Abstract
The ferret cerebellum is anteriorly right-lateralized and posteriorly left-lateralized. This study characterized the left/right difference in ferret cerebellar lobular morphology using 3D-rendered magnetic resonance images of fixed brains from seven male and seven female ferrets on postnatal day 90. Asymmetrical lobular morphology showed asymmetrical sublobular development in the anterior vermis, lobulus simplex, and ansiform lobules and additional grooves asymmetrically appearing in the paramedian lobule, lobule VI, and ansiform lobules. Although we observed these asymmetric hallmarks in four cerebellar transverse domains in both sexes, there was no left/right difference in their incidence in each domain. Males showed a significantly higher incidence of the additional grooves in the left side of the ansiform lobules than in females. Data were combined and classified as per the asymmetry quotient (AQ) into left- (AQ < 0) and right-dominant (AQ > 0) groups. There were significantly higher incidences of poor sublobular development of ansiform lobules and additional groove appearing in lobule VI on the right than on the left in the left-dominant group. Asymmetric hallmarks visible on the cerebellar surface of ferrets are relevant to the left-biased volume asymmetry of the central zone of cerebellar transversus domains containing lobule VI and ansiform lobules.
1. Introduction
Studies have reported cerebellar volume laterality in humans [1], nonhuman primates [2,3], and carnivores [4], including ferrets [5,6]. Interestingly, regional differences in cerebellar volume laterality revealed distinctive torque morphology formed clockwise (anteriorly left-biased and posteriorly right-biased) in humans [2,7] and counterclockwise (anteriorly right-biased and posteriorly left-biased) in ferrets [5,6]. The posterior cerebellum, including lobule VI and ansiform lobules, is functionally lateralized, that is, the right-side controls language and working memory [8,9], while the left side controls cognition [10,11]. Such functional asymmetry is involved in characteristic functional cerebellar organization via cerebrum–cerebellum connections linked contralaterally with the association cortex, not the motor cortex [9]. Thus, cerebellar asymmetry is evaluated volumetrically and functionally but is not examined on the basis of detailed cerebellar morphology. This study characterized the left/right difference in the cerebellar lobular morphology in male and female ferrets using 3D-rendered images obtained from ex vivo magnetic resonance (MR) images.
2. Materials and Methods
2.1. Samples
Anatomical MR images of fixed brains from seven male and seven female ferrets on postnatal day 90 obtained in our previous studies [5,6] were used in this study. A preclinical 7.0 T magnetic resonance imaging (MRI) system with a 400 mm inner-diameter-bore magnet (Kobelco and Jastec, Kobe, Japan) and an AVANCE-I console (Bruker BioSpin, Ettlingen, Germany) acquired 3D MR images covering the entire fixed brains using the rapid acquisition with relaxation enhancement (RARE) sequence with the following parameters: Repetition time (TR) = 300 ms; echo time (TE) = 9.6 ms (effective TE = 19.2 ms); RARE factor = 4; field of view (FOV) = 32 × 32 × 40 mm3; acquisition matrix = 256 × 256 × 256; voxel size = 125 × 125 × 156 µm3; number of acquisitions (NEX) = 2; and total scan time = 2 h 43 min 50 s.
2.2. Volumetry
Volumetric analysis was conducted using 3D MR images of the cerebellum. The left and right cerebellar sides were divided at the midline, which was defined by the position of the cerebral longitudinal fissure. Cerebellar transverse domains were segmented semi-automatically into four zones: Anterior zone (AZ; lobules I–V of the vermis), central zone anterior (CZa; lobules VI and the lobules simplex), central zone posterior (CZp; lobules VII of the vermis and ansiform lobules), and posterior zone (PZ; lobules VIII–IXa of the vermis and paramedian lobule) [5]. Segmented areas of each domain were measured using the SliceOmatic software version 4.3 (TomoVision, Montreal, Canada), and the volume of each domain was calculated by multiplying the combined areas by the slice thickness (156.25 μm). The asymmetry quotient (AQ) was calculated using the formula ((R − L)/{(R + L) × 0.5}) and was used to assess the volume laterality of each domain. The direction of asymmetry was indicated by the AQ values: Positive value = rightward bias and negative value = leftward bias [12].
