1. Introduction
A large body of evidence has shown that brain asymmetry is not an exclusive peculiarity of the human brain, as lateralisation of brain function has been found to be common across vertebrates [
1,
2,
3,
4,
5].
Due to the lack of the corpus callosum and complete decussation of the optic fibres, the hemispheres of the avian brain are largely independent. For this reason, lateralisation phenomena in birds can be easily investigated by covering one eye so as to largely exclude the involvement of the contralateral hemisphere. Therefore, the birds’ visual system became the subject of investigation for the study of brain functional asymmetries in several contexts such as, imprinting, social recognition, visual learning and spatial cognition [
2,
6,
7,
8,
9].
According to the lateralisation pattern delineated by a number of studies on the visual system of birds, the right hemisphere has an advantage in spatial tasks, in global attention and in social recognition, while the left hemisphere takes the control of behaviours requiring the categorization of visual stimuli and the discrimination of visual details of stimuli [
6,
9].
Visual lateralisation in birds engaged in spatial tasks has been investigated in laboratory settings in both hen chicks and pigeons, and in semi-natural and natural settings in homing pigeons [
10,
11,
12,
13]. Laboratory studies in monocularly occluded chicks tested in a cue-conflict task highlighted the different strategies used by the two brain hemispheres. For instance, chicks trained to find food in the centre of a squared arena, near an object constituting a visual beacon, were tested in monocular condition after the beacon was moved to a new position near a wall of the enclosure. The chicks with the right hemisphere in use preferred to rely on the geometrical properties of the arena by consistently searching food in the centre of the arena, while those with the left hemisphere searched in the new position of the object [
14].
In semi-natural and natural settings, natural spatial cues such as the sun compass constitute an important and often dominant source of information for birds challenged to localise a food reward in an outdoor arena, or to orient towards a goal [
15,
16,
17,
18,
19,
20,
21,
22,
23,
24]. Information useful for determining an absolute direction using the sun reference are processed through the visual system. In fact, sun compass orientation requires the observation of the sun azimuth in order to compute an angle to take with it on the basis on the information about the time of the day provided by the internal clock. A clear demonstration of the use of the sun compass by birds is the consistent deviation from the goal direction shown by birds subjected to phase-shift; an altered light-dark cycle is used to manipulate the circadian rhythm of test birds, thereby predictably altering time-compensated interpretations of the sun compass direction [
20,
21,
22].
Despite the importance of the sun compass mechanism in birds’ spatial behaviours [
15,
17,
18,
20,
21,
22], lateralisation phenomena in relation to sun compass-mediated spatial tasks have been poorly investigated. Few exceptions are represented by studies conducted with homing pigeons subjected to unilateral hippocampal lesions. One of these studies on sun compass-mediated spatial learning in an outdoor arena suggested that only an intact left hippocampal formation is sufficient to allow sun compass-based learning [
25]. However, in a cue-conflict situation, in which visual beacons and sun compass information provide conflicting spatial information, the left hippocampal lesioned pigeons, in contrast to controls, often relied on feature cues [
25]. Two conclusions emerged from this study: (i) only the left hippocampal formation is capable of supporting sun compass-based learning; (ii) the integrity of the hierarchy of strategies for spatial learning, that sees in birds a preferential use of the sun compass over visual features, requires an intact right hippocampal formation.
It is important to clarify that, although the hippocampal formation is involved in sun compass-based spatial learning in a confined condition [
19,
24,
26], hippocampal lesions do not affect the use of the sun compass during homing. As well as this, hippocampal lesions, and importantly even unilateral lesions regardless of which side of the hippocampus remained intact, disrupt familiar visual landmark-based navigation, inducing clock-shifted pigeons to totally rely on the erroneous sun compass information rather than on the chain of familiar landmarks leading them home [
27,
28].
Early studies relying on vanishing bearings and homing times found that birds using the right eye (therefore the left brain hemisphere) performed better when relying on visuospatial information [
29,
30]. However, more recent studies using GPS tracking of monocularly trained pigeons suggests that the right hemisphere may play a more important role when establishing and re-tracing routes from familiar sites [
12,
13]. However, some inconsistency in apparent lateralisation of homing performance may be due to the tendency for monocularly occluded birds to fly towards the direction of the open eye, possibly in order to centre the visual field [
13,
31]. For this reason, the deviation induced by clock-shift treatments may be difficult to evaluate in monocularly occluded pigeons during homing, in which the tendency to deviate their flight path towards the side of the open eye also occurs.
In order to investigate the possible lateralisation of the sun compass, we tested pigeons in a food localisation task in an octagonal outdoor arena, in which it is known that birds preferentially rely on sun compass directional information to localise the food reward associated with a sector [
17,
18,
19,
24,
25]. This setting avoids the in-flight issue of flight bias towards the uncovered eye, whilst investigating a sensory system relevant to wider navigational tasks. Birds were trained to find a food reward in one of eight arena compartments, with sun compass information and beacon cues available, under either binocular or monocular conditions. The arena was then rotated 90 degrees anti-clockwise to introduce a directional conflict between the sun compass and beacon information, and the birds were tested in the absence of a food reward. If the sun compass is dominant in one hemisphere, we would expect birds with the corresponding eye occluded to ignore the sun compass in the test phase, relying on the beacon cues in the arena. However, if use of the sun compass occurs in both hemispheres, left and right monocular treatments should show evidence of sun compass use during the test sessions.
