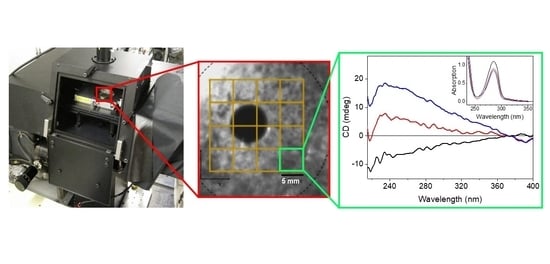
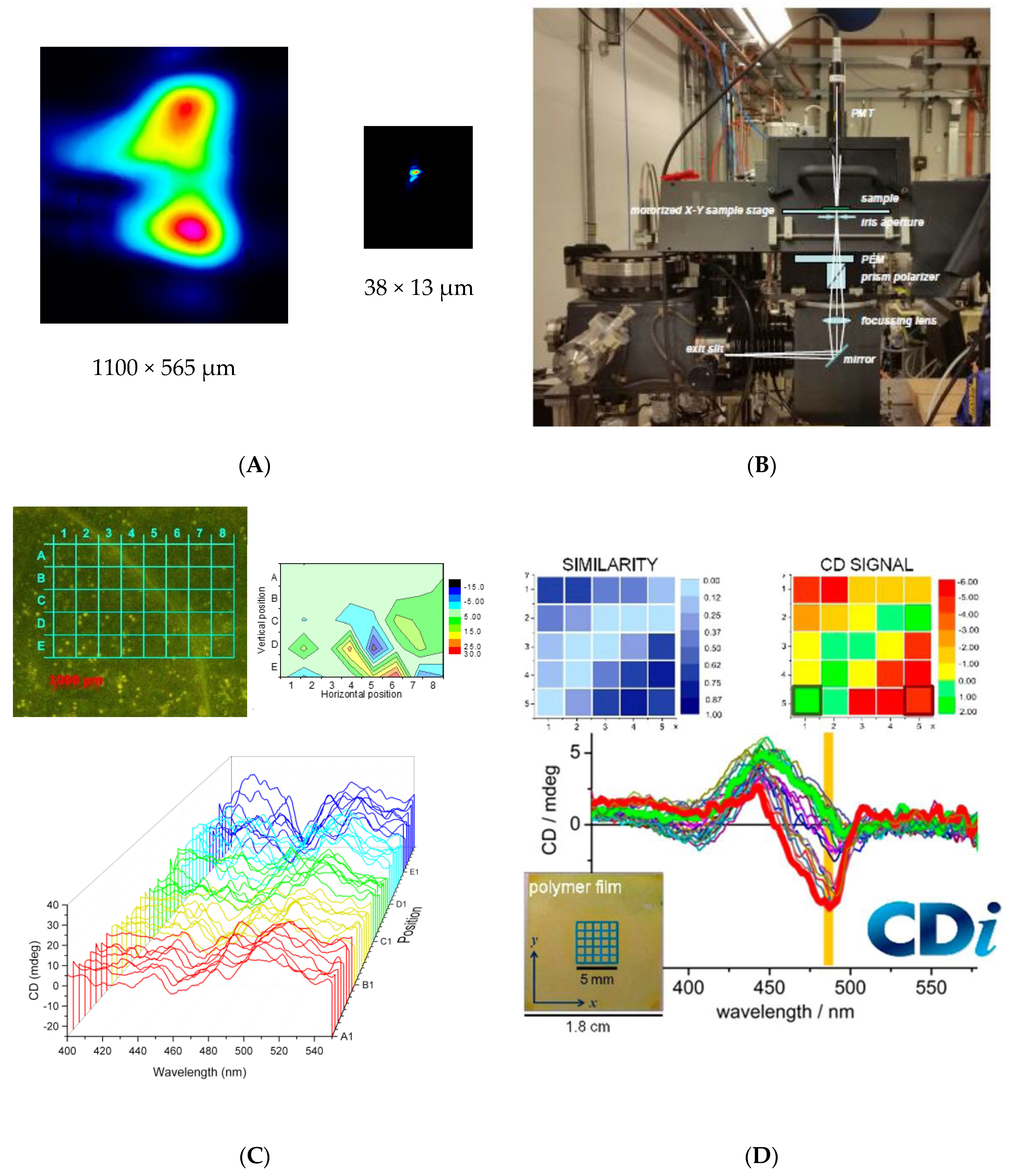
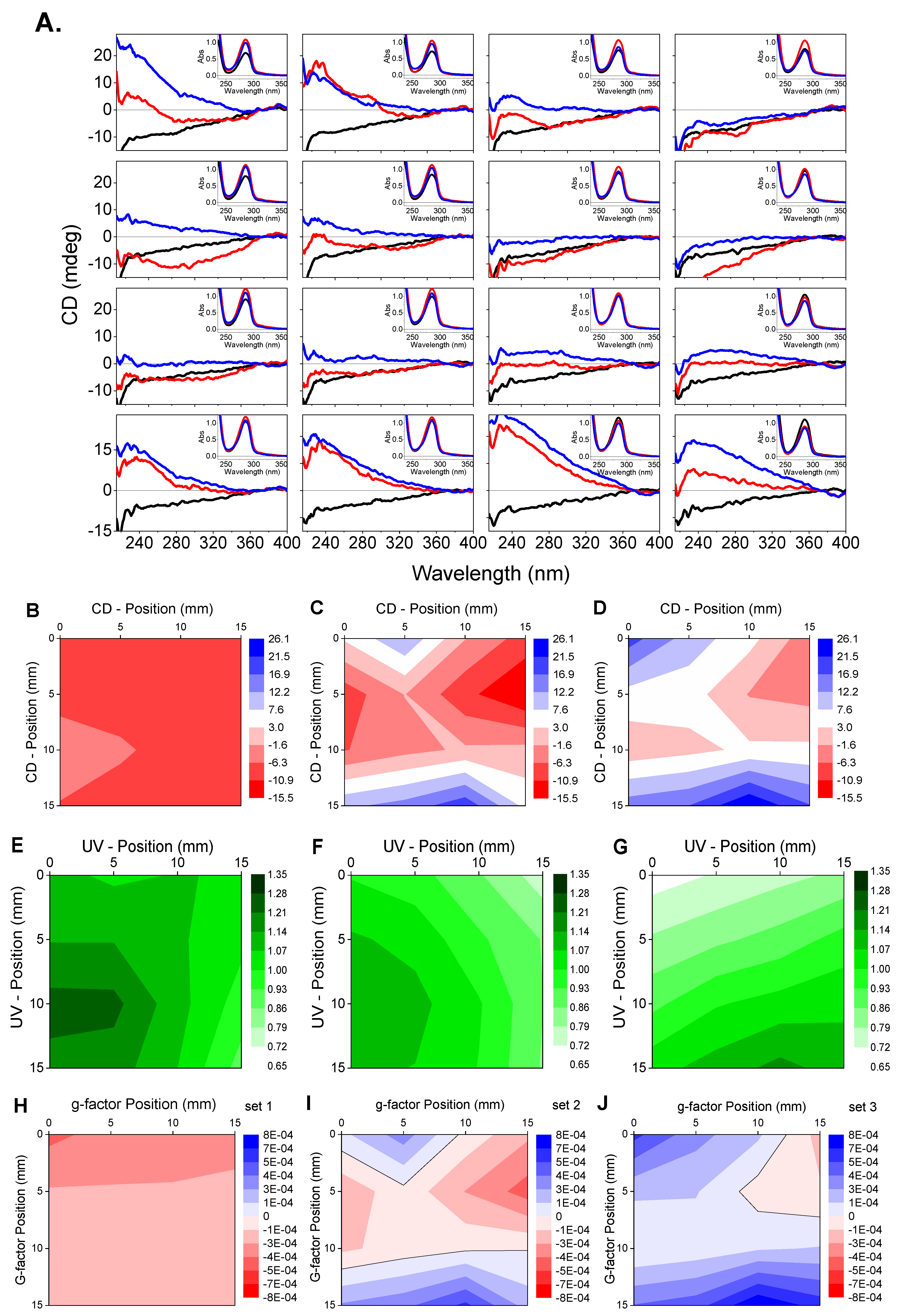
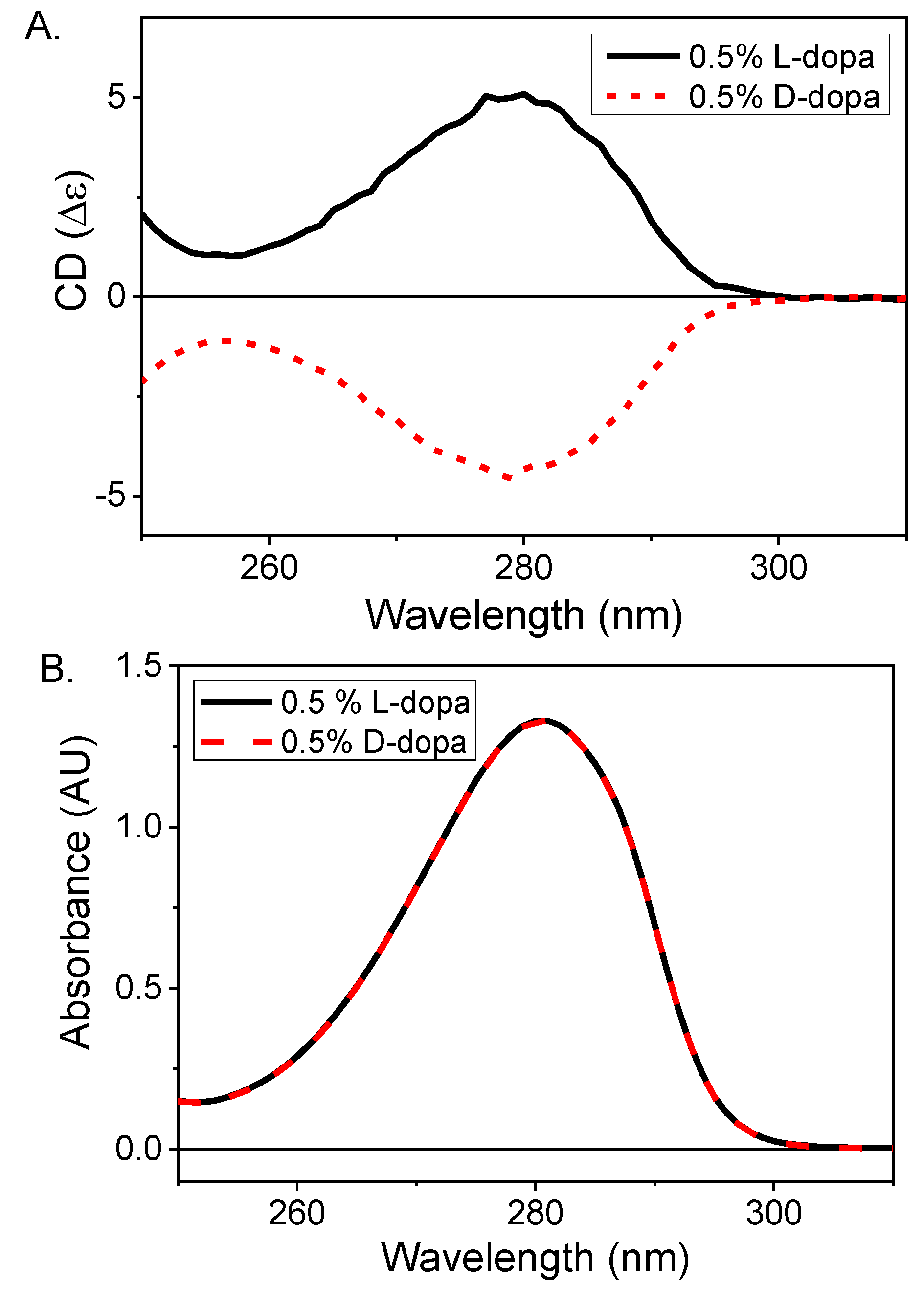
Diamond B23 CD Imaging of Thin Films of Chiral Materials or Achiral Polymers Coated with Chiral Molecules
Abstract
Introduction
- g > 10−2, the transition is magnetically allowed and electrically forbidden;
- g ≈ 10−2, the transition is both electrically and magnetically allowed;
- g < 10−2, the transition is electrically allowed and magnetically forbidden.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Berova, N.; Nakanishi, K.; Woody, R.W. Circular Dichroism Principles and Applications, 2nd ed.; Wiley-VCH: New York, NY, USA, 2000. [Google Scholar]
- Fasman, G.D. Circular Dichroism and Conformational Analysis of Biomolecules; Plenum Press: New York, NY, USA, 1996. [Google Scholar]
- Hussain, R.; Krumpa, N.; Strachan, J.; Clarke, D.; Wagner, U.; Macdonald, B.; Reading, D.; Cobb, T.; Gillingham, I.; Price, A.; et al. Design of B23 circular dichroism beamline at Diamond Light Source. Adv. Synchrotron Radiat. 2008, 1, 265–270. [Google Scholar] [CrossRef]
- Jávorfi, T.; Hussain, R.; Myatt, D.; Siligardi, G. Measuring circular dichroism in a capillary cell using the B23 synchrotron radiation CD beamline at diamond light source. Chirality 2010, 22 (Suppl. 1), E149–E153. [Google Scholar] [CrossRef] [PubMed]
