Abstract
Cyclodextrins (CDs) are cone-shaped molecular rings that have been widely employed in supramolecular/host–guest chemistry because of their low cost, high biocompatibility, stability, wide availability in multiple sizes, and their promiscuity for binding a range of molecular guests in water. Consequently, CD-based host–guest complexes are often employed as templates for the synthesis of mechanically bonded molecules (mechanomolecules) such as catenanes, rotaxanes, and polyrotaxanes in particular. The conical shape and cyclodirectionality of the CD “bead” gives rise to a symmetry-breaking effect when it is threaded onto a molecular “string”; even symmetrical guests are rendered asymmetric by the presence of an encircling CD host. This review focuses on the stereochemical implications of this symmetry-breaking effect in mechanomolecules, including orientational isomerism, mechanically planar chirality, and topological chirality, as well as how they support applications in regioselective and stereoselective chemical synthesis, the design of molecular machine prototypes, and the development of advanced materials.
1. Introduction
Cyclodextrins (CDs) [1] are a class of macrocyclic natural products that were first isolated in 1891 from cultures of starch-fermenting bacteria by Villiers [2]. Structurally, all CDs are oligomeric loops of glucose; different sized rings [3] are denoted by Greek prefixes, beginning with α-CD (6-mer) and continuing up to at least the 26-mer, u-CD [4]. The first three CDs—α-CD, β-CD, and γ-CD (6–8 glucose subunits; Figure 1)—are by far the most common and most widely available. After more than half a century of historically important investigations by Schardinger [5], French [5], Freudenberg [6], Cramer [6], and Pringsheim [7] on the structure and properties of CDs, it was Cramer [8,9] who pioneered the study of cyclodextrin nclusion complexes beginning in the early 1950s—long before the Nobel-recognized work of Pedersen [10], Cram [11], and Lehn [12] of a similar nature. The three main CDs possess a conical shape; the wider “2,3-rim” (secondary face) is reinforced by hydrogen bonds between the secondary alcohol groups at the 2 and 3 positions of the glucose subunits, while the narrower “6-rim” (primary face) displays all of the primary alcohol groups at the 6 position. The hydrophobic cavities of α-CD, β-CD, and γ-CD are approximately 5, 7, and 9 Å in diameter, respectively, and ~8 Å in depth, allowing them to ensconce thousands of appropriately sized lipophilic guests in water [13,14]. While CDs continue to serve as a cornerstone of supramolecular/host–guest chemistry, they are also important compounds in organocatalytic chemistry [15,16,17], analytical chemistry [18], separations [19], medicine [20,21], food science [22], and other industrial applications [23].
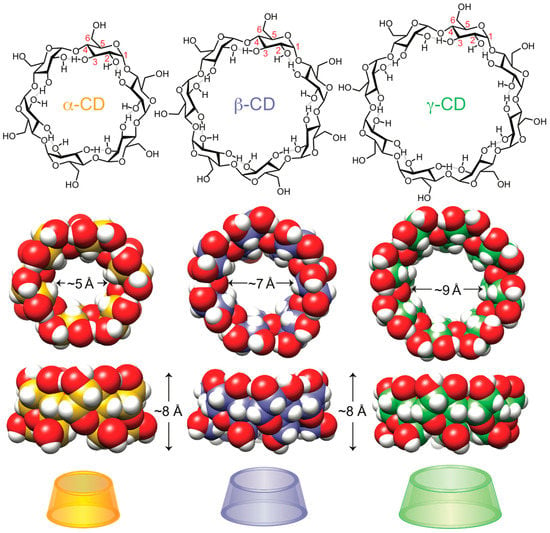
Figure 1.
Structural, space-filling, and graphical representations of the three main cyclodextrin (CD) molecules: α-CD (yellow), β-CD (blue), and γ-CD (green).
CD-based host–guest complexes are convenient templates for the synthesis of molecules with mechanical bonds [24,25,26,27,28,29], also known [30] as mechanomolecules. A mechanical bond is defined [30] as “an entanglement in space between two or more molecular entities (component parts) such that they cannot be separated without breaking or distorting chemical bonds between atoms”. The archetypal mechanomolecules are (i) [n]catenanes, in which n ring-shaped component parts are interlocked, and (ii) [n]rotaxanes, in which the n component parts comprise one or more rings encircling one or more acyclic “dumbbells”, which possess endgroups or “stoppers” that prevent the ring(s) from dissociating. Since complexation is driven by a hydrophobic effect, a simple and convenient template for making mechanomolecules is a hydrocarbon⊂CD pseudo-[2]rotaxane (the prefix pseudo- denotes an unstoppered threaded assembly). In general, CDs exhibit increased affinity for oligomethylenes as chain length increases, assuming the guests are water soluble [31,32]. Indeed, the first successful template-directed synthesis of a [2]rotaxane was carried out by Ogino [33] (Scheme 1) using an amine-terminated dodecane (C12) guest with α-CD or β-CD hosts. Ogino used cis-dichlorobis(ethylenediamine)cobalt(III) chloride [CoCl2(en)2]Cl to stopper CD-threaded H2NC12H24NH2 chains in Me2SO at 75 °C, generating either α-R14+ (19% yield) or β-R14+ (7% yield) upon isolation by size-exclusion chromatography. The higher yield for α-R14+ compared to β-R14+ reflects the alkanes’ higher affinity for α-CD. Note that while the cobalt-capped hydrocarbon chain has a high symmetry (D∞h) by itself, the overall symmetry of the corresponding rotaxanes are reduced (Cn) to that of the encircling cyclodextrin, where each end of the dumbbells become differentiated by their proximity to a different rim of the CD ring.
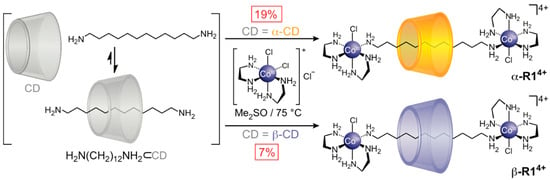
Scheme 1.
Ogino’s seminal template-directed synthesis of [2]rotaxanes α-R14+ and β-R14+ from diaminododecane and α-CD or β-CD, respectively.
This mini-review describes how the symmetry-breaking effect of cyclodextrin can be leveraged to generate mechanomolecules with unique stereochemical and dynamic properties, which may be useful in the generation of novel syntheses, molecular machines, and advanced materials.
2. The Stereochemistry of CD-Based Mechanomolecules
Since stereochemistry [34] is the branch of chemistry that deals with the three-dimensional arrangements of atoms in space, mechanostereochemistry is simply defined [35] as the stereochemistry of molecules with mechanical bonds. Mechanical bonding gives rise to completely new types of molecular isomerism and dynamics that are not observed in other types of compounds. As stereoisomers are molecules that have identical constitutions but different arrangements of atoms in space, mechanostereoisomers [30] are mechanomolecules that have identical co-constitutions, but differences in the arrangement of their component parts in space. The symmetry-breaking effect of CDs—in particular their lack of any mirror planes—can give rise to orientational mechanostereoisomers (see Section 2.1), as well as emergent types of chirality (objects having non-superimposable mirror images), namely, mechanically planar (see Section 2.2) and topological (see Section 2.3) chirality.
2.1. Orientational Mechanostereoisomers
As the name suggests, orientational mechanostereoisomers arise from having different possible ring orientations in mechanomolecules. If a ring lacks mirror symmetry across the plane of its cavity, its rims may be differentiated into a “head” and a “tail”, and it can therefore be threaded onto a string either head-first or tail-first. In the case of cyclodextrins, we refer to the wider 2,3-rim as the “head” and narrower 6-rim as the “tail”. If a second component part is mechanically bonded to a CD, orientational isomerism can arise if it too lacks mirror symmetry across the plane of the CD’s cavity. Orientational mechanostereoisomerism arising from CDs may be observed in [2]rotaxanes (see Section 2.1.1), [3]rotaxanes (see Section 2.1.2), and polyrotaxanes (see Section 2.1.3), as well as [3]catenanes (see Section 2.1.4) and larger [n]catenanes (see Section 2.1.5) in principle. In the case of [2]catenanes, the conditions for achieving orientational isomerism are also sufficient for topological chirality, so these compounds are discussed in Section 2.3.
2.1.1. [2]Rotaxane Orientational Isomers
The earliest rotaxanes to exhibit orientational mechanostereoisomerism were based on α-CD. In 1991, Kaifer [36,37] coupled a carboxylic acid-terminated half-dumbbell with a sulfonated aminonaphthalene stopper in the presence of α-CD to generate (Scheme 2) a mixture of orientational mechanostereoisomers R2a and R2b. In this case, the CD rings had no orientational bias; both isomers were obtained in equal proportion. When the isomers were separated by thin-layer chromatography and isolated as pure compounds, they showed a surprising behavior: the CD ring slowly escaped from the dumbbell in R2a, but not in R2b. This kind of face-selective motion presents a promising opportunity for the design of molecular machine prototypes (see Section 3.2).
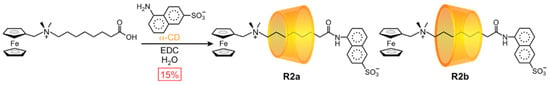
Scheme 2.
Kaifer’s seminal synthesis of a mixture of [2]rotaxane orientational isomers.
While it may seem initially that [2]rotaxane orientational isomers cannot interconvert without the ring either turning inside-out or executing an escape–rotate–rethreading sequence, Kawaguchi and Harada [38] demonstrated an interesting case (Scheme 3) of orientational isomers that exchange by a ring-shuttling mechanism. As the α-CD ring of the [2]rotaxane oscillates between two ends of a symmetrical dumbbell, it alternately exposes its head (R3a6+) and tail (R3b6+) to the bipyridinium barrier at the center of the dumbbell. Rotating R3b6+ 180° such that its stoppers exchange sites can help one see that it is the orientational isomer of R3a6+, owing to the mirror symmetry of the dumbbell.
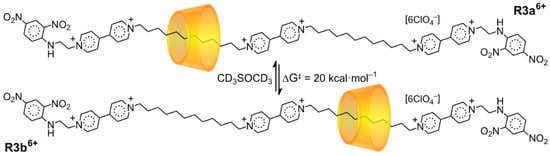
Scheme 3.
A molecular shuttle in which the oscillation of a ring between two sites leads to interconversion between two orientational isomers.
While CD (pseudo)-[2]rotaxanes with desymmetrized dumbbells are often obtained as a mixture of orientational isomers [39,40,41,42,43,44,45,46], it is also common to obtain one orientational isomer selectively (see Section 2.2).
2.1.2. [3]Rotaxane Orientational Isomers
When two or more desymmetrized rings are threaded onto a string, they can access different orientations with respect to one another, so a symmetry-breaking dumbbell is no longer required for orientational isomerism to occur. Such a scenario is exemplified in Scheme 4.
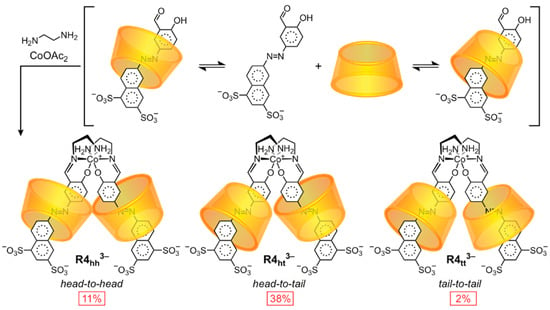
Scheme 4.