2. Materials and Methods
2.1. Subjects
A total of 27 adult homing pigeons Columba livia successfully completed this procedure. All birds had flight experience in the surrounding area of the Arnino field site (Pisa, Italy). For the duration of the experiment, birds were housed in a mesh aviary, open to the elements, and not allowed outside flight. For an individual bird, the experimental duration was generally between 10 and 20 days. Birds had unlimited access to water, but food was restricted to 25–30 g per bird per day during days when the pigeon was being trained in the arena. On days where conditions were unsuitable for training, birds were fed in the aviary. At the end of the experiment, birds were returned to their normal living conditions (food, water and grit ad libitum, outside flight access).
2.2. Experimental Apparatus
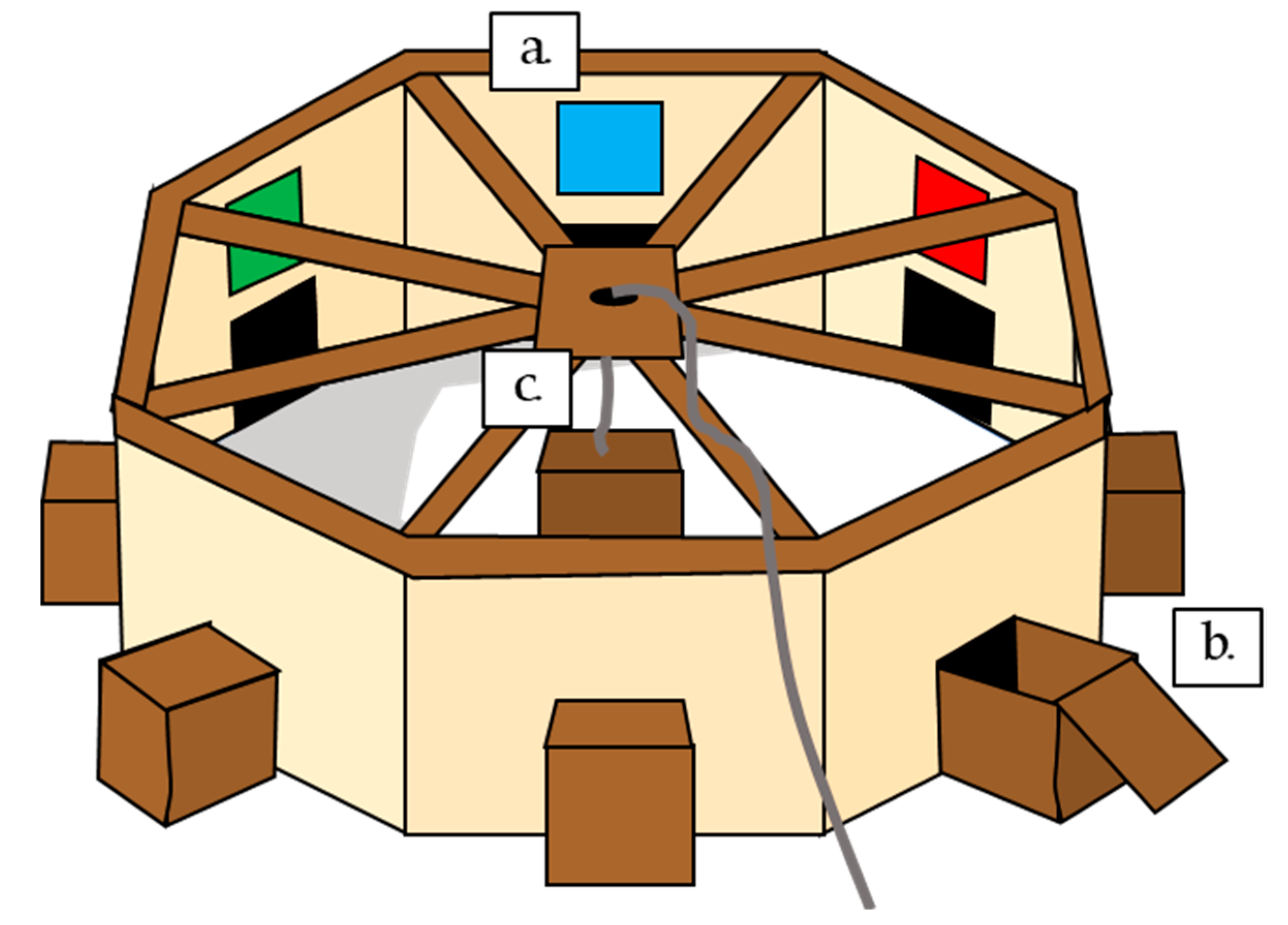
The arena consisted of a large octagon, approximately 2.6 m in diameter, 0.75 m high, with opaque sides to restrict the view of the surrounding landscape, but a mesh top through which the sky and therefore movement of the sun could be clearly seen. The arena was placed in the middle of a field, so no identifiable landmarks could be seen from within the arena. In the middle of each of the eight sides, a 25 × 25 cm square hole gave access to a wooden box, which could contain a food reward. This box had a wooden block in the centre, behind which the food reward could be concealed. Above each box entrance on the arena wall was a coloured beacon, 25 × 25 cm (green, black, barred white, yellow, barred blue, grey, red, and blue). In the centre of the arena was a central supporting pole, with a release box. Birds were placed under this box in the centre of the arena, then released at a distance by the experimenter, via a rope used to lift the box (
Figure 1).
