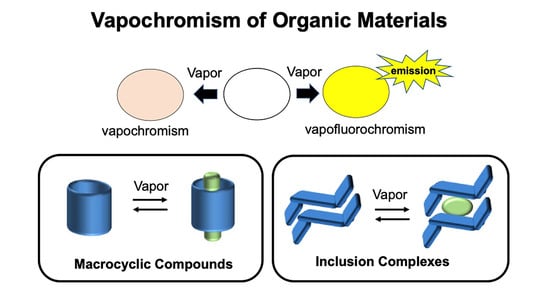
Vapochromism of Organic Crystals Based on Macrocyclic Compounds and Inclusion Complexes
Abstract
1. Introduction
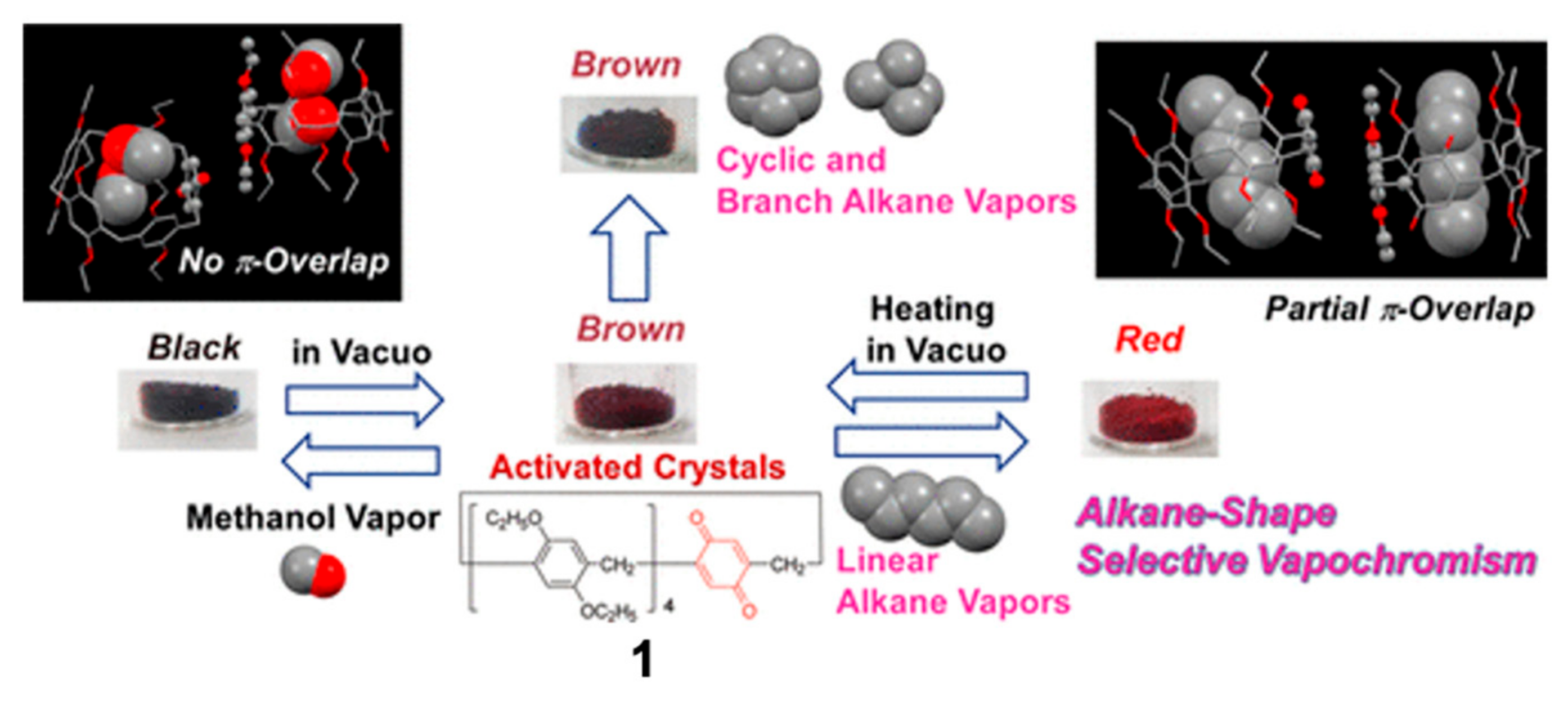
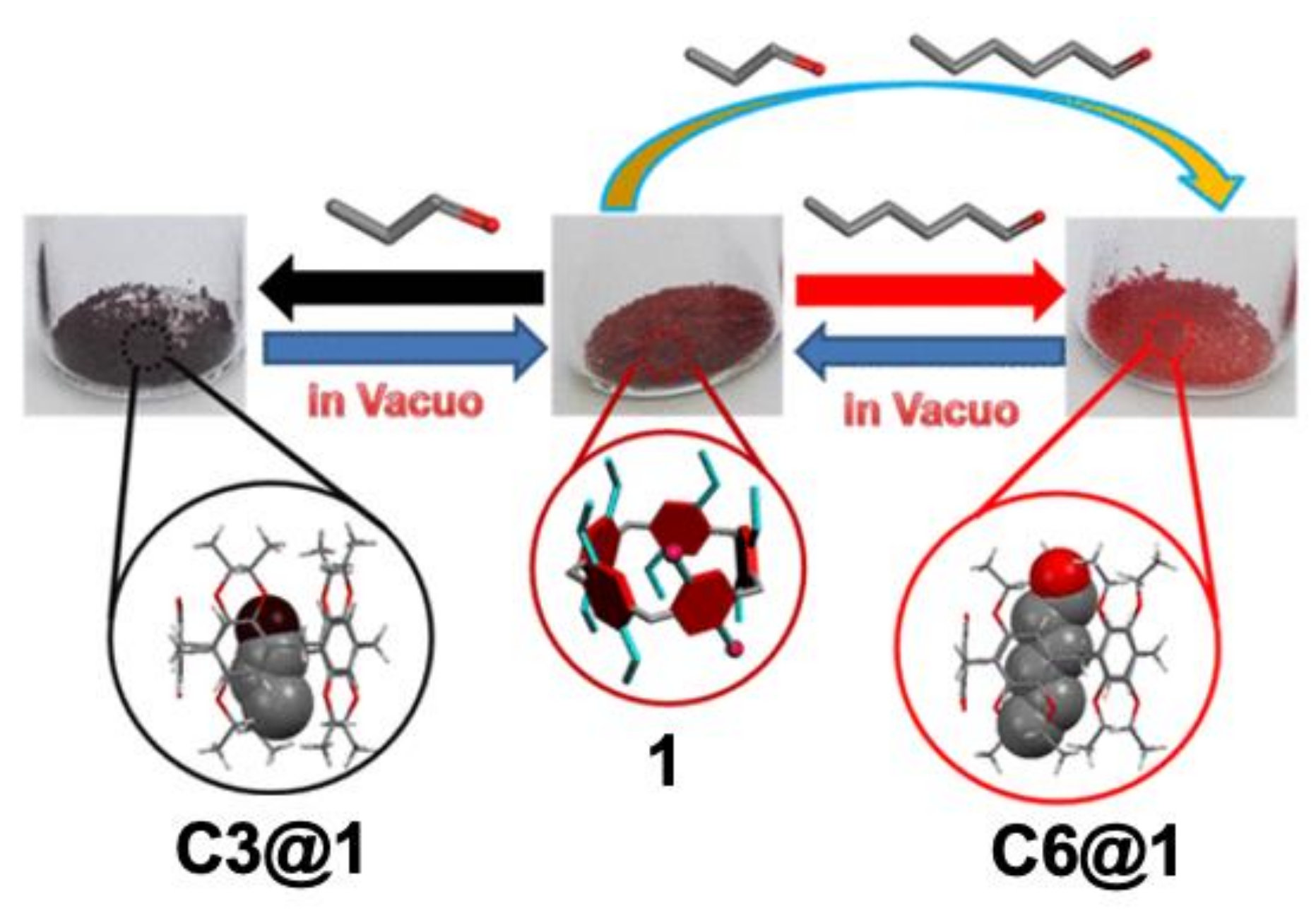
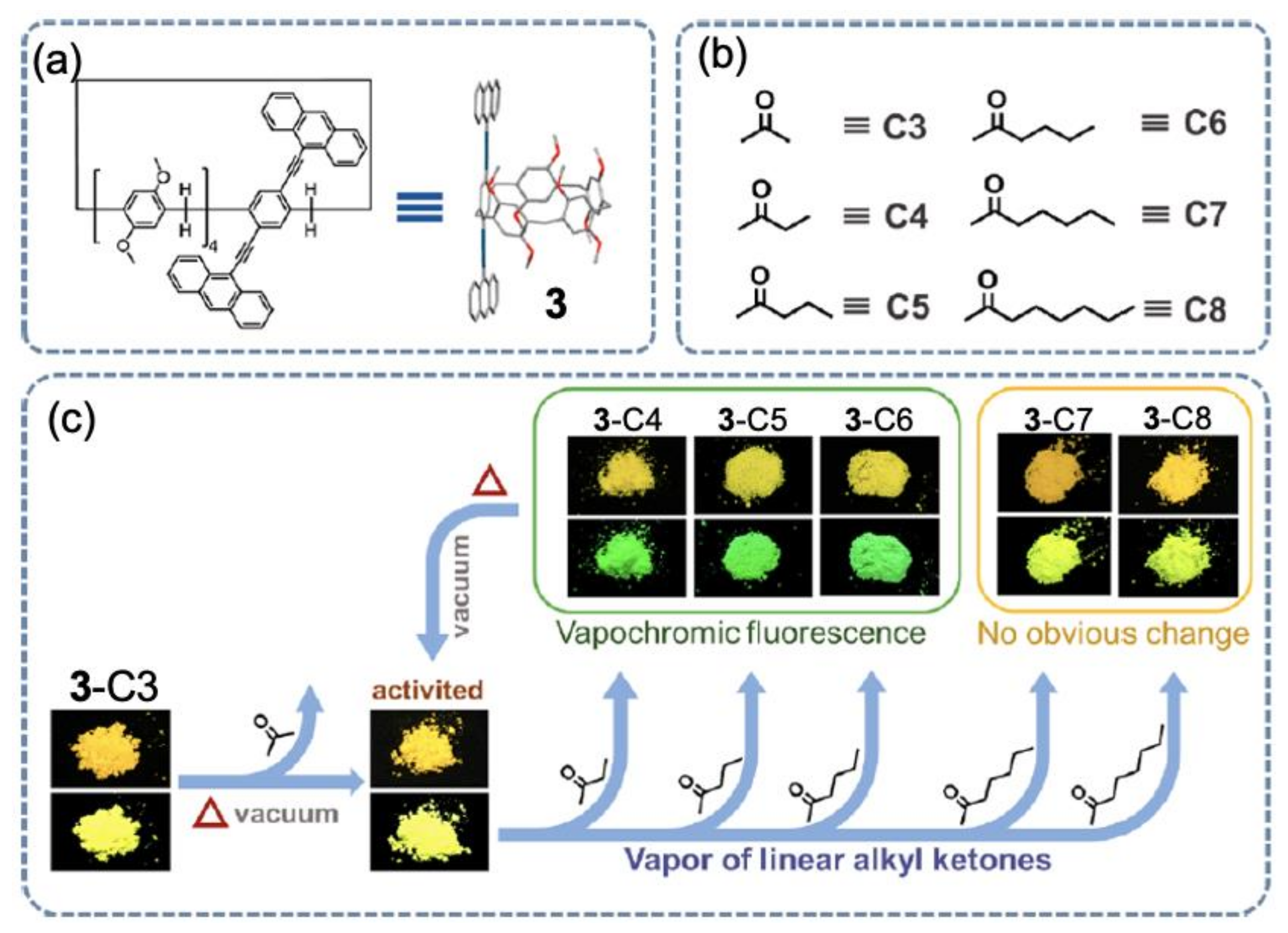
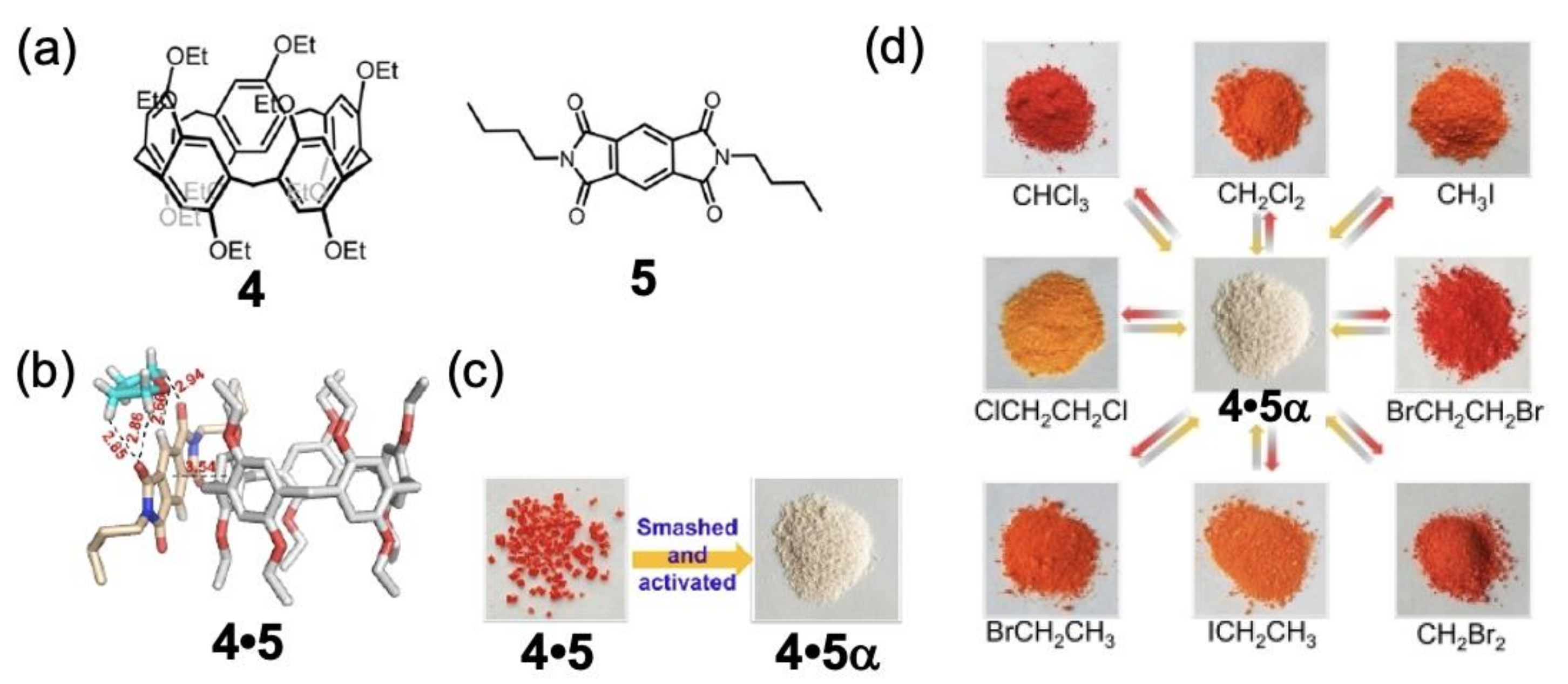
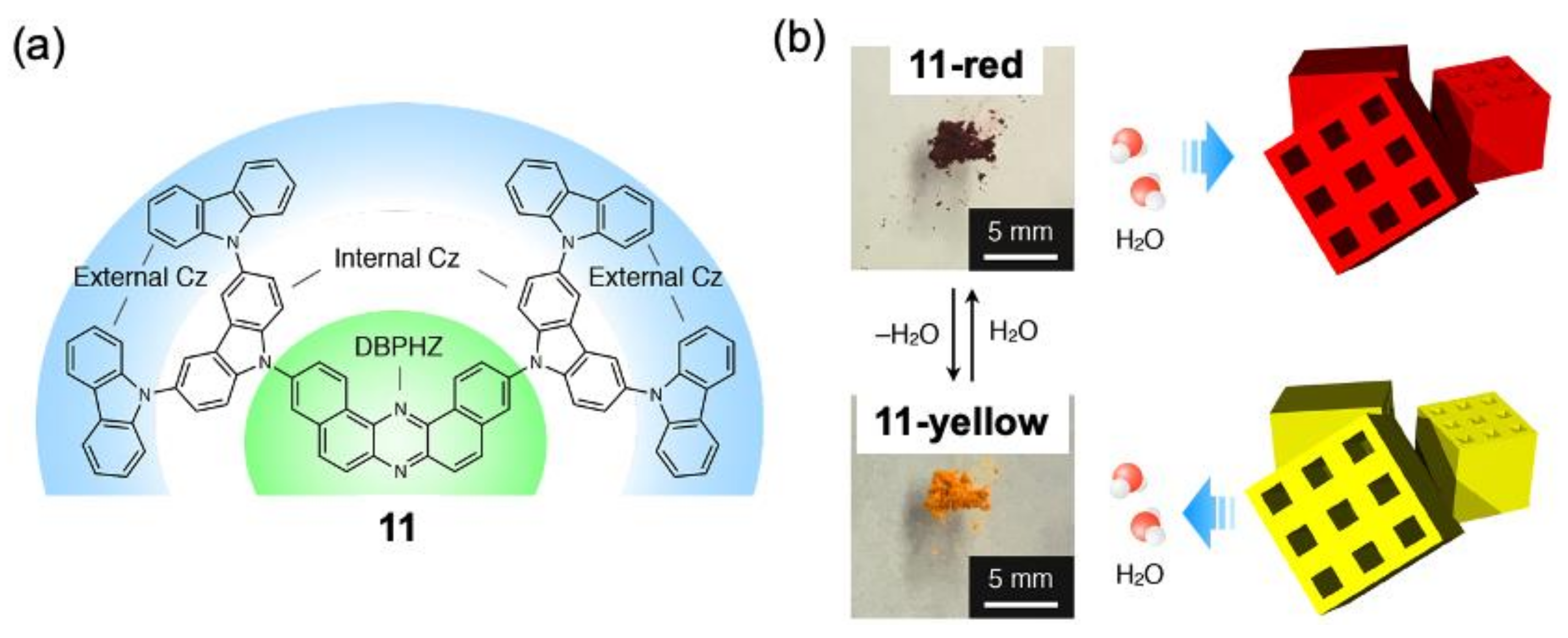
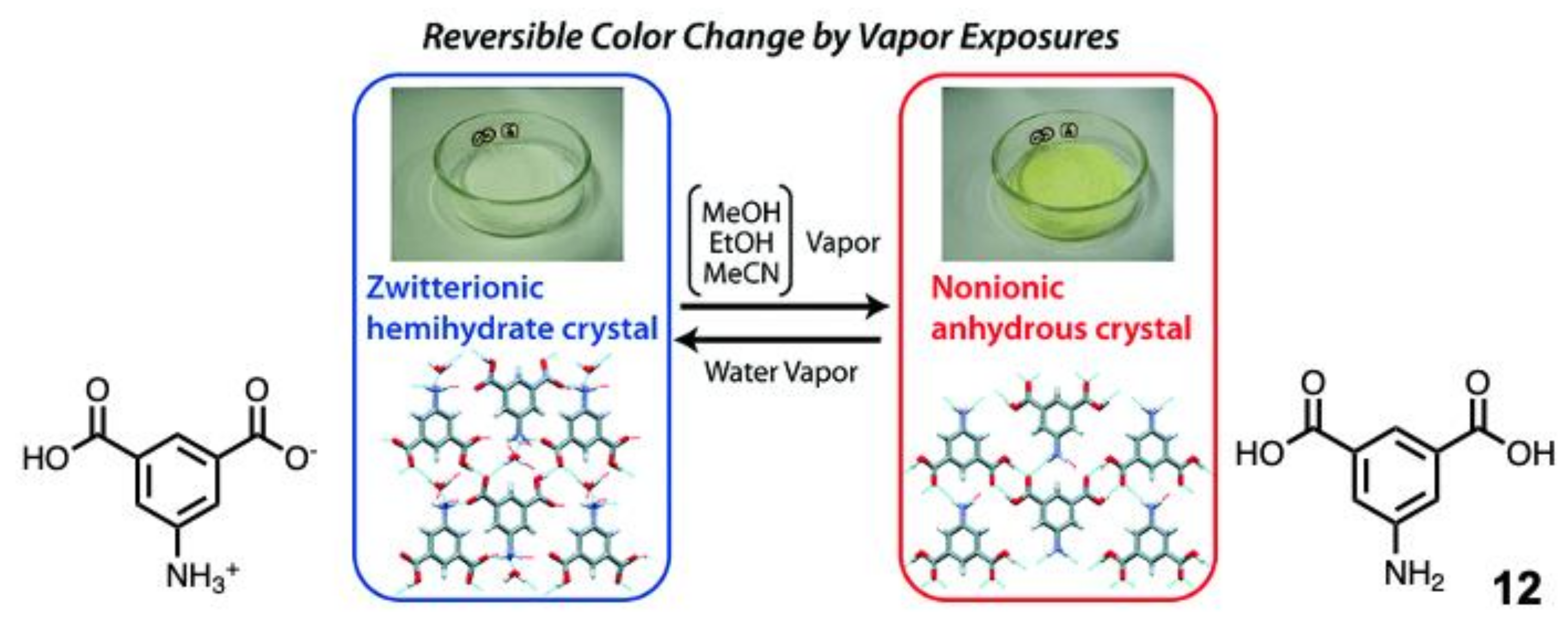
2. Vapochromic Materials Based on Macrocyclic Compounds
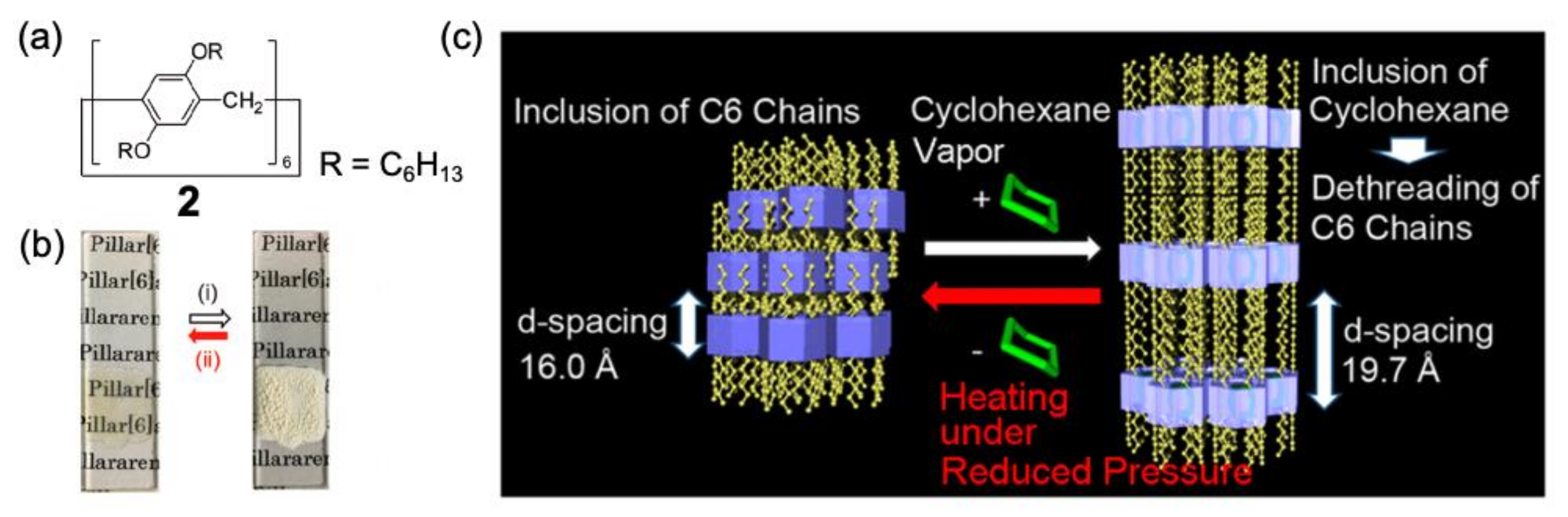
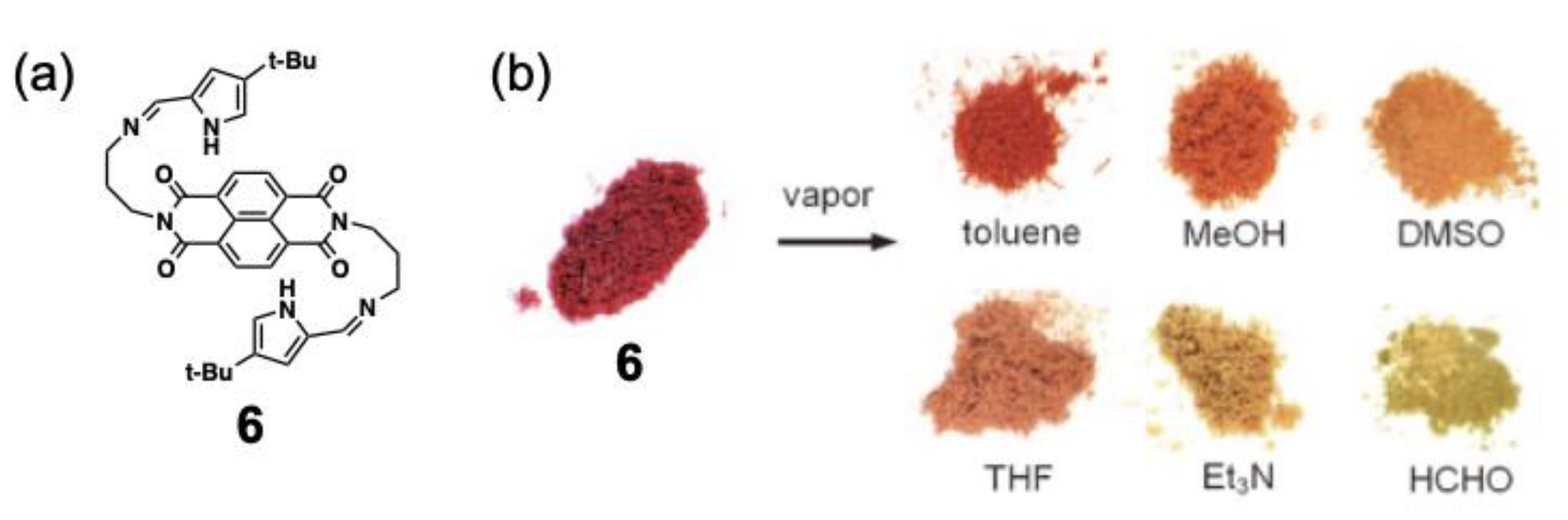
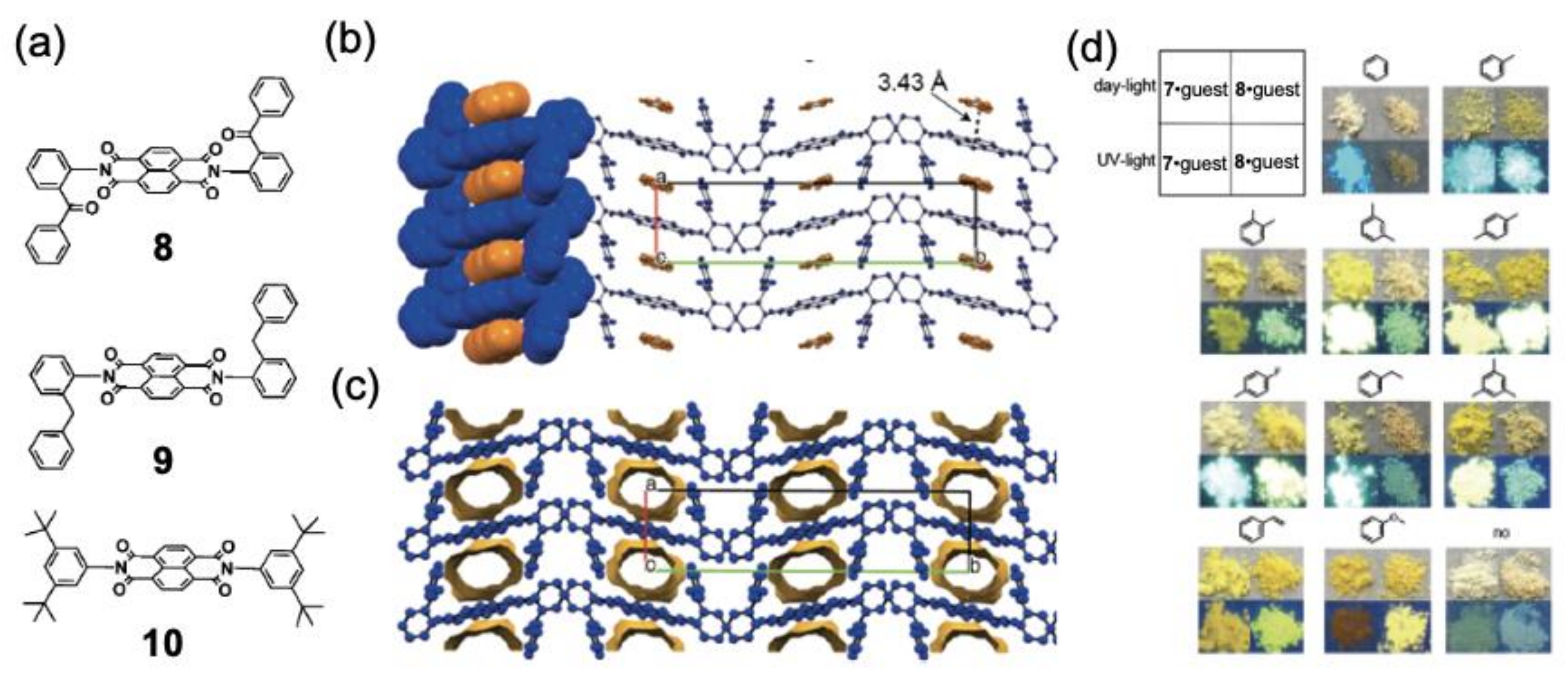
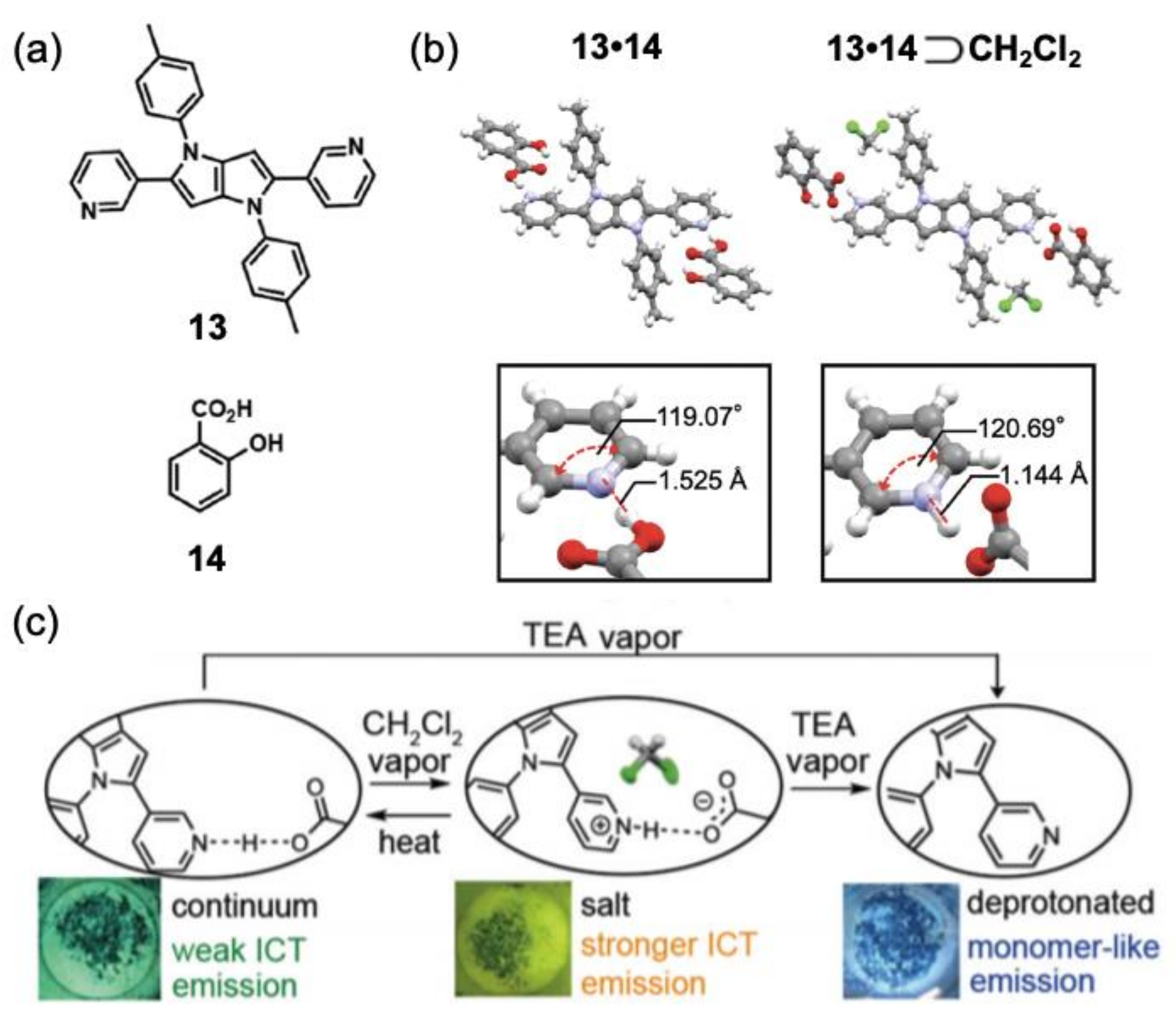
3. Vapochromic Materials Based on Inclusion Crystals
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal-organic framework materials as chemical sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, B.; Chen, Z.-H.; Chen, Z.-N. Luminescence vapochromism in solid materials based on metal complexes for detection of volatile organic compounds (VOCs). J. Mater. Chem. 2012, 22, 11427–11441. [Google Scholar] [CrossRef]
- Wenger, O.S. Vapochromism in organometallic and coordination complexes: Chemical sensors for volatile organic compounds. Chem. Rev. 2013, 113, 3686–3733. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Lee, S.; Xu, Z.; Yoon, J. Recent Progress on the Development of Chemosensors for Gases. Chem. Rev. 2015, 115, 7944–8000. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Ito, H.; Hasegawa, M.; Ishii, K. Soft Crystals: Flexible Response Systems with High Structural Order. Chem. Eur. J. 2019, 25, 5105–5112. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Jie, K.; Liu, M.; Sheng, X.; Zhu, W.; Huang, F. Vapochromic crystals: Understanding vapochromism from the perspective of crystal engineering. Chem. Soc. Rev. 2020, 49, 1517–1544. [Google Scholar] [CrossRef]
- Crini, G. Review: A History of Cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef]
- Ikeda, A.; Shinkai, S. Novel Cavity Design Using Calix[n]arene Skeletons: Toward Molecular Recognition and Metal Binding. Chem. Rev. 1997, 97, 1713–1734. [Google Scholar] [CrossRef]
- Morohashi, N.; Narumi, F.; Iki, N.; Hattori, T.; Miyano, S. Thiacalixarenes. Chem. Rev. 2006, 106, 5291–5316. [Google Scholar] [CrossRef]
- Ogoshi, T.; Yamagishi, T.-A.; Nakamoto, Y. Pillar-shaped macrocyclic hosts pillar [n] arenes: New key players for supramolecular chemistry. Chem. Rev. 2016, 116, 7937–8002. [Google Scholar] [CrossRef]
- Barrow, S.J.; Kasera, S.; Rowland, M.J.; del Barrio, J.; Scherman, O.A. Cucurbituril-Based Molecular Recognition. Chem. Rev. 2015, 115, 12320–12406. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Lu, F.; Wang, J.; Hu, W.; Cao, X.M.; Ma, X.; Tian, H. Amorphous Metal-Free Room-Temperature Phosphorescent Small Molecules with Multicolor Photoluminescence via a Host-Guest and Dual-Emission Strategy. J. Am. Chem. Soc. 2018, 140, 1916–1923. [Google Scholar] [CrossRef] [PubMed]
