(2,38) = 0.02, p = 0.977; η2p = 0.001].
A post-hoc comparison (Duncan) on the cue condition variable revealed significant differences between the three conditions (
p < 0.001) (
Figure 10).
5.3. Discussion
This experiment also presents the illusory effect in the unilateral conditions.
As observed in Experiment 1, an asymmetrical effect (leftward) was also found with a shorter presentation time (96 ms) and with a color to which the superior colliculus is less sensitive.
The absence of significant differences between red and blue conditions allows the involvement of the SC in the right cue attentional saliency to be ruled out.
The presence of ILM, in both the unilateral and bilateral conditions, leads to the conclusion that the effect of movement of the line implies an attentional nature and not a sensory phenomenon, that is, a sensory facilitation around a cue, as claimed by Schmidt and Klein [
40].
Unlike the first two experiments, for the last two experiments, the participants were the same, and a different apparatus has been used, whose performance was more suitable for experiment 4; moreover, the viewing distances varied from 44 to 57 cm. For experiment 4, the presentation time has been reduced to 96 ms, the shape of the cues changed from dot to square, and, finally, the screen background was changed in a flickering gray surface.
Nonetheless, we replicated the result of Experiment 1, showing that the small pseudo-extinction phenomenon is quite constant.
6. General Discussion
The present study investigated ILM under unilateral and bilateral cued conditions with dot cues. Overall, the results show that, while the asymmetrical unilateral cued condition induced an expected illusory motion in the direction away from the cue, the symmetrical bilateral cued condition induced an asymmetrical perception, namely that of leftwards motion. This effect appears to be consistent across experiments with the line oriented horizontally (Experiments 1 and 4), and it is also in line with a similar effect obtained with emotional faces used as cues [
17]. Different orientations of the line (and aligned cues) did not induce the same phenomenon (Experiments 2 and 3).
In our view, the perception of leftward motion perceived in the symmetrical bilateral cued condition should be considered as a new phenomenon of pseudo-extinction. A symmetrical bilateral cue induces an asymmetrical illusory perception of a leftward movement, as does a unilateral right cue. Therefore, as in pseudo-extinction, there is single detection of the right cue with the simultaneous double stimulation of the bilateral cues.
Experiment 2 showed the same tendency to give a downward response for both the no- and the bilateral-cue conditions. The absence of a difference between no cue and bilateral cues suggests that this downward effect is unrelated to the pseudo-extinction phenomenon found in the horizontal plane. Rather, this result is in line with other studies, showing perceptual asymmetries in the vertical plane (e.g., [
41]). For example, [
42] found that, in the judgment of direction discrimination, there is an advantage obtained for moving stimuli presented in inferior visual field quadrants. They suggested that the advantage of LVF in motion perception is pre-attentive and that it could be linked to its role in locomotion and visuomotor coordination, as proposed by Previc [
43].
The oblique conditions resulted in intermediate conditions between horizontal and vertical displacements, with unilateral ILM preserved, but with no significant ILM under the bilateral cued condition.
The asymmetry in bilaterally cued ILM suggests that there is an advantage in the processing of the right vs. left cue because both the right unilateral cue and the bilateral cue induce a leftward illusory motion. In addition, the bilateral cue asymmetrical effect was obtained either with 150 (Experiment 1) or 96 ms (Experiment 4) presentation times, suggesting a fast mechanism.
These two characteristics of the leftward asymmetry observed in our study (i.e., (i) advantage in the processing of the right cue and (ii) short presentation time) leads us to consider it as a form of pseudo-extinction, although at first sight it could be seen as being related to so-called pseudo-neglect.
The term “pseudo-neglect” was originally referred to as a slight, but consistent, leftward bias observed in line-bisection tasks performed by in healthy individuals [
44]. An opposite rightward bias is shown by right-brain-damaged patients affected by unilateral spatial neglect in the performance of the same tasks (e.g., [
45]).
Pseudo-neglect is now a term used to indicate a more general advantage for the processing of the left side of space, in both perceptual and motor tasks, in healthy populations [
46,
47]. It has been associated with a dominance of the right hemisphere visuo-spatial attention and an advantage of the left visual space [
45,
48]. Thus, the effect observed in our study is not related to pseudo-neglect, given that (i) it implies an advantage in the processing of the right cue (whereas pseudo-neglect implies an advantage of the left visual space), and (ii) it is observed with short presentation times (i.e., 150 and 96 ms), whereas pseudo-neglect is typically observed with long presentation times. The asymmetry in bilaterally cued ILM suggests an advantage in the processing of the right vs. left cue because both the right unilateral cue and the bilateral cue induce a leftward illusory motion. It is therefore associated with an exogenous orientation of attention, whereas pseudo-neglect is typically reported as being dependent on an endogenous orientation of attention. In addition, the association of the leftward asymmetry observed with bilaterally cued lines nicely fits the explanations given to explain ILM, where, as discussed in the Introduction, exogenous attention always plays a key role (e.g., [
2,
3,
5]).
Therefore, as much as neglect and extinction are double dissociated (e.g., [
49,
50]), we believe that pseudo-neglect and pseudo-extinction are also due to different mechanisms.
We suggest that the asymmetry observed in our study depends on the competition for simultaneous cues, in line with the results of Reuter-Lorenz, Kinsbourne, and Moscovitch [
51], who found a rightward advantage under very similar conditions. In these studies, a modified version of the perceptual bisection task was used. Simple lines with a brief presentation (of up to 130 ms) were associated with a spatial bias in the direction opposite to the hemifield stimulated by lateral line presentation. When a square stimulus was presented unilaterally to a centered line, it induced a bias both when voluntary attended or not. When bilateral squares were added, a rightward displacement of the subjective center of the line emerged. The authors interpreted their results in terms of an activation-orienting hypothesis, namely the orientation of attention in space is biased in the direction contralateral to the more activated hemisphere. The left hemisphere being dominant for visuo-spatial attention, it “wins” under conditions of conflicting stimuli. A large amount of subsequent research suggests that the general orientation of attention is not biased rightward, given that most of the studies found a leftward advantage (e.g., [
47,
52]). We propose that the rightward advantage is due to the exogenous orienting component of attention only.
We suggest that the activation imbalance produced by a unilateral visual stimulus is also activated with a symmetrical cue because of the competition between simultaneous stimuli and the asymmetry of this system.
In our last experiment, we also aimed at verifying the involvement of the SC in the asymmetrical pseudo-extinction phenomenon of ILM, according to the attentional model of Posner and Peterson [
53]. The model proposes a functional network that comprises the parietal cortex in the disengagement of attention [
54], the superior colliculus in the shifting of attention, and the pulvinar involved in the engagement of attention on a new target [
53]. The SC is one of the subcortical structures involved in both the stimulus- and goal-driven attention [
55,
56,
57,
58] and also in the reorienting of attention [
59].
Experiment 4 shows that blue cues did not modulate either the ILM or the pseudo-extinction phenomenon associated with the bilateral cue condition, suggesting that these illusory and attentional effects may be directed by different visual sub-systems [
60], including the collicular pathway. It is worth mentioning that some concerns have been raised regarding the use of S-cone stimuli to study collicular functions following recent evidence of collicular activation for S-cone stimuli in old world monkeys [
61]. Although such a study did not distinguish whether the activation of the SC arises from a direct retinal or a cortical input [
62], its findings would rule out any conclusion made upon the use of S-cone stimuli to study SC involvement in behavioural responses. Hence, it has been proposed that S-cone stimuli could simply lead to weaker SC neuronal responses and slower responses to the S-cone stimulus, rather than effectively impeding processing in the collicular pathway [
61].