2.3. Experimental Groups
Pigeons were randomly assigned to one of three experimental groups: controls, where no eye caps were attached and pigeons maintained binocular vision; left eye/right hemisphere (LE/RH) where birds had an opaque eye cap attached to cover the right eye; and right eye/left hemisphere (RE/LH), where birds had the eye cap covering the left eye. For the monocular groups, the eye cap was removable using a Velcro attachment. The feathers were trimmed around the assigned eye, and a ring of Velcro attached using a non-toxic glue. The eye cap could then be attached using the corresponding Velcro side when necessary (removed when birds were not in training, e.g., in the aviary at night). This Velcro ring falls off eventually, although some birds learnt to remove the ring, and had to be abandoned from the experiment.
2.4. Experimental Procedure
This experiment consisted of two key phases: training and testing. In the training phase, birds would first become familiar with the arena, then be trained to collect a food reward from a randomly assigned box. Training was divided into the following sessions:
Pre-training one: food was present in all of the eight boxes (eight pieces of corn in each) and was visible from the centre of the arena. The session ended when the bird had eaten the food in all the boxes, or after thirty minutes. The session was repeated until the bird had learnt to eat all the food.
Pre-training two: food was present in all the boxes as before, but this time concealed behind the central blocks, so that the bird would have to enter the box in order to find the food reward. As in pre-training one, the session would continue until all the food had been eaten or after thirty minutes had passed, and the session would be repeated (once per day) until the bird had learnt to eat all the food.
Pre-training three: one box was assigned to each bird, which would contain the food reward for the remainder of the experiment. This training session consisted of ten trials in the same day. In each trial, food was hidden in the assigned box (six pieces of corn per trial), and the trial ended when the pigeon found and ate the food, or after thirty minutes had passed.
Training sessions: each training session consisted of ten trials in the same day. In each trial, food was concealed in the assigned sector, and the trial ended when the pigeon entered any box (i.e., training without correction). If the bird entered the rewarded box, it was allowed to eat all of the food before being removed. If the bird entered a different box, it was removed without a food reward. Training sessions continued until the training threshold was met.
The birds were trained up to a criterion of a minimum of 24 correct choices in three consecutive training sessions, discounting the first training session, with at least 8 correct choices made in the final session. Sessions taken to reach the criterion ranged from the minimum possible of four, to ten. All training was conducted on days when the sun was unobscured by cloud, and between 6 a.m. and 12 a.m., and 4 p.m. and 9 p.m., when the arena was at its coolest.
After training had been passed, birds could be tested. In the test session, the arena was rotated 90 degrees anti-clockwise, so that there was a conflict between the coloured beacons and the sun compass direction identifying the training sector. The training session consisted of five trials, in which no food reward was given, each trial ending when the bird entered the first box. Between test trials, the bird was removed from the arena, with a break of 5–10 minutes between test trials. No other training was performed during this time, and the pigeon was returned to the arena for the next test trial following the break.
2.5. Data Analysis
2.5.1. Training
The number of sessions taken by the three groups of pigeons to reach the criterion and the average time in minutes taken to reach the chosen box was compared with the Kruskal–Wallis test. Finally, a linear mixed model was produced to assess possible differences in the number of errors made by the three groups. The number of errors per session was modelled with session and treatment as fixed effects, and individual as a random effect, allowing for by-individual random slopes for the effect of session.
The five choices made in each test session were compared with the last five choices of the final training session, in order to determine how choices differed between the conditions. For each bird, the assigned training direction was set to 0°, to make each bird comparable. Therefore, when the arena was rotated −90° during the test session, the direction given by the beacon cue was −90°, vs. the 0° of the sun compass direction.
2.5.2. Test
For each bird, the mean vectors for the last five training trials and the five test trials were calculated. These were then used to calculate the mean vector for each experimental group. The paired Hotelling’s test was used to compare the mean vector length and direction between the test and training trials for each group.
For the test session, a V-test was used to test for randomness in the individual median distributions of the three groups of pigeons, considering as expected direction either the sun compass training direction (360°), or the direction of the training beacon (270°). The distributions of the mean vector lengths of the three groups were compared using the Kruskal–Wallis test in order to compare the level of consistency in directional choices during the test session. The Watson–Williams test was applied to the individual median directions in order to compare the orientation of the three groups of birds. As during the test was conducted in absence of food reward, the Watson–William test was also applied to the first and second directional choices, in order to evaluate a possible effect of learning during the following choices. By using the Mann–Whitney U test, a direct comparison between the two monocular groups was also performed by considering the rate of choices for the sector identified by the training direction, the sector identified by the training beacon and the sector located in between.