2.3. 3D-Rendered Images
The cerebellar cortex was segmented semi-automatically on MR images using Amira ver. 5.2 (Visage Imaging, Inc., San Diego, CA, USA) on the basis of image contrast. The images were rendered in 3D using the surface projection algorithm, which best visualizes the surface. Then, the 3D-rendered images were rotated and manipulated to best visualize the cerebellar morphology by linear registration using SliceOmatic software. Finally, asymmetric morphological changes (i.e., poor sublobular development and additional grooves) were noted in the left and right sides of each cerebellar transverse domain on the 3D-rendered images. The cerebellar lobules/sublobules were identified with reference to the textbook of Lawes and Andrews (1998) [13].
2.4. Statistical Analysis
The incidence of asymmetry in sublobular development and additional grooves was estimated in each cerebellar transverse domain segmented with reference to expression patterns of zebrin II/aldolase C in other mammals [14,15]. Left-/right-side or sexual differences were evaluated using the chi-square test. In addition, the relationship between morphological asymmetry and volume laterality in each cerebellar transverse domain was evaluated. Then, data from males and females were combined and classified on the basis of AQ values into left-dominant (AQ < 0) and right-dominant (AQ > 0) groups. The incidence of asymmetry in sublobular development and additional grooves was estimated in left- and right-dominant groups of all transverse domains, and left-/right-side or intergroup differences were evaluated using the chi-square test.
2.5. Ethics
All experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals by the National Institutes of Health (NIH, Bethesda, MD, USA). The study, and all its procedures, was approved by the Institutional Animal Care and Use Committee of Tsukuba International University, Japan. All efforts were made to minimize the number of animals used and their suffering.
3. Results
3.1. Types of Cerebellar Gross Anatomical Asymmetry
The cerebellar morphological asymmetry was roughly classified into two types: Asymmetrical lobular/sublobular development and asymmetrical appearance of additional grooves. Figure 1 illustrates the types of morphological asymmetry of the ferret cerebellum.
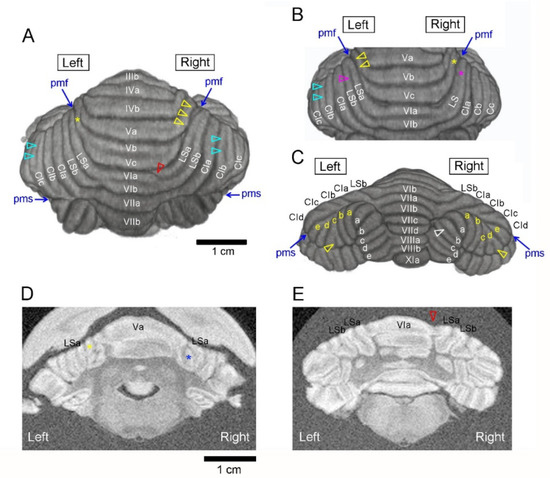
Figure 1.
