3D Printed Personalized Corneal Models as a Tool for Improving Patient’s Knowledge of an Asymmetric Disease
Abstract
1. Introduction
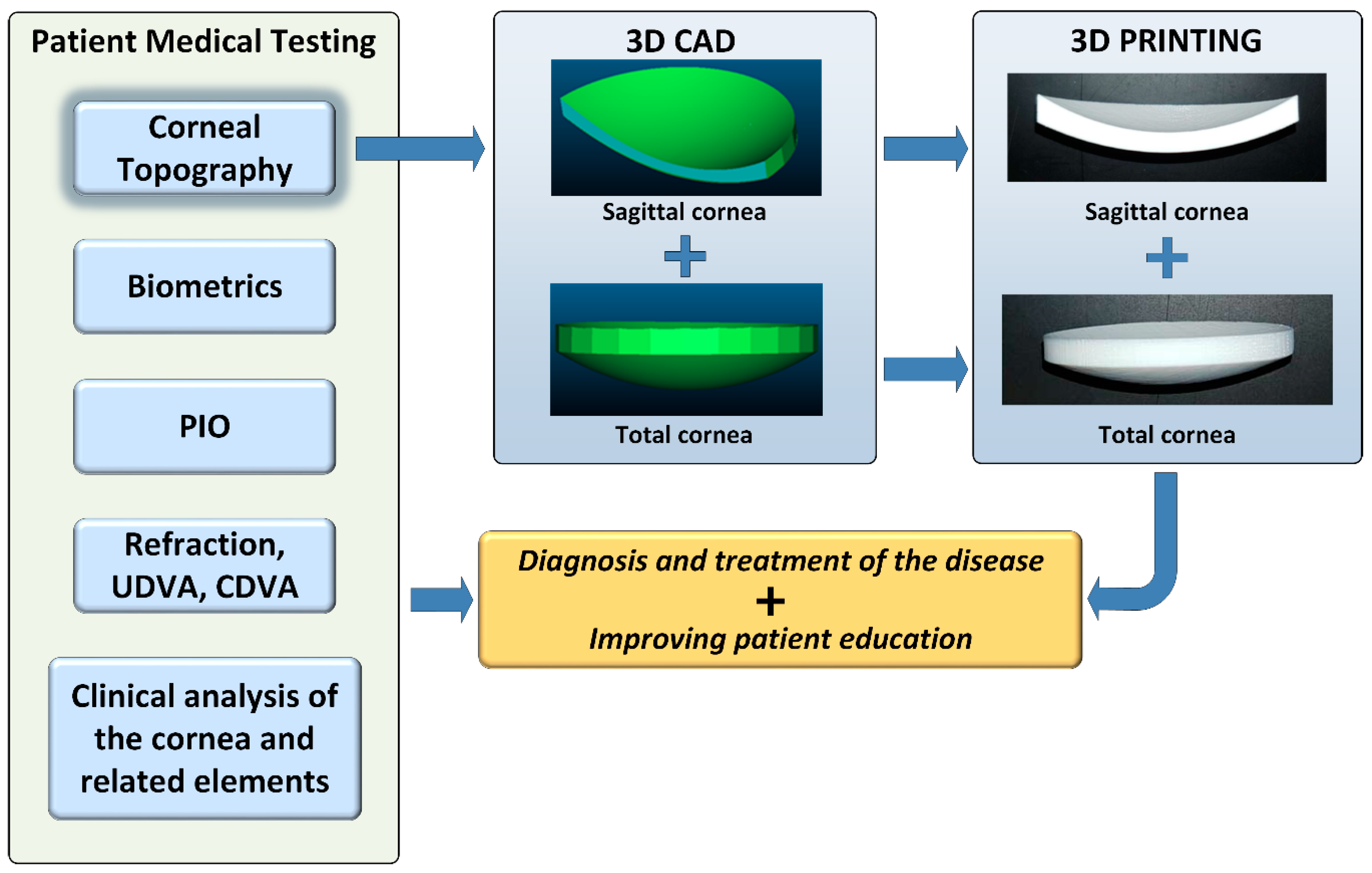
2. Material and Methods
2.1. Patients
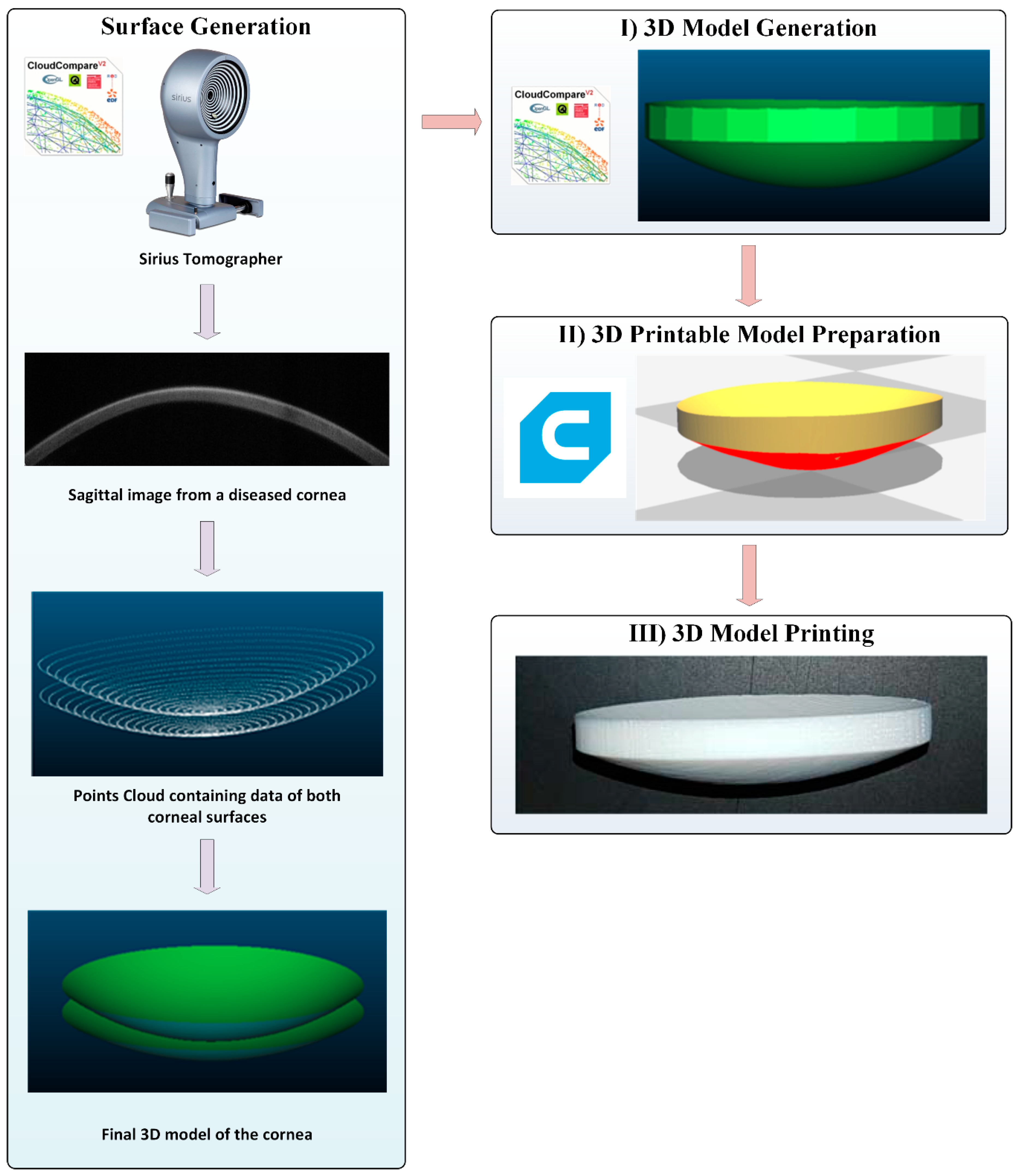
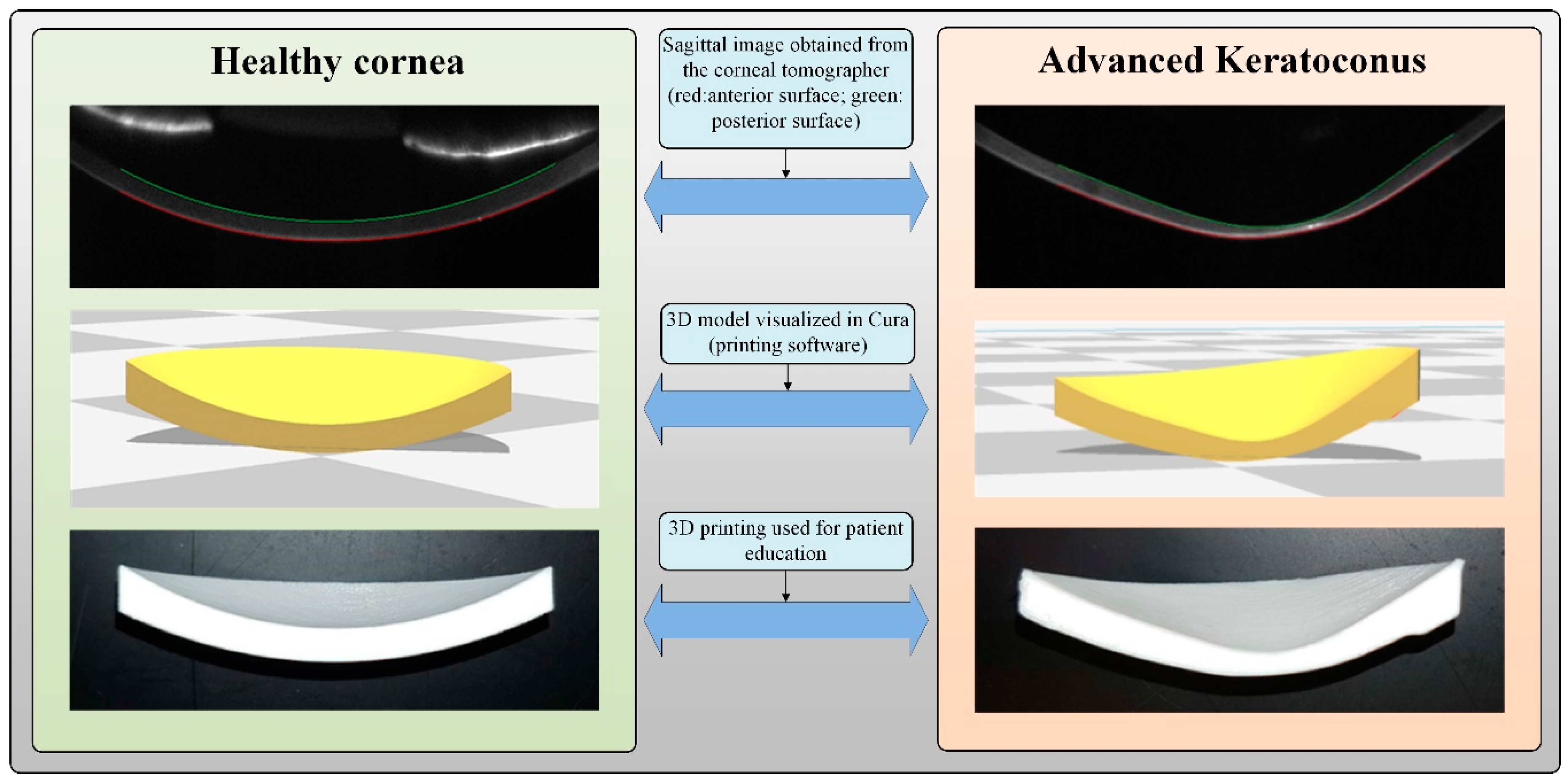
2.2. Methods
2.3. Questionnaire
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rabinowitz, Y.S. Keratoconus. Surv. Ophthalmol. 1998, 42, 297–319. [Google Scholar] [CrossRef]
- Cavas-Martinez, F.; De la Cruz Sanchez, E.; Nieto Martinez, J.; Fernandez Canavate, F.J.; Fernandez-Pacheco, D.G. Corneal topography in keratoconus: State of the art. Eye Vis. (Lond. UK) 2016, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Salisbury, H. Helen Salisbury: The informed patient. BMJ 2019, 364, l638. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhang, X. 3D Printing: Print the future of ophthalmology. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5380–5381. [Google Scholar] [CrossRef]
- Pinero, D.P. Technologies for anatomical and geometric characterization of the corneal structure and anterior segment: A review. Semin. Ophthalmol. 2015, 30, 161–170. [Google Scholar] [CrossRef]
- Bauermeister, A.J.; Zuriarrain, A.; Newman, M.I. Three-Dimensional Printing in Plastic and Reconstructive Surgery: A Systematic Review. Ann. Plast. Surg. 2016, 77, 569–576. [Google Scholar] [CrossRef]
- Preece, D.; Williams, S.B.; Lam, R.; Weller, R. “Let’s get physical”: Advantages of a physical model over 3D computer models and textbooks in learning imaging anatomy. Anat. Sci. Educ. 2013, 6, 216–224. [Google Scholar] [CrossRef]
- Watson, R.A. A low-cost surgical application of additive fabrication. J. Surg. Educ. 2014, 71, 14–17. [Google Scholar] [CrossRef]
- Hoang, D.; Perrault, D.; Stevanovic, M.; Ghiassi, A. Surgical applications of three-dimensional printing: A review of the current literature & how to get started. Ann. Transl. Med. 2016, 4, 456. [Google Scholar] [CrossRef]
- Labonnote, N.; Rønnquist, A.; Manum, B.; Rüther, P. Additive construction: State-of-the-art, challenges and opportunities. Autom. Constr. 2016, 72, 347–366. [Google Scholar] [CrossRef]
- Gibson, I.; Rosen, D.; Stucker, B. Additive Manufacturing Technologies: 3D Printing, Rapid Prototyping, and Direct Digital Manufacturing, 2nd ed.; Springer: New York, NY, USA, 2015; pp. 1–498. [Google Scholar] [CrossRef]