2.6. Ethical Statement
The pigeons were bred and manipulated in accordance with the 57 EU Directive 2010/63/EU on the protection of animals used for scientific purposes. The experiment was approved by the Animal Welfare Committee (OPBA) of the University of Pisa and by the Italian Ministry of Health (permit number 227/2019). All birds used for the procedure were captive-bred, and had experience of experimental conditions from previous release experiments. Birds had free access to water for the duration of their use in the experiment, and were removed from the experiment if they were unable to use the apparatus. For the monocular birds, feather trimming and gluing of the Velcro rings took place using cloth restraints to prevent movement which might cause harm. A non-toxic glue was used, which allows the Velcro ring to fall off over time. If loose enough, the Velcro was removed at the end of the experiment. Eye-caps were only worn during the experimental procedure, and removed during rest periods and overnight to minimise distress. As the experiment was conducted during the summer in an open field, shade was provided for the birds waiting to be tested, and the arena was periodically cooled with water to prevent the floor from reaching an uncomfortable temperature. Birds had a set ration of food for each day, and any which did not reach this ration through use of the arena were fed the remainder at the end of the day. Trials were spaced out so that each bird had a significant break between trials in the arena, as other birds took their turn. Birds were used for an average of one week, and were returned to standard living conditions afterwards.
4. Discussion
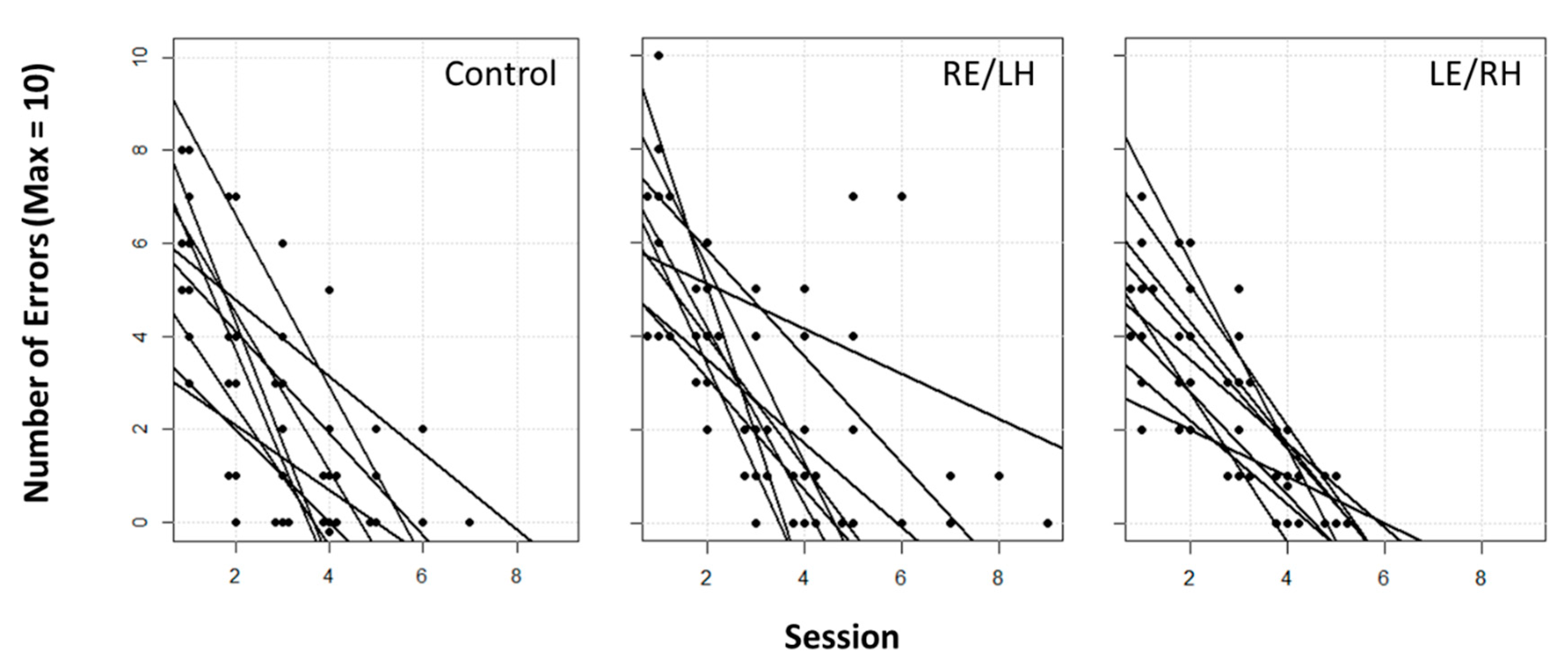
Here we present a study designed to determine whether there is a functional asymmetry in the visual system of pigeons in sun compass processing. Our results suggest that there is no clear lateralisation of the sun compass. In fact, both monocular groups displayed no difference in the use of the sun compass vs. intra-maze visual beacons. During the learning phase, the performances of monocularly occluded pigeons challenged to localise a food reward in an outdoor octagonal arena provided with a distinctive beacon in each sector, were similar to those of control pigeons with binocular view. Monocular occlusion did not hinder task learning, with all three experimental groups showing similar error rate curves (
Figure 2). This suggests that, even if birds in the different groups were learning using different cues, all were similarly able to learn the task.
Past studies have shown that monocular birds using the left hemisphere performed better in grain-grit discrimination tasks [
33], as the left hemisphere has an advantage, through the inhibition on the right hemisphere, in responding in an appropriate way in tasks implying a dual choice. Similarly, pigeons with the right eye/left hemisphere visual system perform better in reversal learning tasks, in which birds are requested to inhibit their response to a previously learned stimulus [
34]. However, while in both grain-grit discrimination and reversal learning tasks birds have to refrain from pecking inedible items or to an unrewarded stimulus previously associated to a reward, in the present experiment birds have to learn to localise the food reward, on the basis of a beacon distinctive of a specific sector of the arena, or on the basis of a specific sun compass direction, or both. Therefore, the lack of an advantage of the left hemisphere in the learning food localisation in the present experiment is consistent with a lack of a dual choice implying inhibition by the left hemisphere on the right one.
