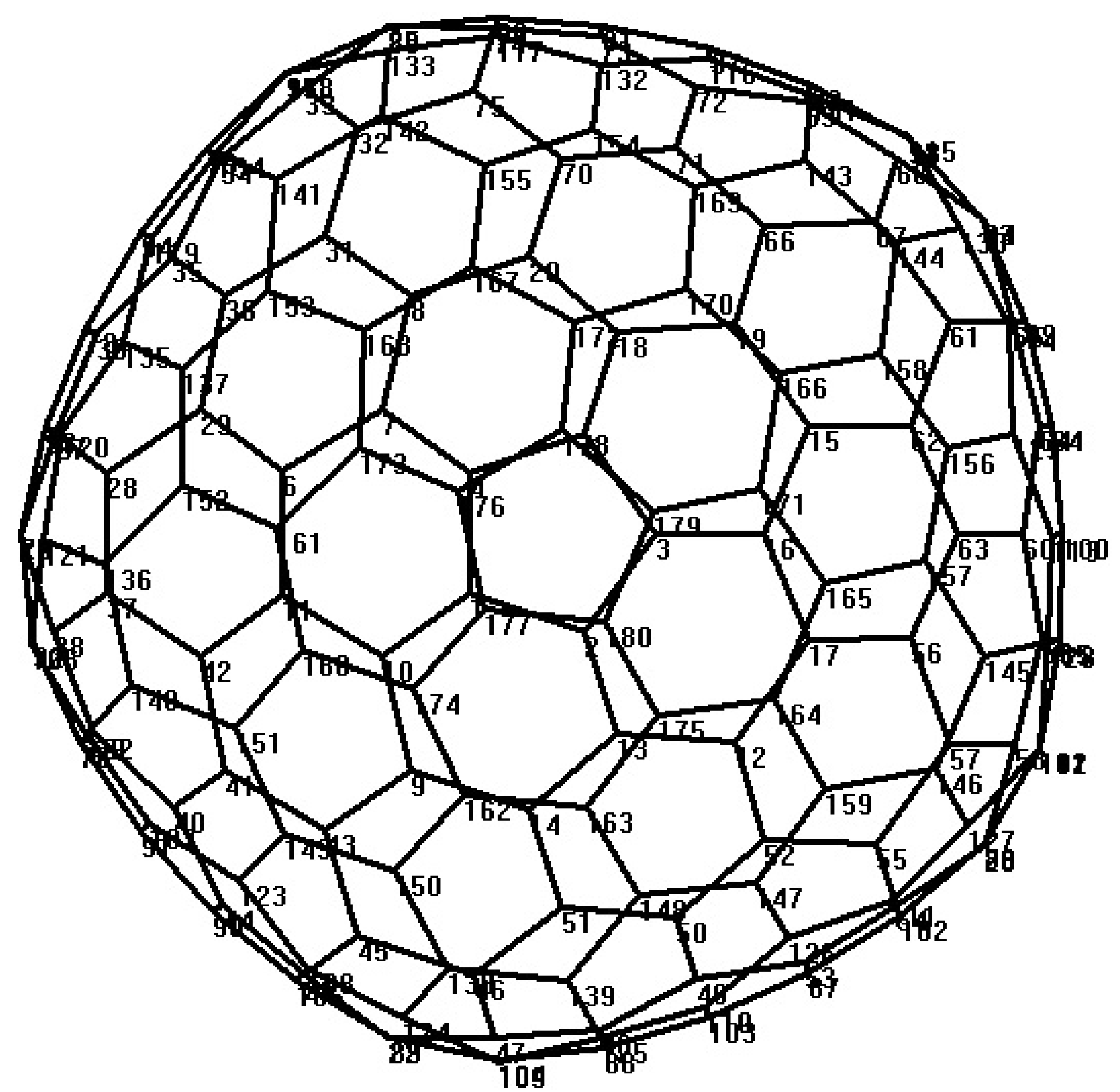
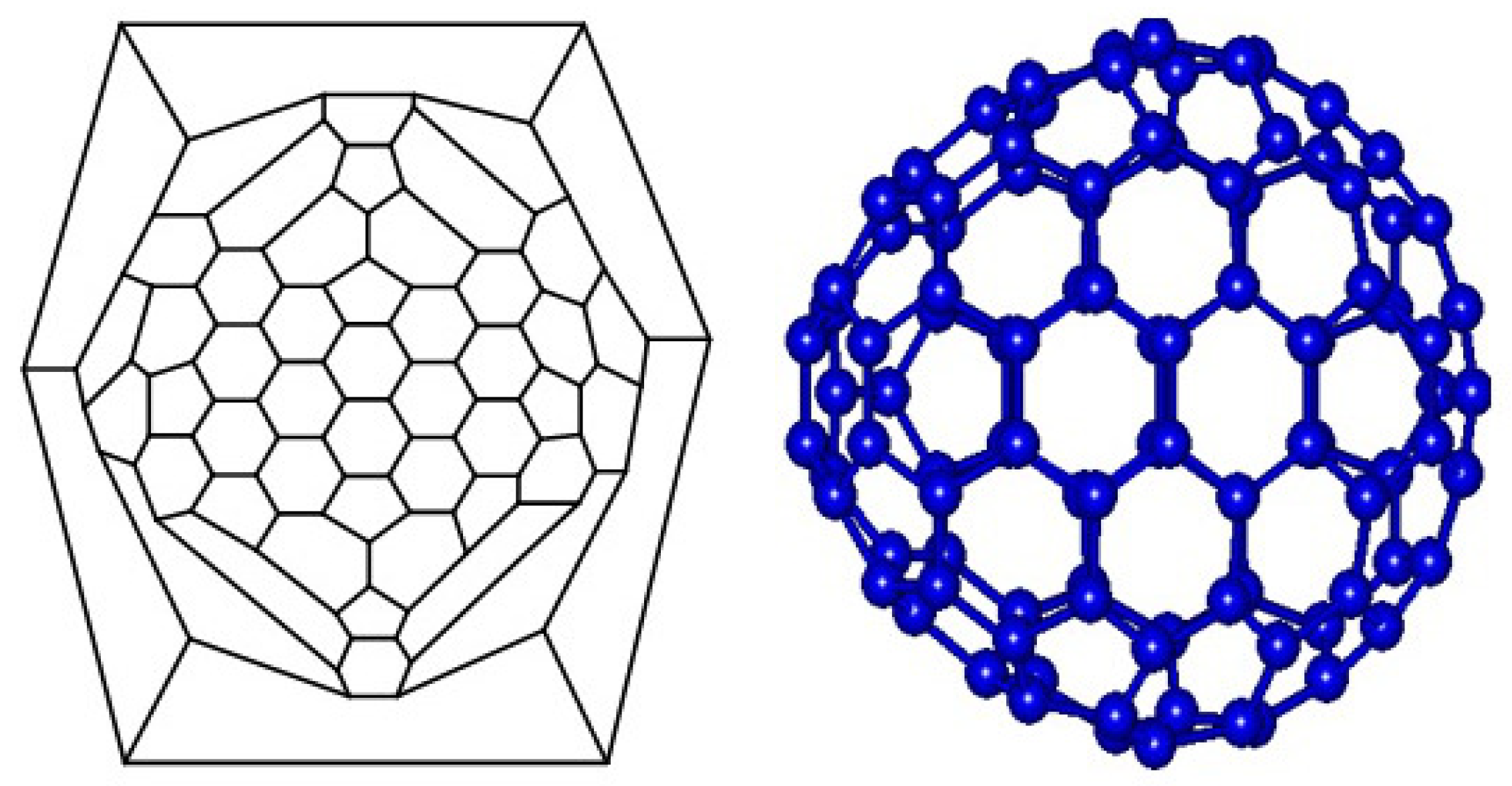
Symmetry has a significant role in the analysis of the structure, bonding and spectroscopy of molecules. A classification of molecules can be done by their symmetry. As we mentioned in the last section, about the fullerene graphs, the symmetry group and the point group are the same. The name point group is also given, because the symmetry elements such as points, lines and planes intersect at a single point. Symmetry elements include mirror planes, axes of rotation, centers of inversion and improper axes of rotation, and all of them are properties related to the structure of the molecule. All molecules have an operation which leaves the molecule where it is and we call it as identity operation. Certain physical properties of molecules are clearly linked to molecular symmetry. For example, molecules with a center of inversion or a mirror plane cannot be chiral. Let F be a fullerene with symmetry group . The orbit of a vertex is . The stabilizer of the vertex is defined as Let , then for , is denoted by . The orbit-stabilizer theorem implies that . For every , let , then we yield the following result.