- Siligardi, G.; Hussain, R.; Myatt, D.; Jávorfi, T. Diam. Light Source Proc. 2010, 1, e104. [Google Scholar] [CrossRef]
- Hussain, R.; Javorfi, T.; Siligardi, G. Circular dichroism beamline B23 at the Diamond Light Source. J. Synchrotron Radiat. 2012, 19, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Hussain, R.; Jávorfi, T.; Siligardi, G. Spectroscopic Analysis: Synchrotron Radiation Circular Dichroism. Compr. Chiral. 2012, 8, 438–448. [Google Scholar]
- Hussain, R.; Siligardi, G. Characterisation of Conformational and Ligand Binding Properties of Membrane Proteins Using Synchrotron Radiation Circular Dichroism (SRCD). In The Next Generation in Membrane Protein Structure Determination, Advances in Experimental Medicine and Biology; Moraes, I., Ed.; Springer: Cham, Switzerland, 2016; Volume 922, pp. 43–60. [Google Scholar]
- Hussain, R.; Jávorfi, T.; Rudd, T.; Siligardi, G. High-throughput SRCD using multi-well plates and its applications. Sci. Rep. 2016, 6, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Siligardi, G.; Hussain, R. CD Spectroscopy, An Essential Tool for Quality Control of Protein Folding. Meth. Molec. Biol. 2015, 1261, 255–276. [Google Scholar]
