Enantiomers of Carbohydrates and Their Role in Ecosystem Interactions: A Review
Abstract
1. Introduction
2. D- and L-Carbohydrates of Plants
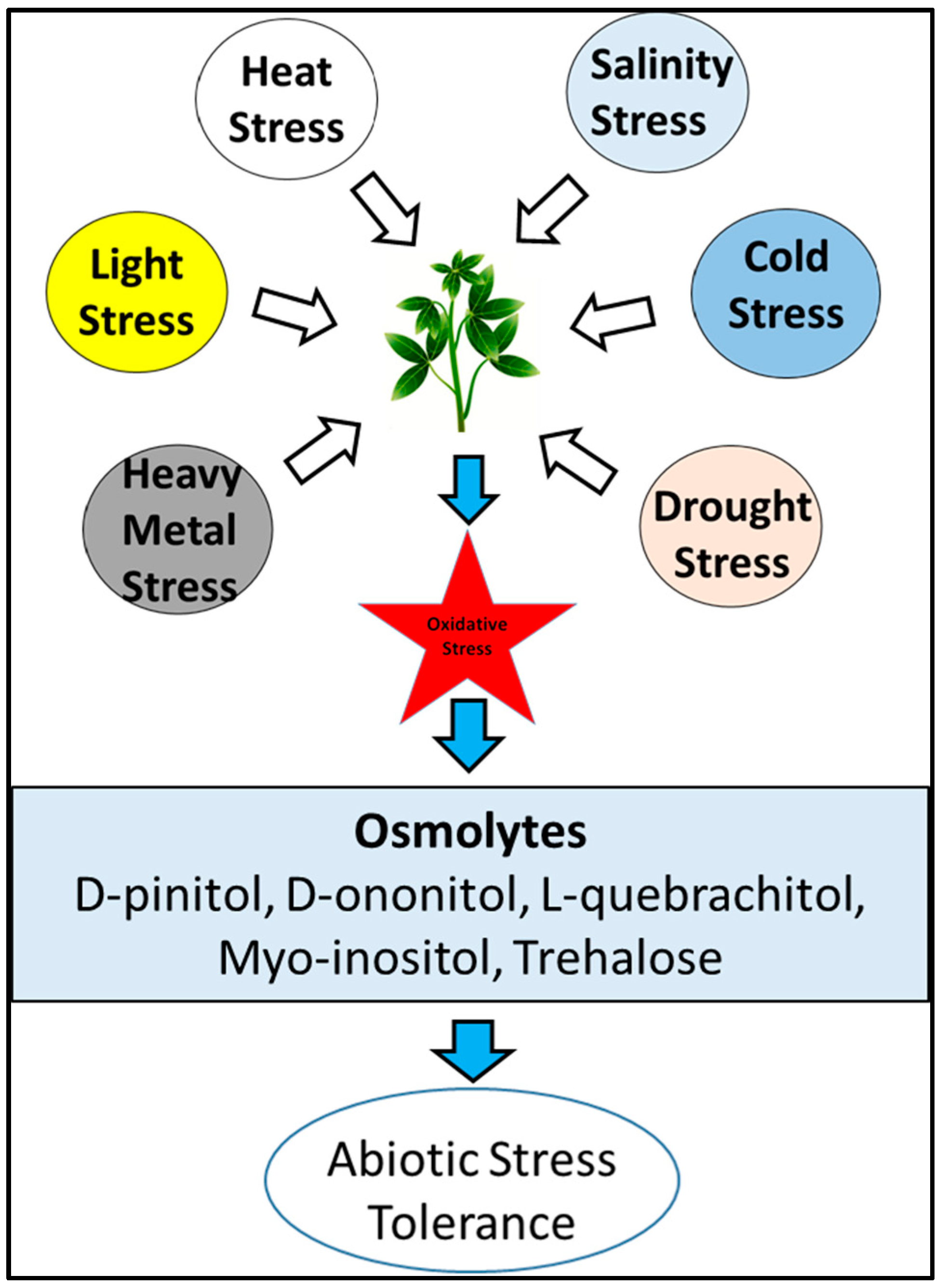
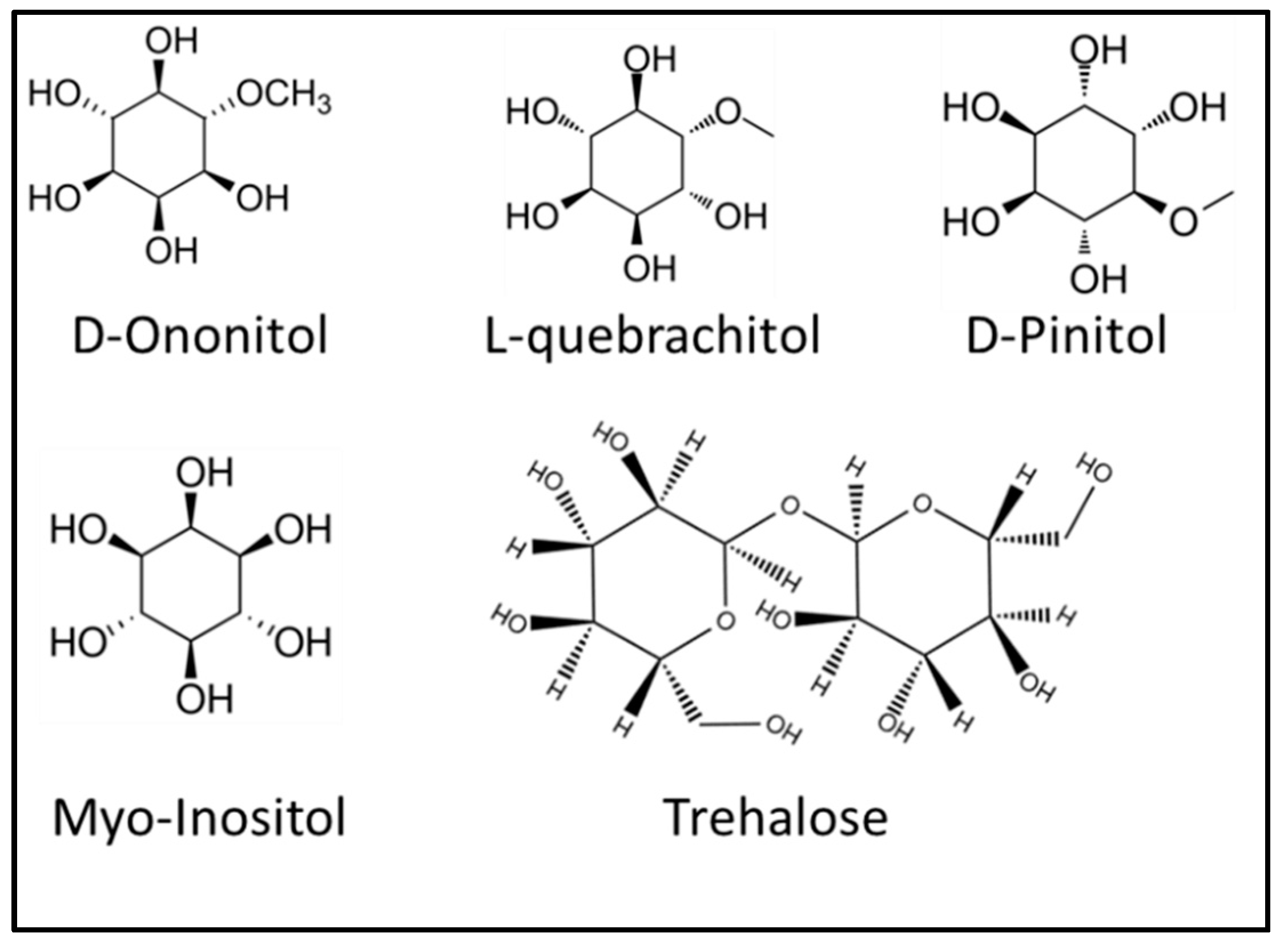
2.1. Role of Carbohydrates under Stress and Protection against Herbivory
2.2. Occurrence Of D- and L-Carbohydrates, Their Metabolism in Plants, and Its Genetic Regulation
2.3. Perspectives for Future Research
3. Significance of Enantiomers of Carbohydrates for Invertebrates and Vertebrates
4. L- and D-Carbohydrates in Soil
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boulineau, F.P.; Wei, A. Mirror-image carbohydrates: synthesis of the unnatural enantiomer of a blood group trisaccharide. J. Org. Chem. 2004, 69, 3391–3399. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-S.; Lee, Y.-S. Effect of reaction pH on enolization and racemization reactions of glucose and fructose on heating with amino acid enantiomers and formation of melanoidins as result of the Maillard reaction. Food Chem. 2008, 108, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Padgett, P.E.; Cook, H.; Bytnerowicz, A.; Heath, R.L. Foliar loading and metabolic assimilation of dry deposited nitric acid air pollutants by trees. J. Environ. Monit. 2009, 11, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, D.; Tilgner, A.; Iinuma, Y.; Herrmann, H. Atmospheric Stability of Levoglucosan: A Detailed Laboratory and Modeling Study. Environ. Sci. Technol. 2010, 44, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.-C.; Wu, Y.-S.; Chu, C.-C.; Huang, S.-H.; Rau, J.-Y. The concentration, dry deposition, composition study of ambient air particulate and metallic pollutants at a traffic sampling site. Toxicol. Ind. Health 2003, 19, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, S.; Matsumoto, K.; Kaneyasu, N.; Shigihara, A.; Katono, K.; Igawa, M. Measurements of particulate sugars at urban and forested suburban sites. Atmos. Environ. 2011, 45, 2335–2339. [Google Scholar] [CrossRef]
- Fuß, W. Biological homochirality as result from a single event. Colloids Surf. B Biointerfaces 2009, 74, 498–503. [Google Scholar] [CrossRef]
- Aluwihare, L.; Repeta, D. A comparison of the chemical characteristics of oceanic DOM and extracellular DOM produced by marine algae. Mar. Ecol. Prog. Ser. 1999, 186, 105–117. [Google Scholar] [CrossRef]
- Guimarães, R.; Barros, L.; Carvalho, A.M.; Sousa, M.J.; Morais, J.S.; Ferreira, I.C.F.R. Aromatic plants as a source of important phytochemicals: Vitamins, sugars and fatty acids in Cistus ladanifer, Cupressus lusitanica and Eucalyptus gunnii leaves. Ind. Crops Prod. 2009, 30, 427–430. [Google Scholar] [CrossRef]
- Oliveira, L.; Cordeiro, N.; Evtuguin, D.V.; Torres, I.C.; Silvestre, A.J.D. Chemical composition of different morphological parts from ‘Dwarf Cavendish’ banana plant and their potential as a non-wood renewable source of natural products. Ind. Crops Prod. 2007, 26, 163–172. [Google Scholar] [CrossRef]
- Sariyildiz, T. Interactions between litter quality, decomposition and soil fertility: A laboratory study. Soil Biol. Biochem. 2003, 35, 391–399. [Google Scholar] [CrossRef]
- Sridhar, K.R.; Seena, S. Nutritional and antinutritional significance of four unconventional legumes of the genus Canavalia – A comparative study. Food Chem. 2006, 99, 267–288. [Google Scholar] [CrossRef]
- Hsu, F.-L.; Chou, C.-J.; Chang, Y.-C.; Chang, T.-T.; Lu, M.-K. Promotion of hyphal growth and underlying chemical changes in Antrodia camphorata by host factors from Cinnamomum camphora. Int. J. Food Microbiol. 2006, 106, 32–38. [Google Scholar] [CrossRef]
- Beleski-Carneiro, E. Structural and biological features of a hydrogel from seed coats of Chorisia speciosa. Phytochemistry 2002, 61, 157–163. [Google Scholar] [CrossRef]
- Janaki, B.; Sashidhar, R.B. Physico-chemical analysis of gum kondagogu (Cochlospermum gossypium): A potential food additive. Food Chem. 1998, 61, 231–236. [Google Scholar] [CrossRef]