A mixture of three [3]rotaxane orientational isomers is obtained upon dimerization of salicylaldehyde-terminated α-CD pseudo-[2]rotaxanes around a CoIII metal center [47].
A team led by Anderson [47] isolated, by anion exchange chromatography, all three orientational isomers—head-to-head R4hh3− (11%), head-to-tail R4ht3− (38%), and tail-to-tail R4tt3− (2%)—of [3]rotaxane upon conjoining a pair of salicylaldehyde-functionalized pseudorotaxanes around a Co3+ ion to create a symmetrical dumbbell in the presence of α-CD. The unequal distribution of products indicates a bias, but not complete selectivity, for the head-to-tail orientation. Later, the team prepared [48] a head-to-tail orientational isomer with high selectively on an oligophenylene(ethynylene) dumbbell on porous glass solid supports. Selective syntheses of tail-to-tail [49,50] and head-to-head [51,52,53,54] [3]rotaxanes have also been reported (see Section 3.1.2). A [3]rotaxane based on oligothiophene-threaded β-CD rings has also been synthesized [55] as a mixture of orientational isomers.
2.1.3. [n]Rotaxane Orientational Isomers
There are a few examples of discrete [n>3]rotaxanes [56,57,58,59] based on CD templates, but the ring orientations are not well characterized. In 1992, Harada [60] reported the first synthesis of a CD polyrotaxane, comprising polyethylene glycol (PEG) threaded by many α-CD rings. It is believed [60,61] that α-CD/PEG poly(pseudo)rotaxanes self-assemble with high mechanostereoselectivity into an arrangement with a repeating sequence of head-to-head/tail-to-tail orientations.
2.1.4. [3]Catenane Orientational Isomers
Although the synthesis of CD-based catenanes was attempted [62] as early as 1958, Stoddart [63] first reported the synthesis (Scheme 5) of catenated cyclodextrins in 1993.
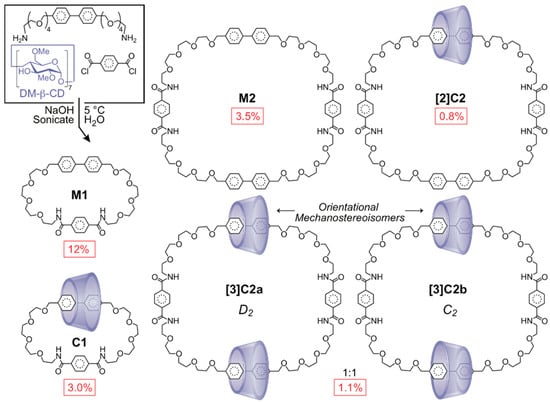
Scheme 5.
The synthesis of catenated cyclodextrins [63].
Macrocyclizing terephthaloyl chloride with a biphenylene unit bis-functionalized with flexible amine-terminated tetraethylene glycol linkers in the presence of heptakis (2,6-di-O-methyl)-β-cyclodextrin (DM-β-CD) afforded a mixture of [1+1] products (macrocycle M1, [2]catenane C1) and [2+2] products (macrocycle M2, [2]catenane [2]C2, and [3]catenanes [3]C2a-b). The pair of [3]catenanes, [3]C2a and [3]C2b, were isolated [64] as a 1:1 mixture of head-to-head/tail-to-tail and head-to-tail orientational isomers, respectively, which may be distinguished by their different time-averaged (D2 and C2) symmetries. A group led by Otto [65] has also observed [3]catenane orientational isomers within a dynamic combinatorial library of catenated cyclodextrins.
2.1.5. [n]Catenane Orientational Isomers
Radial-type [n]catenanes possess a structure in which multiple small rings interlock a single large ring. With the exception of their probable formation [66] as a side-product in the photopolymerization of anthracene-stoppered poly [n]rotaxanes, this type of topology was not realized using CDs until very recently. Higashi et al. reported [67] a one-pot approach to making radial [n]catenanes with many (>10) β-CD rings encircling a polymer macrocycle. While the ring orientations were not characterized (to do so would be very challenging), it is likely that a multitude of different orientations occur, although polydispersity in chain length and ring-threading ratios preclude the presence of true isomers. The number of possible isomers —both orientational and topological—increases exponentially as more and more oriented or cyclodirectional rings are added to either [n]rotaxanes or [n]catenanes.
2.2. Mechanically Planar Chirality
Whereas orientational mechanostereoisomers require rings that are desymmetrized across the plane of their cavity, mechanically planar enantiomers require rings that lack mirror symmetry across any plane orthogonal to the ring’s cavity. Such rings are said to be “cyclodirectional”, denoting a polarity in the sequence of atoms constituting the macrocycle. It is noteworthy that chirality and cyclodirectionality are not equivalent. Although CDs are chiral, an achiral yet cyclodirectional ring may combine with an appropriate achiral yet sufficiently desymmetrized dumbbell to produce this emergent type of chirality.
The mirror-image enantiomers of cartoonized mechanically planar chiral [2]rotaxanes with achiral yet directional component parts are illustrated in Figure 2. Employing vocabulary inspired by Prelog [68], rotaxanes expressing this type of asymmetry have been described [69,70,71,72,73,74] as “cyclochiral”, or having “cycloenantiomers” and “cyclodiastereomers”. This language can be misleading because cycloenantiomers are macrocycles [75], not rotaxanes. After Takata [76] described it as a form of planar chirality, Goldup [77] proposed the term “mechanically planar” to differentiate this kind of rotaxane-specific chirality from more classical forms of planar chirality. Left-handed and right-handed co-configurations of mechanically planar rotaxanes are written as (Rmp) and (Smp), respectively, where the subscript “mp” denotes mechanically planar. The assignment of absolute co-configuration relies on Cahn–Ingold–Prelog [78] rules to define the directionality of each component part.
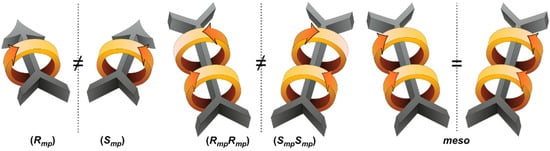
Figure 2.
Illustration of enantiomeric mechanostereoisomers possessing mechanically planar chirality. A [2]rotaxane requires an oriented dumbbell, while a [3]rotaxane does not.
CD cyclodirectionality is attributable to the polarity of the glycosidic bonds linking glucose subunits. This cyclodirectionality means that any CD-based [2]rotaxane capable of orientational isomerism necessarily possesses mechanically planar chirality. A collection of [2]rotaxanes from Anderson [48,79] (R53−, R11), Zhao [80,81,82] (R546a-c2−), Tian [83,84,85] (R73−, R82−, R93−, R102−), Park [86] (R12+), and Mezzina and Lucarini [87] (R13) are shown in Figure 3 as examples of [2]rotaxanes that were isolated as pure orientational isomers (and therefore also as single mechanically planar enantiomers).
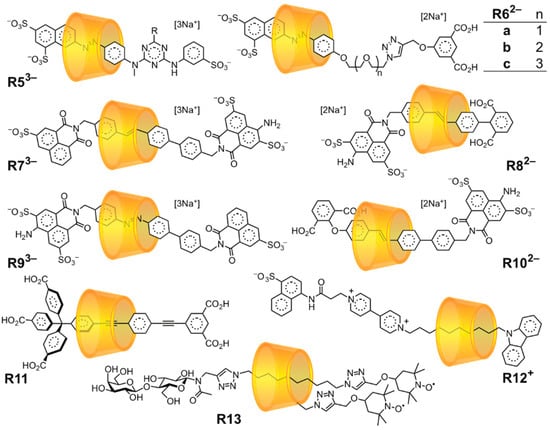
Figure 3.
Examples of mechanically planar chiral [2]rotaxanes based on α-CD, all isolated as single orientational isomers [48,79,80,81,82,83,84,85,86,87].
Although flipping the orientation of the CD ring in these rotaxanes would change the mechanically planar chirality from Rmp to Smp or vice versa, the two orientational isomers are not enantiomers (they are diastereomers). CD rotaxanes are never obtained as mixtures of mechanically planar enantiomers because cyclodextrins are homochiral; the mirror-image cyclodextrin of opposite chirality (and opposite cyclodirectionality) does not exist.
Mechanically planar chirality can arise in [3]rotaxanes even if the dumbbell is not oriented, depending on the relative orientation of the rings (see Figure 2). If both rings are oriented in the same direction, an achiral meso structure is obtained, but two rings with opposing cyclodirectionality will give either of two topological enantiomers. Head-to-head and tail-to-tail orientational isomers of [3]rotaxanes therefore possess a mechanically planar chiral architecture, regardless of the dumbbell’s constitution.
2.3. Topological Chirality
In catenanes, the conceptual equivalent of mechanically planar chirality is topological chirality. A groundbreaking 1961 paper by Frisch and Wasserman [88] marked the birth of a new subfield called “chemical topology”, which applies mathematical topology to molecular structure. Topology characterizes the attributes of an object that remain invariant throughout continuous deformation, which allows bonds to compress, stretch, or bend without breaking, intersecting, or crossing, whereas conventional stereochemistry typically deals only with Euclidean geometry. A number of reviews [69,88,89,90,91,92,93,94,95,96,97,98,99] are available on topological stereochemistry.
In mathematics, the topology of a catenane is called a link. A link can be either conditionally or unconditionally chiral. Conditionally chiral links do not have inherently chiral topologies, as unconditionally chiral links do. The simplest [2]catenane (known in mathematics as a Hopf link) is not a chiral topology. In order for a Hopf link to have conditional chirality, therefore, its rings must be directionally oriented. Interlocking a cyclodirectional CD with a second cyclodirectional ring will therefore lead to conditional topological chirality.
Most examples [63,64,100,101,102] of CD [2]catenanes do not possess a second cyclodirectional ring. However, Kuhnert and Tang [103] have created diastereomeric [2]catenanes (RRRRRR)-C3 and (SSSSSS)-C3 (Figure 4) by [3+3] cyclocondensation of trans-1,2-diaminocyclohexane and terephthalaldehyde in the presence of β-CD to form the hexaimine macrocycle known [104] as trianglimine. The corresponding trianglamine catenanes were also obtained and characterized by reduction of the imine bonds. By using pure (RR)- or (SS)-diaminocyclohexane during cyclocondensation, it is possible to capture either the all-R or all-S enantiomer, respectively, of the trianglimine in β-CD. Since β-CD is also a chiral macrocycle, these two catenanes are diastereomers rather than enantiomers. Although the authors did not comment on the presence of orientational isomers or their distribution, the cyclodirectionality of the trianglimines should lead to two possible orientational isomers for each of these catenanes. Since both rings are cyclodirectional, these orientational isomers are also topological isomers.
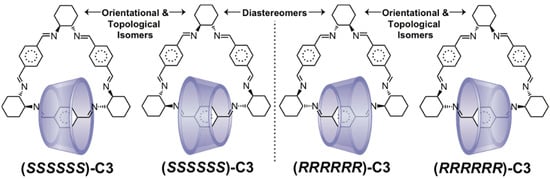
Figure 4.