- Hussain, R.; Benning, K.; Myatt, D.; Javorfi, T.; Longo, E.; Rudd, T.R.; Pulford, B.; Siligardi, G. CDApps: Integrated software for experimental planning and data processing at beamline B23, Diamond Light Source. J. Synchrotron Radiat. 2015, 22, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Borisenko, K.B.; Shanmugam, J.; Williams, B.A.; Ewart, P.; Gholipour, B.; Hewak, D.W.; Hussain, R.; Jávorfi, T.; Siligardi, G.; Kirkland, A.I. Photo-induced optical activity in phase-change memory materials. Sci. Rep. 2015, 5, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Zinna, F.; Resta, C.; Gorecki, M.; Pescitelli, G.; di Bari, L.; Javorfi, T.; Hussain, R.; Siligardi, G. Circular Dichroism Imaging: Mapping the Local Supramolecular Order in Thin Films of Chiral Functional Polymers. Macromolecules 2017, 50, 2054–2060. [Google Scholar] [CrossRef]
- Cseh, L.; Mang, X.; Zeng, X.; Liu, F.; Mehl, G.H.; Ungar, G.; Siligardi, G. Helically Twisted Chiral Arrays of Gold Nanoparticles Coated with a Cholesterol Mesogen. J. Am. Chem. Soc. 2015, 137, 12736–12739. [Google Scholar] [CrossRef] [PubMed]
- Dressel, C.; Liu, F.; Prehm, M.; Zeng, X.; Ungar, G.; Tschierske, C. Dynamic mirror-symmetry breaking in biscontinuous cubic phases. Angew. Chem. Int. Ed. 2014, 53, 13115–13120. [Google Scholar] [CrossRef] [PubMed]