- Benhura, M.A.N.; Chidewe, C. Some properties of a polysaccharide preparation that is isolated from the fruit of Cordia abyssinica. Food Chem. 2002, 76, 343–347. [Google Scholar] [CrossRef]
- Jacobs, A.; Kaiser, K.; Ludwig, B.; Rauber, R.; Joergensen, R.G. Application of biochemical degradation indices to the microbial decomposition of maize leaves and wheat straw in soils under different tillage systems. Geoderma 2011, 162, 207–214. [Google Scholar] [CrossRef]
- Kakegawa, K.; Edashige, Y.; Ishii, T. Xyloglucan from xylem-differentiating zones of Cryptomeria japonica. Phytochemistry 1998, 47, 767–771. [Google Scholar] [CrossRef]
- Naqvi, S.A.; Khan, M.M.; Shahid, M.; Jaskani, M.J.; Khan, I.A.; Zuber, M.; Zia, K.M. Biochemical profiling of mucilage extracted from seeds of different citrus rootstocks. Carbohydr. Polym. 2011, 83, 623–628. [Google Scholar] [CrossRef]
- Elayoubi, F.A.; Fraser, A.; Jenkins, D.J.; Craig, P.S. Partial characterisation of carbohydrate-rich Echinococcus granulosus coproantigens. Int. J. Parasitol. 2003, 33, 1553–1559. [Google Scholar] [CrossRef]
- Dungait, J.A.J.; Docherty, G.; Straker, V.; Evershed, R.P. Seasonal variations in bulk tissue, fatty acid and monosaccharide δ13C values of leaves from mesotrophic grassland plant communities under different grazing managements. Phytochemistry 2010, 71, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Kompella, U.B.; Lee, V.H.L. Delivery systems for penetration enhancement of peptide and protein drugs: design considerations. Adv. Drug Deliv. Rev. 2001, 46, 211–245. [Google Scholar] [CrossRef]
- Kajita, S.; Mashino, Y.; Nishikubo, N.; Katayama, Y.; Omori, S. Immunological characterization of transgenic tobacco plants with a chimeric gene for 4-coumarate:CoA ligase that have altered lignin in their xylem tissue. Plant Sci. 1997, 128, 109–118. [Google Scholar] [CrossRef]
- Tverdislov, V.A.; Malyshko, E.V.; Il’chenko, S.A.; Zhulyabina, O.A.; Yakovenko, L.V. A periodic system of chiral structures in molecular biology. Biophysics 2017, 62, 331–341. [Google Scholar] [CrossRef]
- Malyshko, E.V. On regularities in the spontaneous formation of structural hierarchies in chiral systems of nonliving and living matter. Phys.-Uspekhi 2019, 62, 354–363. [Google Scholar] [CrossRef]
- Sidorova, A.E.; Malyshko, E.V.; Kotov, A.R.; Tverdislov, V.A.; Ustinin, M.N. Quantitative Criteria of Chirality in Hierarchical Protein Structures. Biophysics 2019, 64, 155–166. [Google Scholar] [CrossRef]
- Zhou, Y.; Stuart-Williams, H.; Farquhar, G.D.; Hocart, C.H. The use of natural abundance stable isotopic ratios to indicate the presence of oxygen-containing chemical linkages between cellulose and lignin in plant cell walls. Phytochemistry 2010, 71, 982–993. [Google Scholar] [CrossRef] [PubMed]
- Danso Marfo, T.; Datta, R.; Vranová, V.; Ekielski, A. Ecotone Dynamics and Stability from Soil Perspective: Forest-Agriculture Land Transition. Agriculture 2019, 9, 228. [Google Scholar] [CrossRef]
- Marfo, T.D.; Datta, R.; Pathan, S.I.; Vranová, V. Ecotone Dynamics and Stability from Soil Scientific Point of View. Diversity 2019, 11, 53. [Google Scholar] [CrossRef]
- Hussain, M.; Debnath, B.; Qasim, M.; Bamisile, B.S.; Islam, W.; Hameed, M.S.; Wang, L.; Qiu, D. Role of Saponins in Plant Defense Against Specialist Herbivores. Mol. Basel Switz. 2019, 24, 2067. [Google Scholar] [CrossRef]
- Atkinson, N.J.; Dew, T.P.; Orfila, C.; Urwin, P.E. Influence of Combined Biotic and Abiotic Stress on Nutritional Quality Parameters in Tomato (Solanum lycopersicum). J. Agric. Food Chem. 2011, 59, 9673–9682. [Google Scholar] [CrossRef] [PubMed]
- Constabel, C.P.; Major, I.T. Molecular Biology and Biochemistry of Induced Insect Defense in Populus. Recent Adv. Phytochem. 2005, 119–143. [Google Scholar]
- Datta, R.; Kelkar, A.; Baraniya, D.; Molaei, A.; Moulick, A.; Meena, R.; Formanek, P. Enzymatic degradation of lignin in soil: A review. Sustainability 2017, 9, 1163. [Google Scholar] [CrossRef]