Typical asymmetric morphology in the ferret cerebellum. (A) Dorsal view of 3D-rendered cerebellar image showing asymmetrically poor development of the sublobule in the anterior vermis and additional grooves emerging asymmetrically in the lobulus simplex (LS) and ansiform lobules. Sublobule Vb/c was visible at the left end on the cerebellar surface (yellow asterisk) but not at the right end, which was covered by adjacent sublobules (yarrow arrowheads). Additional grooves were found asymmetrically in sublobule VIa of the right paravermis (red arrowhead) and crus I sublobules (right CIb and left CIc) (light-blue arrowheads). (B) Dorsal view of 3D-rendered cerebellar image showing asymmetrically poor development of the LS. The sulcus demarcating LS sublobules was present on the left (purple arrowhead) but not on the right (purple asterisk). Sublobule Vc was visible at the right end on the cerebellar surface (yellow asterisk) but not at the left end, which was covered by adjacent sublobules (yarrow arrowheads). Additional grooves emerging asymmetrically were seen in the left CIb (light-blue arrowheads). (C) Posterior view of 3D-rendered cerebellar image showing additional grooves emerging asymmetrically in crus II (CII) sublobules (left CIIc and right CIId) (yellow arrowheads) and the paramedian sublobule (right PMb) (white arrowhead). Yellow a–e letters indicate CII sublobules; white a–e letters indicate PM sublobules. (D) Coronal (transaxial) MR image showing asymmetrically poor sublobular development in the anterior vermis. The blue asterisk indicates the poorly developed right Vc sublobule, which was covered by the adjacent sublobules Va and LSa on the cerebellar surface. The yellow asterisk indicates contralateral Vc sublobules, which were visible on the cerebellar surface. (E) Coronal (transaxial) MR image showing an additional groove appearing in sublobule VI of the right paravermis (red arrowhead). CI, crus I of ansiform lobule; CII, crus II of ansiform lobule; LS, lobulus simplex; PM, paramedian lobule; MR, magnetic resonance.
Sublobules were poorly developed on the left or right, which was covered by adjacent lobules/sublobules (Figure 1A,D). Such asymmetry was seen primarily in the anterior vermis and secondarily in the lobulus simplex and ansiform lobules. The asymmetrical lobular development was also characterized by underdeveloped sulci demarcating the sublobules on the left or right (Figure 1B). This asymmetry was found primarily in lobule VI and the lobulus simplex. Additional grooves appeared asymmetrically in sublobules of paravermian lobule VI, ansiform lobules, and the paramedian lobule (Figure 1A,C). Particularly, the additional groove appearing in the right paravermian lobule VI was distinct from those appearing in other lobules; it infolded loosely and made a distinctive asymmetric profile in lobule VI on coronal MR images (Figure 1E).
3.2. Incidence of Cerebellar Asymmetrical Morphology
The highest incidence of asymmetrically poor development of sublobules was observed in the AZ (corresponding to the anterior vermis) on both left and right sides in 57.1% of males and on the right in 57.1% of females. The incidence did not differ between left and right sides and at ipsilateral sides between sexes (Table 1). We also observed no additional grooves asymmetrically appearing in AZ sublobules (Table 2).

Table 1.
Incidences of asymmetrically poor sublobule development in male and female ferrets.

Table 2.
Incidences of asymmetrically appearing additional cerebellum indentations in male and female ferrets.
In the CZa, the lobulus simplex was poorly developed on the right in 42.9% of males and on the left in 42.9% of females (Table 1). Additional grooves appeared in paravermian lobule VI on the right in 42.9% of males and 28.6% of females but not on the left in both sexes (Table 2).
In the CZp, sublobules in ansiform lobules were poorly developed on the right in 28.6% of males and 42.9% of females. Although ansiform sublobules on the left were well developed in both males and females, we did not detect a statistical left/right difference in the incidence of asymmetrically poor development of ansiform sublobules in both sexes (Table 1). Additional grooves were found in ansiform lobules on the left in 57.1% of males and on the right in 28.6% of males (Table 2). However, there were no additional grooves in either the left or the right of ansiform lobules in females (Table 2). We observed a significant sexual difference in the incidence of additional grooves only in the left CZp, which was significantly higher in males compared to females (Table 2).
There were no asymmetrically poorly developed sublobules in the PZ in both males and females (Table 1). Additional grooves appeared asymmetrically on the left and right of the paramedian sublobules in both males and females without a significant sexual difference and a left/right difference (Table 2).
3.3. Relationship with Regional Cerebellar Volume Laterality
Volumes of left and right sides and mean AQ values of each transverse domain are shown in Table S1 in the Supplementary Materials.