Each diastereomer of the trianglimine/β-CD [2]catenane C3 may also exist as a pair of orientational and topological isomers arising from the cyclodirectionality of both interlocked macrocycles.
While linear [3]catenane also represents an achiral topology, [3]catenanes can also display conditional topological isomerism if at least two rings are oriented [105]. Thus, the seminal CD [3]catenanes [63,64] (see Section 2.1.4) provide another example of conditional topological isomers. Compounds [3]C2a and [3]C2b are not only orientational mechanostereoisomers, but also conditional topological isomers, since their peripheral CD rings are oriented by the polarities of their glycosidic bonds.
It is worth noting that the Solomon link [106] is a doubly interlocked [2]catenane with an intrinsically chiral topology. Although Solomon links can be prepared from rings that encapsulate two guests, and γ-CD is known [107,108] to bind two guests, a CD-based Solomon link has not yet been reported.
3. Applications of CD Symmetry Breaking in Mechanomolecules
It is clear that the symmetry-breaking effect of the cyclodextrins has interesting (mechano)stereochemical implications. Here we briefly discuss applications that take advantage of this effect in stereoselective synthesis (see Section 3.1) and the biased directional motion important for molecular machine prototypes (see Section 3.2) and advanced materials (see Section 3.3).
3.1. Stereoselectivc Synthesis
A reaction is said to be stereoselective [34] if one stereoisomer is preferentially formed over another. The symmetry-breaking effect of the cyclodextrins often leads to stereoselective reactions, especially regioselectivity (see Section 3.1.1) in the modification of the CD ring, and orientational selectivity (see Section 3.1.2) with respect to the ring direction.
3.1.1. Regioselectivity
Reactions for the selective modification of cyclodextrins almost always occur via the hydroxyl groups. Regioselective reactions around the cyclodextrin ring are challenging to achieve because the hydroxyl groups at the 2, 3, and 6 positions of the glucopyranose rings may have similar reactivities. Nevertheless, researchers have managed to find ways [109] to achieve mono-, di-, tri-, or per-substitution selectively at either the primary or the secondary face of CDs. These regioselective modifications have afforded an opportunity to create novel covalently bridged rotaxane architectures and exercise even greater control over their structures.
The regioselective mono-functionalization of CDs has facilitated the synthesis of [1]rotaxanes, wherein axles are covalently bonded to the encircling rings. Kaneda [110] and Easton [111] independently first exploited the regioselective alkylation of α-CD to make [1]rotaxanes in 2003. Tian [112] has also prepared a β-CD [1]rotaxane. Terao [27,113,114,115,116,117,118,119,120,121,122] has developed an extensive family (Figure 5) of oligo- and poly[1]rotaxanes R14-R26 with fully π-conjugated phenylene–ethynylene backbones. Polyrotaxanes possessing π-conjugated (semiconducting) backbones are known [123] as insulated molecular wires (IMWs). An advantageous outcome of using CDs to make [1]rotaxane monomers is that their regioselective functionalization leads to the formation of mechanostereochemically pure orientational isomers. Fixing the rings to the backbone allows one to exercise perfect control over the location and orientation of the insulating CD beads that protect the inner molecular wire. Note that all of these compounds also exhibit mechanically planar chirality (see Section 2.2).
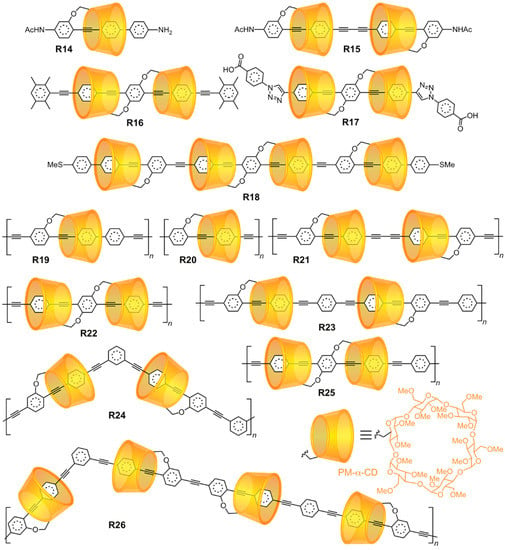
Figure 5.
The regioselective functionalization of α-CD enables the development of [1]rotaxanes that engender a high degree of control over the orientation and location of the rings surrounding phenylene–ethynylene insulated molecular wires (IMWs).
Another group of mechanomolecules enabled by the regioselective functionalization of CD with CD-binding moieties are known [124,125] as daisy chains (Figure 6), in which two or more self-complementary ring-axle dyads are cross-threaded into cyclic (denoted [cn]daisy chain) or acyclic ([an]daisy chain) structures. Kaneda [126] and Harada [127] independently and almost simultaneously introduced the first α-CD daisy chains (Na4R27 and R28) as cyclic dimers and trimers, respectively. Harada [128] also obtained the [an]daisy chain oligomer R29 from 2-cinnamoyl-α-CD monomers, possessing up to 10 repeating units. A number of other CD-based daisy chains with these architectures have followed these seminal works [129,130,131,132,133]. Daisy chains are important compounds in the field of molecular machines (see Section 3.3).
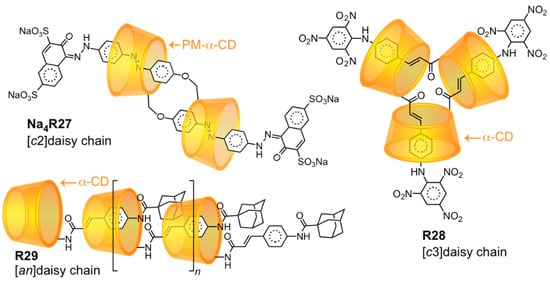
Figure 6.
Daisy chain architectures enabled by the regioselective modification of α-CD with CD-binding axles.
3.1.2. Orientational Selectivity
Another kind of stereoselectivity is possible in the synthesis of mechanomolecules capable of displaying orientational isomerism (see Section 2.1). In many cases, the syntheses of CD-based rotaxanes yield predominantly only one orientational isomer. Note that all of the [2]rotaxanes featured in Figure 3 were obtained with high orientational stereoselectivity.
A number of [3]rotaxanes (Figure 7) have also been obtained with high orientational selectivity. Anderson [49] achieved the first mechanostereoselective synthesis of the [3]rotaxane R30 as the tail-to-tail orientational isomer. Tian [50] has also observed high orientoselectivity in the synthesis of tail-to-tail α-CD [3]rotaxane Na3R31. In contrast, Anderson’s oligophenylene(ethynylene)/α-CD [3]rotaxane was obtained [48] solely as the head-to-tail orientational isomer R32 by solid-phase synthesis. Finally, the head-to-head isomer of R33, as reported by the group of Takata, [51] has been characterized in the solid state (Figure 7e) by X-ray crystallography.
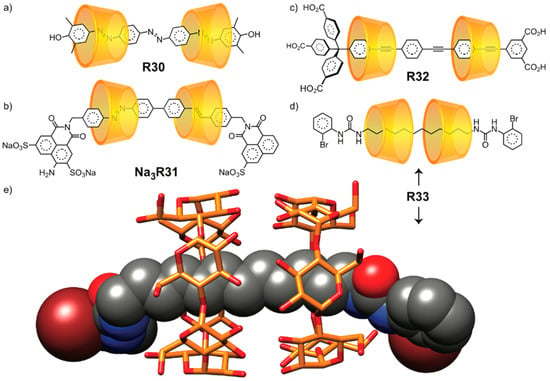
Figure 7.
Examples of [3]rotaxanes obtained as single orientational isomers with high selectivity in tail-to-tail (a,b), head-to-tail (c), and head-to-head (d,e) arrangements.
3.2. Mechanostereoselectivity—Biased Directional Motion
Mechanostereoselectivity is a term introduced by Stoddart [30,134,135] to describe the biased directional motion of component parts in mechanomolecules. An intramolecular motion in a catenane or rotaxane is mechanostereoselective if it happens faster along one pathway than another. The term “unidirectional” is problematic when describing the kind of biased Brownian motion undergone by molecular machines, because a net displacement does not imply a linear path. Indeed, the component parts of a mechanomolecule move incessantly and randomly in many directions at all times. The minimum requirements for biased Brownian/mechanostereoselective motion are non-equilibrium conditions and a source of broken symmetry. Thus, the symmetry-breaking effect of cyclodextrin may lead to mechanostereoselective motion in mechanomolecules if they are made to be stimulus-responsive for the design of artificial molecular machines.
The mechanostereoselective translation of an α-CD ring along the dumbbell of a [2]rotaxane was first observed (Scheme 6) by Anderson [136]. The α-CD ring travels only head-first along the symmetrical isophthalate-stoppered stilbene dumbbell of (E)-R344− upon photoisomerization to (Z)-R344−.
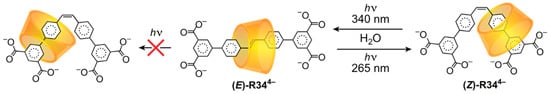
Scheme 6.
The mechanostereoselective translation of α-CD in a head-first direction upon photoisomerization of a trans-stilbene unit in the dumbbell.
When stimulus-responsive features are incorporated into a daisy chain, the mechanostereoselective motion of each component part can lead to a net contraction or extension in molecular length, serving as the basis for rotaxane-based artificial molecular muscles [137]. Several examples of daisy chain molecular muscles based on α-CD are illustrated in Figure 8.
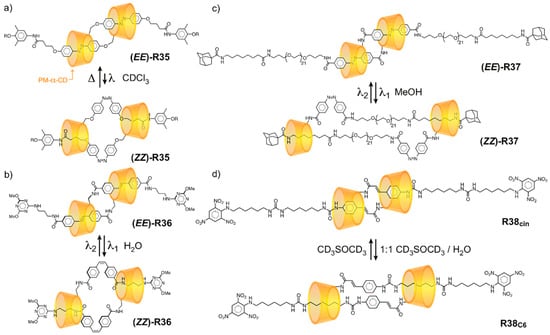
Figure 8.
Switchable [c2]daisy chain “molecular muscles” undergo contractions in molecular length on account of the antiparallel face-selective translation of each α-CD ring.
In Kaneda’s daisy chain (EE)-R35 [131], the self-complementary monomers comprise permethylated a-CD (PM-a-CD) rings linked directly to trans-azobenzene units. The (EZ)-R35 and (ZZ)-R35 isomers emerge (Figure 8a) in 20% and 5% yields, respectively, upon irradiation at 366 nm, owing to the E→Z photoisomerization of one or two azobenzenes. Easton’s daisy chain (EE)-R36 operates [130] analogously (Figure 8b), but the more stable nature of the photoactive stilbene unit allows (EE), (EZ), and (ZZ) isomers to be isolated and characterized as pure compounds. The hydrodynamic radius (RH) in water is shortened from 4.4 nm in Harada’s (EE)-R37 to 3.6 nm in (ZZ)-R37, which is populated (Figure 8c) with up to 85% efficiency in the photostationary state in MeOH [133]. Harada [132] also made a solvent-switchable daisy chain R38 in which the α-CD rings encircle cinnamamide units in CD3SOCD3, but migrate to a peripheral hexamethylene chain upon addition of water, affording a net contraction (Figure 8d) driven by the hydrophobic effect.
