Abstract
This paper firstly studies an SIR (susceptible-infectious-recovered) epidemic model without demography and with no disease mortality under both total and under partial quarantine of the susceptible subpopulation or of both the susceptible and the infectious ones in order to satisfy the hospital availability requirements on bed disposal and other necessary treatment means for the seriously infectious subpopulations. The seriously infectious individuals are assumed to be a part of the total infectious being described by a time-varying proportional function. A time-varying upper-bound of those seriously infected individuals has to be satisfied as objective by either a total confinement or partial quarantine intervention of the susceptible subpopulation. Afterwards, a new extended SEIR (susceptible-exposed-infectious-recovered) epidemic model, which is referred to as an SEIAR (susceptible-exposed-symptomatic infectious-asymptomatic infectious-recovered) epidemic model with demography and disease mortality is given and focused on so as to extend the above developed ideas on the SIR model. A proportionally gain in the model parameterization is assumed to distribute the transition from the exposed to the infectious into the two infectious individuals (namely, symptomatic and asymptomatic individuals). Such a model is evaluated under total or partial quarantines of all or of some of the subpopulations which have the effect of decreasing the number of contagions. Simulated numerical examples are also discussed related to model parameterizations of usefulness related to the current COVID-19 pandemic outbreaks.
1. Introduction
Epidemic mathematical models in different formal frameworks are of crucial interest in recent decades [1,2]. Such mentioned formal frameworks include, for instance, differential, difference and differential/difference hybrid equations, dynamic systems, control theory [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20], computation [7], and information theory [21,22,23,24,25,26,27,28] as well as combined mathematical analysis of epidemic spreading and control design tools [21,22,27,28,29,30,31,32,33,34,35,36]. One of the objectives of such models is to investigate and predict the evolution of epidemic infectious diseases in humans, animals, or plants as well as to elucidate how the interventions, like quarantine actions or regular and impulsive vaccination and treatment controls, can avoid or mitigate their contagious propagation (See References [1,2,11,13,15,17,18,19,20,25,26,27,28,29,30,31,32,33,34,35,36]). It can be pointed out that the quarantine and the confinement interventions are qualitatively similar to using impulsive vaccination and treatment controls since all or a part of a targeted subpopulation (typically, the susceptible one or the infectious one or fractions of them) is removed from its associate compartment and the viewable effect is a re-initialization of the corresponding trajectory solution under fewer contagious contacts. Another beneficial effect is that the transmission rate decreases to lower levels because of the mentioned decrease in the number of contacts. The previously mentioned control intervention are very relevant concerning the disease temporary evolution and its steady-state reachable numbers of the various subpopulations since they can increase the values of the transmission rate being compatible with a basic reproduction number being less than unity. This property implies that the disease-free equilibrium point is a global attractor and the disease becomes asymptotically removed as a result. Those above issues have been important in the last months with regard to the evolution of the COVID-19 pandemic around the world (See References [27,29,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51]). In particular, an “ad hoc” SEIR model parameterization related to the COVID-19 pandemic is investigated in Reference [37] including delayed re-susceptibility caused by the infection. Additionally, a kind of autoregressive model average model (ARMA), known as an ARIMA model for prediction of COVID-19, is presented and simulated in Reference [38] for the data of several countries. The effects of different phases of quarantine actions in the values of the transmission rate are studied in Reference [39]. See Reference [42] for a discussion of related simulated numerical results on the COVID-19 outbreak in Italy. Some results have been recently reported on reaction-diffusion epidemic models of usefulness fore dengue [52], on delayed diffusive epidemic models [53], and on multi-group epidemic discrete models with time delays [54]. (See also related references therein).
It can be pointed out that there are links in the descriptions of epidemic models to symmetry and asymmetry concepts with different interpretations. For instance, in Reference [13], the problem of patchy epidemic environments in the multi-node case where there are in-flux and out-flux of populations between different nodes (for instance, with each node describing distinct towns or regions) is focused on. The most general assumption involved is that the matrices of population travelling interchange are asymmetric in the most general case, but they can be symmetric in particular. In Reference [55], a close problem is focused on for epidemic models describing a network with more than one node and the concepts of symmetry and asymmetry play a role in the mathematical description of the proposed model. On the other hand, in Reference [56], the case of reported versus unreported infective cases is calculated. In the case of time-invariant model parameterizations, such a ratio becomes invariant for both the time-instantaneous tests and the time- accumulated tests interpreted as a certain symmetry property. Generally speaking, symmetry is equivalent to an invariance of a certain quantity under transformations. In that way, a related property associated with a number of common epidemic models is their invariance under certain transformations of variables, which is a property that applies even for models of a single node. Assume, for instance, a true-mass action epidemic model [43] with a typical bilinear incidence of the form where is the transmission rate and S and I are the susceptible and infectious instantaneous numbers and is the total population assumed constant through time. Furthermore, assume that the above model is changed to a normalized one for all the subpopulations leading to the transformed variables , etc. It can be easily seen that the form of the equations remains invariant with the redefined transmission rate . Such an idea is applicable even to epidemic models of a single node.
The related existing bibliography on epidemic modelling is abundant and very rich including a variety of epidemic models with several coupled subpopulations including the susceptible, the infectious, and the recovered ones as an elementary starting basis in the simpler SIR epidemic models. Further generalizations lead to the so-called SEIR models, which include the exposed subpopulation (those who do not have external symptoms yet) as a new subpopulation. More complex models, such as SEIADR-type models, which include the asymptomatic infectious and the infective dead subpopulations, have been designed and discussed, for instance, for Ebola disease [15,16]. On the other hand, Covid-19 is an infectious disease caused by a newly discovered coronavirus at the end of 2019. The first outbreak was reported in Wuhan (China) and then it extended worldwide very fast [57]. At this time, there is no vaccine nor effective treatment available while social distancing, isolation, and quarantine have been revealed to be the most important measurements to counteract its spread. Despite the current death rate of the disease being around 3–4%, acute symptoms may lead to intensive care unities (ICU’s) admission to many seriously infected patients, which provokes the overflow of these units and a situation of stress in the health system of many countries where the situation of their health systems is becoming really difficult over recent months because of the lack of beds, material, and staff for the abundant, seriously infected people.
The main objective of this paper is to propose and to investigate a new SEIAR epidemic model with demography, which is a generalized SEIR (susceptible-exposed-infectious-recovered). Such a model includes asymptomatic infectious (A) and eventual illness mortality. All the formulations are developed in a deterministic framework of differential equations with finite jumps in their solutions. A fraction of the exposed subpopulation has a transition to the symptomatic infectious subpopulation while its complementary to unity fraction has a transition to the asymptomatic one. The main results concern the study of the model under, in general, partial quarantines. Total quarantine actions and confinement interventions are considered as particular cases being governed, in each particular situation, by the choices of the gains, which describe the decrease of subpopulation levels subject to eventual reception or transmission of new contagions. Such gains are designed in such a way that the foreseen fraction of the infectious needing hospital care are upper-bounded by a function, which describes the hospital availability on beds and other means, like staff, available amounts and qualification, number of respirators, etc. Before the presentation and discussion of the new proposed SEIAR model, two simple illustrative examples on partial or total quarantines are given on a simpler SIR model without demography and with no mortality.
The paper is organized as follows. Section 2 states and briefly describes the simple mentioned SIR model under total or partial quarantine of the susceptible and infectious in order to satisfy the objective that a weighted proportion of the infectious subpopulation is less than a certain upper-bound defined by hospital treatment considerations. Section 3 presents the proposed SEIAR model, which includes the exposed and the asymptomatic subpopulations subject to demography and eventual mortality so that it generalizes the previously discussed SIR model. The properties of positivity and boundedness of the state trajectory solution are stated and proved. Section 4 studies the model under partial or total quarantines of some or all the subpopulations so that a hospital management objective on temporary availability of technical means and bed disposal can be fulfilled. Section 5 presents and discusses some numerically worked examples under model parameterizations of the COVID-19 pandemic to test the efficiency of several quarantine interventions. Lastly, conclusions end the paper. Appendix A and Appendix B with details of some auxiliary calculations are also given.
