Asymmetry of Cerebellar Lobular Development in Ferrets
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Volumetry
2.3. 3D-Rendered Images
2.4. Statistical Analysis
2.5. Ethics
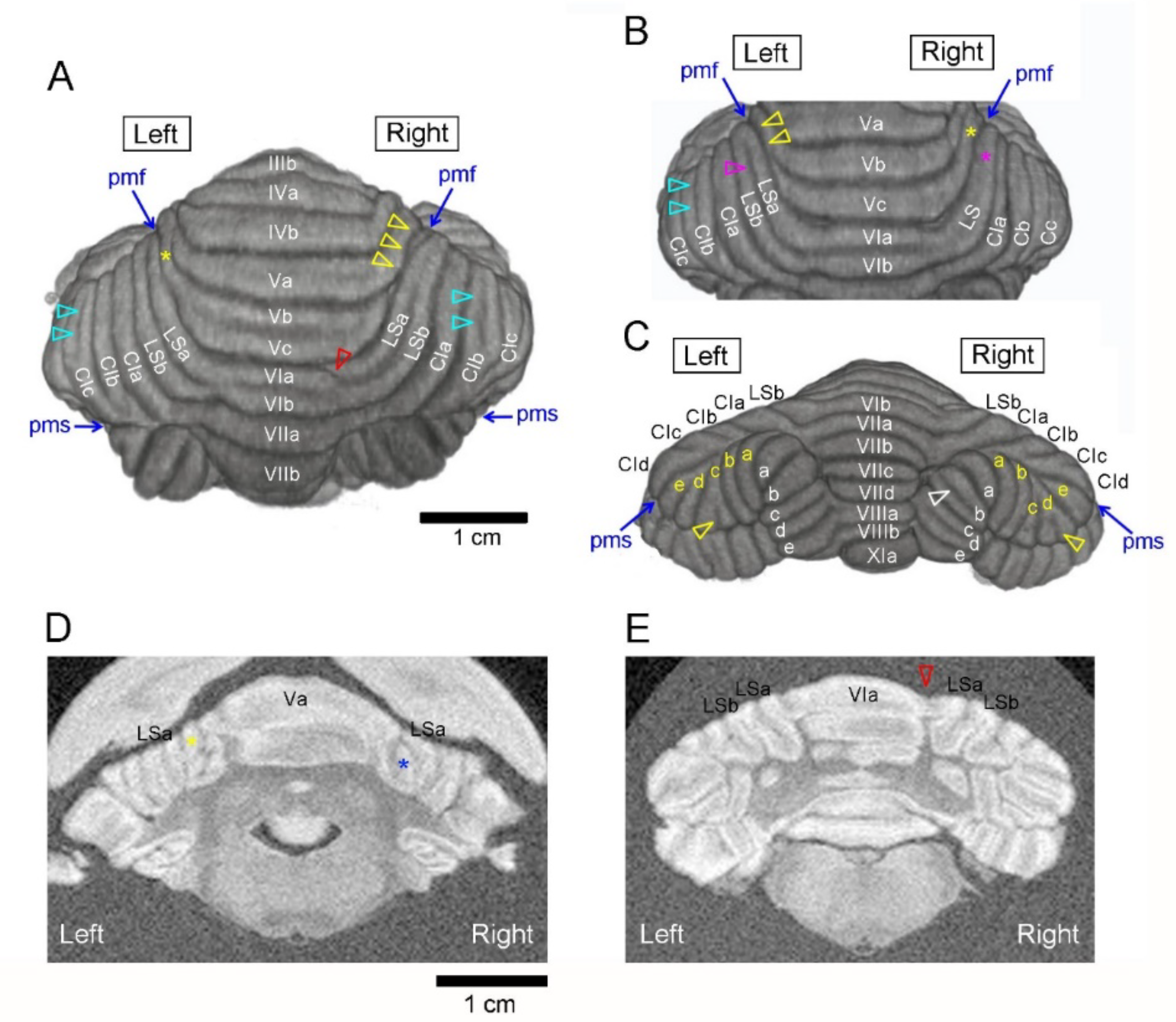
3. Results
3.1. Types of Cerebellar Gross Anatomical Asymmetry
3.2. Incidence of Cerebellar Asymmetrical Morphology
3.3. Relationship with Regional Cerebellar Volume Laterality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Synder, P.J.; Bilder, R.M.; Wu, H.; Bogerts, B.; Lieberman, J.A. Cerebellar volume asymmetries are related to handedness: A quantitative MRI study. Neuropsychologia 1995, 33, 407–419. [Google Scholar] [CrossRef]
- Phillips, K.; Hopkins, W.D. Exploring the relationship between cerebellar asymmetry and handedness in chimpanzees (Pan troglodytes) and capuchins (Cebus apella). Neuropsychologia 2007, 45, 2333–2339. [Google Scholar] [CrossRef] [PubMed]
- Cantalupo, C.; Freeman, H.; Rodes, W.; Hopkins, W. Handedness for tool use correlates with cerebellar asymmetries in chimpanzees (Pan troglodytes). Behav. Neurosci. 2008, 122, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Koyun, N.; Aydinlioğlu, A.; Aslan, K. A morphometric study on dog cerebellum. Neurol. Res. 2011, 33, 220–224. [Google Scholar] [CrossRef]
- Sawada, K.; Horiuchi-Hirose, M.; Saito, S.; Aoki, I. Male prevalent enhancement of leftward asymmetric development of the cerebellar cortex in ferrets (Mustela putorius). Laterality 2015, 20, 723–737. [Google Scholar] [CrossRef]
- Sawada, K.; Aoki, I. Age-dependent sexually-dimorphic asymmetric development of the ferret cerebellar cortex. Symmetry 2017, 9, 40. [Google Scholar] [CrossRef]
- Rosch, R.E.; Ronan, L.; Cherkas, L.; Gurd, J.M. Cerebellar asymmetry in a pair of monozygotic handedness-discordant twins. J. Anat. 2010, 217, 38–47. [Google Scholar] [CrossRef]
- Valera, E.M.; Faraone, S.V.; Biederman, J.; Poldrack, R.A.; Seidman, L.J. Functional neuroanatomy of working memory in adults with attention-deficit/hyperactivity disorder. Biol. Psychiatry 2005, 57, 439–447. [Google Scholar] [CrossRef]
- Wang, D.; Buckner, R.L.; Liu, H. Cerebellar asymmetry and its relation to cerebral asymmetry estimated by intrinsic functional connectivity. J. Neurophysiol. 2013, 109, 46–57. [Google Scholar] [CrossRef]
