Short vs. Standard Length Cone Morse Connection Implants: An In Vitro Pilot Study in Low Density Polyurethane Foam
Abstract
:1. Introduction
- no differences in the in the survival rate between SI and standard length implants (ST);
- no differences in marginal bone loss (MBL);
- lower biological complications in SI;
- good primary stability in SI;
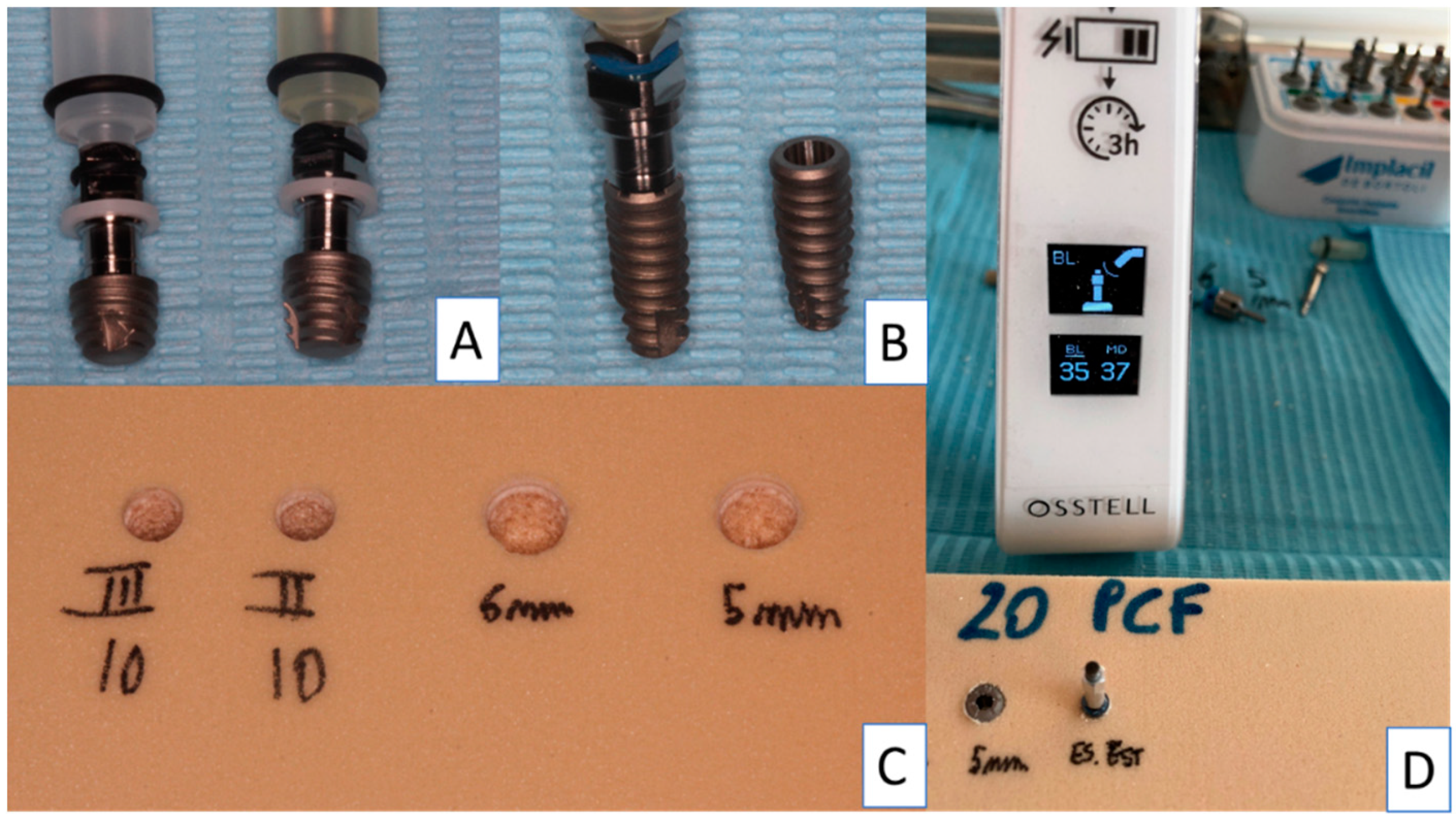
2. Materials and Methods
2.1. Dental Implants
2.2. Study Design
2.3. Implant Drill
2.4. Insertion Torque and Pull-Out Torque
2.5. Resonance Frequency Evaluation
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lombardo, G.; Pighi, J.; Marincola, M.; Corrocher, G.; Simancas-Pallares, M.; Nocini, P.F. Cumulative Success Rate of Short and Ultrashort Implants Supporting Single Crowns in the Posterior Maxilla: A 3-Year Retrospective Study. Int. J. Dent. 2017, 2017, 8434281. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, H.B.; Schou, S.; Isidor, F.; Christensen, A.E.; Starch-Jensen, T. Short implants (≤8 mm) compared to standard length implants (>8 mm) in conjunction with maxillary sinus floor augmentation: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2019, 48, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Cruz, R.S.; Lemos, C.A.A.; Batista, V.E.S.; Oliveira, H.F.F.E.; Gomes, J.M.L.; Pellizzer, E.P.; Verri, F.R. Short implants versus longer implants with maxillary sinus lift. A systematic review and meta-analysis. Braz. Oral Res. 2018, 32, 86. [Google Scholar] [CrossRef] [PubMed]
- Tolentino da Rosa de Souza, P.; Binhame Albini Martini, M.; Reis Azevedo-Alanis, L. Do short implants have similar survival rates compared to standard implants in posterior single crown?: A systematic review and meta-analysis. Clin. Implant. Dent. Relat. Res. 2018, 20, 890–901. [Google Scholar] [CrossRef] [PubMed]
- Lemos, C.A.A.; Ferro-Alves, M.L.; Okamoto, R.; Mendonça, M.R.; Pellizzer, E.P. Short dental implants versus standard dental implants placed in the posterior jaws: A systematic review and meta-analysis. J. Dent. 2016, 47, 8–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- N Dias, F.J.; Pecorari, V.G.A.; Martins, C.B.; Del Fabbro, M.; Casati, M.Z. Short implants versus bone augmentation in combination with standard-length implants in posterior atrophic partially edentulous mandibles: Systematic review and meta-analysis with the Bayesian approach. Int. J. Oral Maxillofac. Surg. 2019, 48, 90–96. [Google Scholar] [CrossRef]
- Markose, J.; Eshwar, S.; Srinivas, S.; Jain, V. Clinical outcomes of ultrashort sloping shoulder implant design: A survival analysis. Clin. Implant Dent. Relat. Res. 2018, 20, 646–652. [Google Scholar] [CrossRef]
- Urdaneta, R.A.; Daher, S.; Leary, J.; Emanuel, K.M.; Chuang, S.K. The survival of ultrashort locking-taper implants. Int. J. Oral Maxillofac. Implant 2012, 27, 644–654. [Google Scholar]