2. A Quarantined SIR Epidemic Model
The effects of total or partial quarantine interventions imply that the transmission rates decrease since the number of contagious contacts decreases. See, for instance, References [38,39]. The quarantines on the susceptible have the qualitative effect of an impulsive vaccination since part of the susceptible population is kept away of infective contacts. This is equivalent to reduce, in practice, along a short time interval (instantaneously in an ideal impulsive model), the susceptible subpopulation. Similarly, quarantines on the infectious are similar to impulsive treatment actions on the infectious subpopulation, which is equivalent to reduce its numbers in short time intervals or instantaneously in the ideal case (See References [11,15,18,19]). Quarantine of infectious agents is also referred to as their isolation. However, in order to unify the nomenclature along the paper, the term quarantine will be used for all populations.
Example 1 below on an SIR model without mortality is given as an introductory one to fix some basic ideas about quarantine decisions. Such decisions might be made on both susceptible and infectious decisions based on the hospital management regarding availability of beds and other hospital technical means necessary for infection treatment on seriously infected individuals. The solution for this model is obtained directly from the differential equations and it is then extended to more general SEIR models including quarantine actions and demography with the presence of exposed and asymptomatic individuals. It can be pointed out that the solution for an SIR model with demography has been obtained in Reference [5] by its reduction to an Abel-type equation by using a power-series perturbation approach.
2.1. Example 1 (An SIR Epidemic Model without Demography Subject to Monitored Quarantine)
Consider the subsequent SIR (Susceptible “S”- Infectious “I”- Recovered “R”) without demography and disease mortality:
with , and with , where and are the transmission rate and the recovery rate, respectively. The total population is ; with being constant in view of (1). The solution of (1) is:
Let the estimated fraction of infectious which require hospital care at time (referred to as the “seriously ill infectious subpopulation”) and the upper-bound of hospital admission availability at time . Note the following simple result which is concerned with an eventual quarantine intervention of all the susceptible individuals:
Proposition 1.
Assume that, for a given and an availability upper-bound , it is possible to hospitalize all the seriously ill infectious subpopulations at time t via susceptible quarantine of all the susceptible subpopulations at time , if .
Proof.
Assume that , that is, a total quarantine of the existing susceptible subpopulation at time , or absence of an initial susceptibility for an eventual contagion. Then from Equation (2) for any given if with initial immune amounts given by . □
In general, it might not be necessarily a total quarantine on the susceptible to accomplish with the hospital management requirements provided that the number of initial infections is small enough according to Proposition 1. In this sense, Proposition 1 might be easily generalized to the case of partial quarantine of the susceptible. It can be necessary to keep the hospital bed availability at time along a time interval of a length of at least , which is the recovery average period or up until some time exceeding along the lasting of the most serious period of the illness force. The subsequent Proposition 2 is based on a partial withdrawal of the initial susceptible amounts from the contagion scenario via a quantifying parameter of the fraction population to withdraw from the contagion environment at the time instant where a quarantine intervention is decided. For instance, if a quarantine is decided on the susceptible subpopulation at time , then , where and and the instantaneous net change of the susceptible subpopulation at is . In the following, we show an alternative and confluent interpretation to the finite jumps in the solution decreases the susceptible candidate individuals available for contagion at the time instant . A Dirac impulse of amplitude , namely , with denoting the Dirac delta distribution is applied to the time–derivative of at , which is physically equivalent to the injection of such an instantaneous impulsive vaccination on the susceptibility [43]. Proposition 2 is concerned with a quarantine intervention at on the susceptible subpopulation, which leads to the eventual existence bounded discontinuity of the solution at and an impulsive corresponding time-derivative as a result. The piecewise continuous solution of the differential system is still unique since the finite jump at the discontinuity is calculated explicitly with its left and right limits at the discontinuity point.
Proposition 2.
Assume that and for some , where is the amount of initial susceptible submitted to quarantine at the initial time instant . Then, the following properties hold.
- (i)
- The constraintis fulfilled for some givenifprovided that.
- (ii)
- The constraintis fulfilled forfor any givenand some prefixedif and only if. Ifand; then the constraintis fulfilled forif.
Proof.
The hospital management availability objective is fulfilled at time t if and only if:
or, equivalently, if and only if
which is satisfied for some if and only if . Property (i) has been proved. Property (ii) is a direct extension of Property (ii). □
Related to Proposition 2, note that and . Then, is the instantaneous relative variation of the susceptible subpopulation at the impulsive time instant where a quarantine intervention is applied on the susceptible. In the same way, is the relative negative decrement of the population at because of the quarantine action. Similar interpretations apply for any quarantined subpopulation as it will be discussed later on in this section and in the remaining sections.
Note that Proposition 1 describes the total initial quarantine of the susceptible as a particular case in Proposition 2 with . Proposition 2 establishes a guaranteed fraction of the susceptible submitted to quarantine for achieving the hospital availability of serious cases at a certain time instant or time interval.
Example 1 is now extended with two combined quarantine interventions on the susceptible and infectious subpopulations, respectively. For exposition simplicity, it is assumed in example 1 and in example 2 below that the transmission rate becomes constant. In practice, such a parameter decreases as the number of infectious-susceptible contacts decreases and increases since such a number increases. This fact would be taken into account along Section 4 and Section 5 where a more general epidemic model will be discussed.
2.2. Example 2 (A Partial Quarantine on the Susceptible Followed by a Later Partial Quarantine on the Infectious)
Example 1 can be generalized by programming a first partial quarantine on the susceptible and a later partial quarantine on the infectious so that certain susceptible amounts can be protected from the infection and some infectious are then taken apart from the successive propagation of the infection. This makes sense if there are relatively few susceptible and relevant infectious numbers in the time where the action starts and it is the considered case. Later on, it is considered the case when there are initially relatively few infectious cases, which is a fraction of known cases submitted to quarantine, while, later on, as the infection progresses, there are relevant numbers of susceptible cases, which need to be protected as the infectious cases are increasing. This has been recently the case in many countries around the world concerning the COVID19 has out-broken from epidemic to pandemic [38,46,47,48,49,50].
Thus, assume that, at a time instant , a fraction is submitted to quarantine on the interval and that, at a time instant , a fraction of the infectious cases is submitted to quarantine on the time interval . Assume, with no loss in generality, that the initial conditions are taken at . The question arises is how to choose reasonably , , T0 = t1 – t0, and such that , for any and some prefixed . The solution trajectory obeys from (1) the following set of equations of which there is at least one per subpopulation, which depends on the infectious evolution only from the initial conditions, in view of Equation (2).
We still keep the notation and term of “recovered” for all the subpopulation while noting the ones which have been transferred as a result of the susceptible quarantine as well as those that do not have permanent or temporary immunity.
2.3. Hospital Management Objective of Example 2 on a Temporary Time Interval
The hospital management availability objective is the fulfilment of ; for any and some prefixed with time intervals to take the quarantine actions defined by and with , that is, one gets from (11) that the objective is fulfilled if:
or equivalently,
which holds if
which still holds under the sufficient condition to be fulfilled on :
or, equivalently, if
under the necessary condition that:
equivalently, if for a given , satisfies the constraints:
equivalently, if for a given , satisfies the constraints:
It becomes clear, as intuitively expected, that the fraction of susceptible allocated in quarantine since time has to be increased as the fraction of infectious in quarantine since time decreases and also as the initial susceptible and infectious subpopulations become larger.
In this case, the quarantine of a fraction of the infectious precedes that of a fraction of the susceptible. The objective is now the fulfilment of the ; for any and some prefixed with time intervals to take the quarantine actions defined by and with ,
for any and some prefixed with time intervals to take the quarantine actions defined by and with . Then,
or,
guaranteed for all , that is for the largest value of Formula (33) if
, which can be always guaranteed if for the objective , that is for the removal of all the infectious cases via quarantine at if the objective is to keep any number of infectious on the time interval since
Assume that the information about the numbers of infections and those susceptible are not precise. Therefore, the number of known infectious cases is at most and that of susceptible cases is at most with known . Therefore, and . We proceed as follows:
2.4. Algorithm 1
It consists of the following main steps:
| Algorithm 1 |
| 1 Step 0. Given are , with ; |
| 2 Fix , |
| 3 Define |
| 4 |
| 5 |
| 6 Step 1. If , then the hospital availability objective is |
| 7 fulfilled without quarantine actions neither on the infectious patients nor |
| 8 on the susceptible individuals. |
| 9 Go to End. |
| 10 Step 2. If , then the hospital availability objective is |
| 11 fulfilled with some quarantine action on the infectious at time . |
| 12 Define. |
| 13 Then,the hospital availability objective is fulfilled with any eventually partial or total quarantine action |
| 14 with fraction at time on the infectious (which is necessarily total if |
| 15 ). Go to End. |
| 16 Step 3. If , then the objective is fulfilled with total |
| 17 quarantine action on the infectious cases at time and some quarantine action on the |
| 18 susceptible ones at the time instant . |
| 19 Define. |
| 20 Then, the hospital availability objective is fulfilled with any eventually partial or total quarantine action |
| 21 with fraction at time on the infectious (which is necessarily total if |
| 22 ). Go to End. |
| 23 Step 4. If , then the hospital availability objective |
| 24 cannot be solved with the time- scheduling specifications of Step 0. |
| 25 Step 5. If possible, decrease one or both of the |
| 26 quarantine time instants to anticipate the quarantine actions. Go to Step 1. Otherwise, go to End. |
The following result holds concerning the feasibility of Step 5 of Algorithm 1.