- Böckin, D.; Tillman, A.M. Environmental assessment of additive manufacturing in the automotive industry. J. Clean. Prod. 2019, 226, 977–987. [Google Scholar] [CrossRef]
- Goh, G.D.; Dikshit, V.; Nagalingam, A.P.; Goh, G.L.; Agarwala, S.; Sing, S.L.; Wei, J.; Yeong, W.Y. Characterization of mechanical properties and fracture mode of additively manufactured carbon fiber and glass fiber reinforced thermoplastics. Mater. Des. 2018, 137, 79–89. [Google Scholar] [CrossRef]
- Espalin, D.; Muse, D.W.; MacDonald, E.; Wicker, R.B. 3D Printing multifunctionality: Structures with electronics. Int. J. Adv. Manuf. Technol. 2014, 72, 963–978. [Google Scholar] [CrossRef]
- Mangum, P., Jr.; Fisher, Z.; Cooksey, K.D.; Mavris, D.; Spero, E.; Gerdes, J.W. An automated approach to the design of small aerial systems using rapid manufacturing. In Proceedings of the ASME 2015 International Design Engineering Technical Conferences and Computers and Information in Engineering Conference, Boston, MA, USA, 2–5 August 2015. [Google Scholar]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Tucker, C.S.; Saint John, D.B.; Behoora, I.; Marcireau, A. Open source 3D scanning and printing for design capture and realization. In Proceedings of the ASME 2014 International Design Engineering Technical Conferences and Computers and Information in Engineering Conference, Buffalo, NY, USA, 17–20 August 2014. [Google Scholar]
- Jones, D.B.; Sung, R.; Weinberg, C.; Korelitz, T.; Andrews, R. Three-Dimensional Modeling May Improve Surgical Education and Clinical Practice. Surg. Innov. 2016, 23, 189–195. [Google Scholar] [CrossRef]
- Abudayyeh, I.; Gordon, B.; Ansari, M.M.; Jutzy, K.; Stoletniy, L.; Hilliard, A. A practical guide to cardiovascular 3D printing in clinical practice: Overview and examples. J. Interv. Cardiol. 2018, 31, 375–383. [Google Scholar] [CrossRef]
- Jastifer, J.R.; Gustafson, P.A. Three-Dimensional Printing and Surgical Simulation for Preoperative Planning of Deformity Correction in Foot and Ankle Surgery. J. Foot Ankle Surg. Off. Publ. Am. Coll. Foot Ankle Surg. 2017, 56, 191–195. [Google Scholar] [CrossRef]
- Pucci, J.U.; Christophe, B.R.; Sisti, J.A.; Connolly, E.S., Jr. Three-dimensional printing: Technologies, applications, and limitations in neurosurgery. Biotechnol. Adv. 2017, 35, 521–529. [Google Scholar] [CrossRef]