Fewer monocular birds that started training completed the process, as some learnt to quickly remove the eye-cap, making them unsuitable for the experiment (seven RE/LH birds, six LE/RH and one control bird which failed to learn the task were abandoned). In terms of orientation in training, the remaining birds successfully learnt the task to the required criteria, showing consistent orientation towards the goal location.
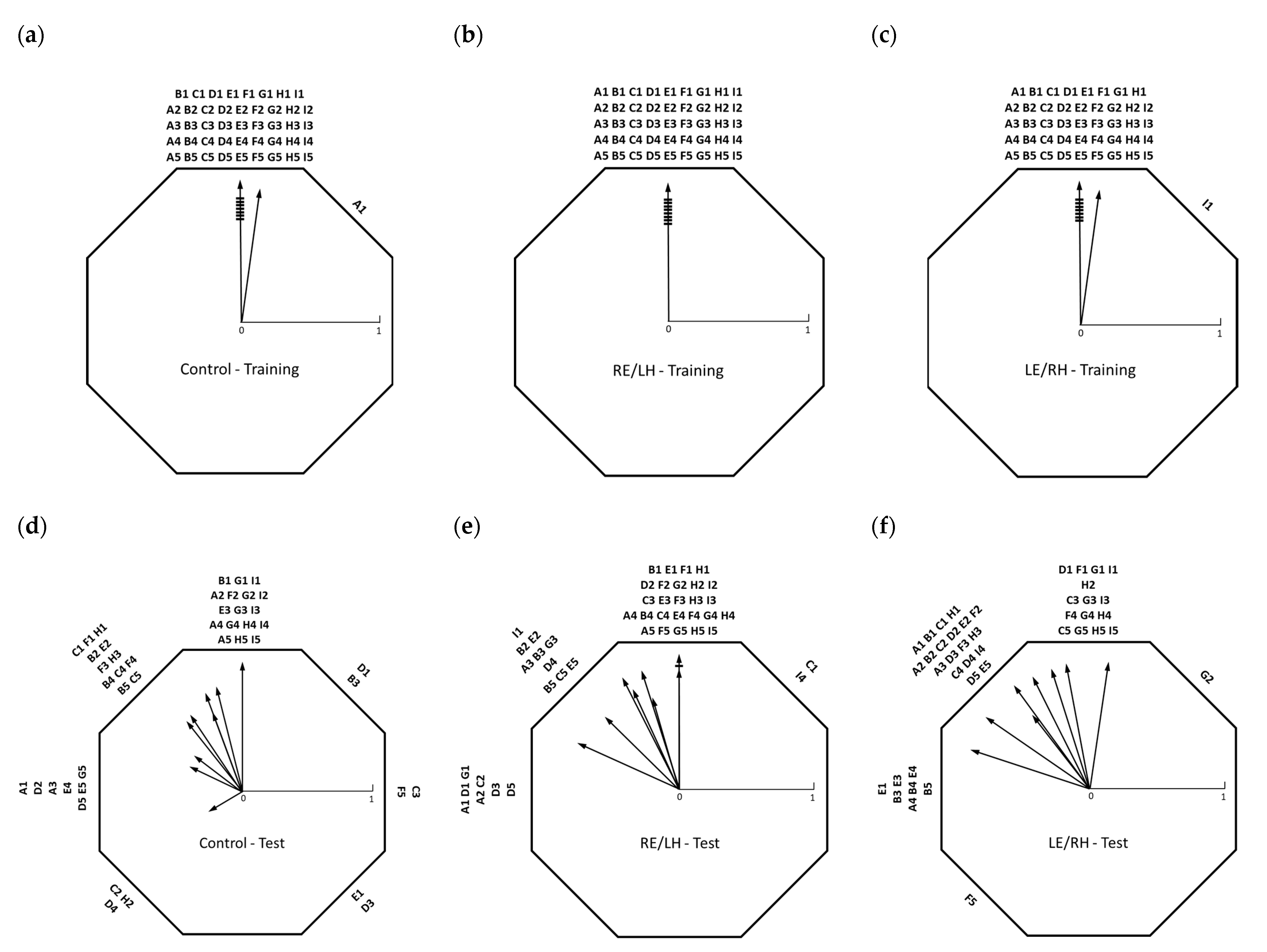
During the test session, following a 90° anti-clockwise rotation of the arena, the sun compass information learned during training were set in conflict with the distinctive beacon associated with the presence of food. Group averages of test orientation showed an intermediate direction between the sun compass direction (360°) and the beacon direction (270°) (see
Table 1), composed of varying individual strategies, many of which showed switching between the sector identified by the training beacon, the sector identified by the training direction and the sector located between the two (
Figure 3). The within-group variation in directional choice and lack of a significant difference in orientation between the test groups suggests that birds in all groups were able to orient using both sun compass and beacon-based strategies, independent of the monocular or binocular conditions. Individual strategies generally showed a high degree of switching, possibly due to the lack of a food reward during the test session, meaning that the birds chose different directions on repeated attempts. However, no significant difference in orientation on the first trial of each test between the groups still supports a lack of treatment-specific effects.
