Abstract
We examine possible temporal variation in a measure of developmental stability, providing insight into the degree of fluctuating asymmetry of several characters of skull morphology, of the common shrew, Sorex araneus L., 1758, in Central Siberia. The level of fluctuating asymmetry during the study period in the beginning of this century (2002–2013) is not correlated with population abundance, while at the end of the last century it was correlated with population abundance, suggesting that high density was the important negative factor affecting breeding females. The absence of an adverse effect of high abundance on developmental stability in the current situation can be related to both an impact of oscillations in environmental conditions and an increase in habitat carrying capacity due to the climate change. Positive correlation of population abundance with the number of adults born last summer and young specimens born this summer indicates the influence of winter and summer conditions on population size. If in the last century developmental stability was correlated with breeding success, indicating that both parameters were affected by the physiological condition of breeding females, in this century these two parameters vary independently, suggesting that breeding success may be affected by other population and habitat factors. Thus, the situation in the population under study is more similar to the noncyclic dynamics than to the four-year cycles, which were revealed for the population in the last century. The results indicate an importance of monitoring possible changes in developmental stability measure, as another population parameter, under climate change.
1. Introduction
Population dynamics is one of the key characteristics of both the population per se and the environmental conditions [1,2,3,4]. Population fluctuations are usually observed under the changes in environmental conditions and abundance increase corresponds to the favorable habitat conditions. In this case, high population abundance is accompanied by high breeding success. Population cycle is commonly observed in the regions with the stable climate and overpopulation is assumed as a limiting factor for a population growth. In this case, peak abundance is accompanied by low breeding success [5,6,7,8]. Developmental stability, which can be estimated by fluctuating asymmetry of morphological characters, is another population parameter. In both laboratory experiments and in natural populations, deterioration of developmental stability takes place under various kinds of environmental stress [9,10,11,12]. In a cyclic population, abundance can reach such a high level that it may adversely affect developmental stability, while population fluctuations are not accompanied by such essential changes in developmental stability [13,14].
Collapses of cyclic dynamics are registered under climate change. Climate stability, including long cold winter and stable high snow cover during the wintertime, is usually considered as an ultimate condition for this type of dynamics, while instability, namely, proves to be a common character of the current climate [5]. Special consideration of possible interaction of developmental stability measure with abundance becomes a challenging task for the population research.
The aim of this study is to assess the developmental stability of the common shrew, Sorex araneus, in Central Siberia (Middle Yenisei taiga) under climate change conditions. High amplitude four-year cycle dynamics were previously observed in the population and developmental stability of the offspring of socially stressed females was impaired in the years of high population abundance [14,15,16]. The working hypothesis is that there is not a significant correlation between developmental stability and population abundance in the population under climate change. Our prediction is that population fluctuations as a result of the oscillations in environmental conditions do not have an adverse effect on developmental stability.
2. Material and Methods
We examined material from the common shrew, Sorex araneus L., 1758, collected in the periods of 2002–2004 and 2007–2013 in Central Siberia (the eastern bank of the river Yenisei, the Yenisei Ecological Station of the Institute of Ecology and Evolution, Russian Academy of Sciences; 62° N, 89° E). The skull collection of the Zoological Museum of Moscow State University was used for the study. The number of individuals studied was 200. Skulls were cleaned enzymatically with papain. Shrews were trapped with the same trap lines annually in August. Each trap line consisted of 20 m dich with two large pitfalls with an alcohol solution at 5 m from the two ends (the study sites and trapping techniques were described in detail in [15,16]).
Two population indices were used in an analysis, including population abundance and breeding success index. The number of animals per 100 trap-day was determined as an indicator of population abundance. We also calculated separately the abundance of adults born last summer and young specimens born this summer. The breeding success index was calculated as the ratio between the number of young individuals born this summer and the number of breeding females.
Developmental stability was estimated by the value of fluctuating asymmetry (as minor deviations from perfect symmetry of morphological characters) [9,10]. Ten morphological characters (as a number of foramina in different parts of the skull) were used [13]. We first calculated the difference between the left and right side in the number of foramina. We did not reveal a significant correlation between the asymmetry of different characters as well as any evidence of directional asymmetry and antisymmetry for their variation [17,18]. Then, we calculated the number of asymmetrical characters per individual. The average frequency of asymmetric manifestations per character was used as an integrated index of developmental stability [14]. Young individuals born this summer were used for the study (sex ratio was 1:1 with an absence of the sex differences for the studied parameters).