- Ravidà, A.; Barootchi, S.; Askar, H.; Suárez-López Del Amo, F.; Tavelli, L.; Wang, H.L. Long-Term Effectiveness of Extra-Short (≤6 mm) Dental Implants: A Systematic Review. Int. J. Oral Maxillofac. Implant. 2019, 34, 68–84. [Google Scholar] [CrossRef]
- Bitaraf, T.; Keshtkar, A.; Rokn, A.R.; Monzavi, A.; Geramy, A.; Hashemi, K. Comparing short dental implant and standard dental implant in terms of marginal bone level changes: A systematic review and meta-analysis of randomized controlled trials. Clin. Implant Dent. Relat. Res. 2019, 21, 796–812. [Google Scholar] [CrossRef]
- Deporter, D. Short and Ultrashort Implants; Quintessence Publishing: New Malden, UK, 2018; pp. 59–74. [Google Scholar]
- Deporter, D.; Ogiso, B.; Sohn, D.S.; Ruljancich, K.; Pharoah, M. Ultrashort sintered porous-surfaced dental implants used to replace posterior teeth. J. Periodontol. 2008, 79, 1280–1286. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Li, Y.; Deng, W.W.; Wu, T.; Zhang, W. Short Implants (5 to 8 mm) Versus Longer Implants (>8 mm) with Sinus Lifting in Atrophic Posterior Maxilla: A Meta-Analysis of RCTs. Clin. Implant Dent. Relat. Res. 2017, 19, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Al-Johany, S.S. Survival Rates of Short Dental Implants (≤6.5 mm) Placed in Posterior Edentulous Ridges and Factors Affecting their Survival after a 12-Month Follow-up Period: A Systematic Review. Int. J. Oral Maxillofac. Implant 2019, 34, 605–621. [Google Scholar] [CrossRef] [PubMed]
- Martinolli, M.; Bortolini, S.; Natali, A.; Pereira, L.J.; Castelo, P.M.; Rodrigues Garcia, R.C.M.; Gonçalves, T.M.S.V. Long-term survival analysis of standard-length and short implants with multifunctional abutments. J. Oral Rehabil. 2019, 46, 640–646. [Google Scholar] [CrossRef]
- Felice, P.; Soardi, E.; Pellegrino, G.; Pistilli, R.; Marchetti, C.; Gessaroli, M.; Esposito, M. Treatment of the atrophic edentulous maxilla: Short implants versus bone augmentation for placing longer implants. Five-month post-loading results of a pilot randomised controlled trial. Eur. J. Oral Implant 2011, 4, 191–202. [Google Scholar]
- Gehrke, S.A.; Guirado, J.L.C.; Bettach, R.; Fabbro, M.D.; Martínez, C.P.A.; Shibli, J.A. Evaluation of the insertion torque, implant stability quotient and drilled hole quality for different drill design: An in vitro Investigation. Clin. Oral Implant Res. 2018, 29, 656–662. [Google Scholar] [CrossRef]
- Romanos, G.E.; Delgado-Ruiz, R.A.; Sacks, D.; Calvo-Guirado, J.L. Influence of the implant diameter and bone quality on the primary stability of porous tantalum trabecular metal dental implants: An in vitro biomechanical study. Clin. Oral Implant Res. 2018, 29, 649–655. [Google Scholar] [CrossRef]