Proposition 3.
The following properties hold:
- (i)
- Ifandthen, .
- (ii)
- The “if” part of Step 5 of algorithm 1 can always be performed by fixing anyandin Step 1 unless Step 0 has been performed for quarantine time actions.
Proof.
Assume that there exists some such that and for and proceed by contradiction arguments. One has from Equation (2) that:
what implies that
so that
and then so that there exists some such that . Thus, there exists some such that , which contradicts the assumption that and for . As a result, ; and the Property (i) is proven. Property (ii) is a direct consequence of Property (i) and Step 0, Step 1, and Step 5 of algorithm 1 and the fact that the initial conditions may be taken at any initial time t0 > 0. □
Remark 1.
It turns out that the average disease transmission rate depends on the number of contagion contacts between susceptible and infectious cases and it increases with the number of average contacts. Therefore, the above example can be directly generalized without difficulty to a piecewise constant disease transmission rate function defined by for (Equations (5)–(8) and (12)–(13)), , (Equations (9)–(11) and (14)–(16)) and , . Similar considerations apply to example 1.
3. An SEIAR Epidemic Model Subject to Confinement or to Partial Quarantines
3.1. The Model
The ideas of the examples of Section 2 are extended to a more general model. The following SEIAR (susceptible “S”, exposed “E”, symptomatically infectious “I”, asymptomatically infectious “A” and recovered “R”) is proposed:
with initial conditions , , , and with . The parameters have the subsequent interpretation.
is the recruitment rate,
is the natural average death rate,
an are the transmission rates of the symptomatic and asymptomatic infectious cases, respectively.
is the average immunity rate,
is the average transition rate from the exposed to all the infectious, i.e., ,
is the average extra mortality associated with the disease,
is the average natural immune response rate for the whole infectious subpopulation ,
and are the fractions of the exposed that become symptomatic and asymptomatic infectious cases, respectively.
The total population obeys the differential equation:
With the initial condition . The above model has two separate transitions from three exposed to the asymptomatic and symptomatic infectious cases so that the two corresponding transition fractions sum-up unity. At the same time, the model considers that the disease transmission rates of the symptomatic and asymptomatic infectious cases to the susceptible cases are eventually distinct. A reason for that is that the symptomatic case can transmit the illness with stronger force such as by means of a stronger and persistent cough.
3.2. Hospital Management Objective on a Temporary Time Interval
The hospital management objective within the time interval is the fulfilment of the following constraint.
for a given . defines the hospital maximum admissible individuals according to its availability on bed disposal and sanitary necessary means for the seriously ill symptomatic infectious individuals who need medical care in the hospital.
The quarantine time instants are defined by the strictly increasing sequence or by a finite subset of this sequence. Any fraction of a subpopulation submitted to quarantine at time translates into the reduction of its numbers for possible contagions, according to where is the fraction of that subpopulation, which is quarantined. Usually, its maximum is less than one or even small enough than one due to precise knowledge of the current numbers belonging to that population. Since the susceptible, exposed, and asymptomatic infectious cases are not mutually identifiable as separable from the others in most of the cases because, due to the lack of efficient tests or availability for their application, they are typically considered together to establish their numbers in quarantine. The problem is more tractable by considering that the asymptomatic infectious cases are a fraction of the symptomatic ones. The proportionality function is obtained from (40)–(41) in the sequel. First, one gets from (40)–(41), the following solutions:
so that
which is also coherent with a proportionality of initial conditions and which can be calculated for any other previous values of the exposed subpopulation as follows:
and Equations (38)–(39) can then be rewritten via Equation (46) as:
The SEIADR model can be alternatively interpreted as a special case of SEIR by removing the explicit evolution of the asymptomatic infectious cases while considering its influence in the susceptible, exposed, and recovered subpopulations via the proportionality function (47). Therefore, the trajectory solution of Equations (38)–(42) in view of Equations (46) and (48)–(49), can be expressed by Equations (44)–(45), subject to Equation (46), together with the subsequent equations:
Proposition 4.
The following properties hold under finite non-negativity of the initial conditions:
- (i)
- All the subpopulations, and then the total population, are non-negative for all time.
- (ii)
- All the subpopulations, and then the total population, are bounded for all time.
Proof.
The proof of property (i) is immediate by inspection of Equations (44), (45), (50), (51), and (56).
The proof of property (ii) follows by contradiction. Assume that is unbounded. Then, there is a strictly increasing sequence such that as and . Then from Equation (43), , which contradicts that as . Then, is bounded. Since all the subpopulations are non-negative for all time from Property (i), then they are bounded as well for all time and property (ii) has been proven. □
The following result proves that is always bounded for all time. Therefore, it cannot happen that converges asymptotically to a positive value or that it oscillates provided that converges asymptotically to zero. This fact is already known as a consequence of fixing and since, in this case, the asymptomatic subpopulation cannot evolve by exceeding the numbers of the infectious subpopulation.
Proposition 5.
is bounded for any finite initial conditions satisfying .
Proof.
First note that if, , then and finite since is finite since the initial conditions are non-negative and finite. Note also from (44)–(45) that, since and , are nonzero, then and cannot be zero at any finite time even though they can potentially converge asymptotically to zero. Now, assume that is not bounded and proceed by contradiction arguments. Note the following: □
Claim 1.
If is unbounded, then there exists a strictly increasing sequence with being bounded such that is strictly increasing.
Proof of Calim 1.
Assume that this is not the case so that is not strictly increasing for any given strictly increasing and is unbounded. However, if ; , and since is continuous, then is bounded for since this interval is bounded and any sequence . Then, is bounded, which contradicts its unboundedness. Then, if is unbounded, then there exists a strictly increasing sequence such that is strictly increasing. The proof follows by proving that there is no such sequence such that is strictly increasing so that is unbounded. □
Claim 2.
From claim 1, one has that for any with the sequence existing from claim 1. Then, one has from Equation (47) that:
so that
and then
Now, since is strictly increasing, then:
Case (a) If is bounded, and since, is unbounded, then
for some subsequence of so that either the claimed bounded leads to as or is unbounded and then is also unbounded. However, being bounded implies from Equation (51) and from continuity arguments (since a continuous function cannot be unbounded in a finite interval, it is bounded on its boundary) that is bounded for the sequence . Since ; , it follows that are bounded. Thus, if is bounded, then as and, from Equation (44), is bounded and, also, from Equation (40), as . However, as a result, Equation (57) is untrue so that if is unbounded then and are unbounded and Case a is not possible, which leads to consider Case b discussed below.
Case (b) If is unbounded, then is unbounded too. Thus, one gets from (39)–(40) that
and the following contradiction arises for any given finite initial conditions since:
As a result, is bounded if is strictly increasing and Case b is not possible. Since neither case a nor case b are possible, one concludes that is not strictly increasing for any sequence and any given with a positive finite separation between consecutive elements. As a result, is bounded as a result of a contradiction to the initial assumption of the proof.
4. Total Confinement or Partial Quarantines to Achieve the Hospital Availability Objective
We consider a sequence (or a finite denumerable set) of isolated time instants at which the quarantine intervention actions will be applied in order to satisfy some defined management hospital objective of intensive care of the seriously ill patients. If is a finite set, then . If is a sequence, its cardinal is infinity, denoted by , since it is a denumerable set of infinity cardinal.