- Milčevičová, R.; Gosch, C.; Halbwirth, H.; Stich, K.; Hanke, M.-V.; Peil, A.; Flachowsky, H.; Rozhon, W.; Jonak, C.; Oufir, M.; et al. Erwinia amylovora-induced defense mechanisms of two apple species that differ in susceptibility to fire blight. Plant Sci. 2010, 179, 60–67. [Google Scholar] [CrossRef]
- Morsy, M.R.; Jouve, L.; Hausman, J.-F.; Hoffmann, L.; Stewart, J.M. Alteration of oxidative and carbohydrate metabolism under abiotic stress in two rice (Oryza sativa L.) genotypes contrasting in chilling tolerance. J. Plant Physiol. 2007, 164, 157–167. [Google Scholar] [CrossRef]
- Wasano, N.; Konno, K.; Nakamura, M.; Hirayama, C.; Hattori, M.; Tateishi, K. A unique latex protein, MLX56, defends mulberry trees from insects. Phytochemistry 2009, 70, 880–888. [Google Scholar] [CrossRef]
- Yancey, P.H. Compatible and Counteracting Solutes: Protecting Cells from the Dead Sea to the Deep Sea. Sci. Prog. 2004, 87, 1–24. [Google Scholar] [CrossRef]
- Anjum, S.A.; Ashraf, U.; Tanveer, M.; Khan, I.; Hussain, S.; Shahzad, B.; Zohaib, A.; Abbas, F.; Saleem, M.F.; Ali, I.; et al. Drought Induced Changes in Growth, Osmolyte Accumulation and Antioxidant Metabolism of Three Maize Hybrids. Front. Plant Sci. 2017, 8, 69. [Google Scholar] [CrossRef]
- Meena, R.S.; Kumar, S.; Datta, R.; Lal, R.; Vijayakumar, V.; Brtnicky, M.; Sharma, M.P.; Yadav, G.S.; Jhariya, M.K.; Jangir, C.K. Impact of Agrochemicals on Soil Microbiota and Management: A Review. Land 2020, 9, 34. [Google Scholar] [CrossRef]
- Ningthoujam, M.; Habib, K.; Bano, F.; Zutshi, S.; Fatma, T. Exogenous osmolytes suppresses the toxic effects of malathion on Anabaena variabilis. Ecotoxicol. Environ. Saf. 2013, 94, 21–27. [Google Scholar] [CrossRef]
- Kampfraath, A.A.; Giesen, D.; van Gestel, C.A.; Le Lann, C. Pesticide stress on plants negatively affects parasitoid fitness through a bypass of their phytophage hosts. Ecotoxicology 2017, 26, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Edmond Ghanem, M.; Han, R.-M.; Classen, B.; Quetin-Leclerq, J.; Mahy, G.; Ruan, C.-J.; Qin, P.; Pérez-Alfocea, F.; Lutts, S. Mucilage and polysaccharides in the halophyte plant species Kosteletzkya virginica: Localization and composition in relation to salt stress. J. Plant Physiol. 2010, 167, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Vetter, J. Plant cyanogenic glycosides. Toxicon 2000, 38, 11–36. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Kumar, V.; Kohli, S.K.; Sidhu, G.P.S.; Bali, A.S.; Handa, N.; Kapoor, D.; Bhardwaj, R.; Zheng, B. Phytohormones Regulate Accumulation of Osmolytes Under Abiotic Stress. Biomolecules 2019, 9, 285. [Google Scholar] [CrossRef]
- Vasconcelos, I.M.; Oliveira, J.T.A. Antinutritional properties of plant lectins. Toxicon 2004, 44, 385–403. [Google Scholar] [CrossRef]
- Applebaum, S.W.; Gestetner, B.; Birk, Y. Physiological aspects of host specificity in the Bruchidae—IV. Developmental incompatibility of soybeans for Callosobruchus. J. Insect Physiol. 1965, 11, 611–616. [Google Scholar] [CrossRef]
- De Geyter, E.; Swevers, L.; Soin, T.; Geelen, D.; Smagghe, G. Saponins do not affect the ecdysteroid receptor complex but cause membrane permeation in insect culture cell lines. J. Insect Physiol. 2012, 58, 18–23. [Google Scholar] [CrossRef]
- kaur, M.; Singh, K.; Rup, P.J.; Saxena, A.K.; Khan, R.H.; Ashraf, M.T.; Kamboj, S.S.; Singh, J. A tuber lectin from Arisaema helleborifolium Schott with anti-insect activity against melon fruit fly, Bactrocera cucurbitae (Coquillett) and anti-cancer effect on human cancer cell lines. Arch. Biochem. Biophys. 2006, 445, 156–165. [Google Scholar] [CrossRef]
- Silva, M.C.P.; Terra, W.R.; Ferreira, C. Absorption of toxic β-glucosides produced by plants and their effect on tissue trehalases from insects. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2006, 143, 367–373. [Google Scholar] [CrossRef]