- Pirondini, L.; Dalcanale, E. Molecular recognition at the gas–solid interface: A powerful tool for chemical sensing. Chem. Soc. Rev. 2007, 36, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Ogoshi, T.; Kanai, S.; Fujinami, S.; Yamagishi, T.-A.; Nakamoto, Y. para-Bridged Symmetrical Pillar[5]arenes: Their Lewis Acid Catalyzed Synthesis and Host–Guest Property. J. Am. Chem. Soc. 2008, 130, 5022–5023. [Google Scholar] [CrossRef]
- Ogoshi, T.; Tsuchida, H.; Kakuta, T.; Yamagishi, T.A.; Taema, A.; Ono, T.; Sugimoto, M.; Mizuno, M. Ultralong Room-Temperature Phosphorescence from Amorphous Polymer Poly(Styrene Sulfonic Acid) in Air in the Dry Solid State. Adv. Funct. Mater. 2018, 28. [Google Scholar] [CrossRef]
- Jie, K.; Zhou, Y.; Li, E.; Huang, F. Nonporous Adaptive Crystals of Pillararenes. Acc. Chem. Res. 2018, 51, 2064–2072. [Google Scholar] [CrossRef]
- Jie, K.; Liu, M.; Zhou, Y.; Little, M.A.; Bonakala, S.; Chong, S.Y.; Stephenson, A.; Chen, L.; Huang, F.; Cooper, A.I. Styrene Purification by Guest-Induced Restructuring of Pillar[6]arene. J. Am. Chem. Soc. 2017, 139, 2908–2911. [Google Scholar] [CrossRef]