Note the following facts:
Fact 1.
with for , . Usually, for since the suitable universal confinement of the fraction of population including the non-symptomatic infectious cases cannot be performed because of the fact that basic services in industry, health, transportation, etc. have to be kept and, furthermore, it is not possible to control precisely all the programmed quarantined individuals.
Fact 2.
Usually in practice, , because all the infectious individuals are not known because of the limitation of appropriate testing and the difficulty of identifying those with a low level of symptoms.
Fact 3.
For quasi-universal quarantines, except for the maintenance of the basic services, it can be programmed for the use of identical fractional values , to get the same proportionality of quarantined numbers for all the non-symptomatic infectious cases because of the practical lack of technical means for identifying them separately from the symptomatic infectious.
Fact 4.
The transmission rate is proportional to the susceptible–infectious contacts so that quarantines or total confinements translate into a decrease in the number of contacts and then into smaller values of the disease transmission rate . For exposition and simplicity in the equations’ presentation, it is assumed that the transmission rate is a piecewise constant function , , where and are any consecutive time instants in .
The quarantine fractions for each subpopulation can be programmed at a set of sampling instants belonging to subject to minimum and maximum interval constraints, namely,
Assume that the subpopulation is an “extended like-recovered” subpopulation, which contains the recovered subpopulation while also incorporating the fractions of susceptible, exposed, and infectious cases, which are quarantined. Let the non-negative real sequences , , , and be the maximum suitable values on for for given values ,, and , namely, , , , , . Assume that the quarantine fractions of the subpopulations for are subject to the constraints , , and for given maximum prescribed nonnegative values , , and . They are selected as follows in such a way that the fractions of quarantined subpopulations are as small as possible to get the hospital management objective.
where
The motivation of taking the quarantined subpopulations , according to Equations (61)–(66) is discussed in Appendix B.
The solution trajectories under partial (or total) quarantines of some or all the subpopulations under different fractioned quarantined subpopulations are given in Appendix A. On the other hand, the proof of the above constraint (66) is given in Appendix B. It is a routine exercise to get the following particular case of Equations (65)–(66).
concerning the situation that all the subpopulations are constrained in identical proportions to quarantine and that the maximum allowed values and for the corresponding fractions. It has to be taken into account to set such maximum thresholds, such as the management of minimal services, maintenance of the industrial and sanitary activities, the food supply and its transportation and distribution, the technical impossibility of fully controlling the confinement, etc.
The feasibility of the hospital management objective is formally addressed in the next result.
Proposition 6.
Let , with , be a finite set or a sequence of quarantine time instants with the in-between quarantine time periods being and assume that the hospital management objective within the time interval is the fulfilment of the following constraint.
Proof.
Note that the objective (68) is fulfilled via Equations (69)–(71) with identical quarantine amounts for all the subpopulations and the maximum admissible being closer as much as possible to the targeted for a given if in Equation (69) fulfils Equations (70)–(71). □
Proposition 7.
Assume that the hospital management objective is only required to be fulfilled at the testing time instants of the set rather than at the time intervals in-between consecutive time instants of . Then, Equation (71) becomes modified as follows.
where .
Proof.
It is similar to Proposition 6 by taking into account Remark B1 in Appendix B. □
Remark 2.
As discussed in Remark 1, the transmission rate depends on the number of contacts susceptible to infections, which can decrease significantly under quarantines or confinements. Therefore, may be replaced by a piecewise constant function ; ; in order to generalize the results of this section (See Equations (62) and (63) and the equations in Appendix A and Appendix B).
5. Numerical Simulations
This section contains some numerical simulation examples aimed at illustrating the theoretical background discussed in previous sections. To this end, parameter values corresponding to COVID-19 are considered. It has to be pointed out that reported data regarding COVID-19 exhibit high variability among outbreaks or are even inconsistent. Thus, parameter values could be subject to changes as knowledge on the infection progress. However, the discussion on the effect of the considered counteracting measurements holds regardless of the particular parameterization of the model. The simulations are performed with the values collected in Table 1 and Table 2 for the specific case of the Madrid Region (“Comunidad de Madrid”).

Table 1.
Parameter values employed in simulations.

Table 2.
Initial conditions for simulations.
From Table 2, it can be concluded that simulation starts with the total population being susceptible and a single exposed case. Figure 1 and Figure 2 display the evolution of all populations in the absence of control actions. It is deduced from Figure 1 that the spreading of the disease would end up affecting the total population if no control action was taken, as similarly concluded in Reference [45].
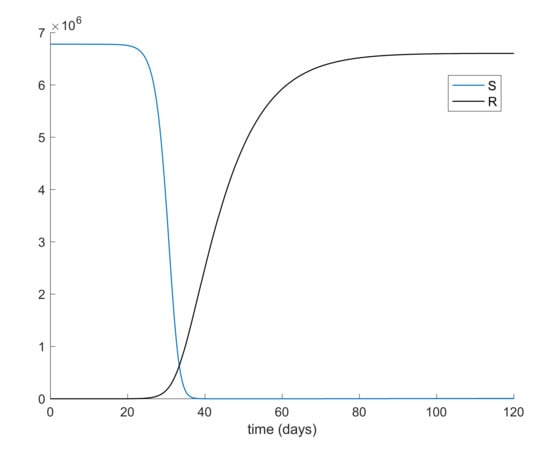
Figure 1.
Evolution of susceptible and recovered in the absence of control actions.
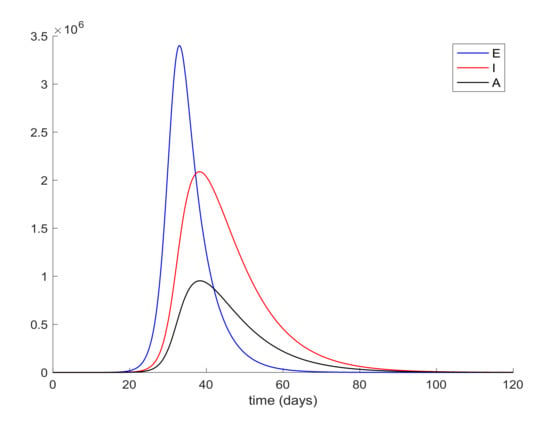
Figure 2.
Evolution of exposed, infectious and asymptomatic in the absence of control actions.
It is also observed in Figure 2 the large number of infected people, I, attained at the infection peak. Such a large number of infected people would definitely overflow the hospital available resources. In order to avoid this situation a quarantine policy will be applied to control the infection spreading. According to [50], it will be considered that the average percentage of infectious cases that require hospitalization is 25%. Thus, the hospital management objective is set as , where denotes the maximum number of available resources. In the Madrid region, the number of available hospital beds is 20,516, [51], so that the management of hospital resources imply that the constraint should be satisfied for all time. This upper-bound is constant since it is the number of installed beds in the health system before the outbreak of the pandemic, which should not be outnumbered by using the proposed control measurements. This situation is depicted in Figure 3 where it is shown that the hospitalized cases may exceed the number of available beds.
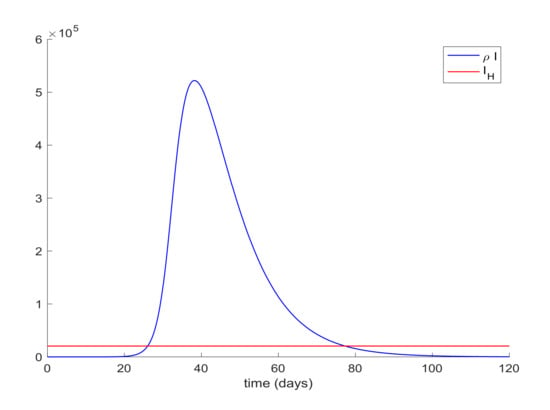
Figure 3.
Graphical representation of the hospital management objective. The infectious curve should lie below the red line representing the number of available beds.
In order to achieve the hospital management objective, quarantine on different populations will be applied. Therefore, the population of infectious cases is assumed t be quarantined (isolated) at different percentages twice per day. This means that every 12 h, a percentage of the infectious population, I, is removed from this compartment due to the fact that they will be isolated and they will no longer spread the infection. Since clinical symptoms may be detected, this population can be quarantined independently from the other ones. Thus, Figure 4 shows the effect of this “isolation of cases” policy. In this way, Figure 4a displays the hospital management objective and the obtained infectious curves for different values of δI ranging from 0.16 to 0.24, so that the percentage of isolated infections range from 16% to 24%. Figure 4b also shows the effect of the impulsive action on the infectious evolution.