3. Results
In the studied period of 2002–2004 there was a sharp increase in population abundance in 2003, with relatively low numbers in 2002 and 2004 (Figure 1). The population dynamics during this time mostly correspond to fluctuations, rather than to the four-year cycle that was common for this population earlier [16]. The breeding success in a year of high abundance, 2003, turned out to be not lower than in years of low abundance and on the contrary was even higher than in 2002 and 2004, which was previously expected precisely for the population fluctuations due to environmental oscillations [11,12,14]. The index of developmental stability did not reveal any significance between year variation, in spite of the high amplitude fluctuations in population abundance, while earlier for this population there was a developmental stability decrease (fluctuating asymmetry increase) at the peak year. In the study period of 2007–2013, the relationship between the dynamics of three main studied indices, including population abundance, breeding success, and developmental stability, proved to be essentially different than in the last century (Figure 1).
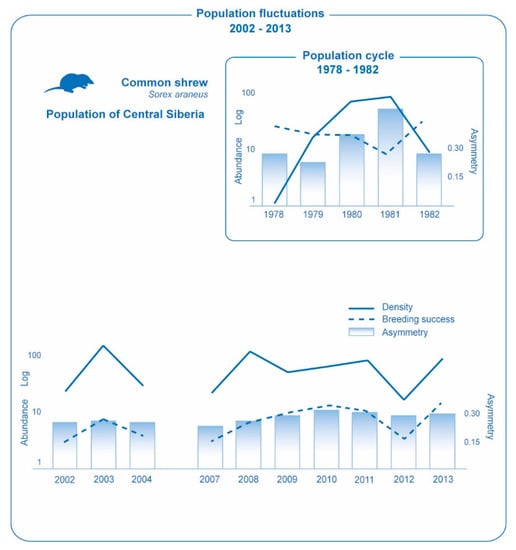
Figure 1.
Values of population indices in a common shrew population in Central Siberia in two studied periods: 1978–1982 [14] and 2002–2013 (original data). Abundance: number of animals per 100 trap-day. Breeding success: ratio between the number of young individuals born this summer and the number of breeding females. Asymmetry: average frequency of asymmetric manifestation per character (for 10 scull characters, number of foramina).
While earlier for this population a negative correlation was observed between the abundance and breeding success, now there is indication for a positive correlation between these parameters (r = 0.63, p < 0.05). There are also indications for a positive correlation of population abundance with both the number of young individuals born this summer (r = 0.95, p <0.05) and with a number of adults born last summer (r = 0.94, p < 0.05). The level of developmental stability (fluctuating asymmetry index) is not correlated with abundance and breeding success, and it proved to be rather stable during the study time (p < 0.1). We can only note that the maximum index value in 2010 (which significantly differs from the minimal value in 2007, p < 0.05) corresponds to the coldest summer (mean summer temperature in this year is 1.6 °C lower than in other years under study) [19]. Thus, all data obtained correspond to the situation described for the population fluctuations where changes in abundance are presumably caused by the influence of external conditions [12,14].
4. Discussion
Among the whole variety of different forms of population dynamics [3,5,20], for this consideration it is crucial to distinguish two basic types. The first type corresponds to population fluctuations and population growth indicates better environmental conditions. The second type corresponds to the population cycle under rather stable conditions where limiting factor is an adverse effect of overpopulation upon reaching a certain threshold level, which leads to a population decline. The difference between these two types of dynamics is that if in the first case high number corresponds to high breeding success and developmental stability, then in the second case peak abundance is accompanied by low breeding success and developmental stability [13,14,21].
The first type of dynamics is common for populations inhabiting the areas of unstable climate and low snow cover, for example, for the coastal populations in southern Finland [12,22]. The second type of dynamics is common for the continental populations of a stable climate and high snow cover, for example, in Finnish Lapland [23]. For these populations, even relatively small alteration in climate can lead to significant consequences, including the collapse of cycles and the transition of the population dynamics from the second type to the first one [5].
A long-term study conducted in Central Siberia in the last century (from 1972 to 1994) showed a four-year cycle of most species of small mammal communities [16,20]. A population growth over three years was replaced by density decline. Long winter with stable conditions and snow cover was crucial for a winter survival [5]. The assumption that overpopulation may lead to population decline was confirmed by a negative correlation of population abundance with breeding success and developmental stability [14]. Developmental stability decrease as a result of overpopulation impact was demonstrated in both natural populations and in laboratory experiments [13,21]. Thus, the peak year in a population of this type of dynamics was characterized by low breeding success and a deterioration of developmental stability [14].