- Jie, K.; Liu, M.; Zhou, Y.; Little, M.A.; Pulido, A.; Chong, S.Y.; Stephenson, A.; Hughes, A.R.; Sakakibara, F.; Ogoshi, T.; et al. Near-Ideal Xylene Selectivity in Adaptive Molecular Pillar[n]arene Crystals. J. Am. Chem. Soc. 2018, 140, 6921–6930. [Google Scholar] [CrossRef]
- Kakuta, T.; Yamagishi, T.-A.; Ogoshi, T. Stimuli-Responsive Supramolecular Assemblies Constructed from Pillar[n]arenes. Acc. Chem. Res. 2018, 51, 1656–1666. [Google Scholar] [CrossRef]
- Ogoshi, T.; Shimada, Y.; Sakata, Y.; Akine, S.; Yamagishi, T.-A. Alkane-Shape-Selective Vapochromic Behavior Based on Crystal-State Host–Guest Complexation of Pillar[5]arene Containing One Benzoquinone Unit. J. Am. Chem. Soc. 2017, 139, 5664–5667. [Google Scholar] [CrossRef]
- Wada, K.; Kakuta, T.; Yamagishi, T.-A.; Ogoshi, T. Obvious vapochromic color changes of a pillar[6]arene containing one benzoquinone unit with a mechanochromic change before vapor exposure. Chem. Commun. 2020, 56, 4344–4347. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Jie, K.; Zhou, Y.; Zhao, R.; Zhang, B.; Wang, Q.; Liu, J.; Huang, F. Aliphatic Aldehyde Detection and Adsorption by Nonporous Adaptive Pillar[4]arene[1]quinone Crystals with Vapochromic Behavior. Acs Appl. Mater. Interfaces 2018, 10, 23147–23153. [Google Scholar] [CrossRef] [PubMed]
- Ogoshi, T.; Maruyama, K.; Sakatsume, Y.; Kakuta, T.; Yamagishi, T.