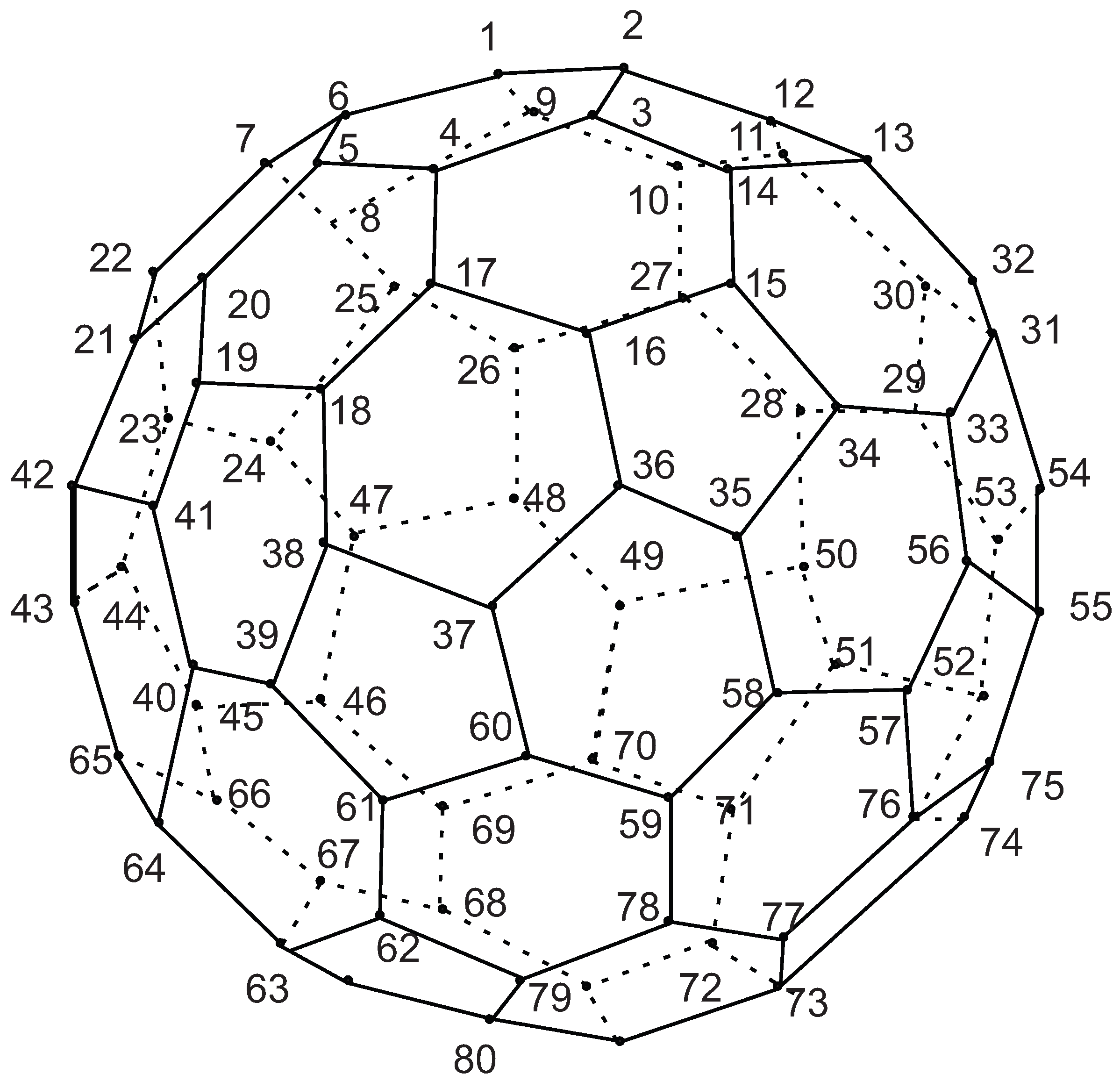
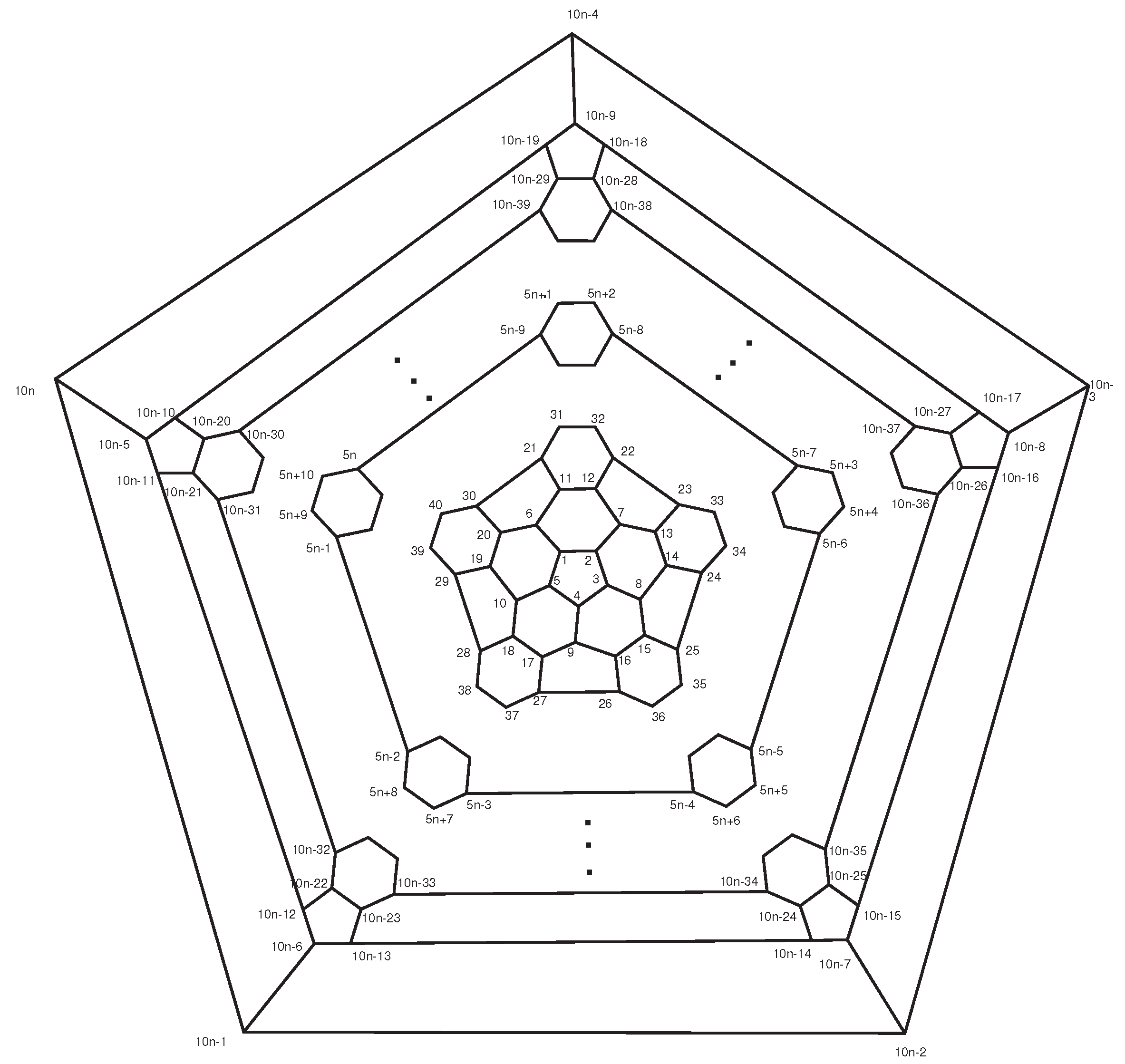
In continuing, we count all orbits of
1,…,10
n} under the action of automorphism group. Again consider the fullerene graph
, where
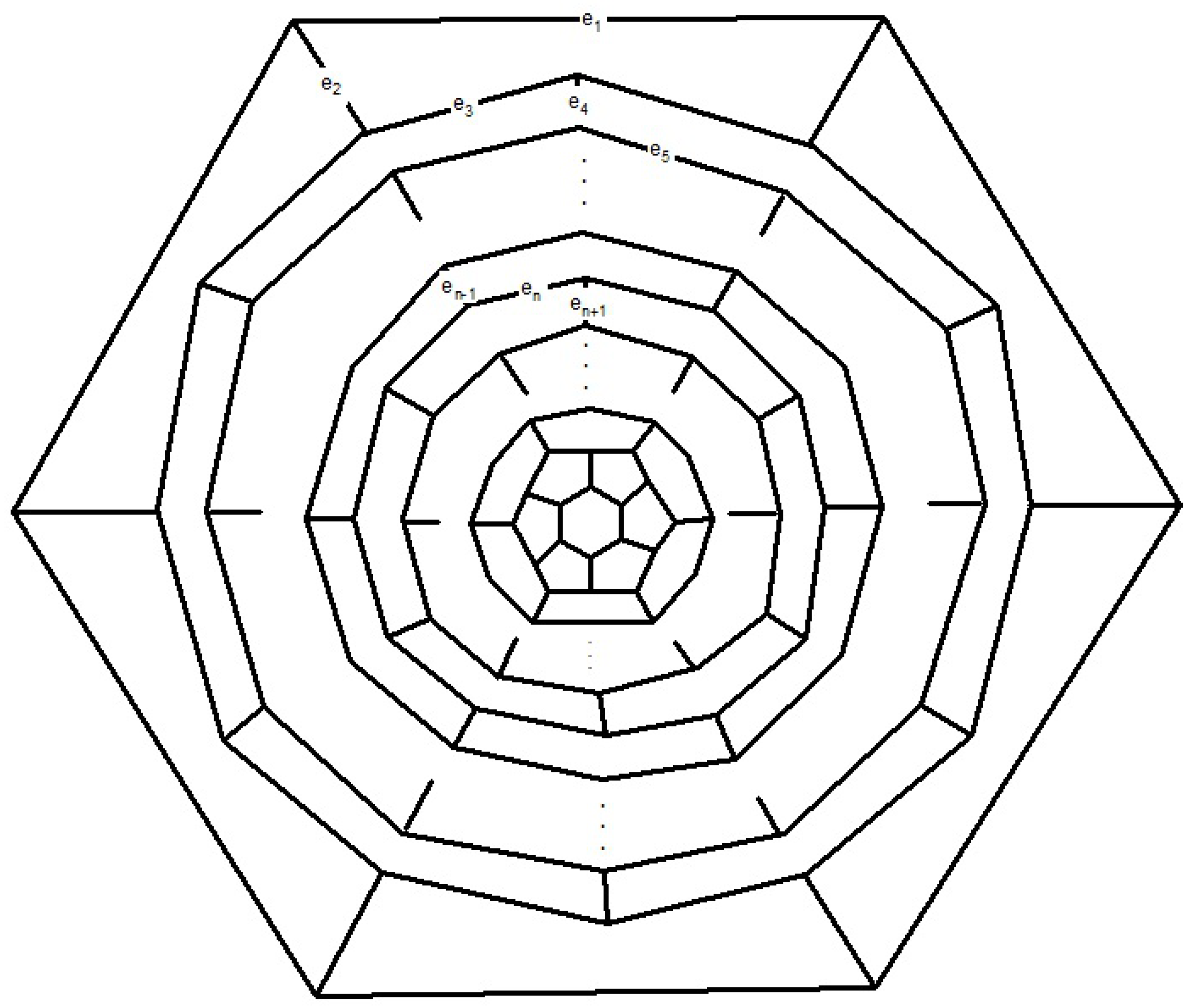
n is even, as depicted in
Figure 8. We find the presentation of elements
. It is not difficult to see that there are five symmetry elements of order 2 in