Changes in population abundance revealed in the recurring study of the population in this century (from 2002 to 2013) correspond more to population fluctuations as a result of oscillations in environmental conditions than to the population cycles [11,12,16,22]. Indication for a positive correlation of abundance with breeding success supposes the suggestion. Positive relation of the population number with the abundance of adults born last summer and young specimens born this summer is also indicative of the influence of environmental conditions both in winter and in summer.
At the same time, there is no evidence for an adverse impact of high abundance on the studied population parameters that were assumed to be the main cause for the subsequent population decrease in the case of the cyclic dynamics. It means that abundance does not reach the certain threshold level for such an impact. Shorter winter and an increased frequency of the “melting–freezing events” become crucial factors for a population regulation [5,24,25,26]. Another reason for the absence of such an effect can be an increase in the richness and carrying capacity of the habitats, due to the temperature increase, manifested in an increase in the productivity of ecosystems and vegetation period in the study area, in Central Siberia [19]. These results correspond to the data obtained for the population fluctuations in southern Finland, where developmental stability is not correlated with abundance. In spite of the fact of the possible adverse impact of the habitat conditions on developmental stability [11,12,18,27,28], some environmental difference between summers during the study period do not reach a critical level to affect developmental stability, there is only some indication for an adverse impact of a cold summer in 2010.
Moreover, in this study, we do not reveal correlated changes in developmental stability and breeding success. It suggests that these two indices can give different characteristics of the population and vary independently. If previously the change in the physiological condition of the breeding females, impacted by overpopulation, influenced both breeding success and developmental stability of the offspring, then now the breeding success mainly depends on some other population and habitat parameters and may vary without essential changes in developmental stability.
Similar population dynamics revealed for both banks of the river Yenisei [29], which are isolated from each other by the water stream of 1.7 km width, also support the assumption of the primary importance of environmental conditions.
Certain changes in the small mammal community were also identified in the study area. An increase in the proportion of the more southern species, the common shrew, S. araneus, is accompanied by a decrease in the proportion of boreal species, the Laxmann’s shrew, S. caecutiens Laxmann, 1788, and the tundra shrew, S. tundrensis Merriam, 1900 [29,30]. Changing community can be another factor affecting the population dynamics of individual species.
A number of studies revealed an alteration from the previous usual population cycle dynamics for different regions, including Central Sweden [31], northern Fennoscandia [5], northern Canada [32], Great Britain [33], Japan on the island of Hokkaido [34], western foothills of the Urals [35], Yakutia [36]. Climate change—climate instability, in particular—was assumed to be the main cause for the alteration revealed. The conclusion was supported through mathematical simulation [32,37]. Some indications for a possible return to cyclic dynamics [38,39] evidenced the necessity of a long-term study as a challenging task for the future research.
Thus, the results indicate the importance of monitoring possible changes in natural population due to climate change. Simultaneous study of various population parameters, including population abundance and developmental stability, may provide certain information supposing possible underlying mechanisms of changes occur.
Author Contributions
The article was prepared at all stages, from the beginning to the end, including the reaction to the reviewers’ comments and suggestions, in collaboration of all three authors V.M.Z., I.E.T. and B.I.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the government program of basic research in Koltzov Institute of Developmental Biology of the Russian Academy of Sciences in 2020 No. 0108–2019–0007.