We observed significant differences in the incidence of asymmetry in sublobular development and additional grooves appearing in the left-dominant group between the left and right sides of the CZa and CZp. Poor sublobular development appeared more frequently in the right CZp compared to the left CZp in the left-dominant group (Table 3). There was a significantly greater incidence of additional grooves in the right CZa compared to the left CZa in the left-dominant group (Table 4). Therefore, the right-dominant appearance of the poor development of CZp sublobules and additional grooves in the CZa might be associated with left-lateralized volumes of these cerebellar domains.

Table 3.
Incidence of asymmetrically poor development of cerebellar sublobules in ferrets with left- or right-dominant volume laterality in cerebellar transverse domains.

Table 4.
Incidence of asymmetrically appearing additional grooves in ferrets with left- or right-dominant volume laterality in cerebellar transverse domains.
4. Discussion
The cerebellum is functionally lateralized by making cerebrum–cerebellum connections linked contralaterally with the association cortex, not the motor cortex [9,16]. Right-handed humans showed mirror linkage of the torque asymmetry between the cerebrum and cerebellum, i.e., counterclockwise versus clockwise [7]. The left-lateralized volume of the CZp in the ferret cerebellum plays a role in poor sublobular development in right ansiform lobules. Such left-lateralized development might be involved in left CZp function, such as place-based navigation [10].
Another asymmetric profile appearing in relation to the cerebellar volume laterality is the additional groove in the right paravermian lobule VI, especially in ferrets with the left-biased volume of the CZa. A meta-analysis of task-based neuroimaging studies has shown that the left lobule VI is involved in spatial processing, while the right lobule VI is involved in language and working memory processing [8]. As cerebellar morphological lateralization is related to cerebrum–cerebellum connections [9], the right lobule VI might infold by an enchantment of connectivity to the left cerebral hemisphere.
There is a significant male-over-female incidence in additional grooves appearing in the left CZp, which mainly emerge in left ansiform lobules, indicating sexual differences in CZp function. Sex-related paw preferences have a significant correlation with cerebellar volume asymmetry in dogs [4]. However, ansiform lobules might not be related to paw preferences. In humans, handedness is not related to the superior posterior cerebellar lobule volume, including crura I and II, in terms of interhemispheric connectivity in cortical systems [17]. In contrast, the emergence of additional indentations, called secondary and tertiary sulci, in the cerebral cortex might be associated with regional cortical growth [18]. A preliminary study revealed the possibility of adult cerebellar neurogenesis in ferrets [19], as reported in humans and mice [20]. As volumetric asymmetry of the ferret cerebellum is evident during puberty to adolescence [6], regional cerebellar growth by adult neurogenesis might be involved in the appearance of additional sex-related grooves. Further studies are needed to elucidate this.
5. Conclusions
A distinctive cerebellar morphological asymmetry has been documented in humans [7], nonhuman primates [2], and carnivores [4,5], although the direction and degree of asymmetry are altered by species and/or sexes [2,3,4,5,6,7] but not by genetic factors [7]. As the rodent cerebellum developed symmetrically [21], ferrets would be useful models for investigating the pathogenesis underlying human neurodevelopmental disorders with disturbances in morphological cerebellar asymmetry, such as autistic spectrum disorder [22], schizophrenia [23,24], dyslexia [25], and attention-deficit hyperactivity disorder [26]. Asymmetric hallmarks of the cortical surface morphology visible on the cerebellar surface of ferrets are relevant to the left-biased volume asymmetry of the CZ of cerebellar transversus domains containing lobule VI and ansiform lobules in ferrets. The findings provide a new insight into assessments of cerebellar asymmetry on the basis of cerebellar surface morphology.
Supplementary Materials
The following is available online at https://www.mdpi.com/2073-8994/12/5/735/s1, Table S1: Volume and asymmetry quotient of cerebellar cortex in male and female ferrets.