- Soon, D.S.; Chae, M.P.; Pilgrim, C.H.; Rozen, W.M.; Spychal, R.T.; Hunter-Smith, D.J. 3D haptic modelling for preoperative planning of hepatic resection: A systematic review. Ann. Med. Surg. 2016, 10, 1–7. [Google Scholar] [CrossRef]
- Zhong, N.; Zhao, X. 3D printing for clinical application in otorhinolaryngology. Eur. Arch. Otorhinolaryngol. 2017, 274, 4079–4089. [Google Scholar] [CrossRef]
- Tanner, J.A.; Jethwa, B.; Jackson, J.; Bartanuszova, M.; King, T.S.; Bhattacharya, A.; Sharma, R. A Three-Dimensional Print Model of the Pterygopalatine Fossa Significantly Enhances the Learning Experience. Anat. Sci. Educ. 2020. [Google Scholar] [CrossRef] [PubMed]
- Barabas, J.I.; Ghimessy, A.K.; Renyi-Vamos, F.; Kocsis, A.; Agocs, L.; Meszaros, L.; Pukacsik, D.; Andi, J.; Laki, A.; Voros, F.; et al. Innovation in medicine: Opportunities of 3D modeling and printing for perioperative care of cardio and thoracic surgical patients. Experiences in Hungary. Orv. Hetil. 2019, 160, 1967–1975. [Google Scholar] [CrossRef] [PubMed]
- Panesar, S.S.; Magnetta, M.; Mukherjee, D.; Abhinav, K.; Branstetter, B.F.; Gardner, P.A.; Iv, M.; Fernandez-Miranda, J.C. Patient-specific 3-dimensionally printed models for neurosurgical planning and education. Neurosurg. Focus 2019, 47, E12. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.S.; Choi, C.H.; Han, I.H.; Lee, J.H.; Choi, H.J.; Lee, J.I. Obtaining Informed Consent Using Patient Specific 3D Printing Cerebral Aneurysm Model. J. Korean Neurosurg. Soc. 2019, 62, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Biro, M.; Kim, I.; Huynh, A.; Fu, P.; Mann, M.; Popkin, D.L. The use of 3-dimensionally printed models to optimize patient education and alleviate perioperative anxiety in Mohs micrographic surgery: A randomized controlled trial. J. Am. Acad. Dermatol. 2019, 81, 1339–1345. [Google Scholar] [CrossRef]
- Zhuang, Y.D.; Zhou, M.C.; Liu, S.C.; Wu, J.F.; Wang, R.; Chen, C.M. Effectiveness of personalized 3D printed models for patient education in degenerative lumbar disease. Patient Educ. Couns. 2019, 102, 1875–1881. [Google Scholar] [CrossRef]
- Gross, B.C.; Erkal, J.L.; Lockwood, S.Y.; Chen, C.; Spence, D.M. Evaluation of 3D printing and its potential impact on biotechnology and the chemical sciences. Anal. Chem. 2014, 86, 3240–3253. [Google Scholar] [CrossRef]