Acknowledgments
We thank members of the laboratory of postnatal ontogenesis of the Koltzov Institute of Developmental Biology of the Russian Academy of Sciences S.G. Dmitriev, F.N. Shkil, N.P. Zhdanova for a fruitful cooperation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hansson, L.; Henttonen, H. Rodent dynamics as community processes. Trends Ecol. Evol. 1988, 3, 195–200. [Google Scholar] [CrossRef]
- Kendall, B.E.; Briggs, C.J.; Murdoch, W.W.; Turchin, P.; Ellner, S.P.; McCauley, E.; Nisbet, R.M.; Wood Simon, N. Why do populations cycle? A synthesis of statistical and mechanistic modeling approaches. Ecology 1999, 80, 1789–1805. [Google Scholar] [CrossRef]
- Henttonen, H.; Wallgren, H. Small rodent dynamics and communities in the birch forest zone of northern Fennoscandia. In Nordic Mountain Birch Ecosystem; Wielgolaski, F.E., Ed.; UNESCO Man and Biosphere Series 27; Paris and Parthenon Publishing Group: New York, NY, USA; London, UK, 2001; pp. 261–278. [Google Scholar]
- Ims, R.A.; Fuglei, E. Trophic Interaction Cycles in Tundra Ecosystems and the Impact of Climate Change. BioScience 2005, 55, 311–322. [Google Scholar] [CrossRef]
- Ims, R.A.; Henden, J.-A.; Killengreen, S.T. Collapsing population cycles. Trends Ecol. Evol. 2008, 23, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Pinot, A.; Gauffre, B.; Bretagnolle, V. The interplay between seasonality and density: Consequences for female breeding decisions in a small cyclic herbivore. BMC Ecol. 2014, 14, 17. [Google Scholar] [CrossRef]
- Zárybnická, M.; Riegert, J.; Bejček, V.; Sedláček, F.; Šťastný, K.; Šindelář, J.; Heroldová, M.; Vilímová, J.; Zima, J. Long-term changes of small mammal communities in heterogenous landscapes of Central Europe. Eur. J. Wildl. Res. 2017, 63, 89. [Google Scholar] [CrossRef]
- Giraudoux, P.; Villette, P.; Quéré, J.P.; Damange, J.P.; Delattre, P. Weather influences M. arvalis reproduction but not population dynamics in a 17-year time series. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Mather, K. Genetical control of stability in development. Heredity 1953, 7, 297–336. [Google Scholar] [CrossRef]
- Soule, M.E. Phenetics of Natural Populations. II. Asymmetry and Evolution in a Lizard. Am. Nat. 1967, 101, 141–160. [Google Scholar] [CrossRef]
- Pankakoski, E. Epigenetic asymmetry as an ecological indicator in muskrats. J. Mammal. 1985, 66, 52–57. [Google Scholar] [CrossRef]
- Pankakoski, E. Relationship between some meteorological factors and population dynamics of Sorex araneus in southern Finland. Acta Zool. Fenn. 1985, 173, 287–289. [Google Scholar]
- Zakharov, V.M.; Yablokov, A.V. (Eds.) Developmental homeostasis in natural populations of mammals: Phenetic approach. Acta Theriol. 1997, 4, 92. [Google Scholar]
- Zakharov, V.M.; Pankakoski, E.; Sheftel, B.I.; Peltonen, A.; Hanski, I. Developmental stability and population dynamics in the common shrew Sorex Araneus. Am. Nat. 1991, 138, 797–810. [Google Scholar] [CrossRef]
- Sheftel, B.I. Zonal features of the population of insectivorous mammals of the Yenisei taiga and forest-tundra. In Fauna of the Yenisei Taiga and Forest-Tundra and Natural Zoning; Science: Moscow, Russia, 1983; pp. 184–203. (In Russian) [Google Scholar]
- Sheftel, B.I. Long-term and seasonal dynamics of shrews in Central Siberia. Ann. Zool. Fenn. 1989, 26, 357–369. [Google Scholar]
- Palmer, A.R.; Strobeck, C. Fluctuating asymmetry: Measurement, Analysis, Patterns. Annu. Rev. Ecol. Syst. 1986, 17, 391–421. [Google Scholar] [CrossRef]
- Zakharov, V.M.; Shadrina, E.G.; Trofimov, I.E. Fluctuating Asymmetry, Developmental Noise and Developmental Stability: Future Prospects for the Population Developmental Biology Approach. Symmetry 2020, 12, 1376. [Google Scholar] [CrossRef]
- Frolov, A.V. (Ed.) Second Roshydromet Assessment Report on Climate Change and Its Consequences in Russian Federation. General Summary; Roshydromet: Moscow, Russia, 2014; 56p. [Google Scholar]
- Sheftel, B.I. Role of different mechanisms in type determination of population dynamics for small mammals from boreal forestry zone. In Biological Diversity and Nature Conservation: Theory and Practice for Teaching; KMK: Moscow, Russia, 2010; pp. 107–117. [Google Scholar]