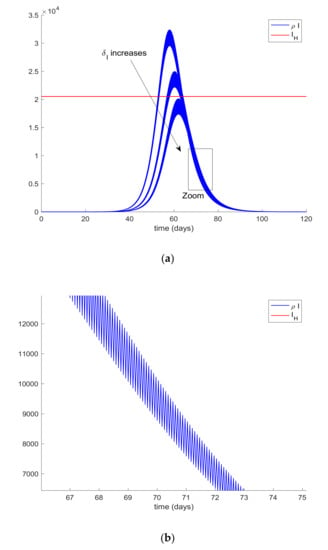
Figure 4.
(a) Isolation (quarantine) of infectious with declared symptoms. (b) Zoom on the plot showing the effect of the impulsive action.
Figure 4 is obtained by assuming that the infectious cases are isolated from the first day of simulation. However, the first day of quarantine (isolation) application may be delayed by a number of days (implying that cases are not isolated and they can still spread the infection for more days) due to several reasons. In this case, the results depicted in Figure 5 are obtained for the same values of δI as before (from 0.16 to 0.24). Thus, Figure 5a displays the evolution of infectious cases when the isolation of cases policy starts 25 days before the first exposed case appears while Figure 5b displays the infectious evolution when the isolation policy starts 30 days after. The isolated individuals are removed from the I -compartment.
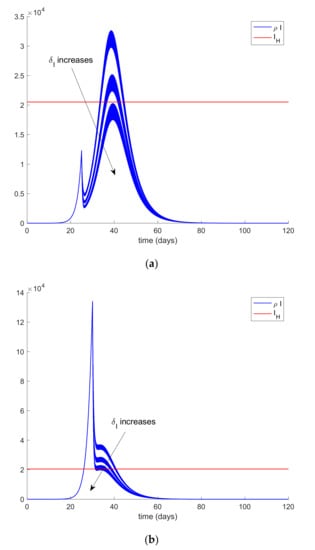
Figure 5.
Effect of delayed infectious quarantine on hospital objective achievability: (a) delay of 25 days, (b) delay of 30 days.
It can be deduced from Figure 5 that the hospital objective may still be accomplished if the quarantine policy is not delayed too much while, if the delay in the isolation application exceeds a certain threshold, then the hospital objective may not be satisfied. Consequently, it is revealed as crucial to isolate the cases as soon as possible. The limit value for the quarantine percentage that allows fulfilling with the hospital objective at all time points can be calculated by trial-error simulation by using a search algorithm such as Algorithm 1 discussed in Section 2 or by using Equations (61)-(66) evaluated at quarantine application time instants. In this case, simulations for different values are performed to analyze the effect of changing the quarantine percentage procedure that, in turn, allows finding its limit value. On the other hand, Figure 6 displays the evolution of the infectious and hospital beds threshold when a quarantine on the general population is applied at day 20 after the first exposed individual is introduced in the population. This situation is modeled as the reduction of the same percentages of individuals from all populations at day 20. This may be the general situation when a universal lock down is decreed as it was the case of Spain. Therefore, the values of δS = δE = δI = δA range between 0.8 and 0.9, which implies that the quarantine involves up to 90% of the whole population. It is observed in Figure 6 that the application of quarantine reduces the peak of infectious cases with respect to not taking any measure but, depending on the percentage, may not be enough to achieve the hospital objective. Figure 7 displays, as an example, the abrupt change on the susceptible when the quarantine is applied at day 20. Furthermore, Figure 8 displays the change in the evolution of the infectious cases when quarantine is applied at different starting times. Thus, we fix δS for all populations and the day when quarantine is applied ranges from 3 to 24 days after the first exposed is introduced in the population. The fact for applying quarantine earlier moves the peak to the right but does not change its value. Therefore, when the quarantine is applied to such a large percentage of population, the time of application is not that crucial. In addition, it is revealed to be more appropriate for early detection and isolation of cases than the quarantine of a large percentage of general population since it allows attaining the hospital management objective without locking down a large amount of individuals.
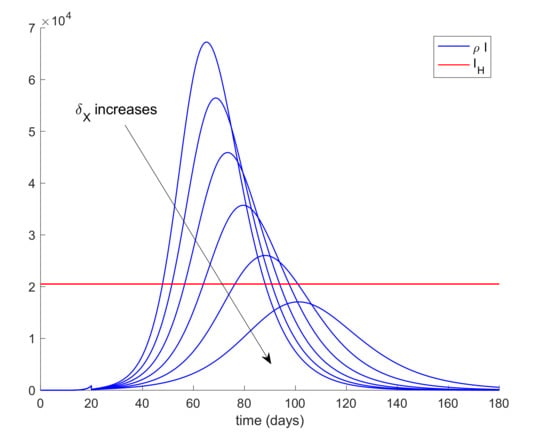
Figure 6.
Effect on the infectious cases of the quarantine of the entire population.
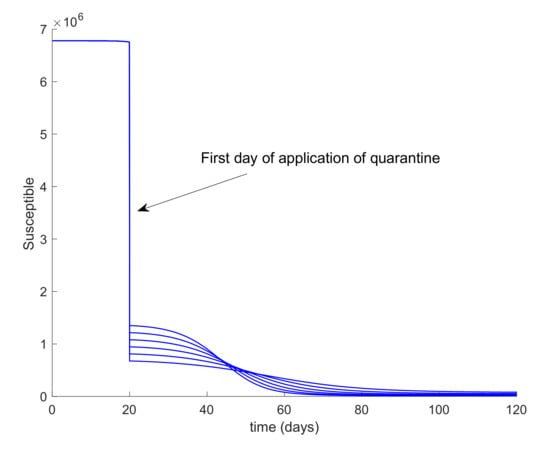
Figure 7.
Evolution of the susceptible cases when quarantine is applied.
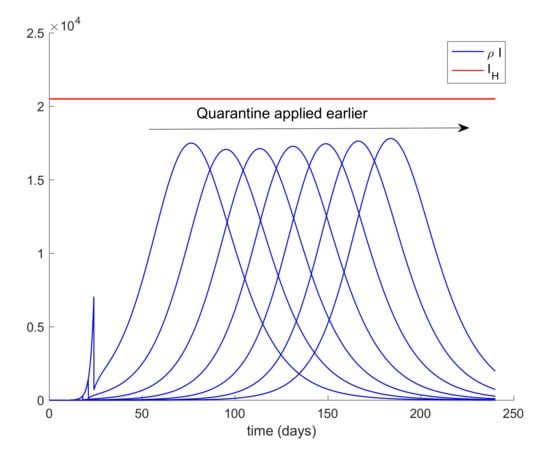
Figure 8.
Effect of the day of application of quarantine in the evolution of the infectious cases.
In addition, Figure 9 displays the evolution of infectious cases when quarantining 80–90% of the entire population is ordered at day 20 and relaxed at day 90 while Figure 10 shows the infectious cases when quarantine is lifted at day 300. In all these cases, all quarantined populations are added to the susceptible population once quarantine finishes. It is deduced from Figure 9 and Figure 10 that, when quarantine is lifted, the number of infectious cases rebounds (end exceeds the hospital availability threshold) no matter how long quarantine had been maintained. Thus, the sole application of a general quarantine is not a sufficient control action to deal with the infection spreading since rebounding may occur at the end of quarantine. Figure 11 shows the effect of isolating 70% of infectious individuals every 6 h after day 40 when a general quarantine for 90% of the population is decreed from day 20 to day 90. It can be concluded from Figure 9, Figure 10 and Figure 11 that an important measure is the early detection and isolation of cases in order to prevent new outbreaks once a situation of quarantine is relaxed.
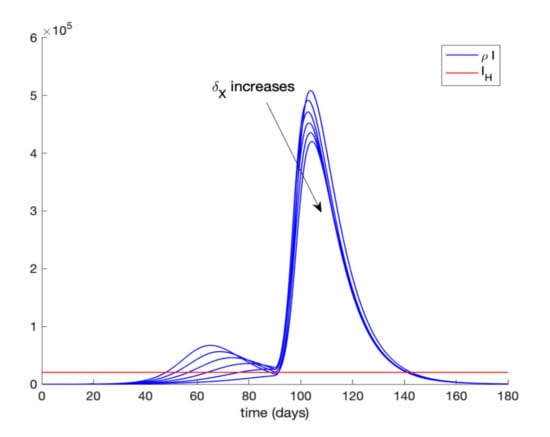
Figure 9.