In 2005, Harada’s group [44] noticed that α-CD passes head-first over a 2-methylpyridinium stopper onto a decamethylene chain much more quickly than it threads tail-first. At lower temperatures, this rim-selective difference in threading kinetics allows one to obtain only one orientational isomer of the corresponding pseudo [2]rotaxane (Scheme 7). At elevated temperatures, however, tail-first threading becomes allowable and the system gradually equilibrates to an equal mixture of orientational isomers. A similar face-selective translation has been observed in several other pseudorotaxanes with similar directionally biased barriers [138,139,140]. The temperature-sensitive process of a ring threading onto an axle over a size-matched barrier is known [141,142] as “slippage”. The mechanostereoselective motion can be utilized for stereoselective synthesis (see Section 3.1); for example, an α-CD [3]rotaxane was obtained [143] as a pure head-to-tail orientational isomer by employing a slippage stopper that favors only a head-first threading.
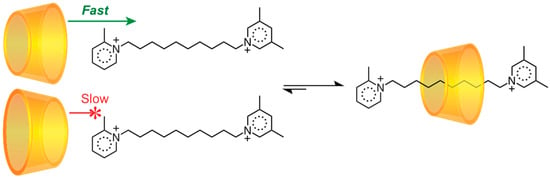
Scheme 7.
Face-selective threading of an α-CD ring over a 2-methylpyridinium barrier [44].
3.3. Networked “Slide-Ring” Materials
A “slide-ring” gel [144,145,146,147] is a material comprising polyrotaxanes with crosslinked rings. Okumura and Ito [148] introduced this concept in 2001 by crosslinking the α-CD rings of a polyrotaxane with a polyethylene glycol (PEG, MW > 10,000) backbone. While slide-ring materials based on PEG/α-CD are most common [149,150,151,152,153,154,155,156], other systems based on polymers such as polyisoprene [157] and polydimethylsiloxane [158] have also been reported. Slide-ring gels exhibit [159,160,161,162,163,164,165,166] remarkable mechanical properties attributable to the so-called “pulley effect”, [167,168,169] where the translational freedom afforded by the mechanically bonded crosslinks equalizes tension throughout the polymer network. The pulley effect makes slide-ring gels soft and stretchable, yet also tough. The original slide-ring gel [148] could be stretched to 24 times its length.
Although the symmetry-breaking effect of CDs has not been utilized significantly in slide-ring gels, there may be ample opportunity for the unusual stereochemical features and mechanostereoselective motions of CD mechanomolecules to impart these materials with new and unusual properties. One recent example from the group of Harada [170,171] involved an artificial muscle material R39 comprising polyether networks crosslinked by photoswitchable [c2]daisy chains. These networks, both in hydrogel and aerogel states, contract and bend in the direction of the light source that causes their internal sliding crosslinks to actuate (Figure 9).
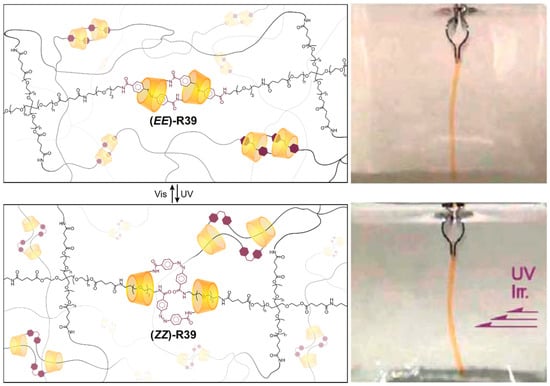
Figure 9.
Graphical representation and photographs of a daisy-chain artificial muscle crosslinked polymer network before and after UV illumination, which drives the contractile motion of the crosslinks [170,171].
4. Conclusions and Outlook
It is clear that the symmetry-breaking effect of cyclodextrins, arising from their differentiated faces that lead to orientational isomerism, as well as their cyclodirectional constitutions that lead to mechanically planar and topological chirality in mechanomolecules, has dramatic implications not only on regio-, stereo-, and orientationally selective synthesis and stereochemical analysis of molecular structure, but also on molecular dynamics such as biased (mechanostereoselective) intramolecular motion.
While the cyclochirality and conical shape of cyclodextrins can be advantageous in mechanomolecular chemistry, CDs are not the only symmetry-breaking hosts available. We can consider CD mechanomolecules as a case study and test bed for investigating and leveraging the effects of symmetry-breaking in other molecular systems as well. Symmetry-breaking hosts have been developed based on motifs including calix[n]arene [172,173,174,175,176,177,178,179,180,181,182], pillar[n]arene [183,184,185,186,187], cucurbit [n]uril [188], and cyanostar [189] motifs, and even transmembrane proteins [190]. These materials have much to contribute to the body of knowledge on symmetry breaking in chemical systems.
Funding
The research was funded by University of Colorado Boulder.
Conflicts of Interest
The author declares no conflict of interest.
References
- Szejtli, J. Introduction and General Overview of Cyclodextrin Chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef] [PubMed]
- Villiers, A. Sur La Transformation De La Fécule en Dextrine Par Le Ferment Butyrique. Compt. Rend. Fr. Acad. Sci. 1891, 112, 435–438. [Google Scholar]
- French, D.; Pulley, A.O.; Effenberger, J.A.; Rougvie, M.A.; Abdullah, M. Studies on the Schardinger Dextrins. XII. the Molecular Size and Structure of the Delta-, Epsilon-, Zeta-, and Eta-Dextrins. Arch. Biochem. Biophys. 1965, 111, 153–160. [Google Scholar] [CrossRef]
- Gessler, K.; Usón, I.; Takaha, T.; Krauss, N.; Smith, S.M.; Okada, S.; Sheldrick, G.M.; Saenger, W. V-Amylose at Atomic Resolution: X-Ray Structure of α-Cycloamylose with 26 Glucose Residues (Cyclomaltohexaicosaose). Proc. Natl. Acad. Sci. USA 1999, 96, 4246–4251. [Google Scholar] [CrossRef] [PubMed]
- French, D. The Schardinger Dextrins. Adv. Carbohydr. Chem. 1957, 12, 189–260. [Google Scholar] [PubMed]
- Freudenberg, K.; Cramer, F. Structure of the Schardinger Dextrins Alpha, Beta, and Gamma. Zeitschrift für Naturforschung 1948, 3b, 464. [Google Scholar] [CrossRef]
- Pringsheim, H. Chemistry of the Polysaccharides. Angew. Chem. Int. Ed. 1922, 35, 345–349. [Google Scholar] [CrossRef]
- Cramer, F. Uber Einschlubverbindungen, I. Mitteil.: Additionsverbuindungen Der Cycloamylosen. Chem. Ber. 1951, 84, 851–854. [Google Scholar] [CrossRef]
- Cramer, F. Über Einschlußverbindungen, II. Mitteil. Die Blauen Jodadditionsverbindungen Organischer Moleküle. Chem. Ber. 1951, 84, 855–859. [Google Scholar] [CrossRef]
- Pedersen, C.J. The Discovery of Crown Ethers (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 1988, 27, 1021–1027. [Google Scholar] [CrossRef]
- Cram, D.J. The Design of Molecular Hosts, Guests, and Their Complexes (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 1988, 27, 1009–1020. [Google Scholar] [CrossRef]
- Lehn, J.-M. Supramolecular Chemistry—Scope and Perspectives Molecules, Supermolecules, and Molecular Devices (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 1988, 27, 89–112. [Google Scholar] [CrossRef]
- Connors, K.A. The Stability of Cyclodextrin Complexes in Solution. Chem. Rev. 1997, 97, 1325–1358. [Google Scholar] [CrossRef] [PubMed]
- Rekharsky, M.V.; Inoue, Y. Complexation Thermodynamics of Cyclodextrins. Chem. Rev. 1998, 98, 1875–1918. [Google Scholar] [CrossRef] [PubMed]
- VanEtten, R.L.; Sebastian, J.F.; Clowes, G.A.; Bender, M.L. Acceleration of Phenyl Ester Cleavage by Cycloamyloses. A Model for Enzymic Specificity. J. Am. Chem. Soc. 1967, 89, 3242–3253. [Google Scholar] [CrossRef]
- VanEtten, R.L.; Clowes, G.A.; Sebastian, J.F.; Bender, M.L. The Mechanism of the Cycloamylose-Accelerated Cleavage of Phenyl Esters. J. Am. Chem. Soc. 1967, 89, 3253–3262. [Google Scholar] [CrossRef]
- Breslow, R. Biomimetic Chemistry and Artificial Enzymes: Catalysis by Design. Acc. Chem. Res. 1995, 28, 146–153. [Google Scholar] [CrossRef]
- Li, S.; Purdy, W.C. Cyclodextrins and Their Applications in Analytical Chemistry. Chem. Rev. 1992, 92, 1457–1470. [Google Scholar] [CrossRef]
- Schneiderman, E.; Stalcup, A.M. Cyclodextrins: A Versatile Tool in Separation Science. J. Chromatogr. B Biomed. Sci. Appl. 2000, 745, 83–102. [Google Scholar] [CrossRef]
- Ke, C.; Smaldone, R.A.; Kikuchi, T.; Li, H.; Davis, A.P.; Stoddart, J.F. Quantitative Emergence of Hetero [4]Rotaxanes by Template-Directed Click Chemistry. Angew. Chem. Int. Ed. 2012, 52, 381–387. [Google Scholar] [CrossRef]
- Szejtli, J. Medicinal Applications of Cyclodextrins. Med. Res. Rev. 1994, 14, 353–386. [Google Scholar] [CrossRef] [PubMed]
- Szente, L.; Szejtli, J. Cyclodextrins as Food Ingredients. Trends Food Sci. Technol. 2004, 15, 137–142. [Google Scholar] [CrossRef]
- Hedges, A.R. Industrial Applications of Cyclodextrins. Chem. Rev. 1998, 98, 2035–2044. [Google Scholar] [CrossRef] [PubMed]
- Stoddart, J.F.; Nepogodiev, S.A. Cyclodextrin-Based Catenanes and Rotaxanes. Chem. Rev. 1998, 98, 1959–1976. [Google Scholar]