denoted by
. Clearly, we have
. In other words, one can easily check that
This means that
. Suppose
is an involution that maps
to
,
to
,
to
,
to
,
to
,
to
,
to
,
to
,
to
,
to
,
to
,
to
,
to
,
to
,
to
,
to
and so on. It is clear that fix
. By continuing this method, all permutation presentations of
’sare as follows:
includes four rotational elements
(
) and four permutations
(
) of order 10 with the following permutation presentations:
Hence, C
(
n is even) has
orbits and each of them has 10 vertices, see
Table 1.
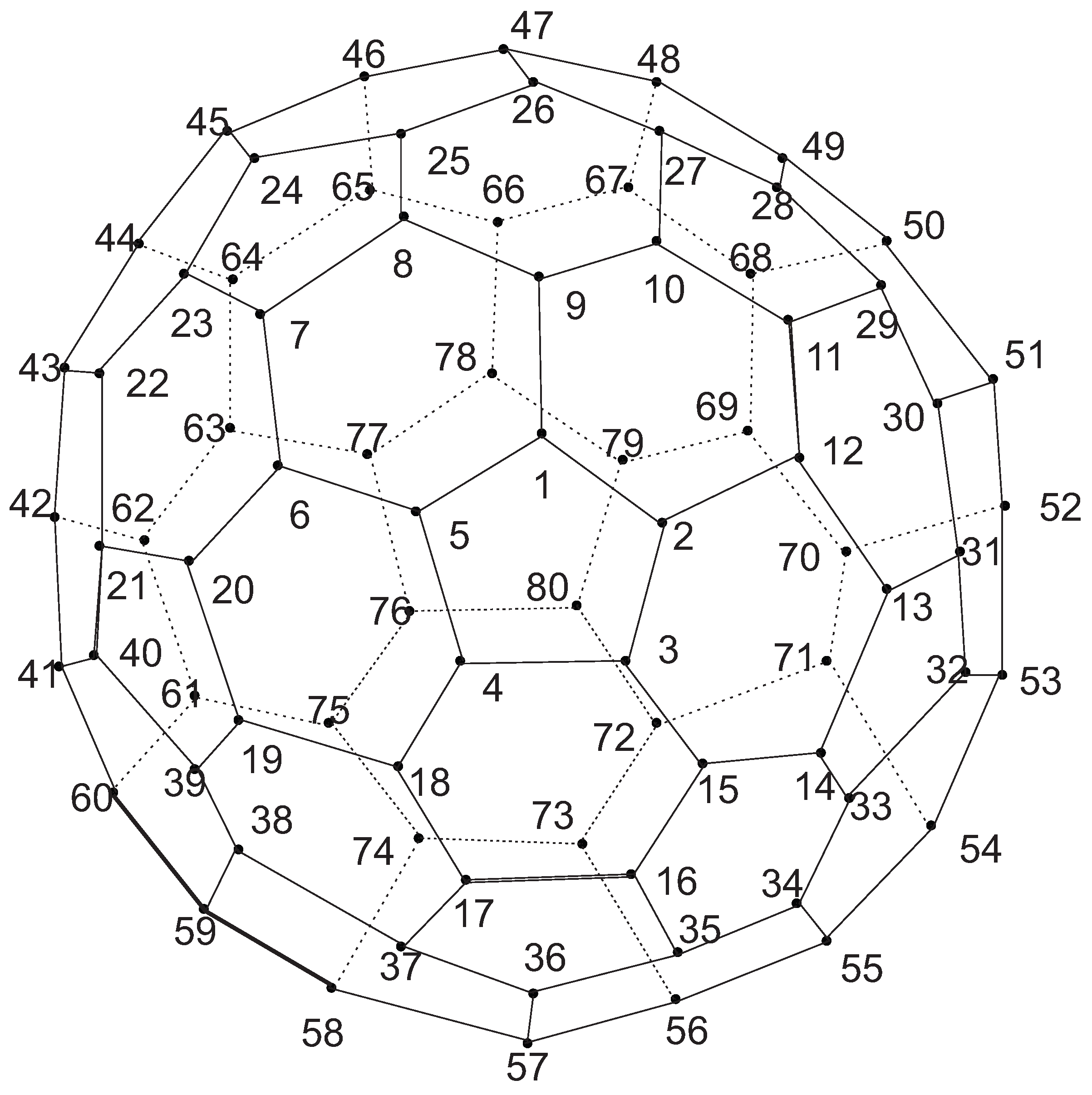
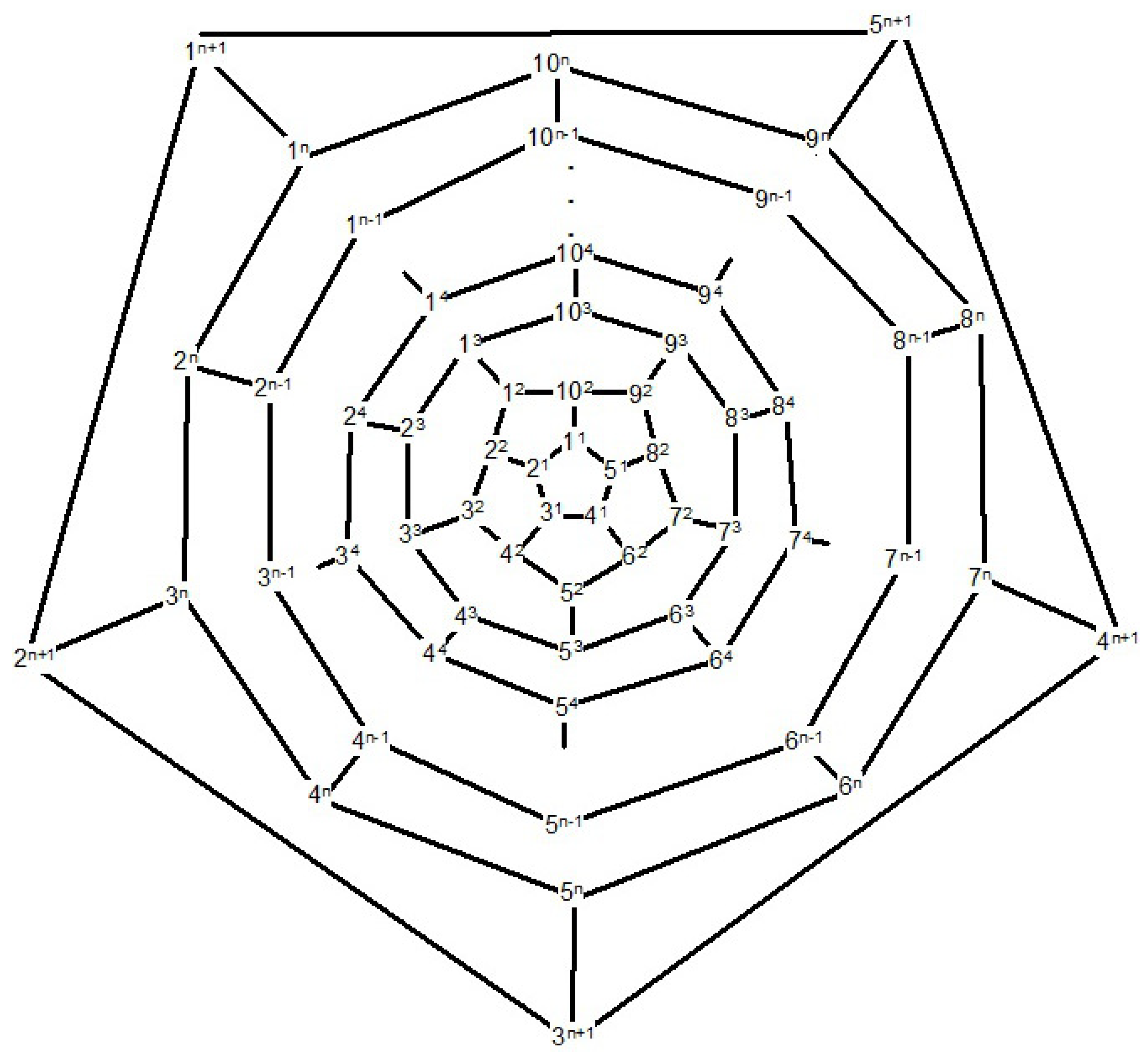
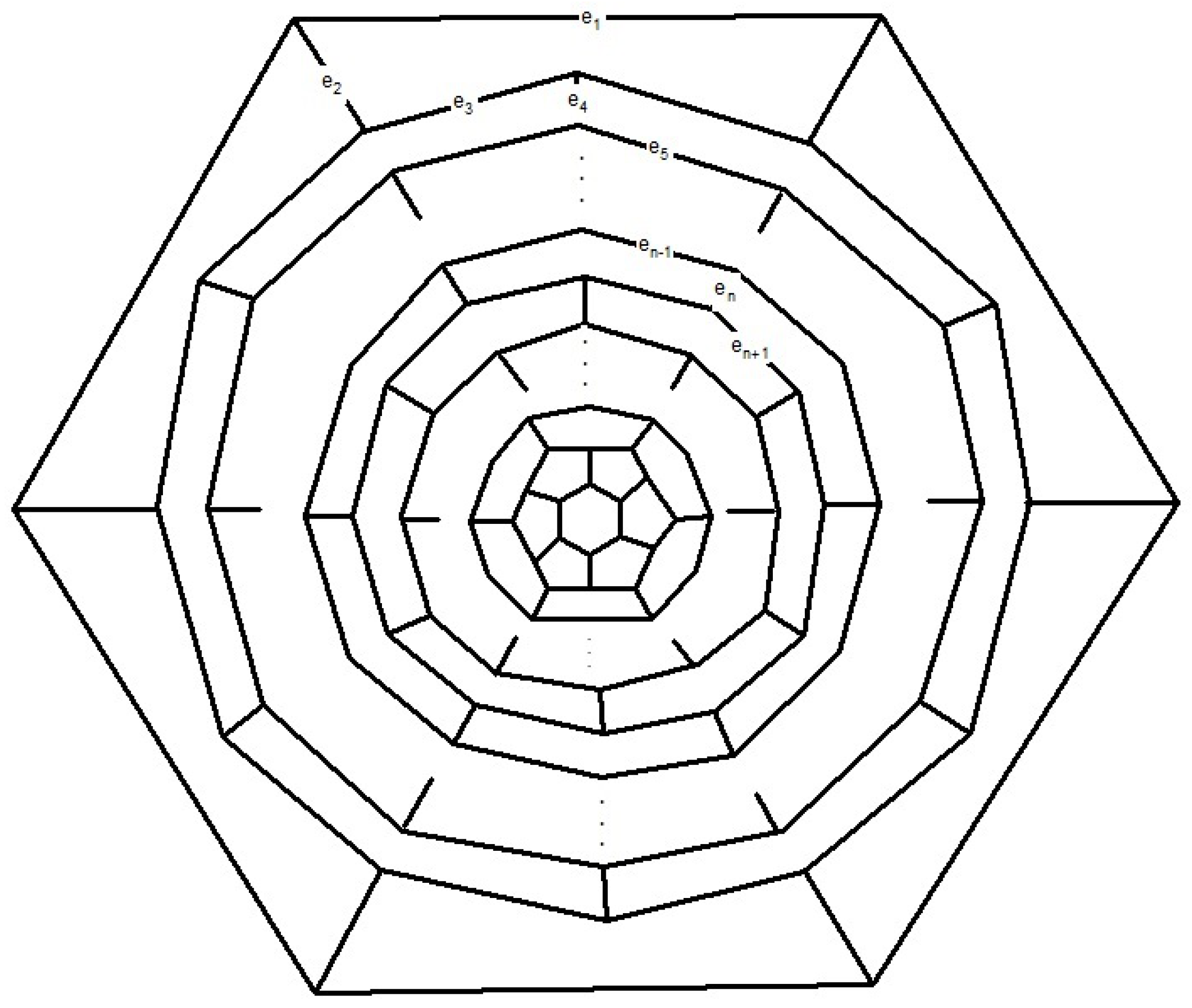
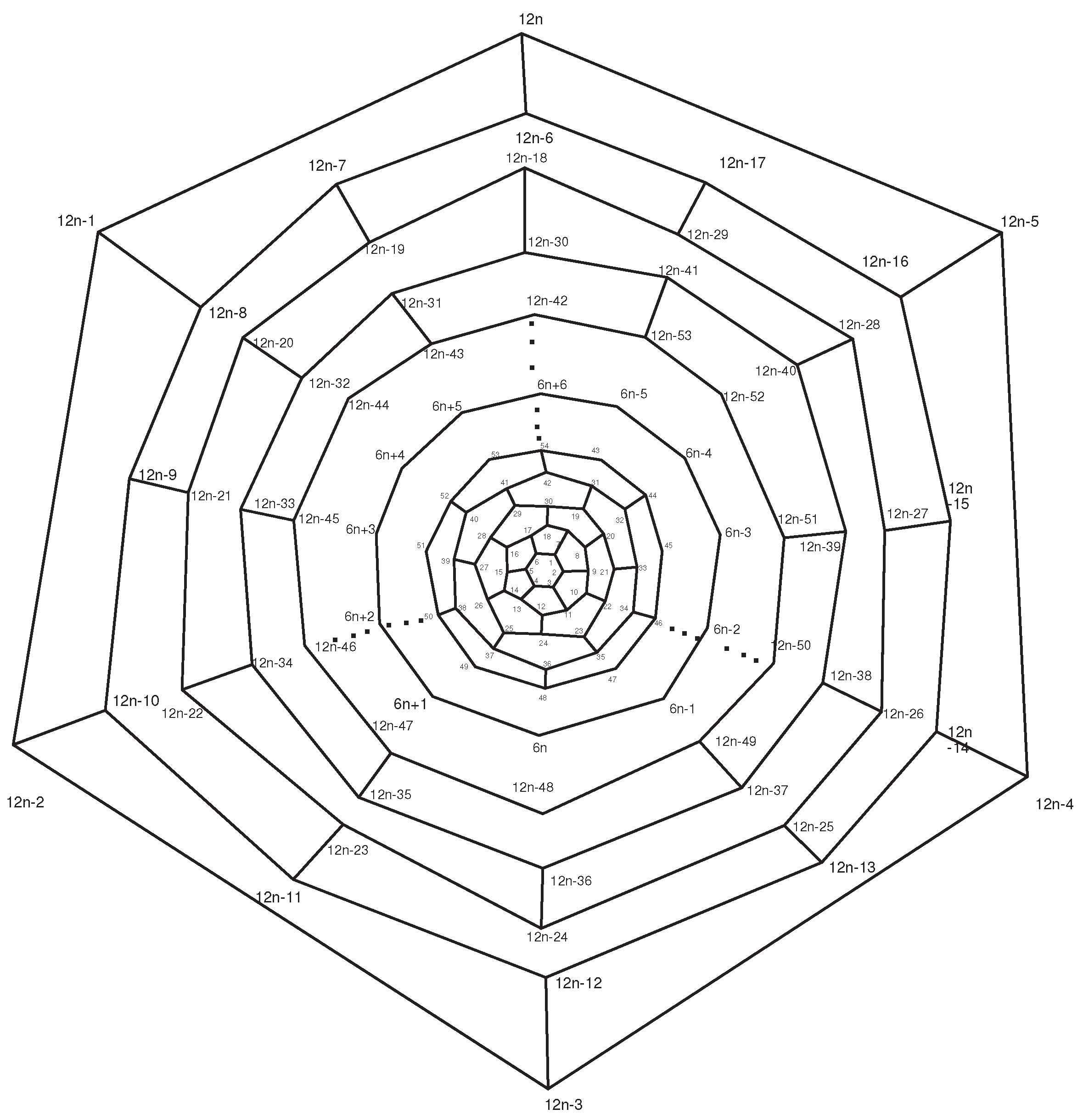
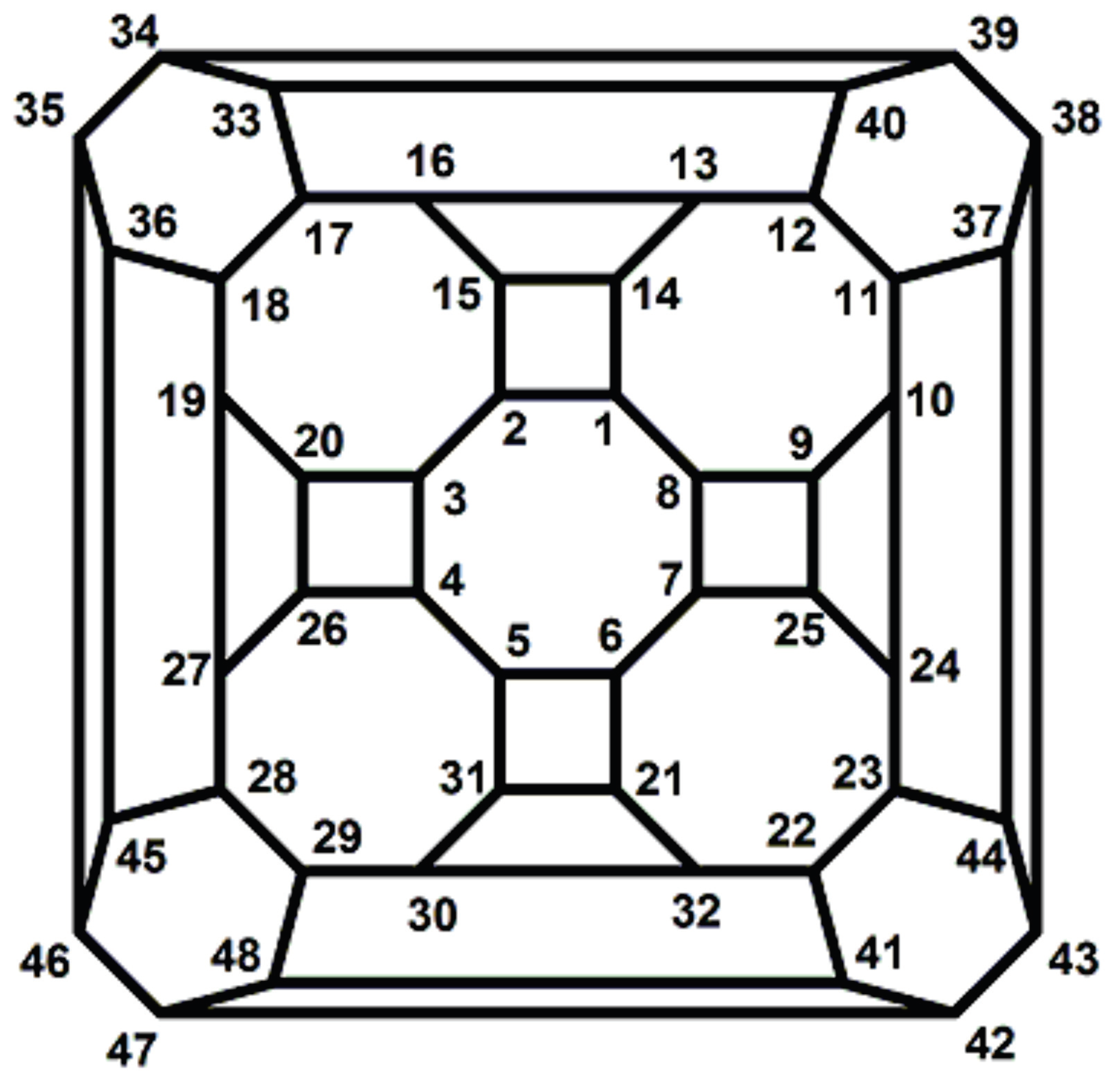
Now consider the fullerene graph
, where
n is odd, as depicted in
Figure 9. There are five symmetry elements of order 2 in
denoted by
. Clearly, we have
. In other words, one can easily check that
This means that
. Similar to the last one, the presentation of other elements of
are as follows:
So, C
(
n is odd) has
orbits which each have 10 vertices, see
Table 2.