- Iglói, K.; Doeller, C.F.; Paradis, A.L.; Benchenane, K.; Berthoz, A.; Burgess, N.; Rondi-Reig, L. Interaction between hippocampus and cerebellum crus I in sequence-based but not place-based navigation. Cereb. Cortex 2015, 25, 4146–4154. [Google Scholar] [CrossRef]
- Sokolov, A.A. The cerebellum in social cognition. Front. Cell Neurosci. 2018, 12, 145. [Google Scholar] [CrossRef]
- Hopkins, W.D.; Marino, L. Asymmetries in cerebral width in nonhuman primate brains as revealed by magnetic resonance imaging (MRI). Neuropsychologia 2000, 38, 493–499. [Google Scholar] [CrossRef]
- Lawes, I.N.C.; Andrews, P.L.R. Neuroanatomy of the ferret brain. In Biology and Diseases of the Ferret, 2nd ed.; Fox, Ed.; Lippincott Williams and Wilkins: Baltimore, MD, USA, 1998; pp. 71–102. [Google Scholar]
- Ozol, K.; Hayden, J.M.; Oberdick, J.; Hawkes, R. Transverse zones in the vermis of the mouse cerebellum. J. Comp. Neurol. 1999, 412, 95–111. [Google Scholar] [CrossRef]
- Sillitoe, R.V.; Hawkes, R. Whole-mount immunohistochemistry: A high-throughput screen for patterning defects in the mouse cerebellum. J. Histochem. Cytochem. 2002, 50, 235–244. [Google Scholar] [CrossRef]
- Siniscalchi, M.; Franchini, D.; Pepe, A.M.; Sasso, R.; Dimatteo, S.; Vallortigara, G.; Quaranta, A. Volumetric assessment of cerebral asymmetries in dogs. Laterality 2011, 16, 528–536. [Google Scholar] [CrossRef]
- Rosch, R.E.; Cowell, P.E.; Gurd, J.M. Cerebellar asymmetry and cortical connectivity in monozygotic twins with discordant handedness. Cerebellum 2018, 17, 191–203. [Google Scholar] [CrossRef]
- Sawada, K.; Fukunishi, K.; Kashima, M.; Imai, N.; Saito, S.; Aoki, I.; Fukui, Y. Regional difference in sulcal infolding progression correlated with cerebral cortical expansion in cynomolgus monkey fetuses. Congenit. Anom. (Kyoto) 2017, 57, 114–117. [Google Scholar] [CrossRef]
- Kamiya, S.; Sawada, K. Immunohistochemical characterization of postnatal changes in cerebellar cortical cytoarchitectures in ferrets. Anat. Rec. (Hoboken). in press.
- Ahlfeld, J.; Filser, S.; Schmidt, F.; Wefers, A.K.; Merk, D.J.; Glaß, R.; Herms, J.; Schüller, U. Neurogenesis from Sox2 expressing cells in the adult cerebellar cortex. Sci. Rep. 2017, 7, 6137. [Google Scholar] [CrossRef]