- Möhlhenrich, S.C.; Heussen, N.; Elvers, D.; Steiner, T.; Hölzle, F.; Modabber, A. Compensating for poor primary implant stability in different bone densities by varying implant geometry: A laboratory study. Int. J. Oral. Maxillofac. Surg. 2015, 44, 1514–1520. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Shiota, M.; FuJii, M.; Sekiya, M.; Ozeki, M. Development and application of a direct method to observe the implant/bone interface using simulated bone. Springerplus 2016, 5, 494. [Google Scholar] [CrossRef] [Green Version]
- Falco, A.; Berardini, M.; Trisi, P. Correlation Between Implant Geometry, Implant Surface, Insertion Torque, and Primary Stability: In Vitro Biomechanical Analysis. Int. J. Oral Maxillofac. Implant 2018, 33, 824–830. [Google Scholar] [CrossRef]
- Di Stefano, D.A.; Arosio, P.; Gastaldi, G.; Gherlone, E. The insertion torque-depth curve integral as a measure of implant primary stability: An in vitro study on polyurethane foam blocks. J. Prosthet. Dent. 2018, 120, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Tsolaki, I.N.; Tonsekar, P.P.; Najafi, B.; Drew, H.J.; Sullivan, A.J.; Petrov, S.D. Comparison of Osteotome and Conventional Drilling Techniques for Primary Implant Stability: An In Vitro Study. J. Oral Implant 2016, 42, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.S.; Rodrigues, J.A.; Shibli, J.A.; Piattelli, A.; Iezzi, G.; Perrotti, V. Influence of osteoporosis on the osteocyte density of human mandibular bone samples: A controlled histological human study. Clin. Oral Implant Res. 2016, 27, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Sennerby, L.; Meredith, N. Implant stability measurements using resonance frequency analysis: Biological and biomechanical aspects and clinical implications. Periodontology 2008, 47, 51–66. [Google Scholar] [CrossRef]
- Comuzzi, L.; Iezzi, G.; Piattelli, A.; Tumedei, M. An In Vitro Evaluation, on Polyurethane Foam Sheets, of the Insertion Torque (IT) Values, Pull-Out Torque Values, and Resonance Frequency Analysis (RFA) of NanoShort Dental Implants. Polymer 2019, 11, 1020. [Google Scholar] [CrossRef]




| Insertion Torque | D4 Density | D3 Density | ||||||
|---|---|---|---|---|---|---|---|---|
| Short 5 mm (A) | Short 6 mm (B) | UN II (C) | UN III (D) | Short 5 mm (E) | Short 6 mm (F) | UN II (G) | UN III (H) | |
| Mean | 26.7 | 30.8 | 17 | 16.8 | 46.5 | 40.4 | 29.1 | 31.1 |
| Std. Deviation | ±1.059 | ±1.033 | ±0.942 | ±1.135 | ±1.269 | ±1.350 | ±0.994 | ±0.994 |
| Multiple Comparison Insertion Torque | 95.00% CI of Diff, | Adjusted p Value |
|---|---|---|
| A-B | −5.577 to −2.623 | <0.0001 |
| B-C | 12.32 to 15.28 | <0.0001 |
| C-D | −1.277 to 1.677 | N.S.D. |
| D-E | −31.18 to −28.22 | <0.0001 |
| A-D | 8.423 to 11.38 | <0.0001 |
| A-C | 8.223 to 11.18 | <0.0001 |
| B-D | 12.52 to 15.48 | <0.0001 |
| E-F | 4.623 to 7.577 | <0.0001 |
| F-G | 9.823 to 12.78 | <0.0001 |
| G-H | −3.477 to −0.5230 | 0.0006 |
| E-H | 13.92 to 16.88 | <0.0001 |
| F-H | 7.823 to 10.78 | <0.0001 |
| E-G | 15.92 to 18.88 | <0.0001 |