- Andrade-Cetto, A.; Vázquez, R.C. Gluconeogenesis inhibition and phytochemical composition of two Cecropia species. J. Ethnopharmacol. 2010, 130, 93–97. [Google Scholar] [CrossRef]
- dos Santos, T.B.; Budzinski, I.G.F.; Marur, C.J.; Petkowicz, C.L.O.; Pereira, L.F.P.; Vieira, L.G.E. Expression of three galactinol synthase isoforms in Coffea arabica L. and accumulation of raffinose and stachyose in response to abiotic stresses. Plant Physiol. Biochem. 2011, 49, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Mandre, M.; Lukjanova, A. Biochemical and structural characteristics of Scots pine (Pinus sylvestris L.) in an alkaline environment. Est. J. Ecol. 2011, 60, 264. [Google Scholar] [CrossRef]
- Ômura, H.; Honda, K. Feeding responses of adult butterflies, Nymphalis xanthomelas, Kaniska canace and Vanessa indica, to components in tree sap and rotting fruits: synergistic effects of ethanol and acetic acid on sugar responsiveness. J. Insect Physiol. 2003, 49, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Mukherjee, S.; Parween, S.; Majumder, A.L. Galactinol synthase across evolutionary diverse taxa: Functional preference for higher plants? FEBS Lett. 2012, 586, 1488–1496. [Google Scholar] [CrossRef] [PubMed]
- Schadel, C.; Blochl, A.; Richter, A.; Hoch, G. Short-term dynamics of nonstructural carbohydrates and hemicelluloses in young branches of temperate forest trees during bud break. Tree Physiol. 2009, 29, 901–911. [Google Scholar] [CrossRef]
- Wróbel-Kwiatkowska, M.; Lorenc-Kukula, K.; Starzycki, M.; Oszmiański, J.; Kepczyńska, E.; Szopa, J. Expression of β-1,3-glucanase in flax causes increased resistance to fungi. Physiol. Mol. Plant Pathol. 2004, 65, 245–256. [Google Scholar] [CrossRef]
- Seifert, G.J. Nucleotide sugar interconversions and cell wall biosynthesis: how to bring the inside to the outside. Curr. Opin. Plant Biol. 2004, 7, 277–284. [Google Scholar] [CrossRef]
- Badejo, A.A.; Fujikawa, Y.; Esaka, M. Gene expression of ascorbic acid biosynthesis related enzymes of the Smirnoff-Wheeler pathway in acerola (Malpighia glabra). J. Plant Physiol. 2009, 166, 652–660. [Google Scholar] [CrossRef]
- Watanabe, K.; Suzuki, K.; Kitamura, S. Characterization of a GDP-d-mannose 3″,5″-epimerase from rice. Phytochemistry 2006, 67, 338–346. [Google Scholar] [CrossRef]
- Bindschedler, L.V.; Tuerck, J.; Maunders, M.; Ruel, K.; Petit-Conil, M.; Danoun, S.; Boudet, A.-M.; Joseleau, J.-P.; Paul Bolwell, G. Modification of hemicellulose content by antisense down-regulation of UDP-glucuronate decarboxylase in tobacco and its consequences for cellulose extractability. Phytochemistry 2007, 68, 2635–2648. [Google Scholar] [CrossRef]
- Minic, Z.; Jouanin, L. Plant glycoside hydrolases involved in cell wall polysaccharide degradation. Plant Physiol. Biochem. 2006, 44, 435–449. [Google Scholar] [CrossRef] [PubMed]
- Karagiannis, E.; Michailidis, M.; Tanou, G.; Samiotaki, M.; Karamanoli, K.; Avramidou, E.; Ganopoulos, I.; Madesis, P.; Molassiotis, A. Ethylene -dependent and -independent superficial scald resistance mechanisms in “Granny Smith” apple fruit. Sci. Rep. 2018, 8, 11436. [Google Scholar] [CrossRef] [PubMed]
- Loewus, F.A.; Murthy, P.P.N. myo-Inositol metabolism in plants. Plant Sci. 2000, 150, 1–19. [Google Scholar] [CrossRef]
- Oomen, R.J.F.J.; Dao-Thi, B.; Tzitzikas, E.N.; Bakx, E.J.; Schols, H.A.; Visser, R.G.F.; Vincken, J.-P. Overexpression of two different potato UDP-Glc 4-epimerases can increase the galactose content of potato tuber cell walls. Plant Sci. 2004, 166, 1097–1104. [Google Scholar] [CrossRef]
- Goulao, L.F.; Santos, J.; de Sousa, I.; Oliveira, C.M. Patterns of enzymatic activity of cell wall-modifying enzymes during growth and ripening of apples. Postharvest Biol. Technol. 2007, 43, 307–318. [Google Scholar] [CrossRef]
- Gantulga, D.; Turan, Y.; Bevan, D.R.; Esen, A. The Arabidopsis At1g45130 and At3g52840 genes encode β-galactosidases with activity toward cell wall polysaccharides. Phytochemistry 2008, 69, 1661–1670. [Google Scholar] [CrossRef]