-A.; Ichikawa, T.; Mizuno, M. Guest Vapor-Induced State Change of Structural Liquid Pillar[6]arene. J. Am. Chem. Soc. 2019, 141, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhu, H.; Huang, F. Alkyl Chain Length-Selective Vapor-Induced Fluorochromism of Pillar[5]arene-Based Nonporous Adaptive Crystals. J. Am. Chem. Soc. 2019, 141, 13290–13294. [Google Scholar] [CrossRef]
- Lei, S.-N.; Xiao, H.; Zeng, Y.; Tung, C.-H.; Wu, L.-Z.; Cong, H. BowtieArene: A Dual Macrocycle Exhibiting Stimuli-Responsive Fluorescence. Angew. Chem. Int. Ed. 2020, 59, 10059–10065. [Google Scholar] [CrossRef]
- Ogoshi, T.; Hamada, Y.; Sueto, R.; Kojima, R.; Sakakibara, F.; Nagata, Y.; Sakata, Y.; Akine, S.; Ono, T.; Kakuta, T.; et al. Vapoluminescence Behavior Triggered by Crystal-State Complexation between Host Crystals and Guest Vapors Exhibiting No Visible Fluorescence. Cryst. Growth Des. 2020. [Google Scholar] [CrossRef]
- Li, B.; Cui, L.; Li, C. Macrocycle Co-Crystals Showing Vapochromism to Haloalkanes. Angew. Chem. Int. Ed. 2020. [Google Scholar] [CrossRef]
- Bishop, R. Designing new lattice inclusion hosts. Chem. Soc. Rev. 1996, 25, 311–319. [Google Scholar] [CrossRef]
- Fei, Z.; Kocher, N.; Mohrschladt, C.J.; Ihmels, H.; Stalke, D. Single Crystals of the Disubstituted Anthracene 9, 10-(Ph2P□S) 2C14H8 Selectively and Reversibly Detect Toluene by Solid-State Fluorescence Emission. Angew. Chem. Int. Ed. 2003, 42, 783–787. [Google Scholar] [CrossRef]