The directional choice distribution of the three groups was significantly different from random when the sun compass training direction was considered as the expected direction. This is consistent with the interpretation that both hemispheres participate in sun compass-mediated spatial learning. Interestingly, the distribution of the choices of the birds processing using the right eye/left hemisphere visual system, and contrarily to both the other two groups, was not different from random if the training beacon direction was considered as the expected direction. This suggests that, although not totally ignoring the learned beacon, they displayed a less consistent reliance on visual feature information.
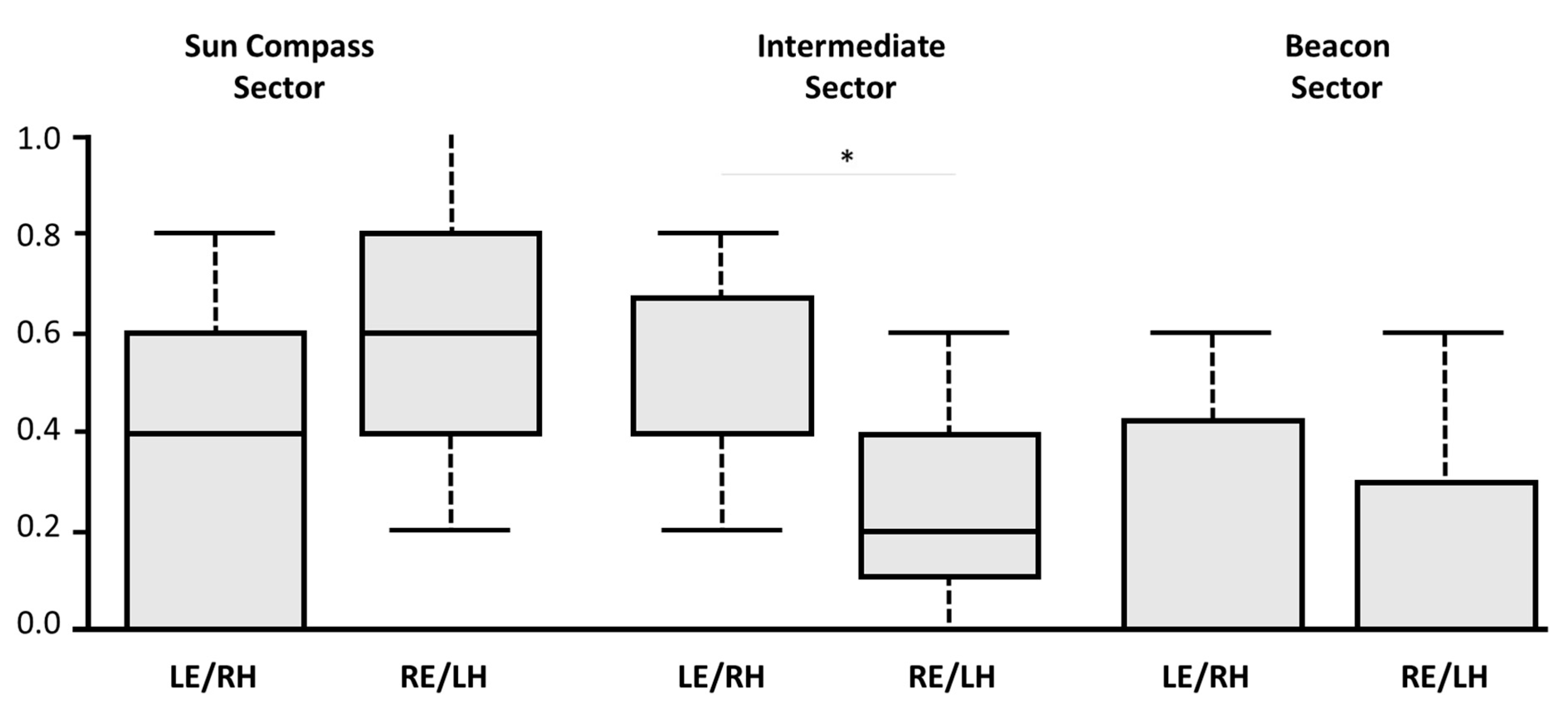
During the test session, the training compass direction and the colour beacon were set in conflict by rotating the arena 90° anti-clockwise. As the lateral visual field of pigeons, as in all diurnal avian species, is wider than 120° a monocularly occluded bird can view at the same time both the training sector associated to the sun compass training direction and the sector identified by the training beacon. An interesting result emerged from the direct comparison between the rate of choices for one of the three relevant sectors: the training direction sector, the sector identified by the training beacon, and the sector located between these two. In fact, the birds with the right hemisphere in use displayed a higher preference for the sector located between the training beacon and the training compass direction, compared to the birds with the left hemisphere in use (
Figure 5). This suggests a possible advantage of the left eye/right hemisphere visual system in integrating information of a different kind, such as the colour beacon and sun compass direction. It is possible that choosing the intermediate sector does not represent higher levels of integration; choice of the intermediate sector could instead be due to an inability to resolve the conflict between the two directions, or as a compromise rather than an integration. It is also possible that, given a range of possibly correct directions indicated by the conflicting cues, the intermediate sector might be chosen randomly as one of three options. However, a random strategy seems unlikely as the birds with the right hemisphere in use display a significant preference for the intermediate sector compared to the birds with the left hemisphere in use.