- Monroe, J.D.; Garcia-Cazarin, M.L.; Poliquin, K.A.; Aivano, S.K. Antisense Arabidopsis plants indicate that an apoplastic α-xylosidase and α-glucosidase are encoded by the same gene. Plant Physiol. Biochem. 2003, 41, 877–885. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W. Application of new biotechnologies for improvements in swine nutrition and pork production. J. Anim. Sci. Biotechnol. 2019, 10, 28. [Google Scholar] [CrossRef]
- Hilz, H.; de Jong, L.E.; Kabel, M.A.; Verhoef, R.; Schols, H.A.; Voragen, A.G.J. Bilberry xyloglucan—novel building blocks containing β-xylose within a complex structure. Carbohydr. Res. 2007, 342, 170–181. [Google Scholar] [CrossRef]
- Hsieh, Y.S.Y.; Harris, P.J. Structures of xyloglucans in primary cell walls of gymnosperms, monilophytes (ferns sensu lato) and lycophytes. Phytochemistry 2012, 79, 87–101. [Google Scholar] [CrossRef]
- Deutschmann, R.; Dekker, R.F.H. From plant biomass to bio-based chemicals: Latest developments in xylan research. Biotechnol. Adv. 2012, 30, 1627–1640. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, P.M.; Andersen, M.R.; Kolenova, K.; vanKuyk, P.A.; Benoit, I.; Gruben, B.S.; Trejo-Aguilar, B.; Visser, H.; van Solingen, P.; Pakula, T. Post-genomic insights into the plant polysaccharide degradation potential of Aspergillus nidulans and comparison to Aspergillus niger and Aspergillus oryzae. Fungal Genet. Biol. 2009, 46, S161–S169. [Google Scholar] [CrossRef] [PubMed]
- Kuivanen, J.; Biz, A.; Richard, P. Microbial hexuronate catabolism in biotechnology. AMB Express 2019, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Rus, E.; Botella, M.A.; Valpuesta, V.; Gomez-Jimenez, M.C. Analysis of genes involved in l-ascorbic acid biosynthesis during growth and ripening of grape berries. J. Plant Physiol. 2010, 167, 739–748. [Google Scholar] [CrossRef]
- Sun, F.; Li, S.; He, D.; Cao, G.; Ni, X.; Tai, G.; Zhou, Y.; Wang, D. Effects of glycoalkaloids from Solanum plants on cucumber root growth. Phytochemistry 2010, 71, 1534–1538. [Google Scholar] [CrossRef]
- Ali, B.H.; Ziada, A.; Blunden, G. Biological effects of gum arabic: A review of some recent research. Food Chem. Toxicol. 2009, 47, 1–8. [Google Scholar] [CrossRef]
- O’Rourke, C.; Gregson, T.; Murray, L.; Sadler, I.H.; Fry, S.C. Sugar composition of the pectic polysaccharides of charophytes, the closest algal relatives of land-plants: presence of 3-O-methyl-D-galactose residues. Ann. Bot. 2015, 116, 225–236. [Google Scholar] [CrossRef]
- Cleveland, L.; Coffman, R.E.; Coon, P.; Davis, L. Role of the copper in galactose oxidase. Biochemistry 1975, 14, 1108–1115. [Google Scholar] [CrossRef]
- Oh, M.-M.; Trick, H.N.; Rajashekar, C.B. Secondary metabolism and antioxidants are involved in environmental adaptation and stress tolerance in lettuce. J. Plant Physiol. 2009, 166, 180–191. [Google Scholar] [CrossRef]
- Cevallos-Cevallos, J.M.; Futch, D.B.; Shilts, T.; Folimonova, S.Y.; Reyes-De-Corcuera, J.I. GC–MS metabolomic differentiation of selected citrus varieties with different sensitivity to citrus huanglongbing. Plant Physiol. Biochem. 2012, 53, 69–76. [Google Scholar] [CrossRef]
- Ma, G.; Zhang, L.; Kato, M.; Yamawaki, K.; Asai, T.; Nishikawa, F.; Ikoma, Y.; Matsumoto, H.; Yamauchi, T.; Kamisako, T. Effect of electrostatic atomization on ascorbate metabolism in postharvest broccoli. Postharvest Biol. Technol. 2012, 74, 19–25. [Google Scholar] [CrossRef][Green Version]
- Boudet, A.M.; Kajita, S.; Grima-Pettenati, J.; Goffner, D. Lignins and lignocellulosics: A better control of synthesis for new and improved uses. Trends Plant Sci. 2003, 8, 576–581. [Google Scholar] [CrossRef]
- Karg, S.R.; Kallio, P.T. The production of biopharmaceuticals in plant systems. Biotechnol. Adv. 2009, 27, 879–894. [Google Scholar] [CrossRef] [PubMed]