- Wenz, G.; Han, B.-H.; Müller, A. Cyclodextrin Rotaxanes and Polyrotaxanes. Chem. Rev. 2006, 106, 782–817. [Google Scholar] [CrossRef] [PubMed]
- Harada, A.; Hashidzume, A.; Yamaguchi, H.; Takashima, Y. Polymeric Rotaxanes. Chem. Rev. 2009, 109, 5974–6023. [Google Scholar] [CrossRef] [PubMed]
- Terao, J. π-Conjugated Molecules Covered by Permethylated Cyclodextrins. Chem. Rec. 2011, 11, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Girek, T. Cyclodextrin-Based Rotaxanes. J. Incl. Phenom. Macrocycl. Chem. 2012, 74, 1–21. [Google Scholar] [CrossRef]
- Hashidzume, A.; Yamaguchi, H.; Harada, A. Cyclodextrin-Based Rotaxanes: From Rotaxanes to Polyrotaxanes and Further to Functional Materials. Eur. J. Org. Chem. 2019, 2019, 3344–3357. [Google Scholar] [CrossRef]
- Bruns, C.J.; Stoddart, J.F. The Nature of the Mechanical Bond: From Molecules to Machines; Wiley: Weinheim, Germany, 2016. [Google Scholar]
- Saito, H.; Yonemura, H.; Nakamura, H.; Matsuo, T. Stability and Exchange Properties of Through-Ring Cyclodextrin Complexes. Effects of Chain Length in Polymethylene Bis(1-pyridinium) as Guest Molecules. Chem. Lett. 1990, 19, 535–538. [Google Scholar] [CrossRef]
- Watanabe, M.; Nakamura, H.; Matsuo, T. Formation of Through-Ring α-Cyclodextrin Complexes with α, Ω-Alkanedicarboxylate Anion. Effects of the Aliphatic Chain Length and Electrostatic Factors on the Complexation Behavior. Bull. Chem. Soc. Jpn. 1992, 65, 164–169. [Google Scholar] [CrossRef]
- Ogino, H. Relatively High-Yield Synthesis of Rotaxanes. Synthesis and Properties of Compounds Consisting of Cyclodextrins Threaded by α, Ω -Diaminoalkanes Coordinated to Cobalt(III) Complexes. J. Am. Chem. Soc. 1981, 103, 1303–1304. [Google Scholar] [CrossRef]
- IUPAC. Compendium of Chemical Terminology, 2nd ed.; (the “Gold Book”); McNaught, A.D., Wilkinson, A., Eds.; Blackwell Scientific Publications: Oxford, UK, 1997. [Google Scholar] [CrossRef]
- Olson, M.A.; Botros, Y.Y.; Stoddart, J.F. Mechanostereochemistry. Pure Appl. Chem. 2010, 82, 1569–1574. [Google Scholar] [CrossRef]
- Isnin, R.; Kaifer, A.E. Novel Class of Asymmetric Zwitterionic Rotaxanes Based on α-Cyclodextrin. J. Am. Chem. Soc. 1991, 113, 8188–8190. [Google Scholar] [CrossRef]
- Isnin, R.; Kaifer, A.E. A New Approach to Cyclodextrin-Based Rotaxanes. Pure Appl. Chem. 1993, 65, 495–498. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Harada, A. A Cyclodextrin-Based Molecular Shuttle Containing Energetically Favored and Disfavored Portions in Its Dumbbell Component. Org. Lett. 2000, 2, 1353–1356. [Google Scholar] [CrossRef] [PubMed]
- Buston, J.E.H.; Young, J.R.; Anderson, H.L. Rotaxane-Encapsulated Cyanine Dyes: Enhanced Fluorescence Efficiency and Photostability. Chem. Commun. 2000, 11, 905–906. [Google Scholar] [CrossRef]
- Buston, J.E.H.; Anderson, H.L.; Marken, F. Enhanced Chemical Reversibility of Redox Processes in Cyanine Dye Rotaxanes. Chem. Commun. 2001, 11, 1046–1047. [Google Scholar] [CrossRef]
- Saudan, C.; Dunand, F.A.; Abou-Hamdan, A.; Bugnon, P.; Lye, P.G.; Lincoln, S.F.; Merbach, A.E. A Model for Sequential Threading of α-Cyclodextrin Onto a Guest: A Complete Thermodynamic and Kinetic Study in Water. J. Am. Chem. Soc. 2001, 123, 10290–10298. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Song, H.J. Isomeric [2]Rotaxanes and Unidirectional [2]Pseudorotaxane Composed of α-Cyclodextrin and Aliphatic Chain-Linked Carbazole-Viologen Compounds. Org. Lett. 2004, 6, 4869–4872. [Google Scholar] [CrossRef] [PubMed]
- Baer, A.J.; Macartney, D.H. Orientational Isomers of α-Cyclodextrin [2]Semi-Rotaxanes with Asymmetric Dicationic Threads. Org. Biomol. Chem. 2005, 3, 1448–1453. [Google Scholar] [CrossRef] [PubMed]
- Oshikiri, T.; Takashima, Y.; Yamaguchi, H.; Harada, A. Kinetic Control of Threading of Cyclodextrins onto Axle Molecules. J. Am. Chem. Soc. 2005, 127, 12186–12187. [Google Scholar] [CrossRef] [PubMed]
- Baer, A.J.; Macartney, D.H. Orientational Isomers of Cyclodextrin Semirotaxanes and Rotaxanes with Organic and Transition Metal Complex Stoppers. Supramol. Chem. 2007, 19, 537–546. [Google Scholar] [CrossRef]
- Yamauchi, K.; Miyawaki, A.; Takashima, Y.; Yamaguchi, H.; Harada, A. A Molecular Reel: Shuttling of a Rotor by Tumbling of a Macrocycle. J. Org. Chem. 2010, 75, 1040–1046. [Google Scholar] [CrossRef] [PubMed]
- Cheetham, A.G.; Claridge, T.D.W.; Anderson, H.L. Metal-Driven Ligand Assembly in the Synthesis of Cyclodextrin [2] and [3]Rotaxanes. Org. Biomol. Chem. 2007, 5, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Daniell, H.W.; Klotz, E.J.F.; Odell, B.; Claridge, T.D.W.; Anderson, H.L. Solid-Phase Synthesis of Oligo (Phenylene Ethynylene) Rotaxanes. Angew. Chem. Int. Ed. 2007, 46, 6845–6848. [Google Scholar] [CrossRef]
- Craig, M.R.; Claridge, T.D.W.; Anderson, H.L.; Hutchings, M.G. Synthesis of a Cyclodextrin Azo Dye [3]Rotaxane as a Single Isomer. Chem. Commun. 1999, 16, 1537–1538. [Google Scholar] [CrossRef]
- Qu, D.-H.; Wang, Q.-C.; Ma, X.; Tian, H. A [3]Rotaxane with Three Stable States That Responds to Multiple-Inputs and Displays Dual Fluorescence Addresses. Chem. Eur. J. 2005, 11, 5929–5937. [Google Scholar] [CrossRef]
- Akae, Y.; Koyama, Y.; Kuwata, S.; Takata, T. Cyclodextrin-Based Size-Complementary [3]Rotaxanes: Selective Synthesis and Specific Dissociation. Chem. Eur. J. 2014, 20, 17132–17136. [Google Scholar] [CrossRef]
- Akae, Y.; Sogawa, H.; Takata, T. Cyclodextrin-Based [3]Rotaxane-Crosslinked Fluorescent Polymer: Synthesis and De-Crosslinking Using Size Complementarity. Angew. Chem. Int. Ed. 2018, 57, 14832–14836. [Google Scholar] [CrossRef]
- Akae, Y.; Sogawa, H.; Takata, T. Synthesis of a Structure-Definite α-Cyclodextrin-Based Macromolecular [3]Rotaxane Using a Size-Complementary Method. Angew. Chem. Int. Ed. 2018, 57, 11742–11746. [Google Scholar] [CrossRef] [PubMed]
- Akae, Y.; Sogawa, H.; Takata, T. Effective Synthesis and Modification of α-Cyclodextrin-Based [3]Rotaxanes Enabling Versatile Molecular Design. Eur. J. Org. Chem. 2019, 22, 3605–3613. [Google Scholar] [CrossRef]
- Sakamoto, K.; Takashima, Y.; Yamaguchi, H.; Harada, A. Preparation and Properties of Rotaxanes Formed by Dimethyl-β-Cyclodextrin and Oligo(Thiophene)s with β-Cyclodextrin Stoppers. J. Org. Chem. 2007, 72, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Chwalek, M.; Auzély, R.; Fort, S. Synthesis and Biological Evaluation of Multivalent Carbohydrate Ligands Obtained by Click Assembly of Pseudo-Rotaxanes. Org. Biomol. Chem. 2009, 7, 1680–1689. [Google Scholar] [CrossRef] [PubMed]
- Taira, T.; Suzaki, Y.; Osakada, K. [5]Rotaxanes Composed of α-Cyclodextrin and Pd or Pt Complexes with Alkylbipyridinium Ligands. Chem. Lett. 2008, 37, 182–183. [Google Scholar] [CrossRef]
- Taira, T.; Suzaki, Y.; Osakada, K. Pd-II and Pt-III Complexes with Amphiphilic Ligands: Formation of Micelles and [5]Rotaxanes with α-Cyclodextrin in Aqueous Solution. Chem. Asian J. 2008, 3, 895–902. [Google Scholar] [CrossRef]
- Taira, T.; Suzaki, Y.; Osakada, K. Metallohydrogel Formed From Amphiphilic Pd Complex and α-Cyclodextrin: Control of Its Sol-Gel Transition. Chem. Lett. 2013, 42, 1062–1064. [Google Scholar] [CrossRef]
- Harada, A.; Li, J.; Kamachi, M. The Molecular Necklace: A Rotaxane Containing Many Threaded α-Cyclodextrins. Nature 1992, 356, 325–327. [Google Scholar] [CrossRef]
- Liu, P.; Chipot, C.; Shao, X.; Cai, W. How Do α-Cyclodextrins Self-Organize on a Polymer Chain? J. Phys. Chem. C 2012, 116, 17913–17918. [Google Scholar] [CrossRef]
- Lüttringhaus, A.; Cramer, F.; Prinzbach, H.; Henglein, F.M. Cyclisationen Von Langkettigen Dithiolen. Versuche Zur Darstellung Sich Umfassender Ringe Mit Hilfe Von Einschlußverbindungen. Liebigs Ann. Chem. 1958, 613, 185–198. [Google Scholar] [CrossRef]
- Armspach, D.; Ashton, P.R.; Moore, C.P.; Spencer, N.; Stoddart, J.F.; Wear, T.J.; Williams, D.J. Self-Assembly of Catenanes with Cyclodextrin Units. Angew. Chem. Int. Ed. Engl. 1993, 32, 854–858. [Google Scholar] [CrossRef]
- Armspach, D.; Ashton, P.R.; Ballardini, R.; Balzani, V.; Godi, A.; Moore, C.; Prodi, L.; Spencer, N.; Stoddart, J.F.; Tolley, M.S.; et al. Catenated Cyclodextrins. Chem. Eur. J. 1995, 1, 33–55. [Google Scholar] [CrossRef]
- Li, J.; Nowak, P.; Fanlo-Virgós, H.; Otto, S. Catenanes from Catenanes: Quantitative Assessment of Cooperativity in Dynamic Combinatorial Catenation. Chem. Sci. 2014, 5, 4968–4974. [Google Scholar] [CrossRef]