Evolution of the infectious cases when general quarantine is applied at day 20 and relaxed at day 90.
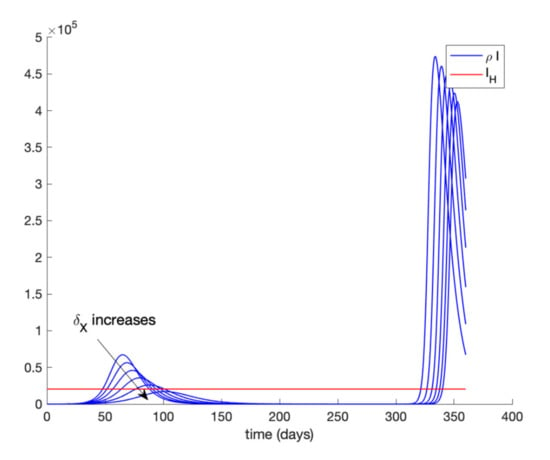
Figure 10.
Evolution of the infectious cases when general quarantine is applied at day 20 and relaxed at day 300.
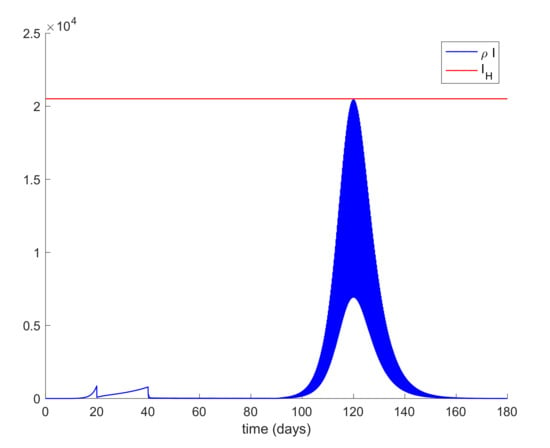
Figure 11.
Evolution of the infectious cases when general quarantine is applied at day 20 and lifted at day 90 while isolation of 70% of infectious individuals is applied every 6 h after day 40.
Lastly, quarantine is commonly implemented with social distancing measurements. Social distancing has the effect of reducing the infectivity factor, . This factor can also be reduced due to the quarantine application discussed before. Therefore, the last simulation will deal with the case when is a piecewise constant function. In this way, the value of displayed in Figure 12 is proposed for simulation. Figure 13 depicts the evolution of the infectious cases with the displayed piecewise constant beta and no other control action. It is observed in Figure 13 that the hospital requirement is fulfilled in this case. Thus, an early reduction of the infectivity rate is crucial to control the infection spreading. Figure 14 shows the effect of joining a reduction of the infectivity rate with quarantine of 50% of the entire population from day 20. It can be concluded that the joint effect is able to attain the hospital requirement in an easier way than by using a single method.
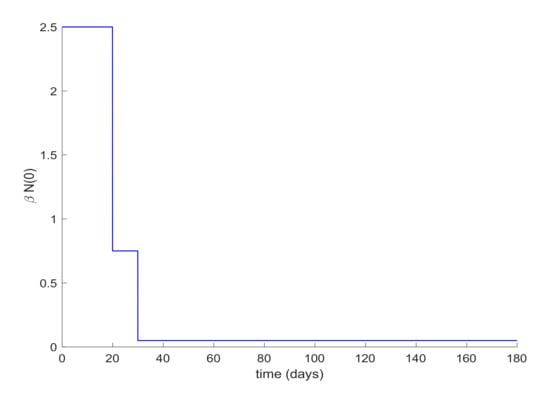
Figure 12.
Piecewise constant β function.
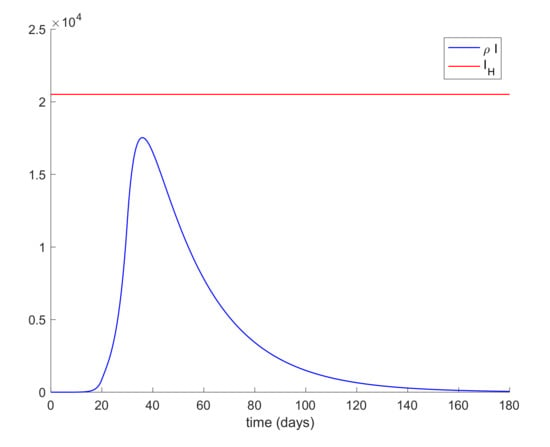
Figure 13.
Evolution of infectious and hospital objective with piecewise constant β.
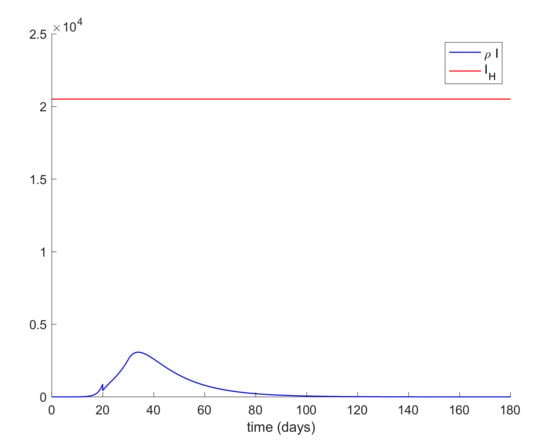
Figure 14.
Evolution of infectious and hospital objective with piecewise constant β and quarantine in all populations of 50% from day 20.
It can be pointed out that current data regarding Covid-19 exhibit high variability between outbreaks and places and many of them include many inconsistencies such as a negative number of deaths in order to regularize incorrectly informed data. It was very common to give erroneous data at the beginning of the infection outbreak since only the seriously infected individuals were tested. Therefore, those with unserious symptoms and those being asymptomatic were not tested. Thus, the confrontation of the model with real data will require an important work of data gathering and analyzing. Therefore, the total number of total infectious cases at any time of the infection evolution can be roughly estimated by the number of deaths caused by the illness with the estimated proportion of 1%-1.5% of deaths from all of the infectious individuals in accordance with recently reported estimations. Basically, the model adequacy analysis could be performed by comparing the actual number of deaths and ICU hospitalized patients from the results obtained from the model. This process will definitely require an appropriate definition of cases and the review of previously informed data. On the other hand, the transmission rate of the symptomatic and asymptomatic infectious subpopulations can be updated through from the corresponding given data by the health system. The remaining parameters of Table 1 can be updated from medical data on hospital records and testing records on populations. This methodology may be useful to adjust the model parameterization from recorded data on the disease evolution.
6. Conclusions
This paper has considered the problem of partial or total quarantines of the susceptible and the susceptible and infectious populations of both a simple SIR model and a more general SEIAR model with mortality and demography. Such a model incorporates the asymptomatic infectious subpopulation to the usual SEIR models. The proposed model is studied under either partial or total quarantines of some or of all of the subpopulations in order to satisfy prescribed hospital availability requirements on bed disposal and other necessary treatment means. The quarantined fractions of one or various involved subpopulations can be mutually distinct. In this way, the total confinement becomes a particular case of quarantine intervention. The hospital objective to be fulfilled is being prescribed through time as a monitored design constraint on the seriously infectious subpopulations, which needs hospital care. Such a subpopulation is assumed to be a fraction of the total infectious individuals while the objective management establishes that their numbers should be kept below a prefixed absolute upper-boundary, which cannot be violated. Some simulated numerical examples are also discussed by using modelling parameterizations related to the current COVID-19 pandemic. Those simulations corroborate that quarantine interventions with enough anticipation related to the pandemic outbreak and the appropriate identification of cases are efficient tools for the fulfilment of required hospital management objectives.
Author Contributions
Conceptualization, M.D.L.S., A.I., and R.P.A. Methodology, M.D.L.S. Software, A.I. Validation, A.I., M.D.L.S., and R.P.A. Formal analysis, M.D.L.S. and R.P.A. Investigation, M.D.L.S. and A.I. Resources, A.I. and R.P.A. Data curation, A.I. Writing—original draft preparation, M.D.L.S. and A.I. Writing—review and editing, M.D.L.S., R.P.A., and A.I. Visualization, A.I. Supervision, R.P.A. Project administration, M.D.L.S. Funding acquisition, M.D.L.S. All authors have read and agreed to the published version of the manuscript.
