Template-Free Synthesis of a Phenanthroline-Containing [2]Rotaxane: A Reversible pH-Controllable Molecular Switch
Abstract
1. Introduction
2. Materials and Methods
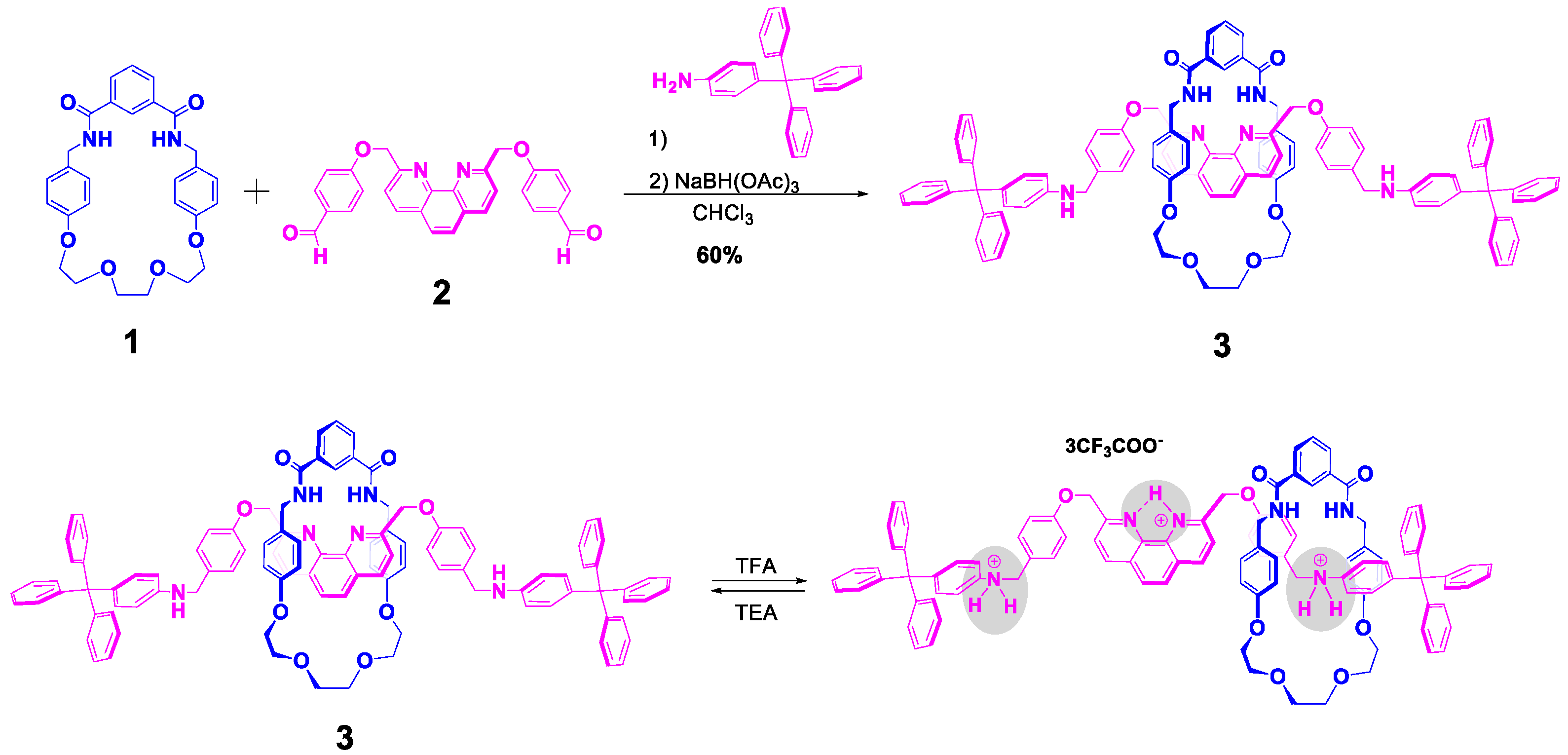
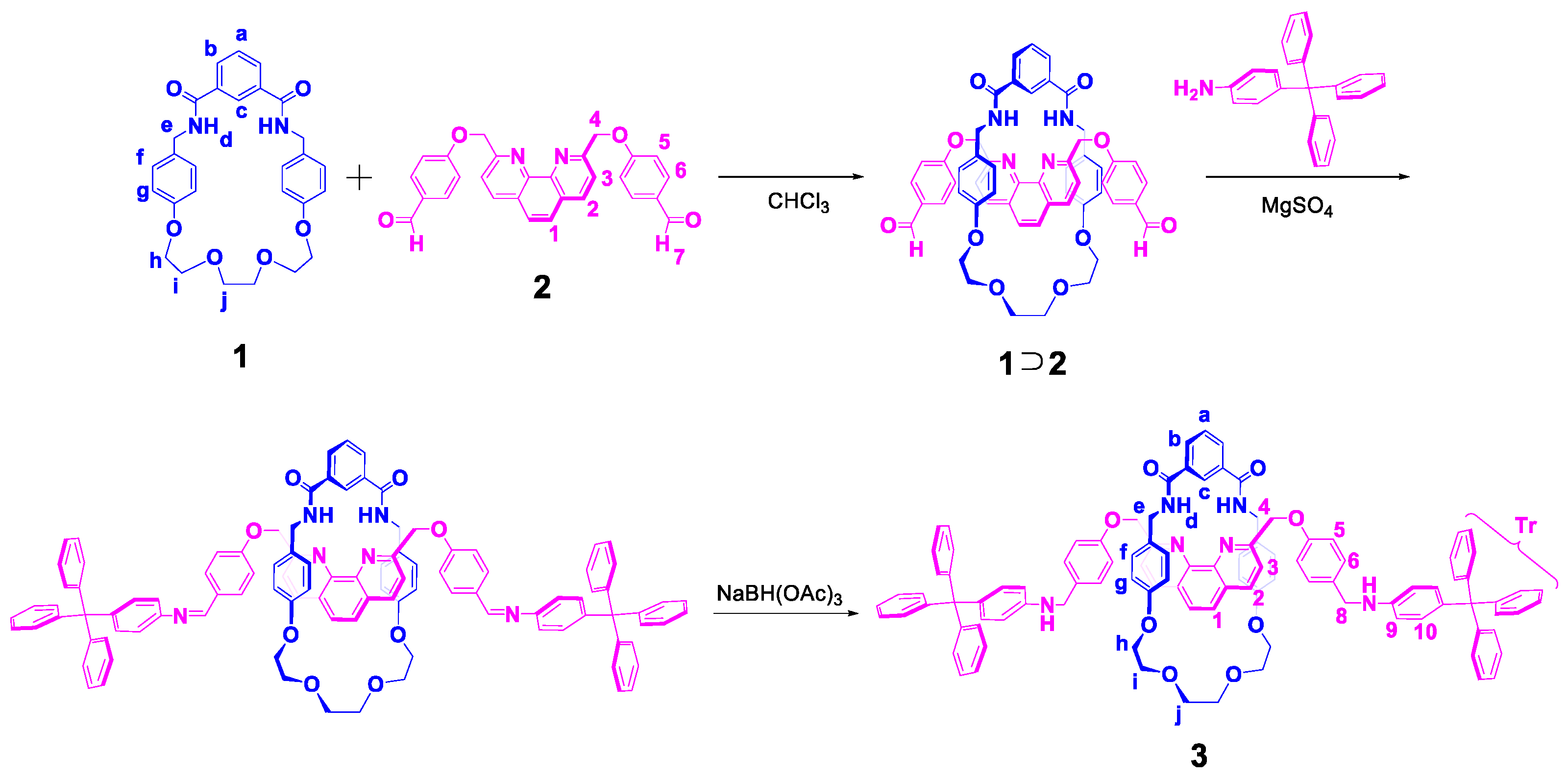
2.1. Synthesis of Rotaxane 3
2.2. Single Crystal X-Ray Analysis
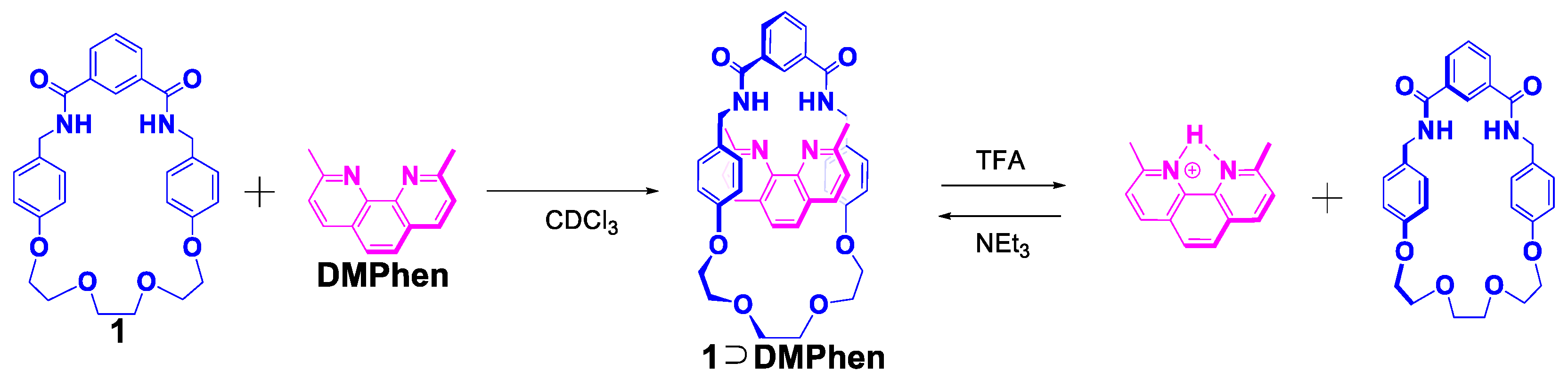
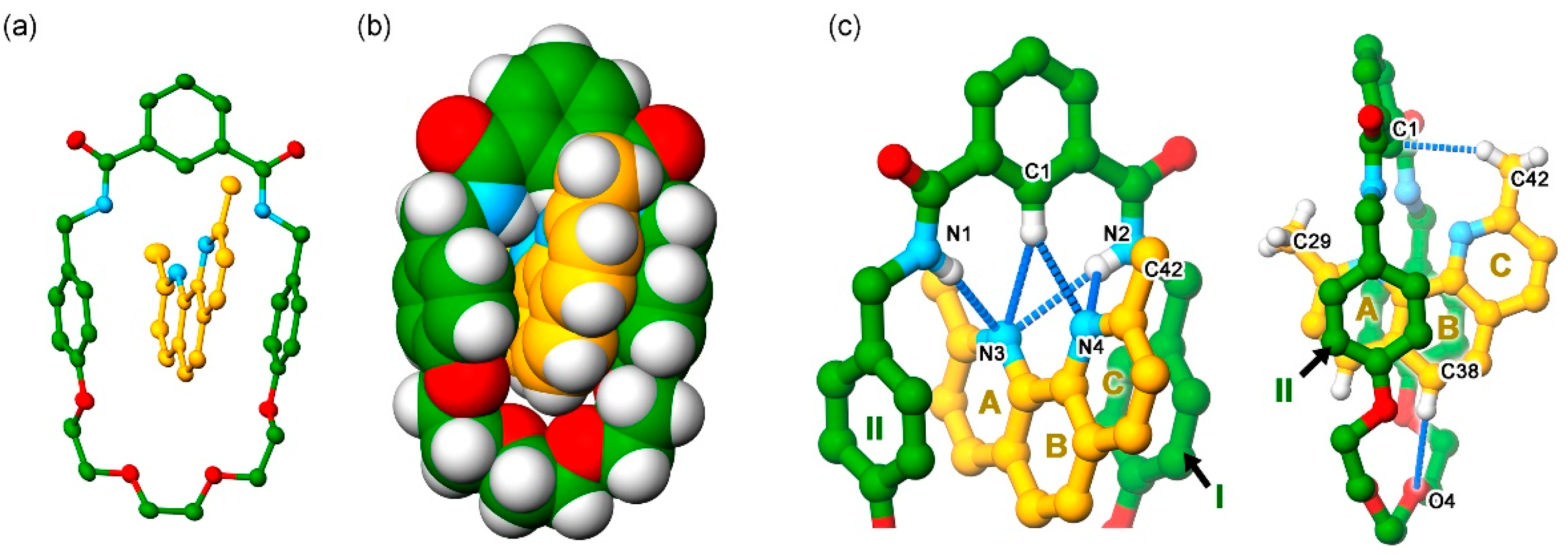
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Appendix A. General
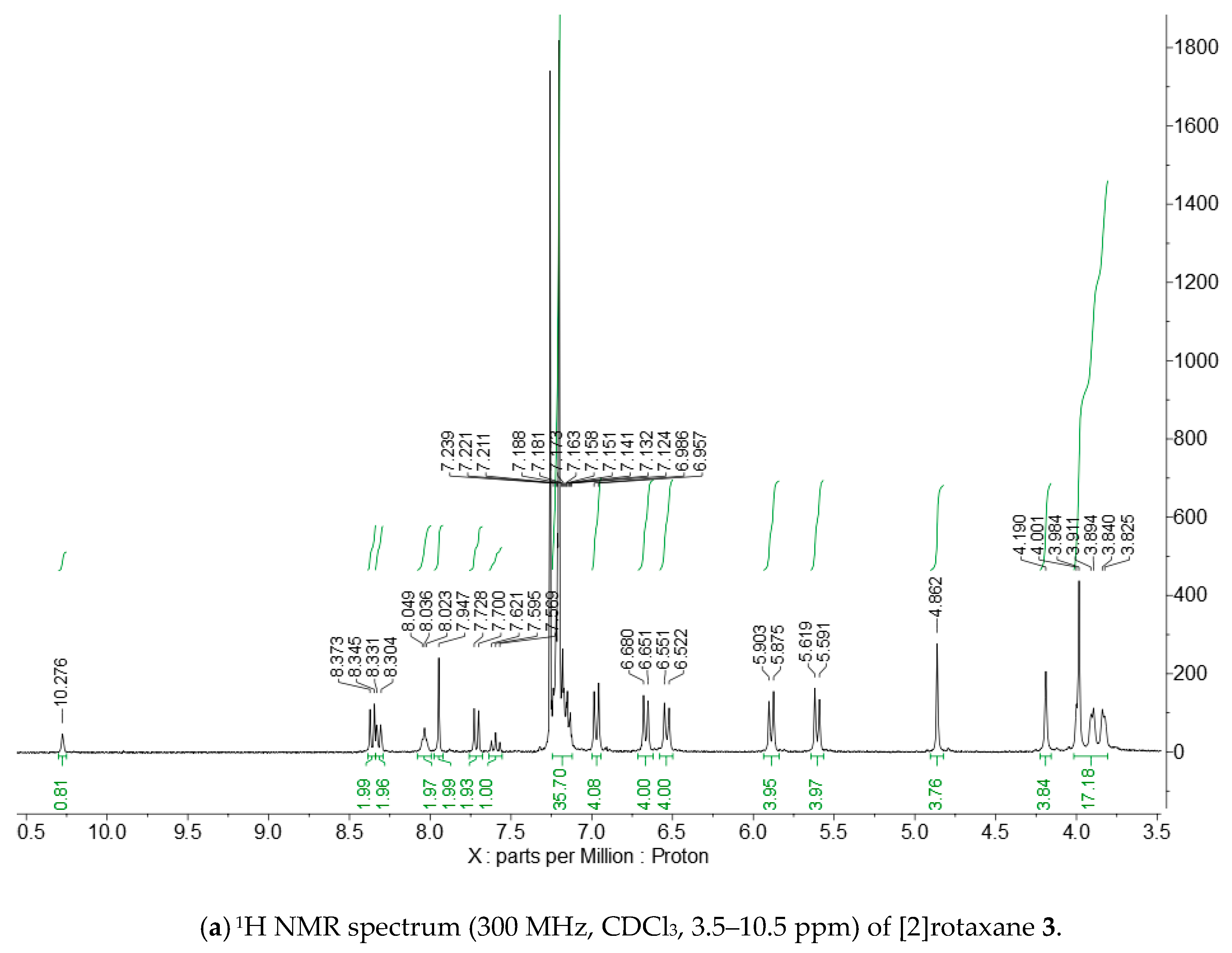
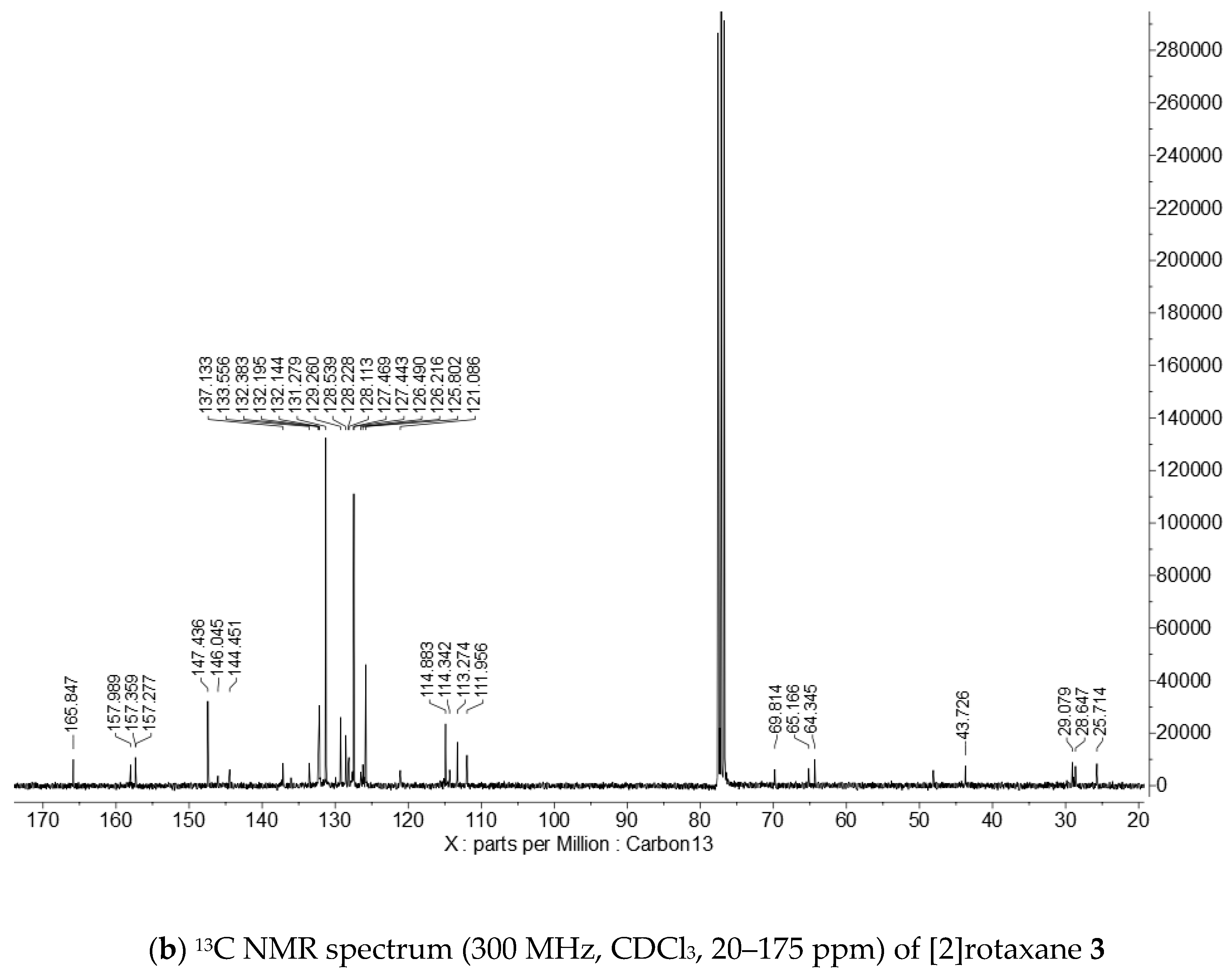
Appendix B. 1H and 13C NMR Spectra of [2]Rotaxane 3
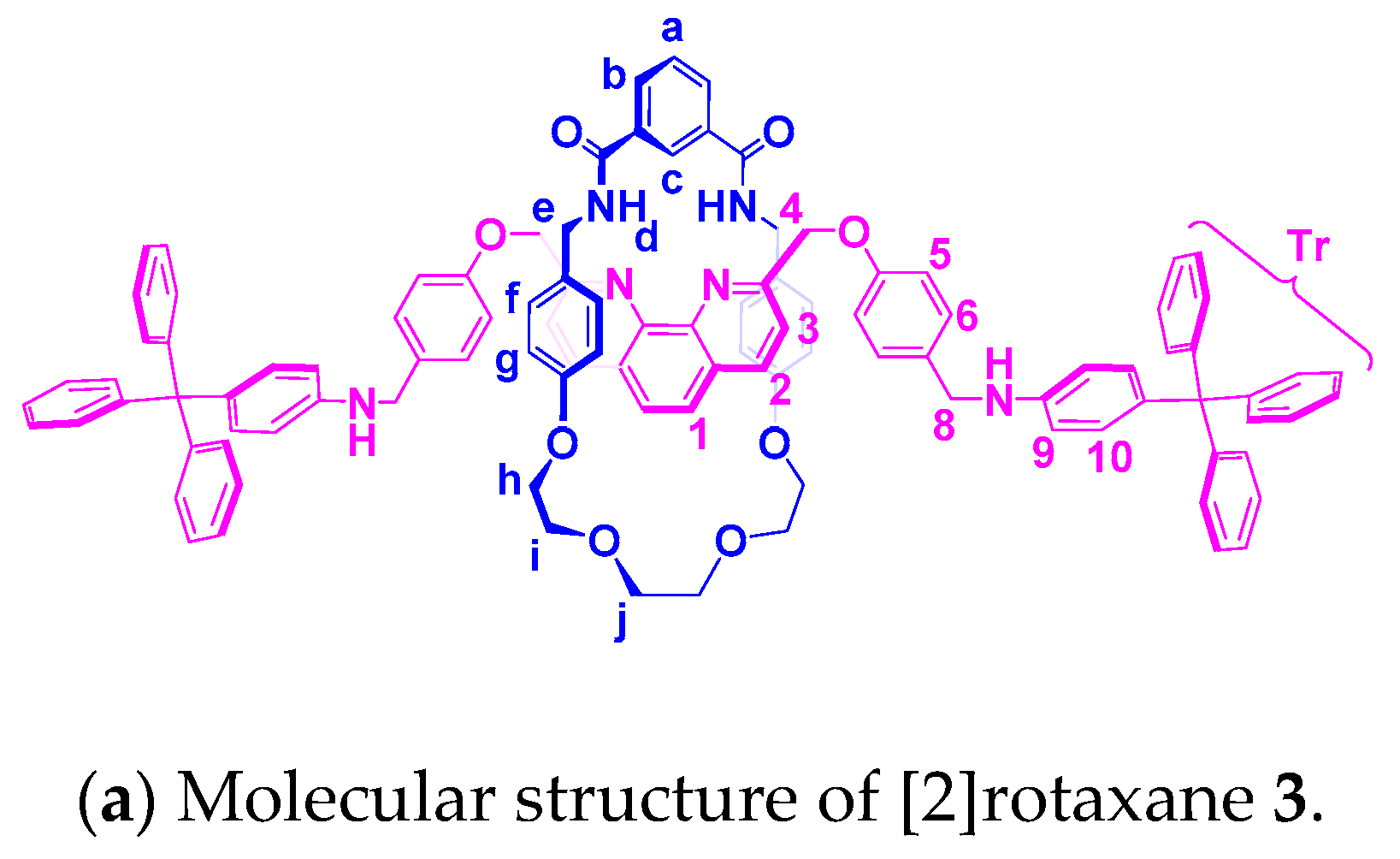
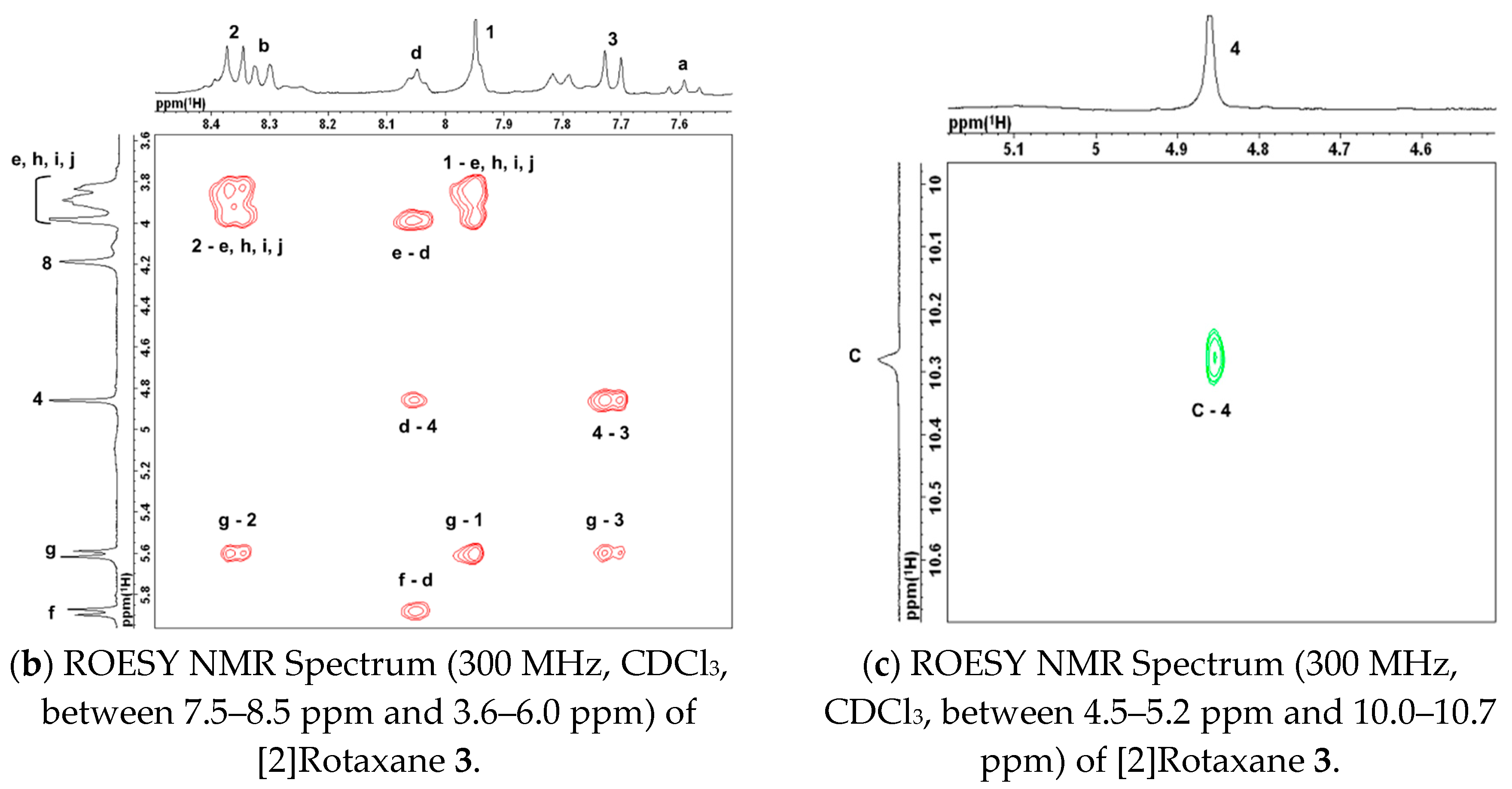
Appendix C. ROESY NMR Spectra of [2]Rotaxane 3
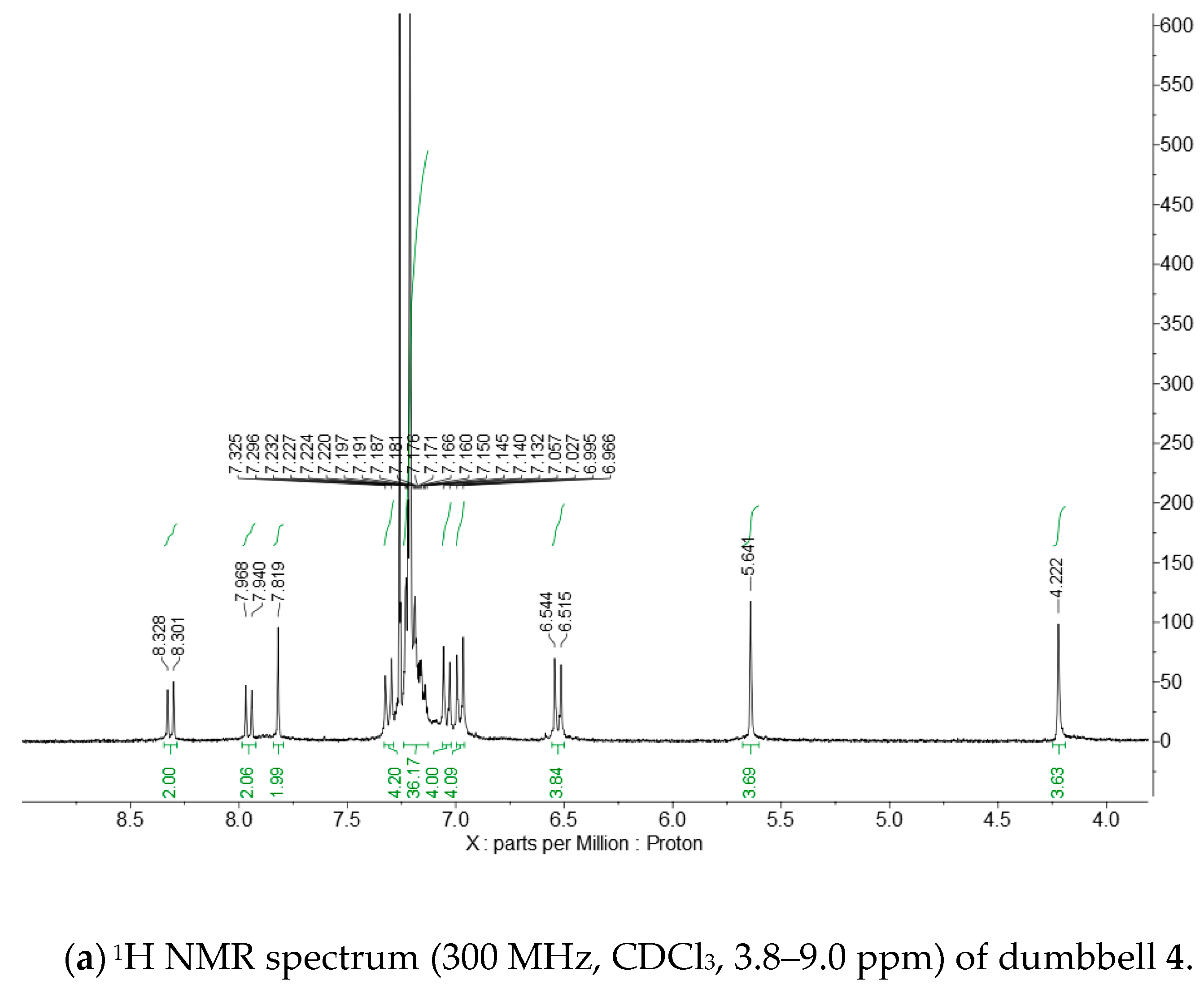
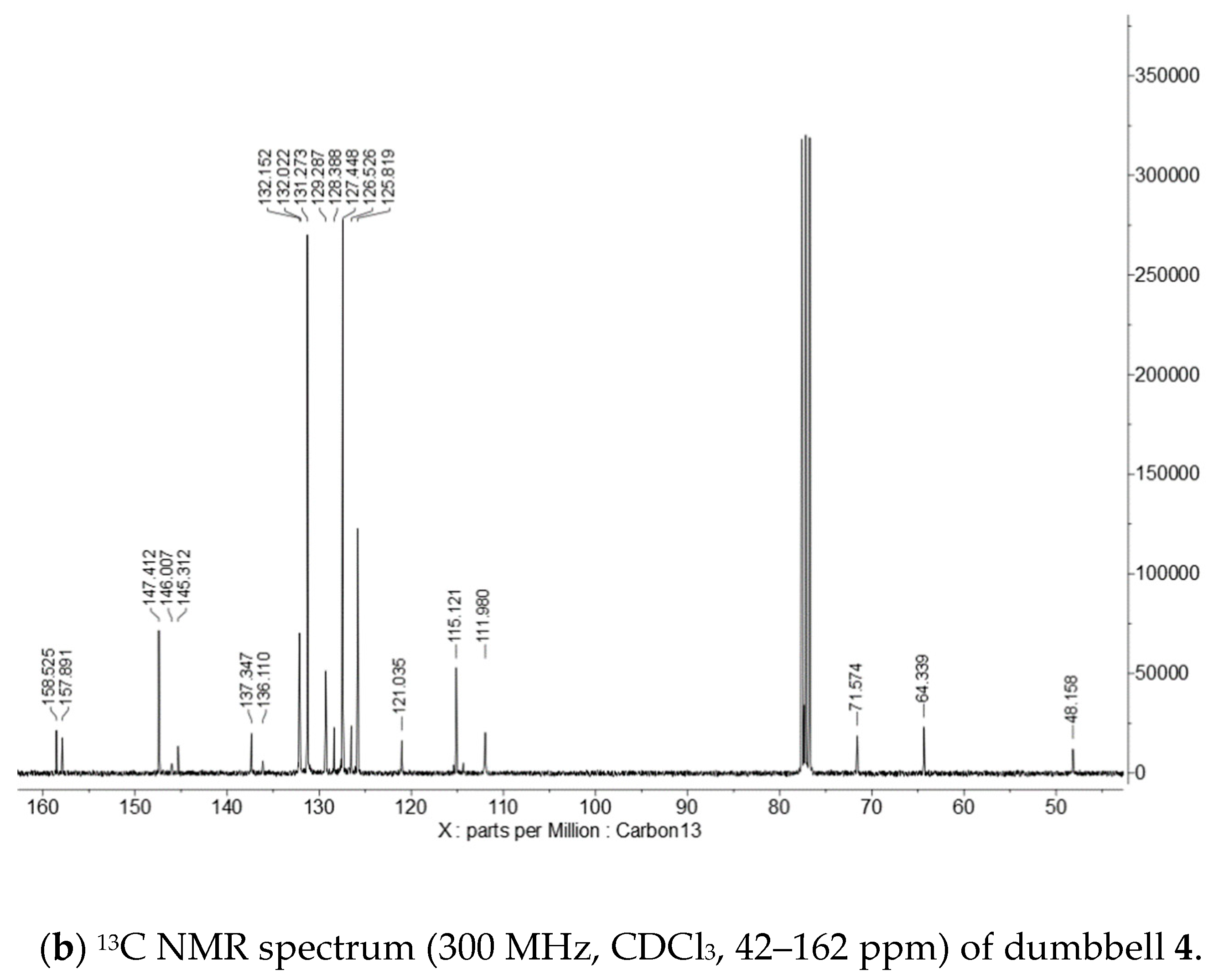
Appendix D. 1H and 13C NMR Spectra of Dumbbell 4
References
- Erbas-Cakmak, S.; Leigh, D.A.; McTernan, C.T.; Nussbaumer, A.L. Artificial Molecular Machines. Chem. Rev. 2015, 115, 10081–10206. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Yang, Y.; Chi, X.; Yan, X.; Huang, F. Development of Pseudorotaxanes and Rotaxanes: From Synthesis to Stimuli-Responsive Motions to Applications. Chem. Rev. 2015, 115, 7398–7501. [Google Scholar] [CrossRef] [PubMed]
- van Dongen, S.F.M.; Cantekin, S.; Elemans, J.A.A.W.; Rowan, A.E.; Nolte, R.J.M. Functional interlocked systems. Chem. Soc. Rev. 2014, 43, 99–122. [Google Scholar] [CrossRef] [PubMed]
- Beves, J.E.; Blight, B.A.; Campbell, C.J.; Leigh, D.A.; McBurney, R.T. Strategies and Tactics for the Metal-Directed Synthesis of Rotaxanes, Knots, Catenanes, and Higher Order Links. Angew. Chem. Int. Ed. 2011, 50, 9260–9327. [Google Scholar] [CrossRef] [PubMed]
- Stodddart, J.F. Mechanically Interlocked Molecules (MIMs)—Molecular Shuttles, Switches, and Machines (Nobel Lecture)†. Angew. Chem. Int. Ed. 2017, 56, 11094–11125. [Google Scholar] [CrossRef] [PubMed]
- Sauvage, J.P. From Chemical Topology to Molecular Machines (Nobel Lecture)†. Angew. Chem. Int. Ed. 2017, 56, 11080–11093. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.E.M.; Galli, M.; Goldup, S.M. Properties and emerging applications of mechanically interlocked ligands. Chem. Commun. 2017, 53, 298–312. [Google Scholar] [CrossRef]
- Langton, M.J.; Beer, P.D. Rotaxane and catenane host structures for sensing charged guest species. Acc. Chem. Res. 2014, 47, 1935–1949. [Google Scholar] [CrossRef]
- Neal, E.A.; Goldup, S.M. Chemical consequences of mechanical bonding in catenanes and rotaxanes: isomerism, modification, catalysis and molecular machines for synthesis. Chem. Commun. 2014, 50, 5128–5142. [Google Scholar] [CrossRef]
- Muraoka, M.; Ohta, M.; Mizutani, Y.; Takezawa, M.; Matsumoto, A.; Nakatsuji, Y. Formation of a pseudorotaxane, capable of sensing cations via dethreading molecular motion, from a cryptand and bipyridinium salts. J. Incl. Phenom. Macrocycl. Chem. 2014, 78, 137–144. [Google Scholar] [CrossRef]
- Nakazono, K.; Kuwata, S.; Takata, T. Crown Ether–tert-Ammonium Salt Complex Fixed as Rotaxane and Its Derivation to Nonionic Rotaxane. Tetrahedron Lett. 2008, 49, 2397–2401. [Google Scholar] [CrossRef]