- Ooyama, Y.; Nagano, S.; Okamura, M.; Yoshida, K. Solid-State Fluorescence Changes of 2-(4-Cyanophenyl)-5-[4-(diethylamino)phenyl]-3H-imidazo[4,5-a]naphthalene upon Inclusion of Organic Solvent Molecules. Eur. J. Org. Chem. 2008, 2008, 5899–5906. [Google Scholar] [CrossRef]
- Hinoue, T.; Miyata, M.; Hisaki, I.; Tohnai, N. Guest-Responsive Fluorescence of Inclusion Crystals with π-Stacked Supramolecular Beads. Angew. Chem. Int. Ed. 2012, 51, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, E.; Takaya, H.; Naota, T. Dynamic vapochromic behaviors of organic crystals based on the open-close motions of S-shaped donor-acceptor folding units. Chem. Eur. J. 2010, 16, 4793–4802. [Google Scholar] [CrossRef] [PubMed]
- Ono, T.; Sugimoto, M.; Hisaeda, Y. Multicomponent Molecular Puzzles for Photofunction Design: Emission Color Variation in Lewis Acid–Base Pair Crystals Coupled with Guest-to-Host Charge Transfer Excitation. J. Am. Chem. Soc. 2015, 137, 9519–9522. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, S.; Ono, T.; Hisaeda, Y. Turn-On Fluorogenic and Chromogenic Detection of Small Aromatic Hydrocarbon Vapors by a Porous Supramolecular Host. Chem. Eur. J. 2016, 22, 10346–10350. [Google Scholar] [CrossRef]
- Ono, T.; Tsukiyama, Y.; Taema, A.; Hisaeda, Y. Inclusion Crystal Growth and Optical Properties of Organic Charge-transfer Complexes Built from Small Aromatic Guest Molecules and Naphthalenediimide Derivatives. Chem. Lett. 2017, 46, 801–804. [Google Scholar] [CrossRef]