Prior et al. found that birds using the right-eye/left-hemisphere visual system were more distracted by changing landmark or beacon cues in an indoor arena setting, suggesting that the left hemisphere may be more involved in the processing of such cues [
35]. However, this result might be in line with the view that the right hemisphere has an advantage in integrating different information and therefore producing a more consistent behavioural output.
The involvement of both hemispheres in processing sun compass information revealed by the present work is in line with previous studies on homing pigeons subjected to unilateral hippocampal lesions. It has been observed that bilateral hippocampal lesions disrupt the ability of clock-shifted pigeons released from familiar sites to re-orient homeward by relying on the spatial relationships among familiar landmarks. The consequence of the impaired familiar landmark-based navigation ability is a marked deflection of the clock-shifted pigeons, consistent with the sun compass orientation [
27,
28]. Interestingly, the total reliance on the site-specific compass orientation strategy, as a consequence of the impaired pilotage strategy, exhibited by birds with bilateral hippocampal lesions is also shown by pigeons subjected to unilateral hippocampal lesions, with no difference between the side of the lesion [
36]. However, as this study was based on the analysis of vanishing bearings, possible differences between the right and the left hippocampal formation in controlling spatial decisions en route are not known.
Ulrich et al. showed that birds using the left eye and therefore the right hemisphere visual system showed a reduced homeward component in overcast, suggesting that they are less able to use alternative landmark (or magnetic) information [
30]. Our birds with the left eye/right hemisphere visual system in use did not show significantly less ability to use the beacon cues, in fact they demonstrate a slight tendency towards the beacons, although we have to make clear that colour beacons in an arena are not the equivalent to familiar landmarks in a large scale navigation context. In fact, hippocampal lesioned pigeons, that are impaired in familiar visual landmarks-based navigation [
27,
28], displayed a total reliance on colour beacons, and not on a specific compass direction, when challenged to locate a food reward in an outdoor octagonal arena [
19]. It has been consistently shown that hippocampal lesions do not affect the pigeons’ ability to orient on the basis of the sun compass in a large scale navigation task, but nevertheless disrupt the ability to associate the presence of food to a compass direction [
19,
24]. Similarly, young hippocampal lesioned pigeons kept confined in an aviary open to winds turned out to be unable to learn an odour-based navigational map, most likely being unable to learn the association “sun compass direction-wind borne odours” [
26]. Of interest for the present study, the left hippocampal formation seems to be critical for the learning process underlying the olfactory map development [
37] and advantaged in learning the association “food reward-sun compass direction” in an outdoor octagonal arena void of colour beacons [
25]. By contrast, in the present study, the right eye/left hemisphere visual system did not show any clear advantage in the preference for a sun compass-dependent spatial strategy, suggesting that such functional lateralisation in the use of the sun compass depends on an asymmetry of the left side of the hippocampal formation in sun compass-mediated learning strategies, rather than in the operation of the sun compass mechanism itself, which according to the present data seems to involve the visual system of both sides.
It is important to mention that after the learning phase we did not test the birds after clock-shift in order to verify that they used the sun compass to learn the training direction. However, consistent evidence from different research groups showed that food localisation in outdoor arenas is a sun compass-mediated spatial task, and that sun compass information is even preferred to other stimuli that might be associated with the food reward, such as intra-maze visual beacons [
17,
18,
19,
24,
25,
38]. One may argue that the pigeons could be using the magnetic compass to locate the food reward, rather than the sun compass. However, a large body of evidence both in field releases and arena experiments has demonstrated that pigeons with access to the magnetic compass and sun compass reliably show deviation under clock-shift, showing that the sun compass is used preferentially to the magnetic compass, even when a cue conflict occurs [
15,
17,
18,
19,
23,
24,
25,
38,
39,
40,
41,
42]. Some studies have shown that the magnetic compass may be involved in re-orientation following clock-shift during flight over unfamiliar areas, after several kilometres of flights either at vanishing to a lesser and variable extent or well beyond the release site area to a greater extent [
43,
44,
45]. GPS-tracking data showed that a complete re-orientation of the birds occurred after several hours and often after the subjective night possibly due to several factors [
45]. This suggests that it is unlikely to have occurred in our arena setting where the test sessions occurred over a narrow time frame [
17,
18,
19,
24,
25,
38]. As previous experiments using an outdoor arena have demonstrated that pigeons do use the sun to learn the location of the food reward [
17,
18,
19,
24,
25,
38], it is reasonable to assume that the pigeons in this experiment used the sun compass in preference of the magnetic compass, especially as the sun’s disc was clearly visible from the centre of the arena at all times.
The asymmetry in the pigeon visual system at neuroanatomical level consists of stronger projection from the right optic tectum to the left nucleus rotundus, which in turn sends fibres to the left entopallium, suggesting an increased passing of information to the left hemisphere [
9] in birds viewing with the right eye, compared to birds viewing with the left eye. However, a previous experiment suggested that the tectofugal pathway does not participate in sun compass-mediated spatial learning, as lesions to the ectopallium did not affect the ability of the pigeons to learn to locate a food reward in an octagonal outdoor arena on the basis of sun compass information [
38]. By contrast, lesions to the visual wulst affected the birds’ performances. Wulst-lesioned pigeons took longer to learn the task, and when subjected to clock-shift treatment displayed inconsistent orientation, suggesting an involvement of the thalamofugal pathway in processing sun compass information [
38].