- Albano, G.; Górecki, M.; Pescitelli, G.; di Bari, L.; Jávorfi, T.; Hussain, R.; Siligardi, G. Electronic circular dichroism imaging (CDi) maps local aggregation modes in thin films of chiral oligothiophenes. New J. Chem. 2019, 43, 14584–14593. [Google Scholar] [CrossRef]
- Dhand, C.; Harini, S.; Venkatesh, M.; Dwivedi, N.; Ng, A.; Liu, S.; Verma, N.K.; Ramakrishna, S.; Beuerman, R.W.; Xian, J.L.; et al. Multifunctional Polyphenols- and Catecholamines-Based Self-Defensive Films for Health Care Applications. ACS Appl. Mater. Interfaces 2016, 8, 1220–1232. [Google Scholar] [CrossRef] [PubMed]
- Arteaga, O.; Freudenthal, J.; Wang, B.; Kahr, B. Mueller matrix polarimetry with four photoelastic modulators: Theory and calibration. Appl. Opt. 2012, 51, 6805–6817. [Google Scholar] [CrossRef] [PubMed]
- Szustakiewicz, P.; Kowalska, N.; Grzelak, D.; Narushima, T.; Gora, M.; Baginski, M.; Pociecha, D.; Okamoto, H.; Liz-Marzan, L.M.; Lewandowski, W. Supramolecular Chirality Synchronization in Thin Films of Plasmonic Nanocomposites. ACS Nano 2020. [Google Scholar] [CrossRef] [PubMed]
- Riva, S. Laccases, Blue Enzymes for Green Chemistry. Trends Biotechnol. 2006, 24, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, W.; Braun, E. Measurement of circular dichroism in the ultra-violet. Z. Physik. Chem. Abt. B 1930, 8, 445–454. [Google Scholar]