- Ono, T.; Tsukiyama, Y.; Hatanaka, S.; Sakatsume, Y.; Ogoshi, T.; Hisaeda, Y. Inclusion crystals as vapochromic chemosensors: Fabrication of a mini-sensor array for discrimination of small aromatic molecules based on side-chain engineering of naphthalenediimide derivatives. J. Mater. Chem. C 2019, 7, 9726–9734. [Google Scholar] [CrossRef]
- Matsunaga, Y.; Yang, J.S. Multicolor Fluorescence Writing Based on Host-Guest Interactions and Force-Induced Fluorescence-Color Memory. Angew. Chem. Int. Ed. 2015, 54, 7985–7989. [Google Scholar] [CrossRef]
- Hsu, L.-Y.; Maity, S.; Matsunaga, Y.; Hsu, Y.-F.; Liu, Y.-H.; Peng, S.-M.; Shinmyozu, T.; Yang, J.-S. Photomechanochromic vs. mechanochromic fluorescence of a unichromophoric bimodal molecular solid: Multicolour fluorescence patterning. Chem. Sci. 2018, 9, 8990–9001. [Google Scholar] [CrossRef]
- Yang, J.-S.; Swager, T.M. Porous Shape Persistent Fluorescent Polymer Films: An Approach to TNT Sensory Materials. J. Am. Chem. Soc. 1998, 120, 5321–5322. [Google Scholar] [CrossRef]
- Yamagishi, H.; Nakajima, S.; Yoo, J.; Okazaki, M.; Takeda, Y.; Minakata, S.; Albrecht, K.; Yamamoto, K.; Badía-Domínguez, I.; Oliva, M.M.; et al. Sigmoidally hydrochromic molecular porous crystal with rotatable dendrons. Commun. Chem. 2020, 3, 118. [Google Scholar] [CrossRef]