- Okada, M.; Harada, A. Poly(Polyrotaxane): Photoreactions of 9-Anthracene-Capped Polyrotaxane. Macromolecules 2003, 36, 9701–9703. [Google Scholar] [CrossRef]
- Higashi, T.; Morita, K.; Song, X.; Zhu, J.; Tamura, A.; Yui, N.; Motoyama, K.; Arima, H.; Li, J. One-Pot Synthesis of Cyclodextrin-Based Radial Poly [n]Catenanes. Commun. Chem. 2019, 2, 78. [Google Scholar] [CrossRef]
- Prelog, V.; Gerlach, H. Cycloenantiomerie Und Cyclodiastereomerie. 1. Mitteilung. Helv. Chim. Acta 1964, 47, 2288–2294. [Google Scholar] [CrossRef]
- Schill, G. Catenanes, Rotaxanes, and Knots; Academic Press: New York, NY, USA, 1971. [Google Scholar]
- Jäger, R.; Händel, M.; Harren, J.; Rissanen, K.; Vögtle, F. Chemistry with Rotaxanes: Intra-and Intermolecularly Covalently Linked Rotaxanes. Liebigs Ann. 1996, 1996, 1201–1207. [Google Scholar] [CrossRef]
- Yamamoto, C.; Okamoto, Y.; Schmidt, T.; Vögtle, F. Enantiomeric Resolution of Cycloenantiomeric Rotaxane, Topologically Chiral Catenane, and Pretzel-Shaped Molecules: Observation of Pronounced Circular Dichroism. J. Am. Chem. Soc. 1997, 119, 10547–10548. [Google Scholar] [CrossRef]
- Schmieder, R.; Hubner, G.; Seel, C.; Vögtle, F. The First Cyclodiasteromeric [3]Rotaxane. Angew. Chem. Int. Ed. 1999, 38, 3528–3530. [Google Scholar] [CrossRef]
- Reuter, C.; Schmieder, R.; Vögtle, F. From Rotaxanes to Knots. Templating, Hydrogen Bond Patterns, and Cyclochirality. Pure Appl. Chem. 2000, 72, 2233–2241. [Google Scholar] [CrossRef]
- Reuter, C.; Seel, C.; Nieger, M.; Vögtle, F. Chiral [1]Rotaxanes: X-Ray Structures and Chiroptical Properties. Helv. Chim. Acta 2000, 83, 630–640. [Google Scholar] [CrossRef]
- Eliel, E.; Wilen, S.; Mander, L. Stereochemistry of Organic Compounds; John Wiley & Sons: New York, NY, USA, 1994. [Google Scholar]
- Makita, Y.; Kihara, N.; Nakakoji, N.; Takata, T.; Inagaki, S.; Yamamoto, C.; Okamoto, Y. Catalytic Asymmetric Synthesis and Optical Resolution of Planar Chiral Rotaxane. Chem. Lett. 2007, 36, 162–163. [Google Scholar] [CrossRef]
- Bordoli, R.J.; Goldup, S.M. An Efficient Approach to Mechanically Planar Chiral Rotaxanes. J. Am. Chem. Soc. 2014, 136, 4817–4820. [Google Scholar] [CrossRef] [PubMed]
- Cahn, R.S.; Ingold, C.; Prelog, V. Specification of Molecular Chirality. Angew. Chem. Int. Ed. Engl. 1966, 5, 385–415. [Google Scholar] [CrossRef]
- Craig, M.R.; Hutchings, M.G.; Claridge, T.D.W.; Anderson, H.L. Rotaxane-Encapsulation Enhances the Stability of an Azo Dye, in Solution and When Bonded to Cellulose. Angew. Chem. Int. Ed. 2001, 40, 1071–1074. [Google Scholar] [CrossRef]
- Yan, H.; Teh, C.; Sreejith, S.; Zhu, L.; Kwok, A.; Fang, W.; Ma, X.; Nguyen, K.T.; Korzh, V.; Zhao, Y. Functional Mesoporous Silica Nanoparticles for Photothermal-Controlled Drug Delivery in Vivo. Angew. Chem. Int. Ed. 2012, 51, 8373–8377. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Zhu, L.; Li, X.; Kwok, A.; Pan, X.; Zhao, Y. A Photoswitchable [2]Rotaxane Array on Graphene Oxide. Asian J. Org. Chem. 2012, 1, 314–318. [Google Scholar] [CrossRef]
- Yan, H.; Zhu, L.; Li, X.; Kwok, A.; Li, X.; Ågren, H.; Zhao, Y. Photothermal-Responsive [2]Rotaxanes. RSC Adv. 2013, 3, 2341. [Google Scholar] [CrossRef]
- Wang, Q.-C.; Qu, D.-H.; Ren, J.; Chen, K.; Tian, H. A Lockable Light-Driven Molecular Shuttle with a Fluorescent Signal. Angew. Chem. Int. Ed. 2004, 43, 2661–2665. [Google Scholar] [CrossRef]
- Qu, D.-H.; Wang, Q.-C.; Ren, J.; Tian, H. A Light-Driven Rotaxane Molecular Shuttle with Dual Fluorescence Addresses. Org. Lett. 2004, 6, 2085–2088. [Google Scholar] [CrossRef]
- Wang, Q.-C.; Ma, X.; Qu, D.-H.; Tian, H. Unidirectional Threading Synthesis of Isomer-Free [2]Rotaxanes. Chem. Eur. J. 2006, 12, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Song, H.J.; Chang, H.-J. Unidirectional α-Cyclodextrin-Based [2]Rotaxanes Bearing Viologen Unit on Axle. Tetrahedron Lett. 2006, 47, 3831–3834. [Google Scholar] [CrossRef]
- Casati, C.; Franchi, P.; Pievo, R.; Mezzina, E.; Lucarini, M. Unraveling Unidirectional Threading of α-Cyclodextrin in a [2]Rotaxane Through Spin Labeling Approach. J. Am. Chem. Soc. 2012, 134, 19108–19117. [Google Scholar] [CrossRef] [PubMed]
- Frisch, H.L.; Wasserman, E. Chemical Topology. J. Am. Chem. Soc. 1961, 83, 3789–3795. [Google Scholar] [CrossRef]
- Prelog, V. Problems in Chemical Topology. Chemistry Britain 1968, 4, 382. [Google Scholar]
- Sokolov, V.I. Topological Ideas in Stereochemistry. Russian Chem. Rev. 1973, 42, 452–463. [Google Scholar] [CrossRef]
- Walba, D.M. Topological Stereochemistry. Tetrahedron 1985, 41, 3161–3212. [Google Scholar] [CrossRef]
- Walba, D.M. A Topological Hierarchy of Molecular Chirality and Other Tidbits in Topological Stereochemistry. In New Developments in Molecular Chirality; Mezey, P.G., Ed.; Kluwer Academic Publishers: Boston, MA, USA, 1991; Volume 5, pp. 119–129. [Google Scholar]
- Chambron, J.-C.; Dietrich-Buchecker, C.; Dietrich-Buchecker, C.; Sauvage, J.-P. From Classical Chirality to Topologically Chiral Catenands and Knots. Top. Curr. Chem. 1993, 165, 131–162. [Google Scholar]
- Liang, C.; Mislow, K. Topological Chirality and Achirality of Links. J. Math. Chem. 1995, 18, 1–24. [Google Scholar] [CrossRef]
- Breault, G.A.; Hunter, C.A.; Mayers, P.C. Supramolecular Topology. Tetrahedron 1999, 55, 5265–5293. [Google Scholar] [CrossRef]
- Siegel, J.S. Chemical Topology and Interlocking Molecules. Science 2004, 304, 1256–1258. [Google Scholar] [CrossRef] [PubMed]
- Fenlon, E.E. Open Problems in Chemical Topology. Eur. J. Org. Chem. 2008, 30, 5023–5035. [Google Scholar] [CrossRef]
- Amabilino, D.B.; Pérez-García, L. Topology in Molecules Inspired, Seen and Represented. Chem. Soc. Rev. 2009, 38, 1562–1571. [Google Scholar] [CrossRef] [PubMed]
- Forgan, R.S.; Sauvage, J.-P.; Stoddart, J.F. Chemical Topology: Complex Molecular Knots, Links, and Entanglements. Chem. Rev. 2011, 111, 5434–5464. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.W.; Sakamoto, S.; Yamaguchi, K.; Hong, J.-I. Versatile Formation of [2]Catenane and [2]Pseudorotaxane Structures; Threading and Noncovalent Stoppering by a Self-Assembled Macrocycle. Org. Lett. 2004, 6, 1079–1082. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Nie, Y.; Saha, M.L.; He, Z.; Jiang, L.; Zhou, Z.; Stang, P.J. Photoreversible [2] Catenane via the Host–Guest Interactions Between a Palladium Metallacycle and β-Cyclodextrin. Inorg. Chem. 2015, 54, 11807–11812. [Google Scholar] [CrossRef] [PubMed]
- Au-Yeung, H.Y.; Yee, C.-C.; Hung Ng, A.W.; Hu, K. Strategies to Assemble Catenanes with Multiple Interlocked Macrocycles. Inorg. Chem. 2017, 57, 3475–3485. [Google Scholar] [CrossRef] [PubMed]
- Kuhnert, N.; Tang, B. Synthesis of Diastereomeric Trianglamine-β-Cyclodextrin-[2]-Catenanes. Tetrahedron Lett. 2006, 47, 2985–2988. [Google Scholar] [CrossRef]
- Gawroński, J.; Kołbon, H.; Kwit, M.; Katrusiak, A. Designing Large Triangular Chiral Macrocycles: Efficient [3+3] Diamine−Dialdehyde Condensations Based on Conformational Bias. J. Org. Chem. 2000, 65, 5768–5773. [Google Scholar] [CrossRef] [PubMed]
- Forgan, R.S.; Blackburn, A.K.; Boyle, M.M.; Schneebeli, S.T.; Stoddart, J.F. The Topological and Chemical Implications of Introducing Oriented Rings to [3]Catenanes. Supramol. Chem. 2014, 26, 192–201. [Google Scholar] [CrossRef]
- Meyer, C.D.; Forgan, R.S.; Chichak, K.; Peters, A.J.; Tangchaivang, N.; Cave, G.W.V.; Khan, S.I.; Cantrill, S.J.; Stoddart, J.F. The Dynamic Chemistry of Molecular Borromean Rings and Solomon Knots. Chem. Eur. J. 2010, 16, 12570–12581. [Google Scholar] [CrossRef] [PubMed]
- Escandar, G.M.; Muñoz de la Peña, A. Room-Temperature Phosphorescence of Acenaphthene in Aerated Solutions in the Presence of Bromoalcohols and γ-Cyclodextrin. Anal. Chim. Acta 1998, 370, 199–205. [Google Scholar] [CrossRef]
- Klotz, E.J.F.; Claridge, T.D.W.; Anderson, H.L. Homo- and Hetero-[3]Rotaxanes with Two Pi-Systems Clasped in a Single Macrocycle. J. Am. Chem. Soc. 2006, 128, 15374–15375. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.R.; Forgo, P.; Stine, K.J.; D’Souza, V.T. Methods for Selective Modifications of Cyclodextrins. Chem. Rev. 1998, 98, 1977–1996. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, G.; Fujimoto, T.; Kaneda, T. Synthesis and Characterization of the First Pair of an Unlocked and a Locked Self-Inclusion Complex From a Permethylated α-Cyclodextrin Derivative. Chem. Lett. 2003, 32, 536–537. [Google Scholar] [CrossRef]