- National Institutes of Health. 3D Print Exchange. Available online: http://3dprint.nih.gov (accessed on 13 May 2019).
- Cavas-Martinez, F.; Fernandez-Pacheco, D.G.; de la Cruz-Sanchez, E.; Nieto Martinez, J.; Canavate, F.J.F.; Alio, J.L. Virtual biomodelling of a biological structure: The human cornea. Dyna 2015, 90, 647–651. [Google Scholar]
- Cavas-Martinez, F.; Bataille, L.; Fernandez-Pacheco, D.G.; Canavate, F.J.F.; Alio, J.L. Keratoconus Detection Based on a New Corneal Volumetric Analysis. Sci. Rep. 2017, 7, 15837. [Google Scholar] [CrossRef]
- Cavas-Martinez, F.; Bataille, L.; Fernandez-Pacheco, D.G.; Canavate, F.J.F.; Alio, J.L. A new approach to keratoconus detection based on corneal morphogeometric analysis. PLoS ONE 2017, 12, e0184569. [Google Scholar] [CrossRef]
- Krumeich, J.H.; Daniel, J.; Knulle, A. Live-epikeratophakia for keratoconus. J. Cataract. Refract. Surg. 1998, 24, 456–463. [Google Scholar] [CrossRef]
- Ariza-Gracia, M.A.; Zurita, J.; Pinero, D.P.; Calvo, B.; Rodriguez-Matas, J.F. Automatized Patient-Specific Methodology for Numerical Determination of Biomechanical Corneal Response. Ann. Biomed. Eng. 2016, 44, 1753–1772. [Google Scholar] [CrossRef] [PubMed]
- Asher, R.; Gefen, A.; Moisseiev, E.; Varssano, D. An analytical approach to corneal mechanics for determining practical, clinically-meaningful patient-specific tissue mechanical properties in the rehabilitation of vision. Ann. Biomed. Eng. 2015, 43, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Lanchares, E.; Del Buey, M.A.; Cristobal, J.A.; Calvo, B. Computational Simulation of Scleral Buckling Surgery for Rhegmatogenous Retinal Detachment: On the Effect of the Band Size on the Myopization. J. Ophthalmol. 2016, 2016, 3578617. [Google Scholar] [CrossRef] [PubMed]
- Simonini, I.; Pandolfi, A. Customized Finite Element Modelling of the Human Cornea. PLoS ONE 2015, 10, e0130426. [Google Scholar] [CrossRef] [PubMed]
- Grimm, T. User’s Guide to Rapid Prototyping; Society of Manufacturing Engineers: Dearborn, MI, USA, 2004. [Google Scholar]
- ETSII UPCT Printer/es. Available online: https://reprap.org/mediawiki/index.php?title=ETSII_UPCT_Printer/es&oldid=156702 (accessed on 3 June 2019).
