Use of Platelet-Rich Fibrin Associated with Xenograft in Critical Bone Defects: Histomorphometric Study in Rabbits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
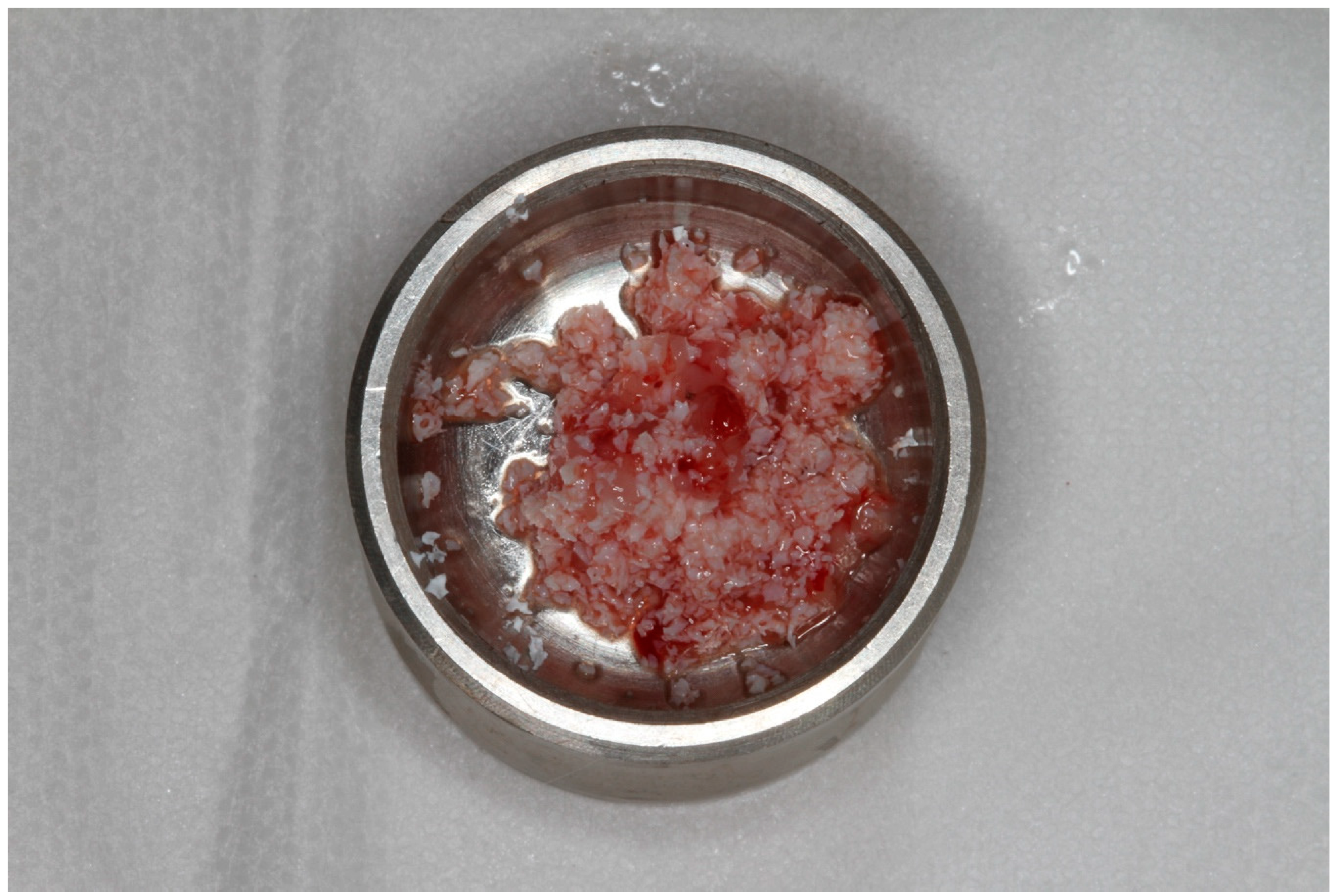
2.2. Preparation and Application of PRF