Author Contributions
K.S. designed the study; K.S. and S.K. performed the experiments and analyzed the data; K.S. and I.A. wrote the manuscript; the final version of the manuscript was approved by all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Grant from Tsukuba International University, and partly supported by the Center of Innovation Program (Japan Science and Technology Agency; JST) for MRI devices and AMED under Grant Number 17dm0107066h for imaging technologies.
Acknowledgments
The authors wish to thank Nobuhiro Nitta (Molecular Imaging Center, National Institute of Radiological Sciences, Chiba, Japan) for the MRI measurements. The authors would like to thank Enago (www.enago.jp) for the English language review.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Synder, P.J.; Bilder, R.M.; Wu, H.; Bogerts, B.; Lieberman, J.A. Cerebellar volume asymmetries are related to handedness: A quantitative MRI study. Neuropsychologia 1995, 33, 407–419. [Google Scholar] [CrossRef]
- Phillips, K.; Hopkins, W.D. Exploring the relationship between cerebellar asymmetry and handedness in chimpanzees (Pan troglodytes) and capuchins (Cebus apella). Neuropsychologia 2007, 45, 2333–2339. [Google Scholar] [CrossRef] [PubMed]
- Cantalupo, C.; Freeman, H.; Rodes, W.; Hopkins, W. Handedness for tool use correlates with cerebellar asymmetries in chimpanzees (Pan troglodytes). Behav. Neurosci. 2008, 122, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Koyun, N.; Aydinlioğlu, A.; Aslan, K. A morphometric study on dog cerebellum. Neurol. Res. 2011, 33, 220–224. [Google Scholar] [CrossRef]
- Sawada, K.; Horiuchi-Hirose, M.; Saito, S.; Aoki, I. Male prevalent enhancement of leftward asymmetric development of the cerebellar cortex in ferrets (Mustela putorius). Laterality 2015, 20, 723–737. [Google Scholar] [CrossRef]
- Sawada, K.; Aoki, I. Age-dependent sexually-dimorphic asymmetric development of the ferret cerebellar cortex. Symmetry 2017, 9, 40. [Google Scholar] [CrossRef]
- Rosch, R.E.; Ronan, L.; Cherkas, L.; Gurd, J.M. Cerebellar asymmetry in a pair of monozygotic handedness-discordant twins. J. Anat. 2010, 217, 38–47. [Google Scholar] [CrossRef]
- Valera, E.M.; Faraone, S.V.; Biederman, J.; Poldrack, R.A.; Seidman, L.J. Functional neuroanatomy of working memory in adults with attention-deficit/hyperactivity disorder. Biol. Psychiatry 2005, 57, 439–447. [Google Scholar] [CrossRef]
- Wang, D.; Buckner, R.L.; Liu, H. Cerebellar asymmetry and its relation to cerebral asymmetry estimated by intrinsic functional connectivity. J. Neurophysiol. 2013, 109, 46–57. [Google Scholar] [CrossRef]
- Iglói, K.; Doeller, C.F.; Paradis, A.L.; Benchenane, K.; Berthoz, A.; Burgess, N.; Rondi-Reig, L. Interaction between hippocampus and cerebellum crus I in sequence-based but not place-based navigation. Cereb. Cortex 2015, 25, 4146–4154. [Google Scholar] [CrossRef]
- Sokolov, A.A. The cerebellum in social cognition. Front. Cell Neurosci. 2018, 12, 145. [Google Scholar] [CrossRef]
- Hopkins, W.D.; Marino, L. Asymmetries in cerebral width in nonhuman primate brains as revealed by magnetic resonance imaging (MRI). Neuropsychologia 2000, 38, 493–499. [Google Scholar] [CrossRef]