- Onagi, H.; Blake, C.J.; Easton, C.J.; Lincoln, S.F. Installation of a Ratchet Tooth and Pawl to Restrict Rotation in a Cyclodextrin Rotaxane. Chem. Eur. J. 2003, 9, 5978–5988. [Google Scholar] [CrossRef]
- Ma, X.; Qu, D.; Ji, F.; Wang, Q.; Zhu, L.; Xu, Y.; Tian, H. A Light-Driven [1]Rotaxane via Self-Complementary and Suzuki-Coupling Capping. Chem. Commun. 2007, 14, 1409–1411. [Google Scholar] [CrossRef]
- Terao, J. Permethylated Cyclodextrin-Based Insulated Molecular Wires. Polym. Chem. 2011, 2, 2444–2452. [Google Scholar] [CrossRef]
- Tsuda, S.; Terao, J.; Kambe, N. Synthesis of an Organic-Soluble π-Conjugated [1]Rotaxane. Chem. Lett. 2009, 38, 76–77. [Google Scholar] [CrossRef]
- Tsuda, S.; Terao, J.; Tsurui, K.; Kambe, N. Synthesis of a Linked [1]-[1] Rotaxane. Chem. Lett. 2009, 38, 190–191. [Google Scholar] [CrossRef]
- Tsuda, S.; Terao, J.; Tanaka, Y.; Maekawa, T.; Kambe, N. Synthesis of Linked Symmetrical [3] and [5]Rotaxanes Having an Oligomeric Phenylene Ethynylene (OPE) Core Skeleton as a π-Conjugated Guest via Double Intramolecular Self-Inclusion. Tetrahedron Lett. 2009, 50, 1146–1150. [Google Scholar] [CrossRef]
- Terao, J.; Ikai, K.; Kambe, N.; Seki, S.; Saeki, A.; Ohkoshi, K.; Fujihara, T.; Tsuji, Y. Synthesis of a Head-to-Tail-Type Cyclodextrin-Based Insulated Molecular Wire. Chem. Commun. 2011, 47, 6816–6818. [Google Scholar] [CrossRef] [PubMed]
- Terao, J.; Tsuda, S.; Tanaka, Y.; Okoshi, K.; Fujihara, T.; Tsuji, Y.; Kambe, N. Synthesis of Organic-Soluble Conjugated Polyrotaxanes by Polymerization of Linked Rotaxanes. J. Am. Chem. Soc. 2009, 131, 16004–16005. [Google Scholar] [CrossRef] [PubMed]
- Terao, J.; Tanaka, Y.; Tsuda, S.; Kambe, N.; Taniguchi, M.; Kawai, T.; Saeki, A.; Seki, S. Insulated Molecular Wire with Highly Conductive π-Conjugated Polymer Core. J. Am. Chem. Soc. 2009, 131, 18046–18047. [Google Scholar] [CrossRef] [PubMed]
- Terao, J.; Tsuda, S.; Tsurui, K.; Kambe, N. Synthesis of Highly Insulated Molecular Wires by Polymerization of Organic-Soluble Symmetrical Linked Inclusion Complex Monomers. Macromol. Symp. 2010, 297, 54–60. [Google Scholar] [CrossRef]
- Terao, J.; Wadahama, A.; Matono, A.; Tada, T.; Watanabe, S.; Seki, S.; Fujihara, T.; Tsuji, Y. Design Principle for Increasing Charge Mobility of π-Conjugated Polymers Using Regularly Localized Molecular Orbitals. Nat. Commun. 2013, 4, 1691. [Google Scholar] [CrossRef]
- Terao, J.; Hosomi, T.; Masai, H.; Matsuda, W.; Seki, S.; Fujihara, T.; Tsuji, Y. Synthesis and Redox Response of Insulated Molecular Wire Elongated Through Iron–Terpyridine Coordination Bonds. Chem. Lett. 2014, 43, 1289–1291. [Google Scholar] [CrossRef]
- Frampton, M.J.; Anderson, H.L. Insulated Molecular Wires. Angew. Chem. Int. Ed. 2007, 46, 1028–1064. [Google Scholar] [CrossRef]
- Baxter, I.; Ashton, P.R.; Cantrill, S.J.; Fyfe, M.C.T.; Glink, P.T.; Stoddart, J.F.; White, A.J.P.; Williams, D.J. Supramolecular Daisy Chains. Angew. Chem. Int. Ed. 1998, 37, 1294–1297. [Google Scholar]
- Rotzler, J.; Mayor, M. Molecular Daisy Chains. Chem. Soc. Rev. 2013, 42, 44–62. [Google Scholar] [CrossRef]
- Fujimoto, T.; Sakata, Y.; Kaneda, T. The First Janus [2]Rotaxane. Chem. Commun. 2000, 21, 2143–2144. [Google Scholar] [CrossRef]
- Hoshino, T.; Miyauchi, M.; Kawaguchi, Y.; Yamaguchi, H.; Harada, A. Daisy Chain Necklace: Tri [2]rotaxane Containing Cyclodextrins. J. Am. Chem. Soc. 2000, 122, 9876–9877. [Google Scholar] [CrossRef]
- Miyawaki, A.; Miyauchi, M.; Takashima, Y.; Yamaguchi, H.; Harada, A. Formation of Supramolecular Isomers; Poly [2]rotaxane and Supramolecular Assembly. Chem. Commun. 2008, 4, 456–458. [Google Scholar] [CrossRef] [PubMed]
- Onagi, H.; Easton, C.J.; Lincoln, S.F. An Hermaphrodite [2]Rotaxane: Preparation and Analysis of Structure. Org. Lett. 2001, 3, 1041–1044. [Google Scholar] [CrossRef] [PubMed]
- Dawson, R.E.; Lincoln, S.F.; Easton, C.J. The Foundation of a Light Driven Molecular Muscle Based on Stilbene and α-Cyclodextrin. Chem. Commun. 2008, 34, 3980–3982. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, S.; Aso, Y.; Kaneda, T. Linear Oligomers Composed of a Photochromically Contractible and Extendable Janus [2]Rotaxane. Chem. Commun. 2006, 29, 3072–3074. [Google Scholar] [CrossRef] [PubMed]
- Tsukagoshi, S.; Miyawaki, A.; Takashima, Y.; Yamaguchi, H.; Harada, A. Contraction of Supramolecular Double-Threaded Dimer Formed by α-Cyclodextrin with a Long Alkyl Chain. Org. Lett. 2007, 9, 1053–1055. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Taura, D.; Hashidzume, A.; Harada, A. Light-Switchable Janus [2]Rotaxanes Based on α-Cyclodextrin Derivatives Bearing Two Recognition Sites Linked with Oligo (Ethylene Glycol). Chem. Asian J. 2010, 5, 2281–2289. [Google Scholar] [CrossRef] [PubMed]
- Fahrenbach, A.C.; Zhu, Z.; Cao, D.; Liu, W.-G.; Li, H.; Dey, S.K.; Basu, S.; Trabolsi, A.; Botros, Y.Y.; Goddard, W.A., III; et al. Radically Enhanced Molecular Switches. J. Am. Chem. Soc. 2012, 134, 16275–16288. [Google Scholar] [CrossRef]
- Fahrenbach, A.C.; Bruns, C.J.; Li, H.; Trabolsi, A.; Coskun, A.; Stoddart, J.F. Ground-State Kinetics of Bistable Redox-Active Donor–Acceptor Mechanically Interlocked Molecules. Acc. Chem. Res. 2014, 47, 482–493. [Google Scholar] [CrossRef]
- Stanier, C.A.; Alderman, S.J.; Claridge, T.D.; Anderson, H.L. Unidirectional Photoinduced Shuttling in a Rotaxane with a Symmetric Stilbene Dumbbell. Angew. Chem. Int. Ed. 2002, 41, 1769–1772. [Google Scholar] [CrossRef]
- Bruns, C.J.; Stoddart, J.F. Rotaxane-Based Molecular Muscles. Acc. Chem. Res. 2014, 47, 2186–2199. [Google Scholar] [CrossRef] [PubMed]
- Oshikiri, T.; Yamaguchi, H.; Takashima, Y.; Harada, A. Face Selective Translation of a Cyclodextrin Ring Along an Axle. Chem. Commun. 2009, 37, 5515–5517. [Google Scholar] [CrossRef] [PubMed]
- Hashidzume, A.; Kuse, A.; Oshikiri, T.; Adachi, S.; Yamaguchi, H.; Harada, A. A Pseudo-Rotaxane of α-Cyclodextrin and a Two-Station Axis Molecule Consisting of Pyridinium and Decamethylene Moieties, and its Deuteration in Deuterium Oxide. Tetrahedron 2017, 73, 4988–4993. [Google Scholar] [CrossRef]
- Hashidzume, A.; Kuse, A.; Oshikiri, T.; Adachi, S.; Okumura, M.; Yamaguchi, H.; Harada, A. Toward a Translational Molecular Ratchet: Face-Selective Translation Coincident with Deuteration in a Pseudo-Rotaxane. Sci. Rep. 2018, 8, 8950. [Google Scholar] [CrossRef] [PubMed]
- Ashton, P.R.; Bělohradský, M.; Philp, D.; Stoddart, J.F. Slippage—An Alternative Method for Assembling [2]Rotaxanes. J. Chem. Soc. Chem. Commun. 1993, 16, 1269–1274. [Google Scholar] [CrossRef]
- Raymo, F.M.; Stoddart, J.F. Slippage—A Simple and Efficient Way to Self-Assemble [n]Rotaxanes. Pure Appl. Chem. 1997, 69, 1987–1997. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Oshikiri, T.; Harada, A. Rotaxanes with Unidirectional Cyclodextrin Array. J. Phys. Condens. Matter 2006, 18, S1809–S1816. [Google Scholar] [CrossRef]
- Ito, K. Novel Cross-Linking Concept of Polymer Network: Synthesis, Structure, and Properties of Slide-Ring Gels with Freely Movable Junctions. Polymer 2007, 39, 489–499. [Google Scholar] [CrossRef]
- Mayumi, K.; Ito, K. Structure and Dynamics of Polyrotaxane and Slide-Ring Materials. Polymer 2010, 51, 959–967. [Google Scholar] [CrossRef]
- Kato, K.; Ito, K. Polymer Networks Characterized by Slidable Crosslinks and the Asynchronous Dynamics of Interlocked Components. React. Funct. Polym. 2013, 73, 405–412. [Google Scholar] [CrossRef]
- Noda, Y.; Hayashi, Y.; Ito, K. From Topological Gels to Slide-Ring Materials. J. Appl. Polym. Sci. 2014, 131, 40509. [Google Scholar] [CrossRef]
- Okumura, Y.; Ito, K. The Polyrotaxane Gel: A Topological Gel by Figure-of-Eight Cross-Links. Adv. Mater. 2001, 13, 485–487. [Google Scholar] [CrossRef]
- Araki, J.; Zhao, C.; Ito, K. Efficient Production of Polyrotaxanes From α-Cyclodextrin and Poly (Ethylene Glycol). Macromolecules 2005, 38, 7524–7527. [Google Scholar] [CrossRef]
- Fleury, G.; Schlatter, G.; Brochon, C.; Hadziioannou, G. From High Molecular Weight Precursor Polyrotaxanes to Supramolecular Sliding Networks. the “Sliding Gels”. Polymer 2005, 46, 8494–8501. [Google Scholar] [CrossRef]
- Sakai, T.; Murayama, H.; Nagano, S.; Takeoka, Y.; Kidowaki, M.; Ito, K.; Seki, T. Photoresponsive Slide-Ring Gel. Adv. Mater. 2007, 19, 2023–2025. [Google Scholar] [CrossRef]