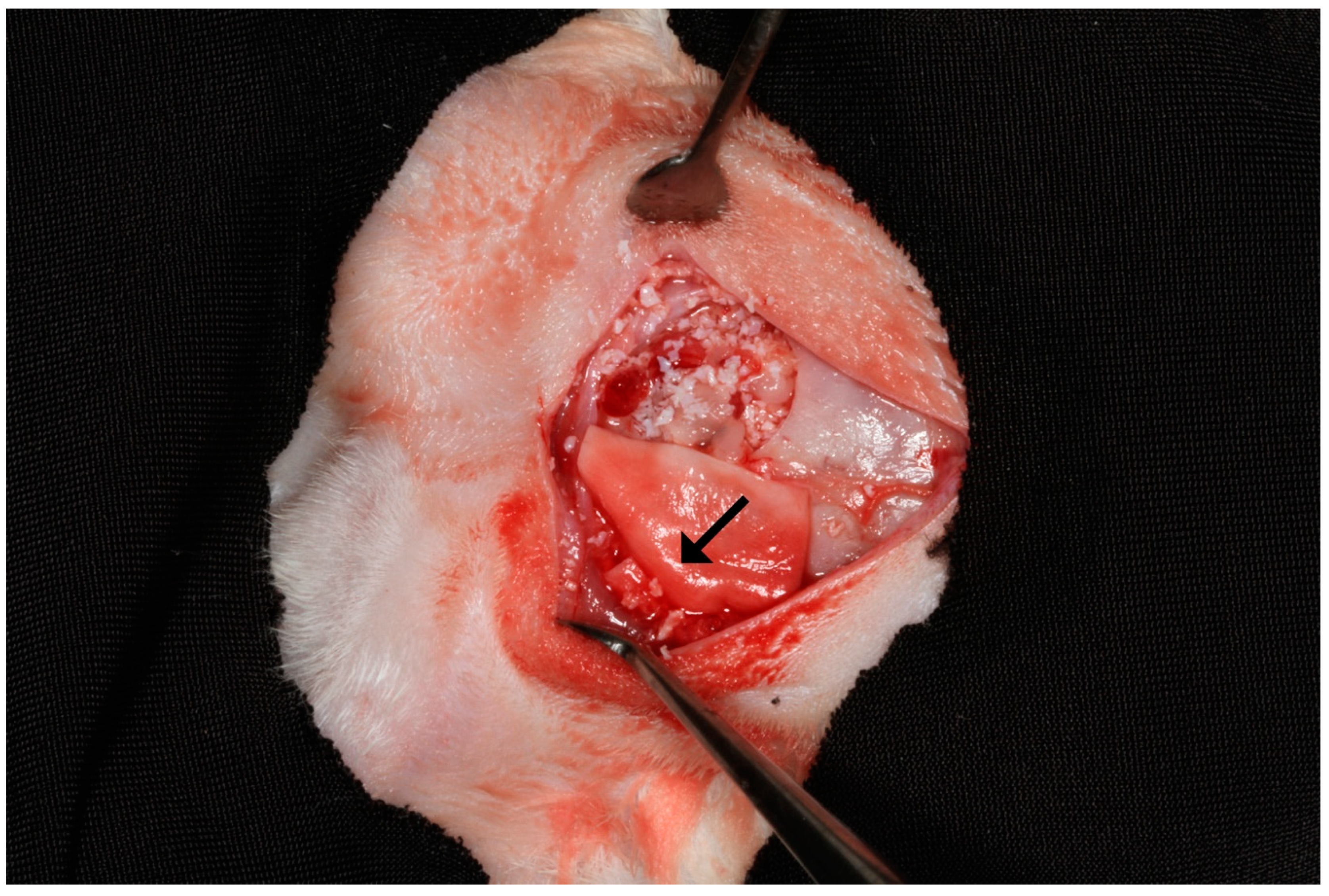
2.3. Biopsies Collection
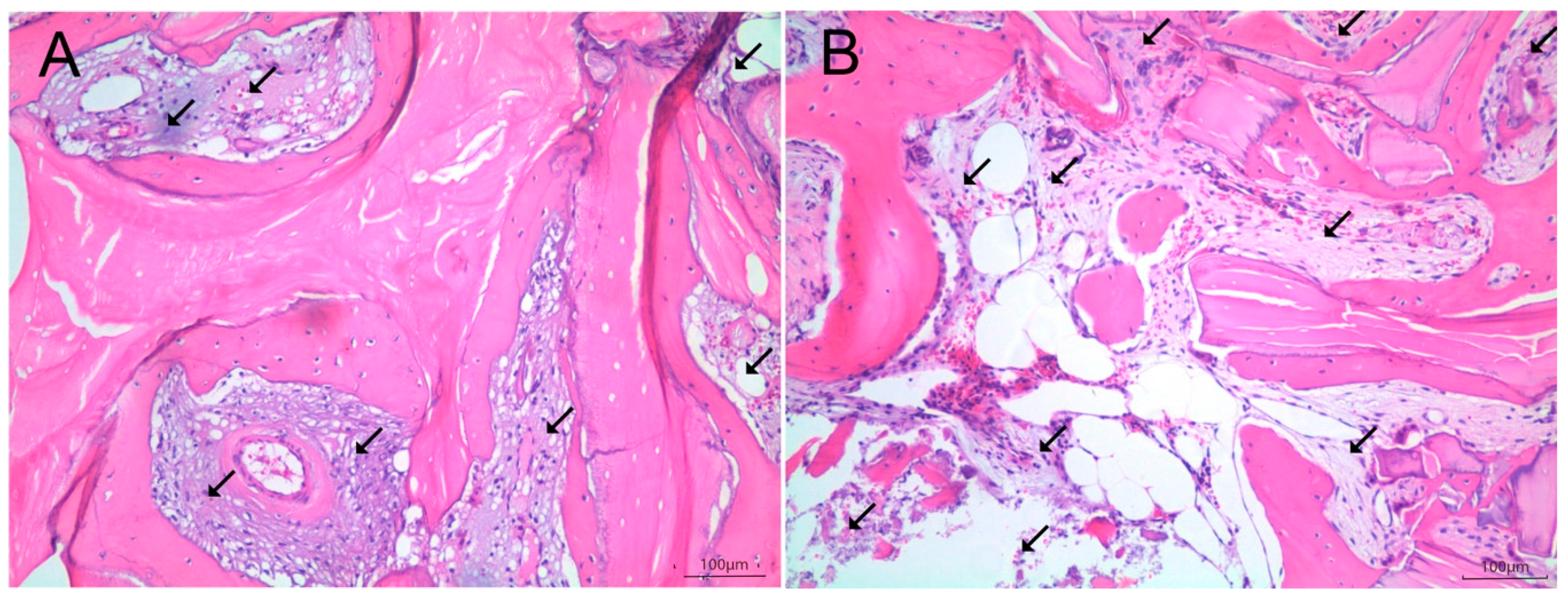
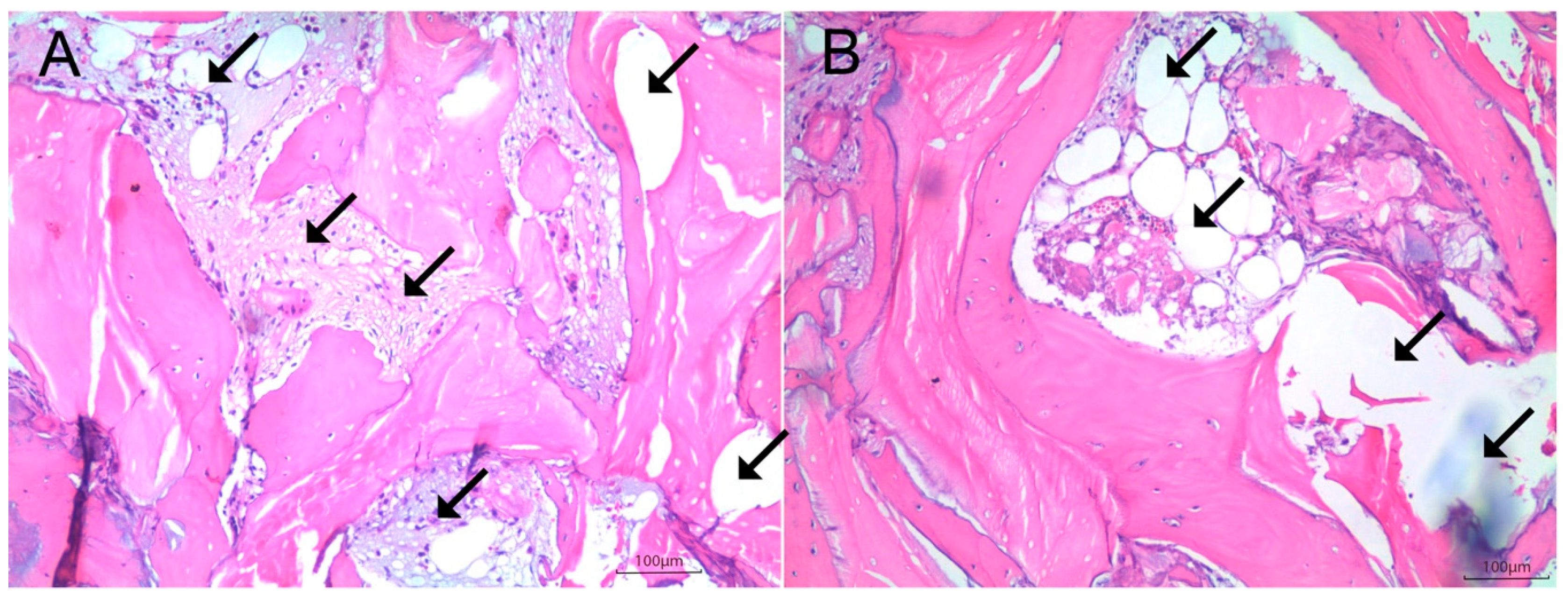
2.4. Histologic and Histomorphometric Analysis
2.5. Statistical Analysis
3. Results
Histomorphometry
4. Discussion
5. Conclusions
- (1)
- Platelet-rich fibrin does not result in higher levels of bone formation when a guided bone regeneration technique (i.e., with membrane coverage) is used.
- (2)
- The usage of the collagen membrane has a synergistic effect on bone healing when associated with a xenograft.
- (3)
- Platelet-rich fibrin may increase the level of newly formed bone only in bone grafting procedures using xenograft without collagen membrane coverage.