- Rao, U.R.; Kwa, B.H.; Nayar, J.K.; Vickery, A.C. Brugia malayi and Brugia pahangi: Transmission blocking activity of ivermectin and brugian filarial infections in Aedes aegypti. Exp. Parasitol. 1990, 71, 259–266. [Google Scholar] [CrossRef]
- Merivee, E.; Märtmann, H.; Must, A.; Milius, M.; Williams, I.; Mänd, M. Electrophysiological responses from neurons of antennal taste sensilla in the polyphagous predatory ground beetle Pterostichus oblongopunctatus (Fabricius 1787) to plant sugars and amino acids. J. Insect Physiol. 2008, 54, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Previato, J.O.; Mendonça-Previato, L.; Lewanczuk, R.Z.; Travassos, L.R.; Gorin, P.A.J. Crithidia spp.: Structural comparison of polysaccharides for taxonomic significance. Exp. Parasitol. 1982, 53, 170–178. [Google Scholar] [CrossRef]
- Schnuch, M.; Hansen, K. Sugar sensitivity of a labellar salt receptor of the blowfly Protophormia terraenovae. J. Insect Physiol. 1990, 36, 409–417. [Google Scholar] [CrossRef]
- Pendland, J.C.; Boucias, D.G. Hemagglutinin activity in the hemolymph of Anticarsiagemmatalis larvae infected with the fungus Nomuraearileyi. Dev. Comp. Immunol. 1985, 9, 21–30. [Google Scholar] [CrossRef]
- Pyle, G.G.; Paaso, B.; Anderson, B.E.; Allen, D.D.; Marti, T.; Li, Q.; Siegel, M.; Khosla, C.; Gray, G.M. Effect of Pretreatment of Food Gluten with Prolyl Endopeptidase on Gluten-Induced Malabsorption in Celiac Sprue. Clin. Gastroenterol. Hepatol. 2005, 3, 687–694. [Google Scholar] [CrossRef]
- Iida, T.; Hayashi, N.; Yamada, T.; Yoshikawa, Y.; Miyazato, S.; Kishimoto, Y.; Okuma, K.; Tokuda, M.; Izumori, K. Failure of d-psicose absorbed in the small intestine to metabolize into energy and its low large intestinal fermentability in humans. Metabolism 2010, 59, 206–214. [Google Scholar] [CrossRef]
- Schwartz, R.M.; Furne, J.K.; Levitt, M.D. Paracellular intestinal transport of six-carbon sugars is negligible in the rat. Gastroenterology 1995, 109, 1206–1213. [Google Scholar] [CrossRef]
- Levantovsky, R.; Allen-Blevins, C.R.; Sela, D.A. Nutritional Requirements of Bifidobacteria. Bifidobact. Relat. Org. 2018, 115–129. [Google Scholar]
- Vranova, V.; Rejsek, K.; Formanek, P. Aliphatic, cyclic, and aromatic organic acids, vitamins, and carbohydrates in soil: A review. Sci. World J. 2013, 2013, 524239. [Google Scholar] [CrossRef] [PubMed]
- Raymer, G.S.; Hartman, D.E.; Rowe, W.A.; Werkman, R.F.; Koch, K.L. An open-label trial of L-glucose as a colon-cleansing agent before colonoscopy. Gastrointest. Endosc. 2003, 58, 30–35. [Google Scholar] [CrossRef]
- Rogers, B.F.; Tate, R.L. Temporal analysis of the soil microbial community along a toposequence in Pineland soils. Soil Biol. Biochem. 2001, 33, 1389–1401. [Google Scholar] [CrossRef]
- Ojha, A.K.; Maiti, D.; Chandra, K.; Mondal, S.; Roy, D.D.S.K.; Ghosh, K.; Islam, S.S. Structural assignment of a heteropolysaccharide isolated from the gum of Cochlospermum religiosum (Katira gum). Carbohydr. Res. 2008, 343, 1222–1231. [Google Scholar] [CrossRef]
- Rana, V.; Kumar, V.; Soni, P.L. Structural characterization of an acidic polysaccharide from Dalbergia sissoo Roxb. leaves. Carbohydr. Polym. 2012, 90, 243–250. [Google Scholar] [CrossRef]
- Laver, M.L.; Chen, C.-H.; Zerrudo, J.V.; Lai, Y.-C.L. Carbohydrates of the inner bark of Pseudotsuga menziesii. Phytochemistry 1974, 13, 1891–1896. [Google Scholar] [CrossRef]
- Carlsson, H.E.; Sundblad, G.; Hammarström, S.; Lönngren, J. Structure of some oligosacharides derived from rat-intestinal glycoproteins. Carbohydr. Res. 1978, 64, 181–188. [Google Scholar] [CrossRef]
- Berman, E. Determination of the structure of three oligosaccharides from normal human urine by using 60-MHz, Carbon-13 nuclear magnetic resonance spectroscopy. Carbohydr. Res. 1983, 118, 9–20. [Google Scholar] [CrossRef]