- Murayama, H.; Imran, A.B.; Nagano, S.; Seki, T.; Kidowaki, M.; Ito, K.; Takeoka, Y. Chromic Slide-Ring Gel Based on Reflection from Photonic Bandgap. Macromolecules 2008, 41, 1808–1814. [Google Scholar] [CrossRef]
- Araki, J. Polyrotaxane Derivatives. II. Preparation and Characterization of Ionic Polyrotaxanes and Ionic Slide-Ring Gels. J. Polym. Sci. Pol. Chem. 2011, 49, 2199–2209. [Google Scholar] [CrossRef]
- Murakami, T.; Schmidt, B.V.K.J.; Brown, H.R.; Hawker, C.J. One-Pot “Click” Fabrication of Slide-Ring Gels. Macromolecules 2015, 48, 7774–7781. [Google Scholar] [CrossRef]
- Kato, K.; Okabe, Y.; Okazumi, Y.; Ito, K. A Significant Impact of Host-Guest Stoichiometry on the Extensibility of Polyrotaxane Gels. Chem. Commun. 2015, 51, 16180–16183. [Google Scholar] [CrossRef] [PubMed]
- Araki, J.; Sainou, N. Amino Acid-Derivatized Slide-Ring Gels: Chemical Crosslinking of Polyrotaxane Conjugates with Different Amino Acid Pendant Groups. Polymer 2015, 74, 133–143. [Google Scholar] [CrossRef]
- Kali, G.; Eisenbarth, H.; Wenz, G. One Pot Synthesis of a Polyisoprene Polyrotaxane and Conversion to a Slide-Ring Gel. Macromol. Rapid Commun. 2015, 37, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Inoue, K.; Kidowaki, M.; Ito, K. Organic−Inorganic Hybrid Slide-Ring Gels: Polyrotaxanes Consisting of Poly (Dimethylsiloxane) and γ-Cyclodextrin and Subsequent Topological Cross-Linking. Macromolecules 2009, 42, 7129–7136. [Google Scholar] [CrossRef]
- Karino, T.; Okumura, Y.; Zhao, C.; Kataoka, T.; Ito, K.; Shibayama, M. SANS Studies on Deformation Mechanism of Slide-Ring Gel. Macromolecules 2005, 38, 6161–6167. [Google Scholar] [CrossRef]
- Fleury, G.; Schlatter, G.; Brochon, C.; Hadziioannou, G. Unveiling the Sliding Motion in Topological Networks: Influence of the Swelling Solvent on the Relaxation Dynamics. Adv. Mater. 2006, 18, 2847–2851. [Google Scholar] [CrossRef]
- Murata, N.; Konda, A.; Urayama, K.; Takigawa, T.; Kidowaki, M.; Ito, K. Anomaly in Stretching-Induced Swelling of Slide-Ring Gels with Movable Cross-Links. Macromolecules 2009, 42, 8485–8491. [Google Scholar] [CrossRef]
- Mayumi, K.; Tezuka, M.; Bando, A.; Ito, K. Mechanics of Slide-Ring Gels: Novel Entropic Elasticity of a Topological Network Formed by Ring and String. Soft Matter 2012, 8, 8179–8183. [Google Scholar] [CrossRef]
- Katsuno, C.; Konda, A.; Urayama, K.; Takigawa, T.; Kidowaki, M.; Ito, K. Pressure-Responsive Polymer Membranes of Slide-Ring Gels with Movable Cross-Links. Adv. Mater. 2013, 25, 4636–4640. [Google Scholar] [CrossRef]
- Kato, K.; Yasuda, T.; Ito, K. Viscoelastic Properties of Slide-Ring Gels Reflecting Sliding Dynamics of Partial Chains and Entropy of Ring Components. Macromolecules 2013, 46, 310–316. [Google Scholar] [CrossRef]
- Bin Imran, A.; Esaki, K.; Gotoh, H.; Seki, T.; Ito, K.; Sakai, Y.; Takeoka, Y. Extremely Stretchable Thermosensitive Hydrogels by Introducing Slide-Ring Polyrotaxane Cross-Linkers and Ionic Groups into the Polymer Network. Nat. Commun. 2014, 5, 5124. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, Y.; Zheng, Z.; Ding, X.; Peng, Y. Periodic Auto-Active Gels with Topologically “Polyrotaxane-Interlocked” Structures. Chem. Commun. 2014, 50, 6372–6374. [Google Scholar] [CrossRef] [PubMed]
- Karino, T.; Shibayama, M.; Okumura, Y.; Ito, K. SANS Study on Pulley Effect of Slide-Ring Gel. Phys. B Condens. Matter 2006, 385, 807–809. [Google Scholar] [CrossRef]
- Fleury, G.; Schlatter, G.; Brochon, C.; Travelet, C.; Lapp, A.; Lindner, P.; Hadziioannou, G. Topological Polymer Networks with Sliding Cross-Link Points: The “Sliding Gels”. Relationship Between Their Molecular Structure and the Viscoelastic as Well as the Swelling Properties. Macromolecules 2007, 40, 535–543. [Google Scholar] [CrossRef]
- Kato, K.; Karube, K.; Nakamura, N.; Ito, K. The Effect of Ring Size on the Mechanical Relaxation Dynamics of Polyrotaxane Gels. Polym. Chem. 2015, 6, 2241–2248. [Google Scholar] [CrossRef]
- Iwaso, K.; Takashima, Y.; Harada, A. Fast Response Dry-Type Supramolecular Artificial Muscles with [c2]Daisy Chains. Nat. Chem. 2016, 8, 625–632. [Google Scholar] [CrossRef]
- Ikejiri, S.; Takashima, Y.; Osaki, M.; Yamaguchi, H.; Harada, A. Solvent-Free Photoresponsive Artificial Muscles Rapidly Driven by Molecular Machines. J. Am. Chem. Soc. 2018, 140, 17308–17315. [Google Scholar] [CrossRef]
- Talotta, C.; Gaeta, C.; Pierro, T.; Neri, P. Sequence Stereoisomerism in Calixarene-Based Pseudo [3]Rotaxanes. Org. Lett. 2011, 13, 2098–2101. [Google Scholar] [CrossRef]
- Pierro, T.; Gaeta, C.; Talotta, C.; Casapullo, A.; Neri, P. Fixed or Invertible Calixarene-Based Directional Shuttles. Org. Lett. 2011, 13, 2650–2653. [Google Scholar] [CrossRef]
- Talotta, C.; Gaeta, C.; Neri, P. Stereoprogrammed Direct Synthesis of Calixarene-Based [3]Rotaxanes. Org. Lett. 2012, 14, 3104–3107. [Google Scholar] [CrossRef]
- Gaeta, C.; Talotta, C.; Mirra, S.; Margarucci, L.; Casapullo, A.; Neri, P. Catenation of Calixarene Annulus. Org. Lett. 2013, 15, 116–119. [Google Scholar] [CrossRef]
- Talotta, C.; Gaeta, C.; Qi, Z.; Schalley, C.A.; Neri, P. Pseudorotaxanes with Self-Sorted Sequence and Stereochemical Orientation. Angew. Chem. Int. Ed. 2013, 52, 7437–7441. [Google Scholar] [CrossRef] [PubMed]
- Gaeta, C.; Talotta, C.; Neri, P. Pseudorotaxane Orientational Stereoisomerism Driven by π-Electron Density. Chem. Commun. 2014, 50, 9917–9920. [Google Scholar] [CrossRef] [PubMed]
- Arduini, A.; Ciesa, F.; Fragassi, M.; Pochini, A.; Secchi, A. Selective Synthesis of Two Constitutionally Isomeric Oriented Calix [6]Arene-Based Rotaxanes. Angew. Chem. Int. Ed. 2005, 44, 278–281. [Google Scholar] [CrossRef]
- Arduini, A.; Calzavacca, F.; Pochini, A.; Secchi, A. Unidirectional Threading of Triphenylureidocalix [6]arene-Based Wheels: Oriented Pseudorotaxane Synthesis. Chem. Eur. J. 2003, 9, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-X.; Meng, Z.; Xiang, J.-F.; Xia, Y.-X.; Sun, Y.; Hu, S.-Z.; Chen, H.; Yao, J.; Chen, C.-F. Guest-Dependent Directional Complexation Based on Triptycene Derived Oxacalixarene: Formation of Oriented Rotaxanes. Chem. Sci. 2016, 7, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.-Z.; Chen, C.-F. Triptycene-Derived Oxacalixarene with Expanded Cavity: Synthesis, Structure and Its Complexation with Fullerenes C60 and C70. Chem. Commun. 2010, 46, 4199–4201. [Google Scholar] [CrossRef]
- Hu, S.-Z.; Chen, C.-F. Triptycene-Derived Oxacalixarenes as New Wheels for the Synthesis of [2]Rotaxanes: Acid-Base- and Metal-Ion-Switchable Complexation Processes. Chem. Eur. J. 2011, 17, 5424–5431. [Google Scholar] [CrossRef]
- Strutt, N.L.; Zhang, H.; Schneebeli, S.T.; Stoddart, J.F. Functionalizing Pillar[n]arenes. Acc. Chem. Res. 2014, 47, 2631–2642. [Google Scholar] [CrossRef]
- Ogoshi, T.; Yamafuji, D.; Aoki, T.; Yamagishi, T.-A. Thermally Responsive Shuttling Behavior of a Pillar[6]arene-Based [2]Rotaxane. Chem. Commun. 2012, 48, 6842–6844. [Google Scholar] [CrossRef]
- Ogoshi, T.; Yamafuji, D.; Aoki, T.; Kitajima, K.; Yamagishi, T.-A.; Hayashi, Y.; Kawauchi, S. High-Yield Diastereoselective Synthesis of Planar Chiral [2]- and [3]Rotaxanes Constructed From Per-ethylated Pillar[5]Arene and Pyridinium Derivatives. Chem. Eur. J. 2012, 18, 7493–7500. [Google Scholar] [CrossRef]
- Ogoshi, T.; Yamafuji, D.; Yamagishi, T.-A.; Brouwer, A.M. Förster Resonance Energy Transfer by Formation of a Mechanically Interlocked [2]Rotaxane. Chem. Commun. 2013, 49, 5468–5470. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Chen, W.; Hou, D.; Meng, Q.; Zheng, R.; Li, C. Novel Binding Regioselectivity in the Interpenetration of a Non-Symmetric Axle Into a Non-Symmetric Pillar[5]arene Wheel. Chem. Commun. 2014, 50, 4820–4823. [Google Scholar] [CrossRef] [PubMed]
- Aav, R.; Mishra, K. The Breaking of Symmetry Leads to Chirality in Cucurbituril-Type Hosts. Symmetry 2018, 10, 98. [Google Scholar] [CrossRef]
- Lee, S.; Chen, C.-H.; Flood, A.H. A Pentagonal Cyanostar Macrocycle with Cyanostilbene CH Donors Binds Anions and Forms Dialkylphosphate [3]Rotaxanes. Nat. Chem. 2013, 5, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Quesada, J.; Saghatelian, A.; Cheley, S.; Bayley, H.; Ghadiri, M.R. Single DNA Rotaxanes of a Transmembrane Pore Protein. Angew. Chem. Int. Ed. 2004, 43, 3063–3067. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).