- Jones, R.; Haufe, P.; Sells, E.; Iravani, P.; Olliver, V.; Palmer, C.; Bowyer, A. RepRap—The replicating rapid prototyper. Robotica 2011, 29, 177–191. [Google Scholar] [CrossRef]
- Sanchez-Tena, M.A.; Alvarez-Peregrina, C. Application of 3D Printing Technology in Scleral Cover Shell Prosthesis. J. Med. Syst. 2019, 43, 149. [Google Scholar] [CrossRef]
- Hughes, A.J.; DeBuitleir, C.; Soden, P.; O’Donnchadha, B.; Tansey, A.; Abdulkarim, A.; McMahon, C.; Hurson, C.J. 3D Printing Aids Acetabular Reconstruction in Complex Revision Hip Arthroplasty. Adv. Orthop. 2017, 2017, 8925050. [Google Scholar] [CrossRef]
- Banks, J. Adding value in additive manufacturing: Researchers in the United Kingdom and Europe look to 3D printing for customization. IEEE Pulse 2013, 4, 22–26. [Google Scholar] [CrossRef]
- Ventola, C.L. Medical Applications for 3D Printing: Current and Projected Uses. P T A Peer-Rev. J. Formul. Manag. 2014, 39, 704–711. [Google Scholar]
- Clemente, C.; Esposito, L.; Speranza, D.; Bonora, N. Firecracker eye exposure: Experimental study and simulation. Biomech. Model. Mechanobiol. 2017, 16, 1401–1411. [Google Scholar] [CrossRef] [PubMed]
- Estomba, C.; González-Fernández, I.; Iglesias-Otero, M. 3D Printing for Biomedical Applications: Where are we now? Eur. Med J. 2017, 2, 16–22. [Google Scholar]
- Isaacson, A.; Swioklo, S.; Connon, C.J. 3D bioprinting of a corneal stroma equivalent. Exp. Eye Res. 2018, 173, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Speranza, D.; Padula, F.; Motyl, B.; Tornincasa, S.; Marcolin, F.; Vezzetti, E.; Martorelli, M. Parenthood Perception Enhancement Through Interaction with 3D Printed Fetal Face Models. In Advances on Mechanics, Design Engineering and Manufacturing II; Springer: Cham, Switzerland, 2019; pp. 527–535. [Google Scholar]
- Bassnett, S.; Shi, Y.; Vrensen, G.F. Biological glass: Structural determinants of eye lens transparency. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2011, 366, 1250–1264. [Google Scholar] [CrossRef]
- Debellemaniere, G.; Flores, M.; Montard, M.; Delbosc, B.; Saleh, M. Three-dimensional Printing of Optical Lenses and Ophthalmic Surgery: Challenges and Perspectives. J. Refract. Surg. (Thorofare NJ 1995) 2016, 32, 201–204. [Google Scholar] [CrossRef]
- Donaldson, P.J.; Grey, A.C.; Maceo Heilman, B.; Lim, J.C.; Vaghefi, E. The physiological optics of the lens. Prog. Retinal Eye Res. 2017, 56, e1–e24. [Google Scholar] [CrossRef]