| Pull Out | D4 Density | D3 Density | ||||||
|---|---|---|---|---|---|---|---|---|
| Short 5 mm (A) | Short 6 mm (B) | UN II (C) | UN III (D) | Short 5 mm (E) | Short 6 mm (F) | UN II (G) | UN III (H) | |
| Mean | 27.3 | 31 | 11.1 | 12 | 37.8 | 38.1 | 27.1 | 27.1 |
| Std. Deviation | ±1.059 | ±1.054 | ±0.994 | ±1.247 | ±0.918 | ±0.875 | ±1.101 | ±1.287 |
| Multiple Comparison Pull Out | 95.00% CI of Diff, | Adjusted p Value |
|---|---|---|
| A-B | −5.124 to −2.276 | <0.0001 |
| B-C | 18.48 to 21.32 | <0.0001 |
| C-D | −2.324 to 0.5237 | N.S.D. |
| A-D | 13.88 to 16.72 | <0.0001 |
| B-D | 17.58 to 20.42 | <0.0001 |
| A-C | 14.78 to 17.62 | <0.0001 |
| D-E | −27.24 to −24.36 | <0.0001 |
| E-F | −1.724 to 1.124 | >0.9999 |
| F-G | 9.576 to 12.42 | <0.0001 |
| G-H | −1.424 to 1.424 | N.S.D. |
| E-H | 9.276 to 12.12 | <0.0001 |
| F-H | 9.576 to 12.42 | <0.0001 |
| E-G | 9.276 to 12.12 | <0.0001 |
| A-B | −5.124 to −2.276 | <0.0001 |
| ISQ | D4 Density | D3 Density | ||||||
|---|---|---|---|---|---|---|---|---|
| Short 5 mm (A) | Short 6 mm (B) | UN II (C) | UN III (D) | Short 5 mm (E) | Short 6 mm (F) | UN II (G) | UN III (H) | |
| Mean | 76.6 | 78.85 | 58.05 | 57.9 | 78.45 | 80 | 76.65 | 76.6 |
| Std. Deviation | ±0.966 | ±0.747 | ±0.283 | ±0.809 | ±0.550 | ±1.491 | ±1.001 | ±0.774 |
| Multiple Comparison RFA | 95.00% CI of Diff, | Adjusted p Value |
|---|---|---|
| A-B | −3.430 to −1.070 | <0.0001 |
| B-C | 19.62 to 21.98 | <0.0001 |
| C-D | −1.030 to 1.330 | N.S.D. |
| A-D | 17.52 to 19.88 | <0.0001 |
| B-D | 19.77 to 22.13 | <0.0001 |
| A-C | 17.37 to 19.73 | <0.0001 |
| D-E | −21.74 to −19.36 | <0.0001 |
| E-F | −2.730 to −0.3703 | 0.0027 |
| F-G | 2.170 to 4.530 | <0.0001 |
| G-H | −1.130 to 1.230 | N.S.D. |
| E-H | 0.6703 to 3.030 | 0.0002 |
| F-H | 2.220 to 4.580 | <0.0001 |
| E-G | 0.6203 to 2.980 | 0.0003 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Comuzzi, L.; Tumedei, M.; Piattelli, A.; Iezzi, G. Short vs. Standard Length Cone Morse Connection Implants: An In Vitro Pilot Study in Low Density Polyurethane Foam. Symmetry 2019, 11, 1349. https://doi.org/10.3390/sym11111349
Comuzzi L, Tumedei M, Piattelli A, Iezzi G. Short vs. Standard Length Cone Morse Connection Implants: An In Vitro Pilot Study in Low Density Polyurethane Foam. Symmetry. 2019; 11(11):1349. https://doi.org/10.3390/sym11111349
Chicago/Turabian StyleComuzzi, Luca, Margherita Tumedei, Adriano Piattelli, and Giovanna Iezzi. 2019. "Short vs. Standard Length Cone Morse Connection Implants: An In Vitro Pilot Study in Low Density Polyurethane Foam" Symmetry 11, no. 11: 1349. https://doi.org/10.3390/sym11111349
APA StyleComuzzi, L., Tumedei, M., Piattelli, A., & Iezzi, G. (2019). Short vs. Standard Length Cone Morse Connection Implants: An In Vitro Pilot Study in Low Density Polyurethane Foam. Symmetry, 11(11), 1349. https://doi.org/10.3390/sym11111349