- Cheng, H.; Wang, B.; Lv, J.; Jiang, M.; Lin, S.; Deng, Z. Xylitol production from xylose mother liquor: A novel strategy that combines the use of recombinant Bacillus subtilis and Candida maltosa. Microb. Cell Factories 2011, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Lemonnier, M.; Fournet, B.; Bourillon, R. Isolation and characterization of monofucosyl monosialyl derivative of lacto N-neohexaose obtained from human pregnancy urine. Biochem. Biophys. Res. Commun. 1977, 77, 767–774. [Google Scholar] [CrossRef]
- Chookaew, T.; O-Thong, S.; Prasertsan, P. Fermentative production of hydrogen and soluble metabolites from crude glycerol of biodiesel plant by the newly isolated thermotolerant Klebsiella pneumoniae TR17. Int. J. Hydrog. Energy 2012, 37, 13314–13322. [Google Scholar] [CrossRef]
- Datta, R.; Vranová, V.; Pavelka, M.; Rejšek, K.; Formánek, P. Effect of soil sieving on respiration induced by low-molecular-weight substrates. Int. Agrophysics 2014, 28, 119–124. [Google Scholar] [CrossRef]
- Zheng, H.; Ouyang, Z.Y.; Wang, X.K.; Fang, Z.G.; Zhao, T.Q.; Miao, H. Effects of regenerating forest cover on soil microbial communities: A case study in hilly red soil region, Southern China. For. Ecol. Manag. 2005, 217, 244–254. [Google Scholar] [CrossRef]
- Garland, J.L. Patterns of potential C source utilization by rhizosphere communities. Soil Biol. Biochem. 1996, 28, 223–230. [Google Scholar] [CrossRef]
- Pathan, S.I.; Větrovský, T.; Giagnoni, L.; Datta, R.; Baldrian, P.; Nannipieri, P.; Renella, G. Laura Giagnoni, Rahul Datta, Petr Baldrian, Paolo Nannipieri, Giancarlo Renella Microbial expression profiles in the rhizosphere of two maize lines differing in N use efficiency. Plant Soil 2018, 433, 401–413. [Google Scholar] [CrossRef]
- Yadav, G.; Datta, R.; Imran Pathan, S.; Lal, R.; Meena, R.; Babu, S.; Das, A.; Bhowmik, S.; Datta, M.; Saha, P. Effects of Conservation Tillage and Nutrient Management Practices on Soil Fertility and Productivity of Rice (Oryza sativa L.)–Rice System in North Eastern Region of India. Sustainability 2017, 9, 1816. [Google Scholar] [CrossRef]
- Castillo, M.A.; Felis, N.; Aragón, P.; Cuesta, G.; Sabater, C. Biodegradation of the herbicide diuron by streptomycetes isolated from soil. Int. Biodeterior. Biodegrad. 2006, 58, 196–202. [Google Scholar] [CrossRef]
- Saibi, W.; Gargouri, A. Hydroxyl distribution in sugar structure and its contributory role in the inhibition of Stachybotrys microspora β-glucosidase (bglG). Carbohydr. Res. 2011, 346, 1848–1854. [Google Scholar] [CrossRef]
- El-Ashry, E.-S.H.; El Nemr, A. Synthesis of mono- and di-hydroxylated prolines and 2-hydroxymethylpyrrolidines from non-carbohydrate precursors. Carbohydr. Res. 2003, 338, 2265–2290. [Google Scholar] [CrossRef]
- Datta, R.; Baraniya, D.; Wang, Y.-F.; Kelkar, A.; Meena, R.; Yadav, G.; Teresa Ceccherini, M.; Formanek, P. Amino Acid: Its Dual Role as Nutrient and Scavenger of Free Radicals in Soil. Sustainability 2017, 9, 1402. [Google Scholar] [CrossRef]
- Kaci, Y.; Heyraud, A.; Barakat, M.; Heulin, T. Isolation and identification of an EPS-producing Rhizobium strain from arid soil (Algeria): characterization of its EPS and the effect of inoculation on wheat rhizosphere soil structure. Res. Microbiol. 2005, 156, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Dastager, S.G.; Deepa, C.K.; Pandey, A. Isolation and characterization of novel plant growth promoting Micrococcus sp. NII-0909 and its interaction with cowpea. Plant Physiol. Biochem. 2010, 48, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Datta, R.; Anand, S.; Moulick, A.; Baraniya, D.; Pathan, S.I.; Rejsek, K.; Vranova, V.; Sharma, M.; Sharma, D.; Kelkar, A.; et al. How enzymes are adsorbed on soil solid phase and factors limiting its activity: A Review. Int. Agrophysics 2017, 31, 287–302. [Google Scholar] [CrossRef]
- Molaei, A.; Lakzian, A.; Datta, R.; Haghnia, G.; Astaraei, A.; Rasouli-Sadaghiani, M.; Ceccherini, M.T. Impact of chlortetracycline and sulfapyridine antibiotics on soil enzyme activities. Int. Agrophysics 2017, 31, 499–505. [Google Scholar] [CrossRef]