- Hejtmancik, J.F.; Shiels, A. Overview of the Lens. Prog. Mol. Biol. Transl. Sci. 2015, 134, 119–127. [Google Scholar] [CrossRef]
- Zhao, F.; Zhao, G.; Weijie, F.; Chen, L. Application of 3D printing technology in RGPCL simulation fitting. Med. Hypotheses 2018, 113, 74–76. [Google Scholar] [CrossRef]
- Callahan, A.B.; Campbell, A.A.; Petris, C.; Kazim, M. Low-Cost 3D Printing Orbital Implant Templates in Secondary Orbital Reconstructions. Ophthalmic Plast. Reconstr. Surg. 2017, 33, 376–380. [Google Scholar] [CrossRef]
- Dave, T.V.; Tiple, S.; Vempati, S.; Palo, M.; Ali, M.J.; Kaliki, S.; Naik, M.N. Low-cost three-dimensional printed orbital template-assisted patient-specific implants for the correction of spherical orbital implant migration. Indian J. Ophthalmol. 2018, 66, 1600–1607. [Google Scholar] [CrossRef]
- Fan, B.; Chen, H.; Sun, Y.J.; Wang, B.F.; Che, L.; Liu, S.Y.; Li, G.Y. Clinical effects of 3-D printing-assisted personalized reconstructive surgery for blowout orbital fractures. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 2051–2057. [Google Scholar] [CrossRef] [PubMed]
- Ruiters, S.; Sun, Y.; de Jong, S.; Politis, C.; Mombaerts, I. Computer-aided design and three-dimensional printing in the manufacturing of an ocular prosthesis. Br. J. Ophthalmol. 2016, 100, 879–881. [Google Scholar] [CrossRef] [PubMed]
- Furdova, A.; Sramka, M.; Thurzo, A.; Furdova, A. Early experiences of planning stereotactic radiosurgery using 3D printed models of eyes with uveal melanomas. Clin. Ophthalmol. (Auckl. N. Z.) 2017, 11, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.W.; Paxton, L.; Dawes, K.; Burlak, K.; Quayle, M.; McMenamin, P.G. 3D printed reproductions of orbital dissections: A novel mode of visualising anatomy for trainees in ophthalmology or optometry. Br. J. Ophthalmol. 2015, 99, 1162–1167. [Google Scholar] [CrossRef] [PubMed]
- Scawn, R.L.; Foster, A.; Lee, B.W.; Kikkawa, D.O.; Korn, B.S. Customised 3D Printing: An Innovative Training Tool for the Next Generation of Orbital Surgeons. Orbit (Amst. Neth.) 2015, 34, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jang, J.; Park, J.; Lee, K.P.; Lee, S.; Lee, D.M.; Kim, K.H.; Kim, H.K.; Cho, D.W. Shear-induced alignment of collagen fibrils using 3D cell printing for corneal stroma tissue engineering. Biofabrication 2019, 11, 035017. [Google Scholar] [CrossRef]