- Dorr, A.E.; Lerch, J.P.; Spring, S.; Kabani, N.; Henkelman, R.M. High resolution three-dimensional brain atlas using an average magnetic resonance image of 40 adult C57Bl/6J mice. Neuroimage 2008, 42, 60–69. [Google Scholar] [CrossRef]
- Hodge, S.M.; Makris, N.; Kennedy, D.N.; Caviness, V.S., Jr.; Howard, J.; McGrath, L.; Steele, S.; Frazier, J.A.; Tager-Flusberg, H.; Harris, G.J. Cerebellum, language, and cognition in autism and specific language impairment. J. Autism Dev. Disord. 2010, 40, 300–316. [Google Scholar] [CrossRef] [PubMed]
- Levitt, J.J.; McCarley, R.W.; Nestor, P.G.; Petrescu, C.; Donnino, R.; Hirayasu, Y.; Kikinis, R.; Jolesz, F.A.; Shenton, M.E. Quantitative volumetric MRI study of the cerebellum and vermis in schizophrenia: Clinical and cognitive correlates. Am. J. Psychiatry 1999, 156, 1105–1107. [Google Scholar] [PubMed]
- Sheng, J.; Zhu, Y.; Lu, Z.; Liu, N.; Huang, N.; Zhang, Z.; Tan, L.; Li, C.; Yu, X. Altered volume and lateralization of language-related regions in first-episode schizophrenia. Schizophr. Res. 2013, 148, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Kibby, M.Y.; Fancher, J.B.; Markanen, R.; Hynd, G.W. A quantitative magnetic resonance imaging analysis of the cerebellar deficit hypothesis of dyslexia. J. Child Neurol. 2008, 23, 368–380. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, F.X.; Giedd, J.N.; Marsh, W.L.; Hamburger, S.D.; Vaituzis, A.C.; Dickstein, D.P.; Sarfatti, S.E.; Vauss, Y.C.; Snell, J.W.; Lange, N.; et al. Quantitative brain magnetic resonance imaging in attention-deficit hyperactivity disorder. Arch. Gen. Psychiatry 1996, 53, 607–616. [Google Scholar] [CrossRef] [PubMed]
| Transverse Domains | Left Side | Right Side | |||
|---|---|---|---|---|---|
| Anterior zone | - | ||||
| Male | 57.1% | (4/7) | 57.1% | (4/7) | |
| Female | 14.3% | (1/7) | 57.1% | (4/7) | |
| Central zone anterior | |||||
| Male | 0% | (0/7) | 42.9% | (3/7) | |
| Female | 42.9% | (3/7) | 0% | (0/7) | |
| Central zone posterior | |||||
| Male | 0% | (0/7) | 28.6% | (2/7) | |
| Female | 0% | (0/7) | 42.9% | (3/7) | |
| Posterior zone | |||||
| Male | 0% | (0/7) | 0% | (0/7) | |
| Female | 0% | (0/7) | 0% | (0/7) | |
| Transverse Domains | Left Side | Right Side | |||
|---|---|---|---|---|---|
| Anterior zone | - | ||||