- Molaei, A.; Lakzian, A.; Haghnia, G.; Astaraei, A.; Rasouli-Sadaghiani, M.; Ceccherini, M.T.; Datta, R. Assessment of some cultural experimental methods to study the effects of antibiotics on microbial activities in a soil: An incubation study. PLoS ONE 2017, 12, e0180663. [Google Scholar] [CrossRef]
- Vidanarachchi, J.K.; Iji, P.A.; Mikkelsen, L.L.; Sims, I.; Choct, M. Isolation and characterization of water-soluble prebiotic compounds from Australian and New Zealand plants. Carbohydr. Polym. 2009, 77, 670–676. [Google Scholar] [CrossRef]
- Zhang, Z.; Srichuwong, S.; Kobayashi, T.; Arakane, M.; Park, J.; Tokuyasu, K. Bioconversion of l-arabinose and other carbohydrates from plant cell walls to α-glucan by a soil bacterium, Sporosarcina sp. N52. Bioresour. Technol. 2010, 101, 9734–9741. [Google Scholar] [CrossRef]
- Brtnicky, M.; Dokulilova, T.; Holatko, J.; Pecina, V.; Kintl, A.; Latal, O.; Vyhnanek, T.; Prichystalova, J.; Datta, R. Long-term effects of biochar-based organic amendments on soil microbial parameters. Agronomy 2019, 9, 747. [Google Scholar] [CrossRef]
| Plant/Animal | Type of Material | Dominant Carbohydrate | References |
|---|---|---|---|
| Acacia sp. | Water-extract | Galactose | [8] |
| Algea | Exopolysaccharides | Glucose, arabinose, mannose, rhamnose, galactose and xylose | [9] |
| Aromatic plants | Leaves | Glucose, fructose | [10] |
| Banana plants | Petioles | Arabinose, galactose, glucose | [11] |
| Beech, Oak | Litter | Xylose, glucose, galactose, arabinose | [12] |
| Canavalia sp. | Fiber | Uronic acid, glucose, arabinose | [13] |
| Cannamonum sp. | Extracted polysaccharide | Mannose, galactosamine | [14] |
| Chorisia speciosa | Seed coat | Galactose | [15] |
| Cochlospermum gossipium | Gum | Uronic acid | [16] |
| Cordia abyssinica | Fruits | Galactose, rhamnose | [17] |
| Corn | Litter | Xylose, glucose | [18] |
| Cryptomeria japonica | Xylem | Glucose, galactose, xylose and fucose | [19] |
| Different plants | Gums | Arabinose, galactose | [20] |
| Seed mucilage | Maltose | ||
| Eucalyptus sp. | Wood | Glucose | [21] |
| Grassland community | Leaves | Arabinose, xylose, mannose, arabinose and glucose | [22] |
| Transgenic plants | Cell walls | Glucose, xylose | [23] |
| Plant/Organism | Material | L/D Carbohydrates | Dominant Carbohydrates | References |
|---|---|---|---|---|
| American larch | Arabinogalactans of wood | 6 | D-galactose, L-arabinose | [96] |
| Cochlospermum religiosum | Heteropolysaccharides from gum | 1 | L-rhamnose, D-galactose, D-galacturonic acid | [97] |
| Dalbergia sissoo | Acid polysaccharide | 0.16 | L-rhamnose, D-glucose, D-galactose | [98] |
| Human | Urine | 0.13 | D-glucose, L-fucose | [99,100] |
| Rat | Feces | 0.5 | D-galactose, L-fucose | [101] |
| Soil | Humic substances | 0.13 | D-glucose, L-fucose | [94] |
| Composts | - | 0.07–0.09 | D-glucose, L-fucose | [94] |
| Pseudotsuga menziesii | Bark holocellulose | 0.04 | L-arabinose, D-glucose | [102] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lojkova, L.; Vranová, V.; Formánek, P.; Drápelová, I.; Brtnicky, M.; Datta, R. Enantiomers of Carbohydrates and Their Role in Ecosystem Interactions: A Review. Symmetry 2020, 12, 470. https://doi.org/10.3390/sym12030470
Lojkova L, Vranová V, Formánek P, Drápelová I, Brtnicky M, Datta R. Enantiomers of Carbohydrates and Their Role in Ecosystem Interactions: A Review. Symmetry. 2020; 12(3):470. https://doi.org/10.3390/sym12030470
Chicago/Turabian StyleLojkova, Lea, Valerie Vranová, Pavel Formánek, Ida Drápelová, Martin Brtnicky, and Rahul Datta. 2020. "Enantiomers of Carbohydrates and Their Role in Ecosystem Interactions: A Review" Symmetry 12, no. 3: 470. https://doi.org/10.3390/sym12030470
APA StyleLojkova, L., Vranová, V., Formánek, P., Drápelová, I., Brtnicky, M., & Datta, R. (2020). Enantiomers of Carbohydrates and Their Role in Ecosystem Interactions: A Review. Symmetry, 12(3), 470. https://doi.org/10.3390/sym12030470