- Fujii, K.; Sakon, A.; Sekine, A.; Uekusa, H. Reversible Color Switching of an Organic Crystal Induced by Organic Solvent Vapors. Cryst. Growth Des. 2011, 11, 4305–4308. [Google Scholar] [CrossRef]
- Sakon, A.; Sekine, A.; Uekusa, H. Powder Structure Analysis of Vapochromic Quinolone Antibacterial Agent Crystals. Cryst. Growth Des. 2016, 16, 4635–4645. [Google Scholar] [CrossRef]
- Yano, Y.; Ono, T.; Hatanaka, S.; Gryko, D.T.; Hisaeda, Y. Salt–cocrystal continuum for photofunction modulation: Stimuli-responsive fluorescence color-tuning of pyridine-modified intramolecular charge-transfer dyes and acid complexes. J. Mater. Chem. C 2019, 7, 8847–8854. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ono, T.; Hisaeda, Y. Vapochromism of Organic Crystals Based on Macrocyclic Compounds and Inclusion Complexes. Symmetry 2020, 12, 1903. https://doi.org/10.3390/sym12111903
Ono T, Hisaeda Y. Vapochromism of Organic Crystals Based on Macrocyclic Compounds and Inclusion Complexes. Symmetry. 2020; 12(11):1903. https://doi.org/10.3390/sym12111903
Chicago/Turabian StyleOno, Toshikazu, and Yoshio Hisaeda. 2020. "Vapochromism of Organic Crystals Based on Macrocyclic Compounds and Inclusion Complexes" Symmetry 12, no. 11: 1903. https://doi.org/10.3390/sym12111903
APA StyleOno, T., & Hisaeda, Y. (2020). Vapochromism of Organic Crystals Based on Macrocyclic Compounds and Inclusion Complexes. Symmetry, 12(11), 1903. https://doi.org/10.3390/sym12111903