- Suzuki, S.; Nakazono, K.; Takata, T. Selective transformation of a crown ether/sec-ammonium salt-type rotaxane to N-alkylated rotaxanes. Org. Lett. 2010, 12, 712–715. [Google Scholar] [CrossRef] [PubMed]
- Chmielewski, M.J.; Davis, J.J.; Beer, P.D. Interlocked host rotaxane and catenane structures for sensing charged guest species via optical and electrochemical methodologies. Org. Biomol. Chem. 2009, 7, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.W., III; Joly, G.D.; Swager, T.M. Chemical sensors based on amplifying fluorescent conjugated polymers. Chem. Rev. 2007, 107, 1339–1386. [Google Scholar] [CrossRef] [PubMed]
- McQuade, D.T.; Pullen, A.E.; Swager, T.M. Conjugated Polymer-Based Chemical Sensors. Chem. Rev. 2000, 100, 2537–2574. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Tian, H. Bright functional rotaxanes. Chem. Soc. Rev. 2010, 39, 70–80. [Google Scholar] [CrossRef]
- Wenz, G.; Han, B.-H.; Müller, A. Cyclodextrin rotaxanes and polyrotaxanes. Chem. Rev. 2006, 106, 782–817. [Google Scholar] [CrossRef]
- Leigh, D.A.; Thomson, A.R. Switchable dual binding mode molecular shuttle. Org. Lett. 2006, 8, 5377–5379. [Google Scholar] [CrossRef]
- Vidonne, A.; Philp, D. Integrating replication processes with mechanically interlocked molecular architectures. Tetrahedron 2008, 64, 8464–8475. [Google Scholar] [CrossRef]
- Kosikova, T.; Hassan, N.I.; Cordes, D.B.; Slawin, A.M.Z.; Philp, D. Orthogonal recognition processes drive the assembly and replication of a [2]Rotaxane. J. Am. Chem. Soc. 2015, 137, 16074–16083. [Google Scholar] [CrossRef]
- Fletcher, B.E.; Peach, M.J.G.; Evans, N.H. Rapidly accessible “click” rotaxanes utilizing a single amide hydrogen bond templating motif. Org. Biomol. Chem. 2017, 15, 2797–2803. [Google Scholar] [CrossRef] [PubMed]
- Muraoka, M.; Irie, H.; Nakatsuji, Y. Acid/base controllable molecular switch based on a neutral phenanthroline guest penetrated pseudorotaxane. Org. Biomol. Chem. 2010, 8, 2408–2413. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yee, C.C.; Au-Yeung, H.Y. Facile syntheses of [3]-, [4]- and [6] catenanes templated by orthogonal supramolecular interactions. Chem. Sci. 2016, 7, 2787–2792. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT-integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Rigaku. CrystalStructure. Ver. 4.2; Rigaku Corporation: Tokyo, Japan, 2015. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- The Cambridge Crystallographic Data Centre. Crystal data of [2] pseudorotaxane 1⊃DMPhen: (C28H30N2O6)•(C14H12N2)•0.5(C4H8O2)•H2O, Fw = 760.89, a = 8.83401(16) Å, b = 12.5026(2) Å, c = 18.7047(3) Å, α = 103.5820(9)°, β = 94.5117(9)°, γ = 103.4930(9)°, V = 1933.14(6) Å3, T = 153 K, triclinic, space group P-1, Z = 2, 13886 collected, 6848 unique (Rint = 0.0536) reflections, the final R1 and wR2 values 0.0504 (I > 2.0σ(I)) and 0.1459 (all data), respectively. (CCDC-1947111) contain the supplementary crystallographic data for this paper. Available online: www.ccdc.cam.ac.uk/ (accessed on 14 August 2019).
- Hirose, K. A Practical Guide for the Determination of Binding Constants. J. Incl. Phenom. Macrocycl. Chem. 2001, 39, 193–209. [Google Scholar] [CrossRef]
- Cantrill, S.J.; Rowan, S.J.; Stoddart, J.F. Rotaxane formation under thermodynamic control. Org. Lett. 1999, 1, 1363–1366. [Google Scholar] [CrossRef]
- Rowan, S.J.; Stoddart, J.F. Thermodynamic synthesis of rotaxanes by imine exchange. Org. Lett. 1999, 1, 1913–1916. [Google Scholar] [CrossRef]
- De Bo, G.; Dolphijn, G.; McTernan, C.T.; Leigh, D.A. [2]Rotaxane Formation by Transition State Stabilization. J. Am. Chem. Soc. 2017, 139, 8455–8457. [Google Scholar] [CrossRef]
- Zheng, H.; Zhou, W.; Lv, J.; Yin, X.; Li, Y.J.; Liu, H.; Li, Y.L. A Dual-Response [2] Rotaxane Based on a 1,2,3-Triazole Ring as a Novel Recognition Station. Chem. Eur. J. 2009, 15, 13253–13262. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muraoka, M.; Aoyama, K.; Fujihara, S.; Yamane, R.; Hisaki, I.; Miyata, M.; Murata, M.; Nakatsuji, Y. Template-Free Synthesis of a Phenanthroline-Containing [2]Rotaxane: A Reversible pH-Controllable Molecular Switch. Symmetry 2019, 11, 1137. https://doi.org/10.3390/sym11091137
Muraoka M, Aoyama K, Fujihara S, Yamane R, Hisaki I, Miyata M, Murata M, Nakatsuji Y. Template-Free Synthesis of a Phenanthroline-Containing [2]Rotaxane: A Reversible pH-Controllable Molecular Switch. Symmetry. 2019; 11(9):1137. https://doi.org/10.3390/sym11091137
Chicago/Turabian StyleMuraoka, Masahiro, Kakeru Aoyama, Sae Fujihara, Risa Yamane, Ichiro Hisaki, Mikiji Miyata, Michihisa Murata, and Yohji Nakatsuji. 2019. "Template-Free Synthesis of a Phenanthroline-Containing [2]Rotaxane: A Reversible pH-Controllable Molecular Switch" Symmetry 11, no. 9: 1137. https://doi.org/10.3390/sym11091137
APA StyleMuraoka, M., Aoyama, K., Fujihara, S., Yamane, R., Hisaki, I., Miyata, M., Murata, M., & Nakatsuji, Y. (2019). Template-Free Synthesis of a Phenanthroline-Containing [2]Rotaxane: A Reversible pH-Controllable Molecular Switch. Symmetry, 11(9), 1137. https://doi.org/10.3390/sym11091137