| Male | 0% | (0/7) | 0% | (0/7) | |
| Female | 0% | (0/7) | 0% | (0/7) | |
| Central zone anterior | |||||
| Male | 0% | (0/7) | 42.9% | (3/7) | |
| Female | 0% | (0/7) | 42.9% | (3/7) | |
| Central zone posterior | |||||
| Male | 57.1% | (4/7) * | 28.6% | (2/7) | |
| Female | 0% | (0/7) | 0% | (0/7) | |
| Posterior zone | |||||
| Male | 42.9% | (3/7) | 57.1% | (4/7) | |
| Female | 28.6% | (2/7) | 28.6% | (2/7) | |
| Transverse Domains | Left-Dominant (AQ < 0) | Right-Dominant (AQ > 0) | ||
|---|---|---|---|---|
| Anterior zone | ||||
| Left side | 66.7% | (2/3) | 27.3% | (3/11) |
| Right side | 33.3% | (1/3) | 63.6% | (7/11) |
| Central zone anterior | ||||
| Left side | 30.0% | (3/10) | 50.0% | (2/4) |
| Right side | 20.0% | (2/10) | 25.0% | (1/4) |
| Central zone posterior | ||||
| Left side | 0% | (0/10) | 0% | (0/4) |
| Right side | 40.0% | (4/10) * | 25.0% | (1/4) |
| Posterior zone | ||||
| Left side | 0% | (3/8) | 0% | (0/6) |
| Right side | 0% | (4/8) | 0% | (0/6) |
| Transverse Domains | Left-Dominant (AQ < 0) | Right-Dominant (AQ > 0) | ||
|---|---|---|---|---|
| Anterior zone | ||||
| Left side | 0% | (0/3) | 0% | (0/11) |
| Right side | 0% | (0/3) | 0% | (0/11) |
| Central zone anterior | ||||
| Left side | 0% | (0/10) | 0% | (0/4) |
| Right side | 40.0% | (4/10) * | 50.0% | (2/4) |
| Central zone posterior | ||||
| Left side | 30.0% | (3/10) | 25.0% | (1/4) |
| Right side | 20.0% | (2/10) | 0% | (0/4) |
| Posterior zone | ||||
| Left side | 37.5% | (3/8) | 33.3% | (2/6) |
| Right side | 50.0% | (4/8) | 33.3% | (2/6) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawada, K.; Kamiya, S.; Aoki, I. Asymmetry of Cerebellar Lobular Development in Ferrets. Symmetry 2020, 12, 735. https://doi.org/10.3390/sym12050735
Sawada K, Kamiya S, Aoki I. Asymmetry of Cerebellar Lobular Development in Ferrets. Symmetry. 2020; 12(5):735. https://doi.org/10.3390/sym12050735
Chicago/Turabian StyleSawada, Kazuhiko, Shiori Kamiya, and Ichio Aoki. 2020. "Asymmetry of Cerebellar Lobular Development in Ferrets" Symmetry 12, no. 5: 735. https://doi.org/10.3390/sym12050735
APA StyleSawada, K., Kamiya, S., & Aoki, I. (2020). Asymmetry of Cerebellar Lobular Development in Ferrets. Symmetry, 12(5), 735. https://doi.org/10.3390/sym12050735