Funding
The Spanish Institute of Health Carlos III under Grant COV 20/01213, the Spanish Government and the European Commission under Grant RTI2018-094336-B-I00 (MCIU/AEI/FEDER, UE), and the Basque Government under Grant IT1207-19 funded this research. The Spanish Institute of Health Carlos III funded the APC.
Acknowledgments
The authors are grateful to the Spanish Government for Grant RTI2018-094336-B-I00 (MCIU/AEI/FEDER, UE) and to the Basque Government for Grant IT1207-19. They also thank the Spanish Institute of Health Carlos III for its support through Grant COV 20/01213. Finally, they thank the referees and editor for their useful suggestions.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A. Evolution Equations for Quarantines In-between to Consecutive Time Instants and Their Counterparts for Accumulated Quarantines
Let be a sequence of confinement time instants. One has from Fact 1 and (38)–(42) that:
The above result can be generalized to the contributions for a set of intermediate confinement time instants. To proceed with such a generalization, we first prove the following result for time-varying, impulsive, real scalar differential equations.
Theorem A1.
Consider the scalar differential equation.
where are bounded piecewise continuous functions, with , ; , is a finite or (denumerable) infinite set or real numbers and is given. Then,
is the unique solution of the above impulsive differential equation for ; , and, for all, if is the maximum element of if such a set has a finite cardinal.
Proof.
It is organized by induction. Take a time instant ; . Then,
where ; . Note that for :
By using recursion to compute from the solution at the preceding and so on until reaching yields:
and the proof is complete. □
In general, for a sequence of potential confinement time instants, by taking with no loss in generality, one obtains the following set of equations for the various subpopulations by proceeding recursively from the above Equations (A1)–(A11) and Theorem A1.
Appendix B. Discussion of the Quarantined Choices According to (61)–(66)
One gets from (A1)–(A9) that
with provided that
provided that
provided that
provided that
and from (A10)–(A11), one gets:
Remark A1.
If in (A20), (A22), (A24), and (A26), one replaces , , and so that the hospital objective is only required at the time instants of . Then the decreased fractions of (A21), (A23), (A25), and (A28) of the involved subpopulations become modified with the replacements:
. and , and
according to (A2), (A5), (A7) and (A9).
References
- Rass, L.; Radcliffe, J. Spatial Deterministic Epidemics, Mathematical Surveys and Monographs; American Mathematical Society: Providence, RI, USA, 2003. [Google Scholar]
- Keeling, M.J.; Rohani, P. Modeling Infectious Diseases; Princeton University Press: Princeton, NJ, USA, 2008. [Google Scholar]
- Shuai, Z.S.; Van der Driessche, P. Global stability of infectious disease models using Lyapunov functions. SIAM J. Appl. Math. 2013, 73, 1513–1532. [Google Scholar] [CrossRef]
- De la Sen, M.; Nistal, R.; Alonso-Quesada, S.; Ibeas, A. Some formal results on positivity, stability and endemic steady-state attainability based on linear algebraic tools for a class of epidemic models with eventual incommensurate delays. Discret. Dyn. Nat. Soc. 2019, 2019, 1–22. [Google Scholar] [CrossRef]
- Harko, T.; Lobo, F.S.N.; Mak, M.K. Exact analytical solutions of the susceptible-infected-recovered (SIR) epidemic model and on SIR model with equal death and birth rates. Appl. Math. Comput. 2014, 236, 184–194. [Google Scholar] [CrossRef]
- Hethcote, H.W. The mathematics of infectious diseases. SIAM Rev. 2000, 42, 599–653. [Google Scholar] [CrossRef]
- Verma, R.; Sehgal, V.K.; Nitin, V. Computational stochastic modelling to handle the crisis occurred during community epidemic. Ann. Data. Sci. 2016, 3, 119–133. [Google Scholar] [CrossRef]
- Iggidr, A.; Souza, M.O. State estimators for some epidemiological systems. J. Math. Biol. 2018, 78, 225–256. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Goel, K.; Nilam. A deterministic time-delayed SIR epidemic model: Mathematical modelling and analysis. Theory Biosci. 2020, 139, 67–76. [Google Scholar] [CrossRef]
- Taylor, M.I.; Carr, T.W. An SIR epidemic model with partial temporary immunity modelled with delay. J. Math. Biol. 2009, 59, 841–880. [Google Scholar] [CrossRef]
- Bai, L.; Nieto, J.J.; Uzal, J.M. On a delayed epidemic model with non-instantaneous impulses. Commun. Pure Appl. Anal. 2020, 19, 1915–1930. [Google Scholar] [CrossRef]
- McCluskey, C.C. Global stability of an SIR epidemic model with delay and general nonlinear incidence. Math. Biosci. Eng. 2010, 7, 837–850. [Google Scholar]
- De la Sen, M.; Ibeas, A.; Alonso-Quesada, S.; Nistal, R. On a SIR model in a patchy environment under constant and feedback decentralized controls with asymmetric parameterizations. Symmetry 2019, 11, 430. [Google Scholar] [CrossRef]
- Cui, S.B.; Bai, M. Mathematical analysis of population migration and its effects to spread of epidemics. Discret. Contin. Dyn. Syst. Ser. B 2015, 20, 2819–2858. [Google Scholar] [CrossRef]
- De la Sen, M.; Alonso-Quesada, S.; Ibeas, A.; Nistal, R. On an SEIADR epidemic model with vaccination, treatment and dead-infectious corpses removal controls. Math. Comput. Simul. 2019, 163, 47–79. [Google Scholar] [CrossRef]
- Al-Darabsah, I.; Yuan, Y. A time-delayed epidemic model for Ebola disease transmission. Appl. Math. Comput. 2016, 290, 307–325. [Google Scholar] [CrossRef]
- He, Z.L.; Nie, L.F. The effect of pulse vaccination and treatment on SIR epidemic model with media impact. Discret. Dyn. Nat. Soc. 2015, 2015. [Google Scholar] [CrossRef]
- Hou, J.; Teng, Z.D. Continuous and impulsive vaccination of SEIR epidemic models with saturation incidence rates. Math. Comput. Simul. 2009, 79, 3038–3054. [Google Scholar] [CrossRef]
- D’Onofrio, A. Mixed pulse vaccination strategy in epidemic model with realistically distributed infectious and latent times. Appl. Math. Comput. 2004, 151, 181–187. [Google Scholar] [CrossRef]
- Ameen, I.; Baleanu, D.; Ali, H.M. An efficient algorithm for solving the fractional optimal control of SIRV epidemic model with a combination of vaccination and treatment. Chaos Solitons Fractals 2020, 137. [Google Scholar] [CrossRef]
- Boonyaprapasorn, A.; Natsupakpong, N.; Ngiamsunthorn, P.S.; Thung-Od, K. An application of finite time synergetic control for vaccination in epidemic systems. In Proceedings of the 2017 IEEE Conference on Systems, Process and Control (ICSPC), Melaka, Malaysia, 14–17 December 2017. [Google Scholar]
- Boonyaprapasorn, A.; Natsupakpong, N.; Ngiamsunthorn, P.S.; Thung-Od, K. Fractional order sliding mode control for vaccination in epidemic systems. In Proceedings of the 2017 2nd International Conference on Control and Robotics Engineering (ICCRE 2017), Bangkok, Thailand, 3–7 April 2017. [Google Scholar]
- Sethaput, T.; Boonyaprapasorn, A. Fractional order sliding mode control applying on the HIV infection system. In Proceedings of the 2018 International Conference on Artificial Life and Robotics, Beppu, Japan, 1–4 February 2018; pp. 157–160. [Google Scholar]
- Ibeas, A.; De la Sen, M.; Alonso-Quesada, S. Robust sliding control of SEIR epidemic models. Math. Probl. Eng. 2014, 2014, 104764. [Google Scholar] [CrossRef]
- De la Sen, M.; Nistal, R.; Ibeas, A.; Garrido, A.J. On the use of entropy issues to evaluate and control the transients in some epidemic models. Entropy 2020, 22, 534. [Google Scholar] [CrossRef]