Author Contributions
Funding
Conflicts of Interest
References
- Chrcanovic, B.; Albrektsson, T.; Wennerberg, A. Bone Quality and Quantity and Dental Implant Failure: A Systematic Review and Meta-analysis. Int. J. Prosthodont. 2017, 30, 219–237. [Google Scholar] [CrossRef] [PubMed]
- Bagher, Z.; Rajaei, F.; Shokrgozar, M. Comparative Study of Bone Repair Using Porous Hydroxyapatite/β-Tricalcium Phosphate and Xenograft Scaffold in Rabbits with Tibia Defect. Iran. Biomed. J. 2012, 16, 18–24. [Google Scholar] [PubMed]
- Stevenson, S.; Emery, S.E.; Goldberg, V.M. Factors Affecting Bone Graft Incorporation. Clin. Orthop. Relat. Res. 1996, 324, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Gultekin, B.A.; Bedeloglu, E.; Kose, T.E.; Mijiritsky, E. Comparison of Bone Resorption Rates after Intraoral Block Bone and Guided Bone Regeneration Augmentation for the Reconstruction of Horizontally Deficient Maxillary Alveolar Ridges. BioMed Res. Int. 2016, 2016, 4987437. [Google Scholar] [CrossRef] [PubMed]
- Titsinides, S.; Agrogiannis, G.; Karatzas, T. Bone grafting materials in dentoalveolar reconstruction: A comprehensive review. Jpn. Dent. Sci. Rev. 2019, 55, 26–32. [Google Scholar] [CrossRef] [PubMed]
- De Lacerda, P.E.; Pelegrine, A.A.; Teixeira, M.L.; Montalli, V.A.M.; Rodrigues, H.; Napimoga, M.H. Homologous transplantation with fresh frozen bone for dental implant placement can induce HLA sensitization: A preliminary study. Cell Tissue Bank. 2016, 17, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Pelegrine, A.A.; Aloise, A.C.; Zimmermann, A.; de Mello E Oliveira, R.; Ferreira, L.M. Repair of critical-size bone defects using bone marrow stromal cells: A histomorphometric study in rabbit calvaria. Part I: Use of fresh bone marrow or bone marrow mononuclear fraction. Clin. Oral Implant. Res. 2014, 25, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Aloise, A.C.; Pelegrine, A.A.; Zimmermann, A.; Oliveira, R.D.M.E.; Ferreira, L.M. Repair of critical-size bone defects using bone marrow stem cells or autogenous bone with or without collagen membrane: A histomorphometric study in rabbit calvaria. Int. J. Oral Maxillofac. Implant. 2015, 30, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, P.J.; Teixeira, M.L.; De Oliveira, T.A.; De Macedo, L.G.S.; Aloise, A.C.; Pelegrine, A.A. Maxillary Sinus Augmentation Combining Bio-Oss with the Bone Marrow Aspirate Concentrate: A Histomorphometric Study in Humans. Int. J. Biomater. 2015, 2015, 121286. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, T.A.; Aloise, A.C.; Orosz, J.E.; Oliveira, R.D.M.E.; De Carvalho, P.; Pelegrine, A.A. Double Centrifugation Versus Single Centrifugation of Bone Marrow Aspirate Concentrate in Sinus Floor Elevation: A Pilot Study. Int. J. Oral Maxillofac. Implant. 2016, 31, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Chieruzzi, M.; Pagano, S.; Moretti, S.; Pinna, R.; Milia, E.; Torre, L.; Eramo, S. Nanomaterials for Tissue Engineering in Dentistry. Nanomaterials 2016, 6, 134. [Google Scholar] [CrossRef] [PubMed]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101, e37–e44. [Google Scholar] [CrossRef] [PubMed]
- Bolukbasi, N.; Ersanli, S.; Keklikoglu, N.; Basegmez, C.; Özdemir, T. Sinus augmentation with platelet-rich fibrin in combination with bovine bone graft versus bovine bone graft in combination with collagen membrane. J. Oral Implant. 2015, 41, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Borie, E.; Oliví, D.G.; Orsi, I.A.; Garlet, K.; Weber, B.; Beltrán, V.; Fuentes, R. Platelet-rich fibrin application in dentistry: A literature review. Int. J. Clin. Exp. Med. 2015, 8, 7922–7929. [Google Scholar] [PubMed]