- Lawes, I.N.C.; Andrews, P.L.R. Neuroanatomy of the ferret brain. In Biology and Diseases of the Ferret, 2nd ed.; Fox, Ed.; Lippincott Williams and Wilkins: Baltimore, MD, USA, 1998; pp. 71–102. [Google Scholar]
- Ozol, K.; Hayden, J.M.; Oberdick, J.; Hawkes, R. Transverse zones in the vermis of the mouse cerebellum. J. Comp. Neurol. 1999, 412, 95–111. [Google Scholar] [CrossRef]
- Sillitoe, R.V.; Hawkes, R. Whole-mount immunohistochemistry: A high-throughput screen for patterning defects in the mouse cerebellum. J. Histochem. Cytochem. 2002, 50, 235–244. [Google Scholar] [CrossRef]
- Siniscalchi, M.; Franchini, D.; Pepe, A.M.; Sasso, R.; Dimatteo, S.; Vallortigara, G.; Quaranta, A. Volumetric assessment of cerebral asymmetries in dogs. Laterality 2011, 16, 528–536. [Google Scholar] [CrossRef]
- Rosch, R.E.; Cowell, P.E.; Gurd, J.M. Cerebellar asymmetry and cortical connectivity in monozygotic twins with discordant handedness. Cerebellum 2018, 17, 191–203. [Google Scholar] [CrossRef]
- Sawada, K.; Fukunishi, K.; Kashima, M.; Imai, N.; Saito, S.; Aoki, I.; Fukui, Y. Regional difference in sulcal infolding progression correlated with cerebral cortical expansion in cynomolgus monkey fetuses. Congenit. Anom. (Kyoto) 2017, 57, 114–117. [Google Scholar] [CrossRef]
- Kamiya, S.; Sawada, K. Immunohistochemical characterization of postnatal changes in cerebellar cortical cytoarchitectures in ferrets. Anat. Rec. (Hoboken). in press.
- Ahlfeld, J.; Filser, S.; Schmidt, F.; Wefers, A.K.; Merk, D.J.; Glaß, R.; Herms, J.; Schüller, U. Neurogenesis from Sox2 expressing cells in the adult cerebellar cortex. Sci. Rep. 2017, 7, 6137. [Google Scholar] [CrossRef]
- Dorr, A.E.; Lerch, J.P.; Spring, S.; Kabani, N.; Henkelman, R.M. High resolution three-dimensional brain atlas using an average magnetic resonance image of 40 adult C57Bl/6J mice. Neuroimage 2008, 42, 60–69. [Google Scholar] [CrossRef]
- Hodge, S.M.; Makris, N.; Kennedy, D.N.; Caviness, V.S., Jr.; Howard, J.; McGrath, L.; Steele, S.; Frazier, J.A.; Tager-Flusberg, H.; Harris, G.J. Cerebellum, language, and cognition in autism and specific language impairment. J. Autism Dev. Disord. 2010, 40, 300–316. [Google Scholar] [CrossRef] [PubMed]
- Levitt, J.J.; McCarley, R.W.; Nestor, P.G.; Petrescu, C.; Donnino, R.; Hirayasu, Y.; Kikinis, R.; Jolesz, F.A.; Shenton, M.E. Quantitative volumetric MRI study of the cerebellum and vermis in schizophrenia: Clinical and cognitive correlates. Am. J. Psychiatry 1999, 156, 1105–1107. [Google Scholar] [PubMed]
- Sheng, J.; Zhu, Y.; Lu, Z.; Liu, N.; Huang, N.; Zhang, Z.; Tan, L.; Li, C.; Yu, X. Altered volume and lateralization of language-related regions in first-episode schizophrenia. Schizophr. Res. 2013, 148, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Kibby, M.Y.; Fancher, J.B.; Markanen, R.; Hynd, G.W. A quantitative magnetic resonance imaging analysis of the cerebellar deficit hypothesis of dyslexia. J. Child Neurol. 2008, 23, 368–380. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, F.X.; Giedd, J.N.; Marsh, W.L.; Hamburger, S.D.; Vaituzis, A.C.; Dickstein, D.P.; Sarfatti, S.E.; Vauss, Y.C.; Snell, J.W.; Lange, N.; et al. Quantitative brain magnetic resonance imaging in attention-deficit hyperactivity disorder. Arch. Gen. Psychiatry 1996, 53, 607–616. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).