A previous GPS study suggested that the left eye/right hemisphere visual system has an advantage in developing route fidelity and memorising familiar visual landmarks [
13]. Interestingly, birds with navigational experience had an enlarged right optic tectum, which projects to the ipsilateral entopallium, when compared with birds without homing experience [
7]. This might suggest a critical involvement of the tectofugal pathway in the familiar landmark-based navigation.
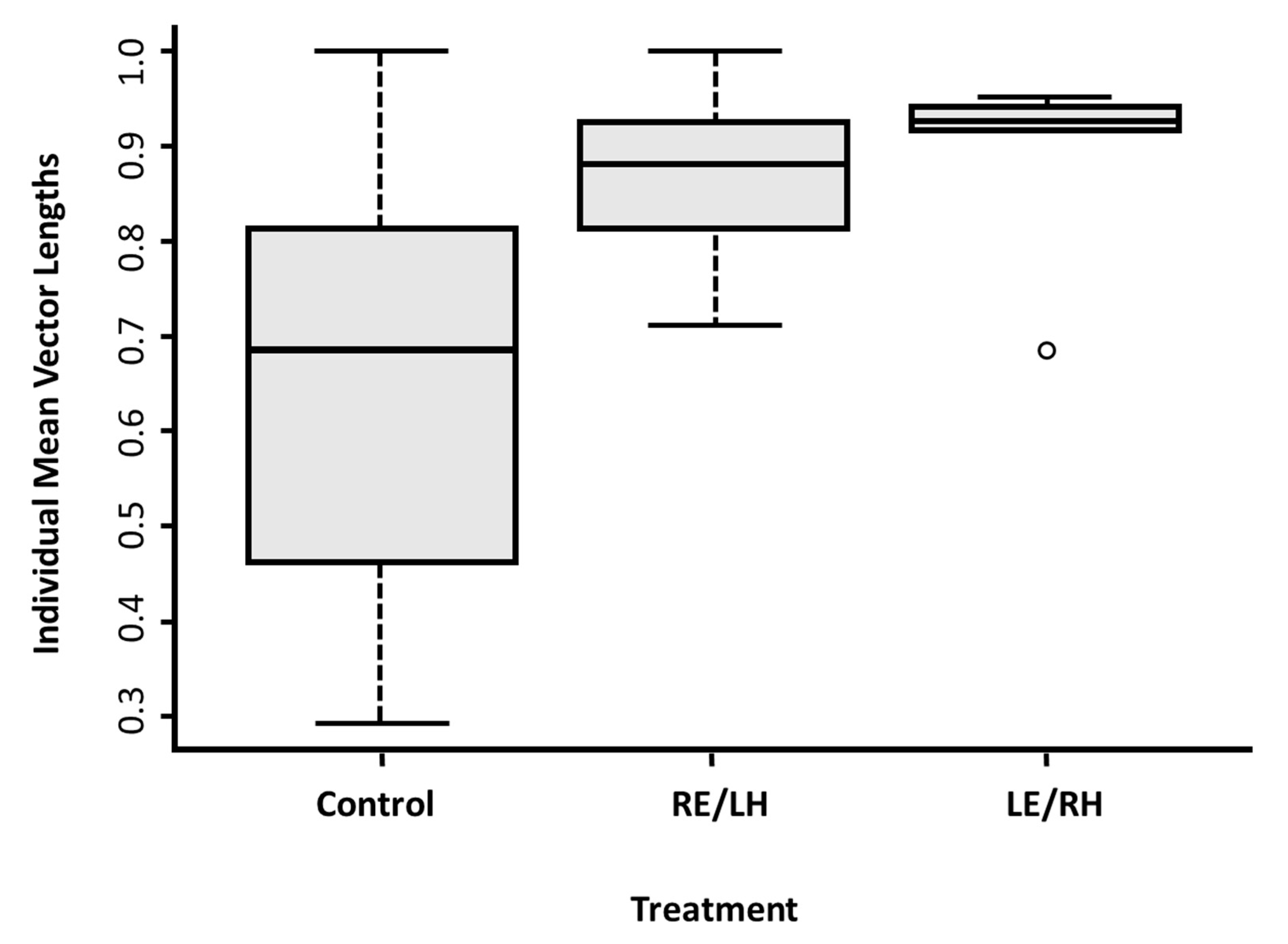
The control pigeons showed a higher level of inconsistency in their directional choices in comparison to both monocular groups in the cue-conflict test, as shown by the shorter lengths of the individual mean vectors (
Figure 3d and
Figure 4). This could be due to increased inter-hemispheric communication relative to the monocular treatments [
45], suggesting that in a conflict condition the two hemispheres might process different information at the same time, but that the processing of a particular item of information is not largely segregated in one hemisphere. It may otherwise be that binocular birds, having a wider field of vision, are able to collect the discrepant information more easily.
Overall, these results do not support complete lateralisation of the sun compass use to the left eye/right hemisphere or the right eye/left hemisphere visual system, as both left and right monocularly occluded pigeons displayed a comparable level of preference for the training compass direction in a cue-conflict situation. However, birds processing visual information with the left eye/right hemisphere visual system may be more capable to integrate both directional and feature cues, than the birds with the right eye/left hemisphere in use, as shown by their preferential choice for the intermediate sector.