- Pelegrine, A.A.; Teixeira, M.L.; Sperandio, M.; Almada, T.S.; Kahnberg, K.E.; Pasquali, P.J.; Aloise, A.C. Can bone marrow aspirate concentrate change the mineralization pattern of the anterior maxilla treated with xenografts? A preliminary study. Contemp. Clin. Dent. 2016, 7, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Lucarelli, E.; Beretta, R.; Dozza, B.; Tazzari, P.; O’Connell, S.; Ricci, F.; Pierini, M.; Squarzoni, S.; Pagliaro, P.; Oprita, E.; et al. A recently developed bifacial platelet-rich fibrin matrix. Eur. Cells Mater. 2010, 20, 13–23. [Google Scholar] [CrossRef]
- Zimmermann, A.; Pelegrine, A.A.; Peruzzo, D.; Martinez, E.F.; Oliveira, R.D.M.E.; Aloise, A.C.; Ferreira, L.M. Adipose mesenchymal stem cells associated with xenograft in a guided bone regeneration model: A histomorphometric study in rabbit calvaria. Int. J. Oral Maxillofac. Implant. 2015, 30, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Nacopoulos, C.; Dontas, I.; Lelovas, P.; Galanos, A.; Vesalas, A.-M.; Raptou, P.; Mastoris, M.; Chronopoulos, E.; Papaioannou, N. Enhancement of Bone Regeneration with the Combination of Platelet-Rich Fibrin and Synthetic Graft. J. Craniofacial Surg. 2014, 25, 2164–2168. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.-S.; Lee, S.-H.; Yoon, H.-J. The influence of platelet-rich fibrin on angiogenesis in guided bone regeneration using xenogenic bone substitutes: A study of rabbit cranial defects. J. Cranio-Maxillofac. Surg. 2014, 42, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Pistilli, R.; Felice, P.; Piatelli, M.; Nisii, A.; Barausse, C.; Esposito, M. Blocks of autogenous bone versus xenografts for the reha- bilitation of atrophic jaws with dental implants: Preliminary data from a pilot randomised controlled trial. Eur. J. Oral Implantol. 2014, 7, 153–171. [Google Scholar] [PubMed]

| Tissue | With Membrane | p-Value | Without Membrane | p-Value | ||
|---|---|---|---|---|---|---|
| Control Group | Test Group | Control Group | Test Group | |||
| NVMT | 13.50 ± 0.09 | 17.88 ± 7.57 | 0.2971 | 13.09 ± 0.06 | 16.59 ± 3.54 | 0.0542 |
| VMT | 13.29 ± 0.16 | 13.88 ± 5.68 | 0.5211 | 6.57 ± 0.08 | 9.67 ± 0.60 | 0.0039 * |
| NMT | 73.21 ± 0.12 | 68.24 ± 5.05 | 0.0776 | 80.34 ± 0.05 | 73.74 ± 3.48 | 0.0038 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maia, P.W.; Teixeira, M.L.; Scavone de Macedo, L.G.; Aloise, A.C.; Passos Junior, C.A.; Aragoneses, J.M.; Calvo-Guirado, J.L.; Pelegrine, A.A. Use of Platelet-Rich Fibrin Associated with Xenograft in Critical Bone Defects: Histomorphometric Study in Rabbits. Symmetry 2019, 11, 1293. https://doi.org/10.3390/sym11101293
Maia PW, Teixeira ML, Scavone de Macedo LG, Aloise AC, Passos Junior CA, Aragoneses JM, Calvo-Guirado JL, Pelegrine AA. Use of Platelet-Rich Fibrin Associated with Xenograft in Critical Bone Defects: Histomorphometric Study in Rabbits. Symmetry. 2019; 11(10):1293. https://doi.org/10.3390/sym11101293
Chicago/Turabian StyleMaia, Paulo Wilson, Marcelo Lucchesi Teixeira, Luís Guilherme Scavone de Macedo, Antonio Carlos Aloise, Celio Amaral Passos Junior, Juan Manuel Aragoneses, José Luis Calvo-Guirado, and André Antonio Pelegrine. 2019. "Use of Platelet-Rich Fibrin Associated with Xenograft in Critical Bone Defects: Histomorphometric Study in Rabbits" Symmetry 11, no. 10: 1293. https://doi.org/10.3390/sym11101293
APA StyleMaia, P. W., Teixeira, M. L., Scavone de Macedo, L. G., Aloise, A. C., Passos Junior, C. A., Aragoneses, J. M., Calvo-Guirado, J. L., & Pelegrine, A. A. (2019). Use of Platelet-Rich Fibrin Associated with Xenograft in Critical Bone Defects: Histomorphometric Study in Rabbits. Symmetry, 11(10), 1293. https://doi.org/10.3390/sym11101293