- Kim, H.; Park, M.N.; Kim, J.; Jang, J.; Kim, H.K.; Cho, D.W. Characterization of cornea-specific bioink: High transparency, improved in vivo safety. J. Tissue Eng. 2019, 10, 2041731418823382. [Google Scholar] [CrossRef]
- Ludwig, P.E.; Huff, T.J.; Zuniga, J.M. The potential role of bioengineering and three-dimensional printing in curing global corneal blindness. J. Tissue Eng. 2018, 9, 2041731418769863. [Google Scholar] [CrossRef]
- Navajas, E.V.; Ten Hove, M. Three-Dimensional Printing of a Transconjunctival Vitrectomy Trocar-Cannula System. Ophthalmologica 2017, 237, 119–122. [Google Scholar] [CrossRef]
- Sommer, A.C.; Blumenthal, E.Z. Implementations of 3D printing in ophthalmology. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019. [Google Scholar] [CrossRef]
- Ayyildiz, O. Customised spectacles using 3-D printing technology. Clin. Exp. Optom. 2018, 101, 747–751. [Google Scholar] [CrossRef]
- Kamali, P.; Dean, D.; Skoracki, R.; Koolen, P.G.L.; Paul, M.A.; Ibrahim, A.M.S.; Lin, S.J. The Current Role of Three-Dimensional (3D) Printing in Plastic Surgery. Plast. Reconstr. Surg. 2016. [Google Scholar] [CrossRef] [PubMed]
- Sukindar, N.A. Optimization of the Parameters for Surface Quality of the Open-source 3D Printing. J. Mech. Eng. 2017, SI3, 33–43. [Google Scholar]
- Speranza, D.; Citro, D.; Padula, F.; Motyl, B.; Marcolin, F.; Calí, M.; Martorelli, M. Additive Manufacturing Techniques for the Reconstruction of 3D Fetal Faces. Appl. Bionics Biomech. 2017, 2017, 9701762. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, J.C.; Isotani, S.; Matsugasumi, T.; Duddalwar, V.; Hung, A.J.; Suer, E.; Baco, E.; Satkunasivam, R.; Djaladat, H.; Metcalfe, C.; et al. Personalized 3D printed model of kidney and tumor anatomy: A useful tool for patient education. World J. Urol. 2016, 34, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Andolfi, C.; Plana, A.; Kania, P.; Banerjee, P.P.; Small, S. Usefulness of three-dimensional modeling in surgical planning, resident training, and patient education. J. Laparoendosc. Adv. Surg. Tech. 2017, 27, 512–515. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Values/Settings |
|---|---|
| Material | PLA |
| Quality: layer height | 0.2–0.3 mm |
| Fusing material density | 1.25 g/cm3 |
| Fusing material fusion point | 160 °C |