By similar argument, we have the following theorem.
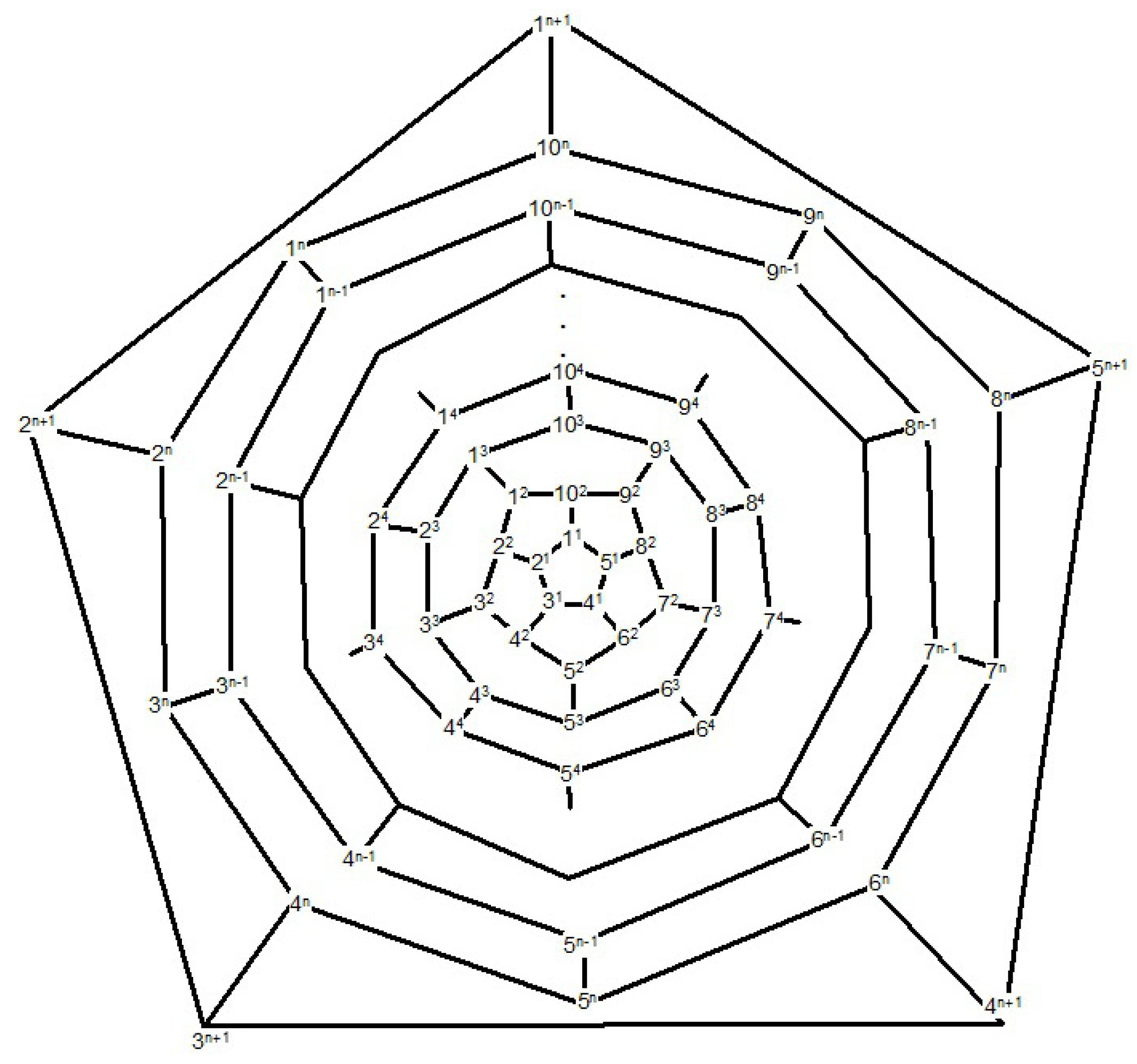
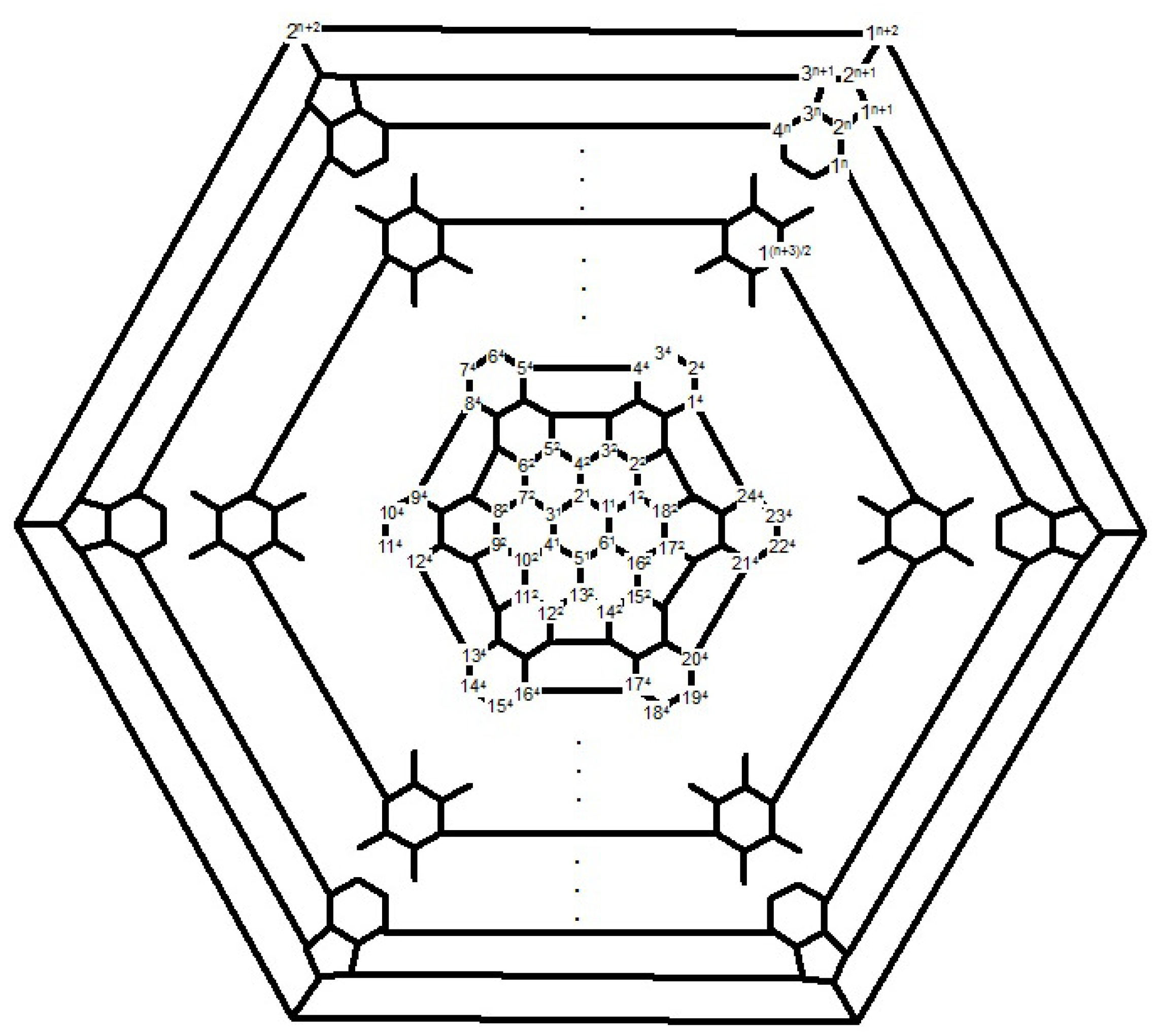
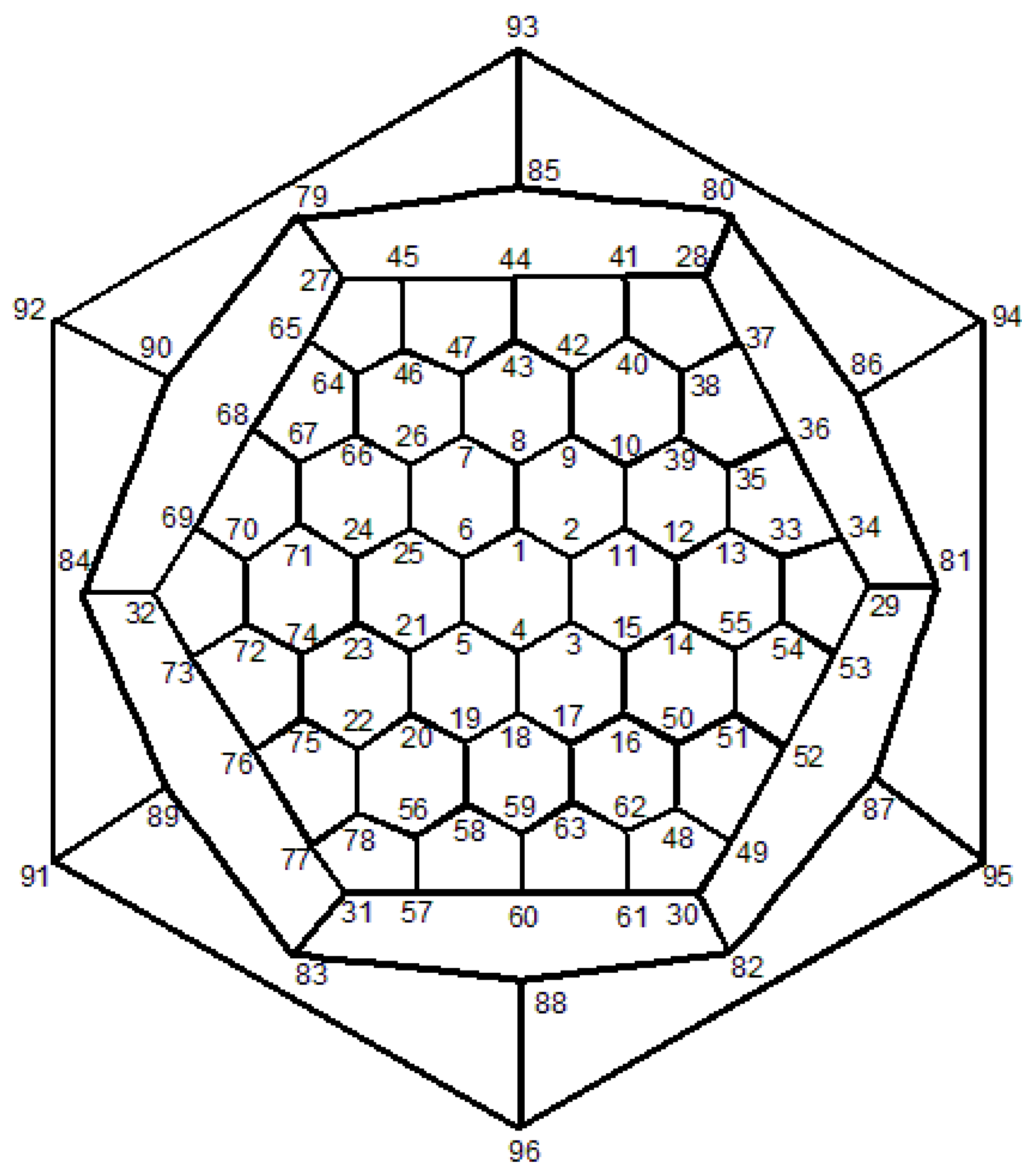
Proof. First, we compute the full automorphism group
G =
of fullerene C
, where
n is even as depicted in
Figure 12. Suppose
a is a symmetry element in which
and
b denotes the element of order 12 such that permutation presentation of
b is as follows:
b = (1, 1, 2, 2, 2, 2, 2, 2, 2, 2, 2, 2) (1, 2, 4, 5, 7, 8, 10, 11, …, 16, 17 (2, 3, 5, 6, 8, 9, 11, 12, …, 17, 18) (3, 4, 6, 7, 9, 10, 12, 13, …, 18, 1) (1, 3, 5, 7, 9, 11, 13, 15, …, 21, 23) (2, 4, 6, 8, 10, 12, 14,1 6, …, 22, 24) (3, 5, 7, 9, 11, 13, 15, 17, …, 23, 1) (4, 6, 8, 10, 12, 14, 16, 18, …, 24, 2), where (3≤i≤(n+2)/2).