- De la Sen, M.; Ibeas, A.; Nistal, R. On the entropy of events under eventually inflated or deflated probability constraints. Application to the supervision of epidemic models under vaccination controls. Entropy 2020, 22, 284. [Google Scholar] [CrossRef]
- Wang, W.B.; Wu, Z.N.; Wang, C.F.; Hu, R.F. Modelling the spreading rate of controlled communicable epidemics through and entropy-based thermodynamic model. Sci. China Phys. Mech. Astron. 2013, 56, 2143–2150. [Google Scholar] [CrossRef] [PubMed]
- Koivu-Jolma, M.; Annila, A. Epidemic as a natural process. Math. Biosci. 2018, 299, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, J. A mathematical model for the novel coronavirus epidemic in Wuhan, China. AIMS Math. Biosci. Eng. 2020, 17, 2708–2724. [Google Scholar] [CrossRef]
- Huo, H.F.; Ma, Z.P. Dynamics of a delayed epidemic model with non-monotonic incidence rate. Commun. Nonlinear Sci. Numer. Simul. 2010, 15, 459–468. [Google Scholar] [CrossRef]
- Li, X.; Liu, W.Y.; Zhao, C.L.; Zhang, X.; Yi, D.Y. Locating multiple sources of contagion in complex networks under the SIR model. Appl. Sci. 2019, 9, 4472. [Google Scholar] [CrossRef]
- Yang, H.M.; Freitas, A.R. Biological view of vaccination described by mathematical modellings: From rubella to dengue vaccines. Math. Biosci. Eng. 2019, 16, 3195–3214. [Google Scholar] [CrossRef]
- Thakare, P.R.; Mathurkar, S.S. Modeling of epidemic spread by social interactions. In Proceedings of the 2016 IEEE International Conference on Recent Trends in Electronics, Information & Communication Technology (RTEICT), Bangalore, India, 20–26 May 2016; pp. 1320–1324. [Google Scholar] [CrossRef]
- Darabi Sahneh, F.; Scoglio, C. Epidemic spread in human networks. In Proceedings of the 2011 50th IEEE Conference on Decision and Control and European Control Conference, Orlando, FL, USA, 12–15 December 2011; pp. 3008–3013. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H. Epidemic source tracing on social contact networks. In Proceedings of the 2015 17th International Conference on E-Health Networking, Application & Services (HealthCom), Boston, MA, USA, 14–17 October 2015; pp. 354–357. [Google Scholar] [CrossRef]
- Zha, W.T.; Pang, F.R.; Zhou, N.; Wu, B.; Liu, Y.; Du, Y.B.; Hong, X.Q.; Lv, Y. Research about the optimal strategies for prevention and control of varicella outbreak in a school in a central city of China: Based on an SEIR dynamic model. Epidemiol. Infect. 2020, 148, e56. [Google Scholar] [CrossRef]
- Ng, K.Y.; Gui, M.M. COVID-19: Development of a robust mathematical model and simulation package with consideration for ageing population and time delay for control action and resusceptibility. Phys. D Nonlinear Phenom. 2020, 411, 132599. [Google Scholar] [CrossRef]
- Kumar, R.K.; Rani, M.; Bhagavathula, A.S.; Sah, R.; Rodriguez-Morales, A.J.; Kalita, H.; Nanda, C.; Sharma, S.; Sharma, Y.D.; Rabaan, A.A.; et al. Prediction of the COVID-19 pandemic for the top 15 affected countries: Advanced autoregressive integrated moving average (ARIMA) model. JMIR Public Health Surveill. 2020, 6, e19115. [Google Scholar] [CrossRef]
- Kuniya, T.; Inaba, H. Possible effects of mixed prevention strategy for COVID-19 epidemic: Massive testing, quarantine and social distance. AIMS Public Health 2020, 7, 490–503. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. Death Toll Estimation for COVID-19: Is the Curve Flattened Yet? Available online: https://ssrn.com/abstract=3592343 (accessed on 24 September 2020).
- Mortality Rate of COVID-19 in Spain as of May 22, 2020, by Age Group. Available online: https://www.statista.com/statistics/1105596/covid-19-mortality-rate-by-age-group-in-spain-march (accessed on 9 June 2020).
- Abdulrahman, I.K. SimCOVID: An Open Source Simulation Program for the COVID-19 Outbreak. medRxiv. Paper in Collection COVID-19 SARS-CoV-2 Preprints from medRxiv and bioRxiv 2020. Available online: https://www.medrxiv.org/content/10.1101/2020.04.13.20063354v2 (accessed on 24 September 2020).
- De la Sen, M.; Agarwal, R.P.; Ibeas, A.; Alonso-Quesada, S. On a generalized time-varying SEIR epidemic model with mixed point and distributed delays and combined regular and impulsive vaccination controls. Adv. Differ. Equ. 2010, 2010, 281612. [Google Scholar] [CrossRef]
- Demographic data of Madrid. Available online: http://www.madrid.org/iestadis/fijas/estructu/demograficas/mnp/estructuespevida.htm (accessed on 29 June 2020).
- Mishra, A.M.; Purohit, S.D.; Owolabi, K.M.; Sharma, Y.D. A nonlinear epidemiological model considering asymptomatic and quarantine classes for SARS CoV-2 virus. Chaos Solitons Fractals 2020, 138, 109953. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Xu, Y.; Sun, C.; Wang, X.; Guo, Y.; Qiu, S.; Ma, K. A systematic review of asymptomatic infections with COVID-19. J. Microbiol. Immunol. Infect. 2020. [Google Scholar] [CrossRef] [PubMed]
- Ivorra, B.; Fernandez, M.R.; Vela-Pérez, M.; Ramos, A.M. Mathematical modeling of the spread of the coronavirus disease 2019 (COVID-19) taking into account the undetected infections. The case of China. Commun. Nonlinear Sci. Numer. Simul. 2020, 88, 105303. [Google Scholar] [CrossRef]
- De la Sen, M.; Ibeas, A.; Garrido, A.J. On the estimation of some relevant parameters in the COVID-19 pandemic. In Proceedings of the 9th International Conference on Mathematical Modeling in Physical Sciences, Paper ID C01-Y20-P167, Tinos Island, Greece, 7–10 September 2020. Journal of Physics Conference Series. [Google Scholar]
- Hiroshi, N.; Kobayashi, T.; Miyama, T.; Suzuki, A.; Jung, S.; Hayashi, K.; Kinoshita, R. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID- 19). Int. J. Infect. Dis. 2020, 94, 154–155. [Google Scholar] [CrossRef]
- Percentage of COVID-19 Cases in the United States from February 12 to March 16, 2020 That Resulted in Hospitalization, by Age Group. Available online: https://www.statista.com/statistics/1105402/covid-hospitalization-rates-us-by-age-group (accessed on 29 June 2020).
- Distribución del Número de Camas en Hospitales en España en 2019, por Comunidad Autónoma. Available online: https://es.statista.com/estadisticas/578785/numero-total-de-camas-en-hospitales-en-espana-por-comunidad-autonoma (accessed on 29 June 2020).
- Ahmed, N.; Malik, M.R.; Baleanu, D.; Alshomrani, A.S.; Rehman, M. Positive explicit and implicit computational techniques for reaction-diffusion epidemic model of dengue disease dynamics. Adv. Differ. Equ. 2020, 2020, 1–22. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Lu, D.C.; Zhou, J.B.; Wei, J.D. Existence of travelling wave solutions with critical speed in a delayed diffusive epidemic model. Adv. Differ. Equ. 2019, 2019, 1–21. [Google Scholar] [CrossRef]
- Xu, J.H.; Geng, Y. A non-standard finite difference scheme for a multi-group epidemic model with time delay. Adv. Differ. Equ. 2017. [Google Scholar] [CrossRef]
- Keeling, M.J.; Eames, K.T.D. Networks and epidemic models. J. R. Soc. Interface 2005, 2, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Magal, P.; Webb, G. The parameter identification problem for SIR epidemic models: Identifying unreported cases. J. Math. Biol. 2018, 77, 1629–1648. [Google Scholar] [CrossRef]
- Liu, W.; Yue, X.G.; Tchounwou, P.B. Response to the COVID-19. Epidemic: The chinese experience and implications for other countries. Int. J. Environ. Res. Public Health 2020, 17, 2304. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).