| Printing temperature | 225 °C |
| Nozzle diameter | 1 mm |
| Flow rate | 100% |
| Print speed | 500 mm/s |
| Travel speed | 130 mm/s |
| Printing area | 22 cm × 23 cm × 20 cm |
| Number | Question Test | Possible Answer |
|---|---|---|
| Q1 | What usefulness do you attribute to this custom 3D model? | From 1 = not useful at all to 10 = very useful |
| Q2 | How much did the custom 3D model help you to better understand your condition? | From 1 = nothing at all to 10 = a huge lot |
| Q3 | Would you like to take the custom 3D model with you after the consultation? | Yes/No/Neutral |
| Q4 | Would you consider that using this custom 3D model improves the quality of our clinical service? | Yes/No/Neutral |
| Q5 | How much do you consider that patients would benefit from the use of these custom 3D models in consultations? | From 1 = nothing at all to 10 = a huge lot |
| Number | Possible Answer | Percentage | Average | Standard Deviation (SD) |
|---|---|---|---|---|
| Q1 | From 1 = not useful at all to 10 = very useful | - | 9.67 | 0.53 |
| Q2 | From 1 = nothing at all to 10 = a huge lot | - | 9.74 | 0.45 |
| Q3 | Yes | 100.0 | ||
| No | 0.0 | |||
| Neutral | 0.0 | |||
| Q4 | Yes | 95.2 | ||
| No | 0.0 | |||
| Neutral | 4.8 | |||
| Q5 | From 1 = nothing at all to 10 = a huge lot | - | 8.62 | 0.58 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velázquez, J.S.; Cavas, F.; Bolarín, J.M.; Alió, J.L. 3D Printed Personalized Corneal Models as a Tool for Improving Patient’s Knowledge of an Asymmetric Disease. Symmetry 2020, 12, 151. https://doi.org/10.3390/sym12010151
Velázquez JS, Cavas F, Bolarín JM, Alió JL. 3D Printed Personalized Corneal Models as a Tool for Improving Patient’s Knowledge of an Asymmetric Disease. Symmetry. 2020; 12(1):151. https://doi.org/10.3390/sym12010151
Chicago/Turabian StyleVelázquez, Jose S., Francisco Cavas, José M. Bolarín, and Jorge L. Alió. 2020. "3D Printed Personalized Corneal Models as a Tool for Improving Patient’s Knowledge of an Asymmetric Disease" Symmetry 12, no. 1: 151. https://doi.org/10.3390/sym12010151
APA StyleVelázquez, J. S., Cavas, F., Bolarín, J. M., & Alió, J. L. (2020). 3D Printed Personalized Corneal Models as a Tool for Improving Patient’s Knowledge of an Asymmetric Disease. Symmetry, 12(1), 151. https://doi.org/10.3390/sym12010151