Clearly,
and the orbit-stabilizer property shows that
. By considering the action of subgroup
G on the set of vertices, it can be found that any symmetry of fullerene C
which fixes two vertices
and
must also fixes the opposite vertices
and
. Applying again the orbit-stabilizer property yields
It is easy to prove that
= 2,
and
. Hence |
G| = 24. On the other hand,
and this leads us to conclude that
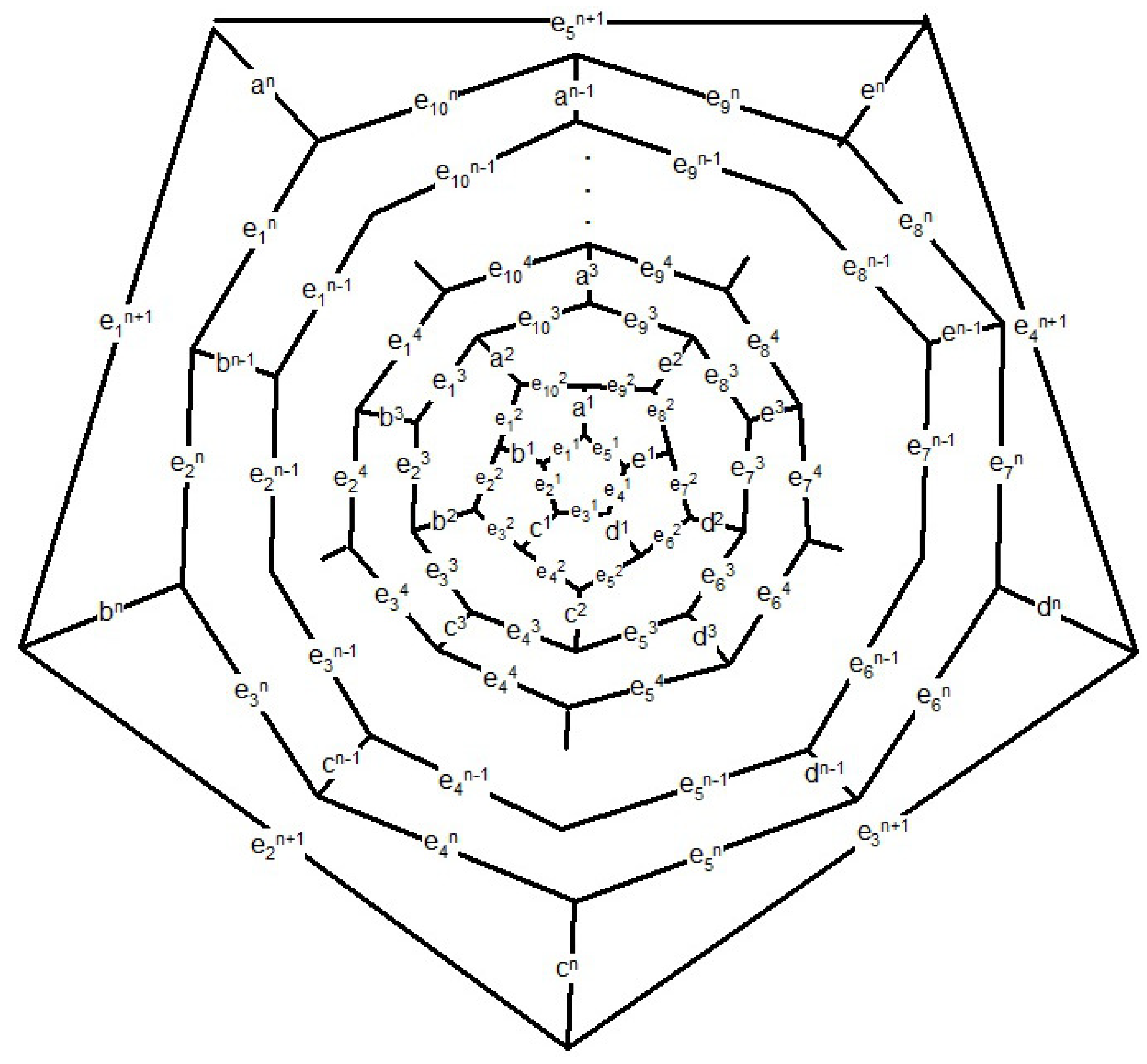
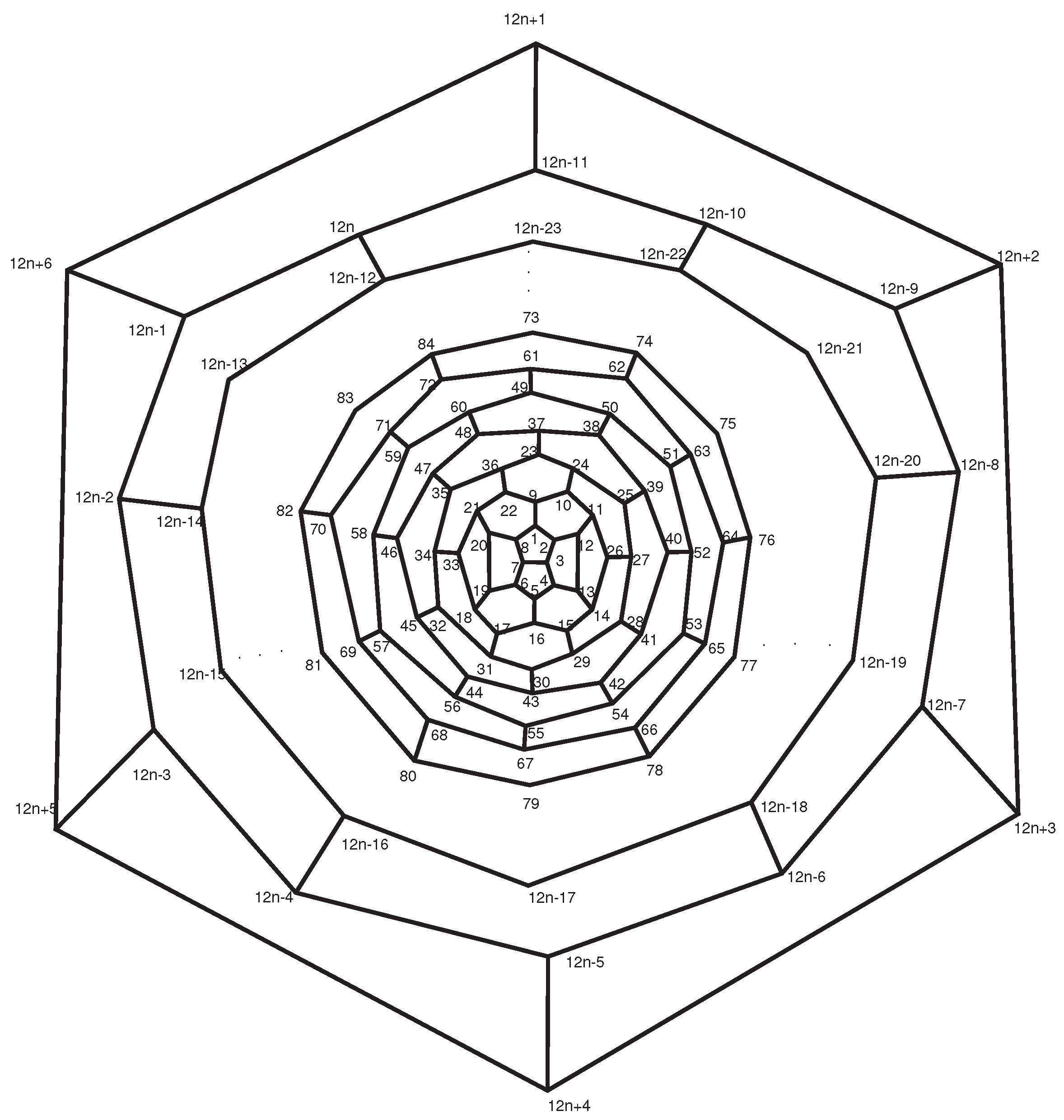
. Now suppose
n is odd (see
Figure 13),
and b = (1
, 1
, 2
, 2
, 2
, 2
, 2
, 2
, 2
, 2
, 2
, 2
) (1
, 2
, 4
, 5
, 7
, 8
, 10
, 11
, …, 16
, 17
) (2
, 3
, 5
, 6
, 8
, 9
, 11
, 12
, …, 17
, 18
) (3
, 4
, 6
, 7
, 9
, 10
, 12
, 13
, …, 18
, 1
) (1
, 3
, 5
, 7
, 9
, 11
, 13
, 15
, …., 21
, 23
) (2
, 4
, 6
, 8
, 10
, 12
, 14
, 16
, …, 22
, 24
) (3
, 5
, 7
, 9
, 11
, 13
, 15
, 17
, …, 23
, 1
) (4
, 6
, 8
, 10
, 12
, 14
, 16
, 18
, …, 24
, 2
) (1
, 3
, 5
, 7
, …, 21
, 22
) (2
, 4
, 6
, 8
,…, 22
, 24
), where (3≤i≤(n-3)/2). By similar argument
. In general, we can conclude that the symmetry elements C
have the cycle types as given in
Table 3. ☐