- Snatzke, G. Circular Dichrosim and Optical Rotatory Dispersion—Principles and Application to the Investigation of the Stereochemistry of Natural Products. Angew. Chem. Int. Ed. Engl. 1968, 7, 14–25. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, R.; Jávorfi, T.; Hughes, C.S.; Sriram, H.; Lashminarayanan, R.; Siligardi, G. Diamond B23 CD Imaging of Thin Films of Chiral Materials or Achiral Polymers Coated with Chiral Molecules. Symmetry 2020, 12, 1847. https://doi.org/10.3390/sym12111847
Hussain R, Jávorfi T, Hughes CS, Sriram H, Lashminarayanan R, Siligardi G. Diamond B23 CD Imaging of Thin Films of Chiral Materials or Achiral Polymers Coated with Chiral Molecules. Symmetry. 2020; 12(11):1847. https://doi.org/10.3390/sym12111847
Chicago/Turabian StyleHussain, Rohanah, Tamás Jávorfi, Charlotte S Hughes, Harini Sriram, Rajamani Lashminarayanan, and Giuliano Siligardi. 2020. "Diamond B23 CD Imaging of Thin Films of Chiral Materials or Achiral Polymers Coated with Chiral Molecules" Symmetry 12, no. 11: 1847. https://doi.org/10.3390/sym12111847
APA StyleHussain, R., Jávorfi, T., Hughes, C. S., Sriram, H., Lashminarayanan, R., & Siligardi, G. (2020). Diamond B23 CD Imaging of Thin Films of Chiral Materials or Achiral Polymers Coated with Chiral Molecules. Symmetry, 12(11), 1847. https://doi.org/10.3390/sym12111847