- Zakharov, V.M.; Zhdanova, N.P.; Kirik, E.F.; Shkil’, F.N. Ontogenesis and Population: Evaluation of Developmental Stability in Natural Populations. Russ. J. Dev. Biol. 2001, 32, 336–351. [Google Scholar] [CrossRef]
- Solonen, T. Overwinter population change of small mammals in southern Finland. Ann. Zool. Fenn. 2006, 43, 295–302. [Google Scholar]
- Hansson, L.; Henttonen, H. Gradients in density variations of small rodents: The importance of latitude and snow cover. Oecologia 1985, 67, 394–402. [Google Scholar] [CrossRef]
- Aars, J.; Ims, R.A. Intrinsic and climatic determinants of population demography: The winter dynamics of tundra voles. Ecology 2002, 83, 3449–3456. [Google Scholar] [CrossRef]
- Putkonen, J.; Roe, G. Rain-on-snow events impact soil temperatures and affect ungulate survival. Geophys. Res. Lett. 2003, 30. [Google Scholar] [CrossRef]
- Korslund, L.; Steen, H. Small rodent winter survival: Snow conditions limit access to food resources. J. Anim. Ecol. 2006, 75, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Badyaev, A.V.; Foresman, K.R.; Fernandes, M.V. Rapid environmental change and developmental stability. Vegetation removal causes increased fluctuating asymmetry in a free-living shrew population. Ecology 2000, 81, 336–345. [Google Scholar] [CrossRef]
- Wojcik, J.M.; Polly, P.D.; Wojcik, A.M.; Sikorski, M.D. Epigenetic variation of the common shrew, Sorex araneus, in different habitats. Russ. J. 2007, 6, 43–49. [Google Scholar] [CrossRef]
- Sheftel, B.I. Changes in the species composition in the community of shrews (Soricidae) of the Middle Yenisei taiga. In Theriofauna of Russia and Adjacent Territories, Proceedings of the International Meeting X Congress of the Theriological Society at the Russian Academy of Sciences, Moscow, Russia, 1–5 February 2016; KMK: Moscow, Russia, 2016; p. 460. [Google Scholar]
- Zakharov, V.M.; Sheftel, B.I.; Dmitriev, S.G. Climate change and population dynamics: Possible consequences (with particular references to study of small mammals in Central Siberia). Uspekhi Sovrem. Biol. 2011, 131, 435–439. [Google Scholar]
- Hörnfeldt, B. Long-term decline in numbers of cyclic voles in boreal Sweden: Analysis and presentation of hypotheses. Oikos 2004, 107, 376–392. [Google Scholar] [CrossRef]
- Berteaux, D.; Humphries, M.M.; Krebs, C.J.; Lima, M.; McAdam, A.G.; Pettorelli, N.; Reale, D.; Saitoh, T.; Tkadlec, E.; Weladji, R.B.; et al. Constraints to projecting the effects of climate change on mammals. Clim. Res. 2006, 32, 151–158. [Google Scholar] [CrossRef]
- Bierman, S.M.; Fairbairn, J.P.; Petty, S.J.; Elston, D.A.; Tidhar, D.; Lambin, X. Changes over time in the spatiotemporal dynamics of cyclic populations of field voles (Microtus agrestis L.). Am. Nat. 2006, 167, 583–590. [Google Scholar] [CrossRef]
- Saitoh, T.; Cazelles, B.; Vik, J.O.; Viljugrein, H.; Stenseth, N.C. Effects of regime shifts on the population dynamics of the grey-sided vole in Hokkaido, Japan. Clim. Res. 2006, 32, 109–118. [Google Scholar] [CrossRef]
- Bobretsov, A.V. Population Ecology of Small Mammals of Plain and Mountain Landscapes in the North-East of the European Part of Russia (Populyatsionnaya Ekologiya Melkikh Mlekopitayushchikh Ravninnykh i Gornykh Landshaftov Severo-Vostoka Evropeyskoy Chasti Rossii); KMK: Moscow, Russia, 2016; 381p. (In Russian) [Google Scholar]
- Safronov, V.M. Climate change and mammals of Yakutia. Biol. Bull. Russ. Acad. Sci. 2016, 43, 1256–1270. [Google Scholar] [CrossRef]
- Mertens, S.K.; Yearsley, J.M.; van den Bosch, F.; Gilligan, C.A. Transient population dynamics in periodic matrix models: Methodology and effects of cyclic permutations. Ecology 2006, 87, 2338–2348. [Google Scholar] [CrossRef]
- Brommer, J.E.; Pietiäinen, H.; Ahola, K.; Karell, P.; Karstinen, T.; Kolunen, H. The return of the vole cycle in southern Finland refutes the generality of the loss of cycles through ‘climatic forcing’. Glob. Chang. Biol. 2010, 16, 577–586. [Google Scholar] [CrossRef]
- Korpela, K.; Delgado, M.; Henttonen, H.; Korpimäki, E.; Koskela, E.; Ovaskainen, O.; Pietiäinen, H.; Sundell, J.; Yoccoz, N.G.; Huitu, O. Nonlinear effects of climate on boreal rodent dynamics: Mild winters do not negate high-amplitude cycles. Glob. Chang. Biol. 2013, 19, 697–710. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).