A Crucial Role of Attention in Lateralisation of Sound Processing?
Abstract
1. Introduction
2. Results
2.1. Study 1: Behavioural Responses of Campbell’s Monkeys to Familiar or Novel Sound Stimuli
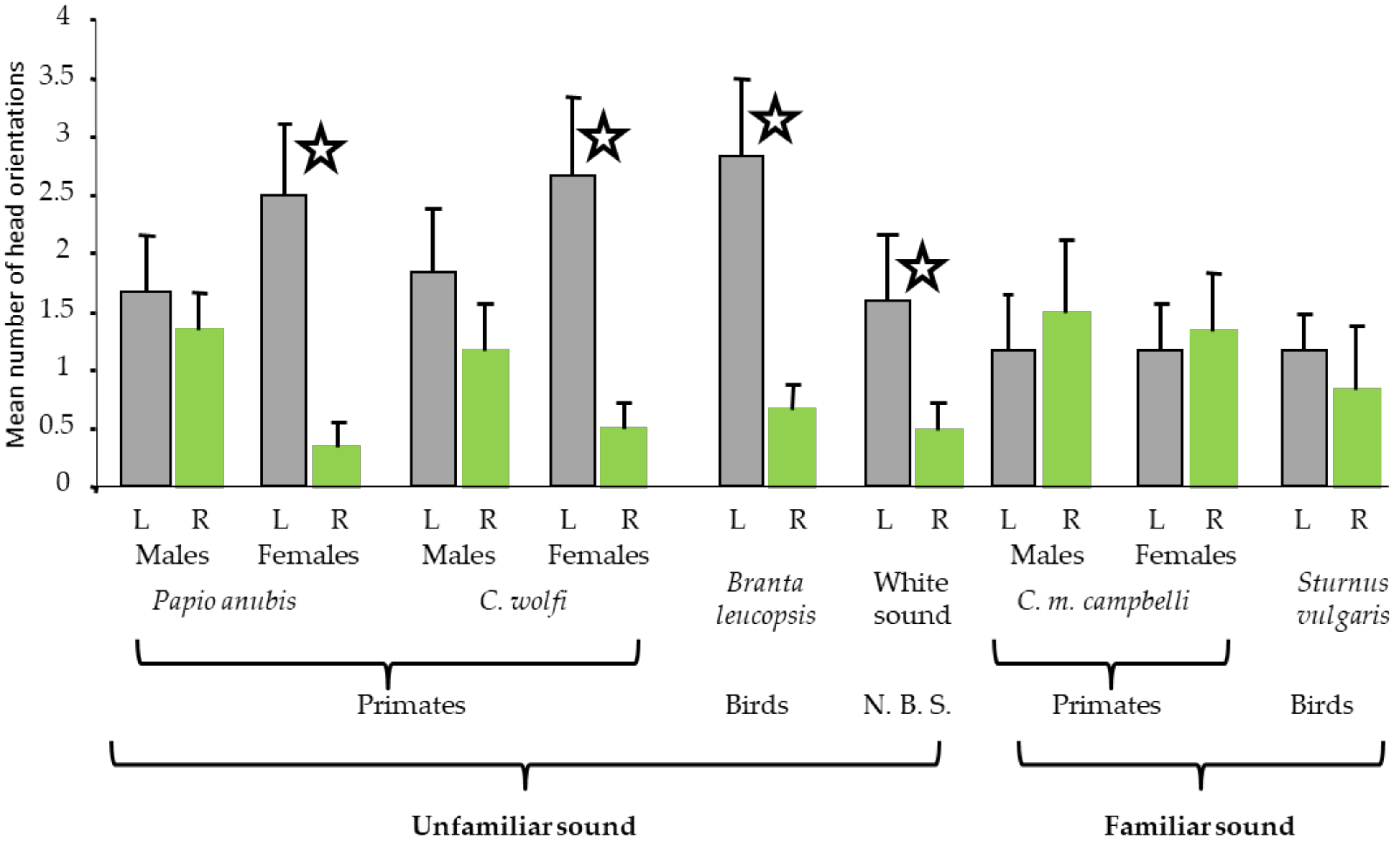
2.1.1. Results of Study 1
2.1.2. Discussion of Study 1
2.2. Study 2: Electrophysiological Responses of Auditory Neurons to Different Sounds in European Starlings
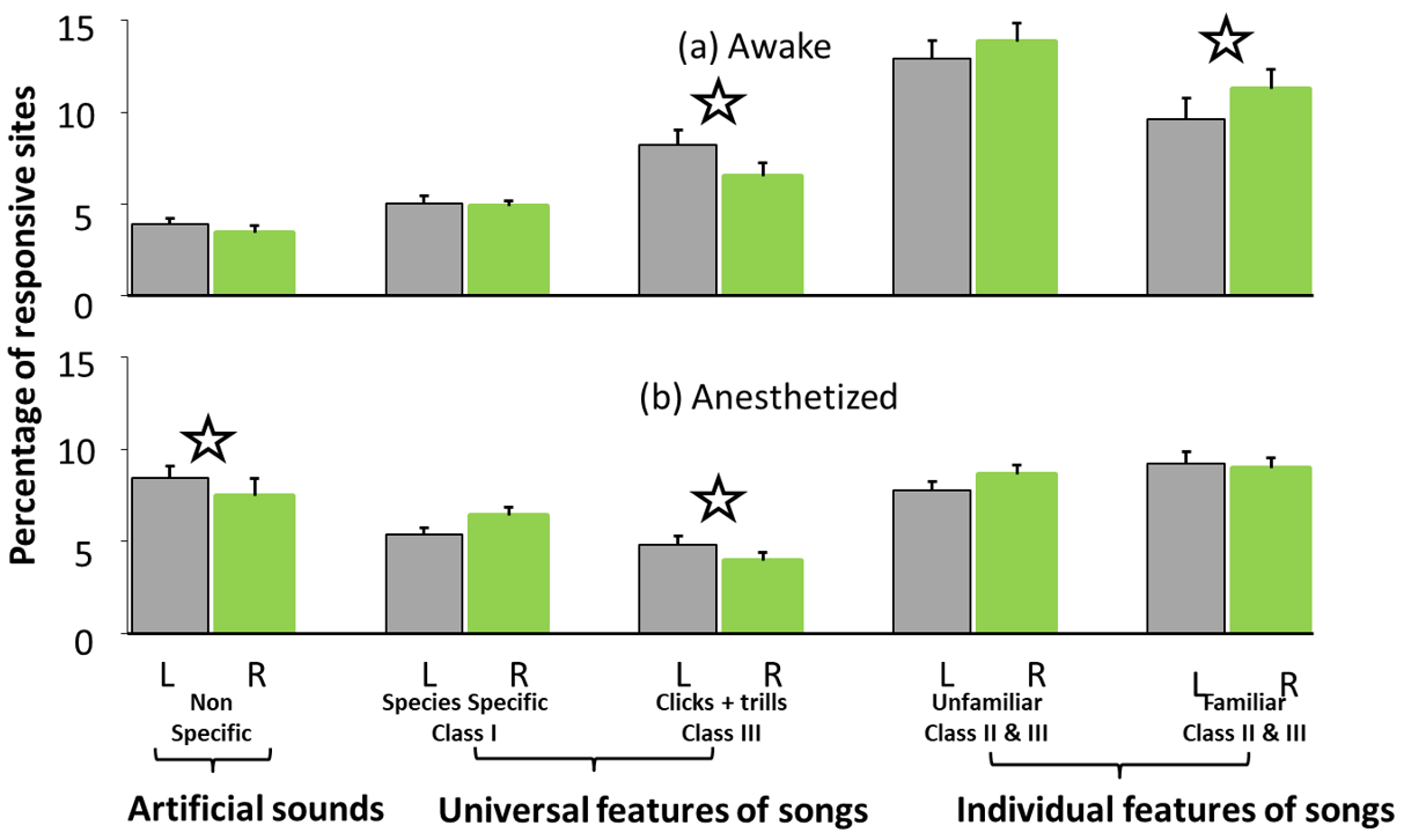
2.2.1. Results of Study 2
2.2.2. Discussion of Study 2
3. General Discussion
4. Materials and Methods
4.1. Study 1: Behavioural Responses of Campbell’s Monkeys to Familiar or Novel Sound Stimuli
4.1.1. Subjects
4.1.2. Procedure
4.1.3. Auditory Stimuli
4.1.4. Statistical Analysis
4.2. Study 2: Electrophysiological Responses of Auditory Neurons to Different Sounds in European Starlings (see also [38,93])
4.2.1. Subjects
4.2.2. Electrophysiological Recordings
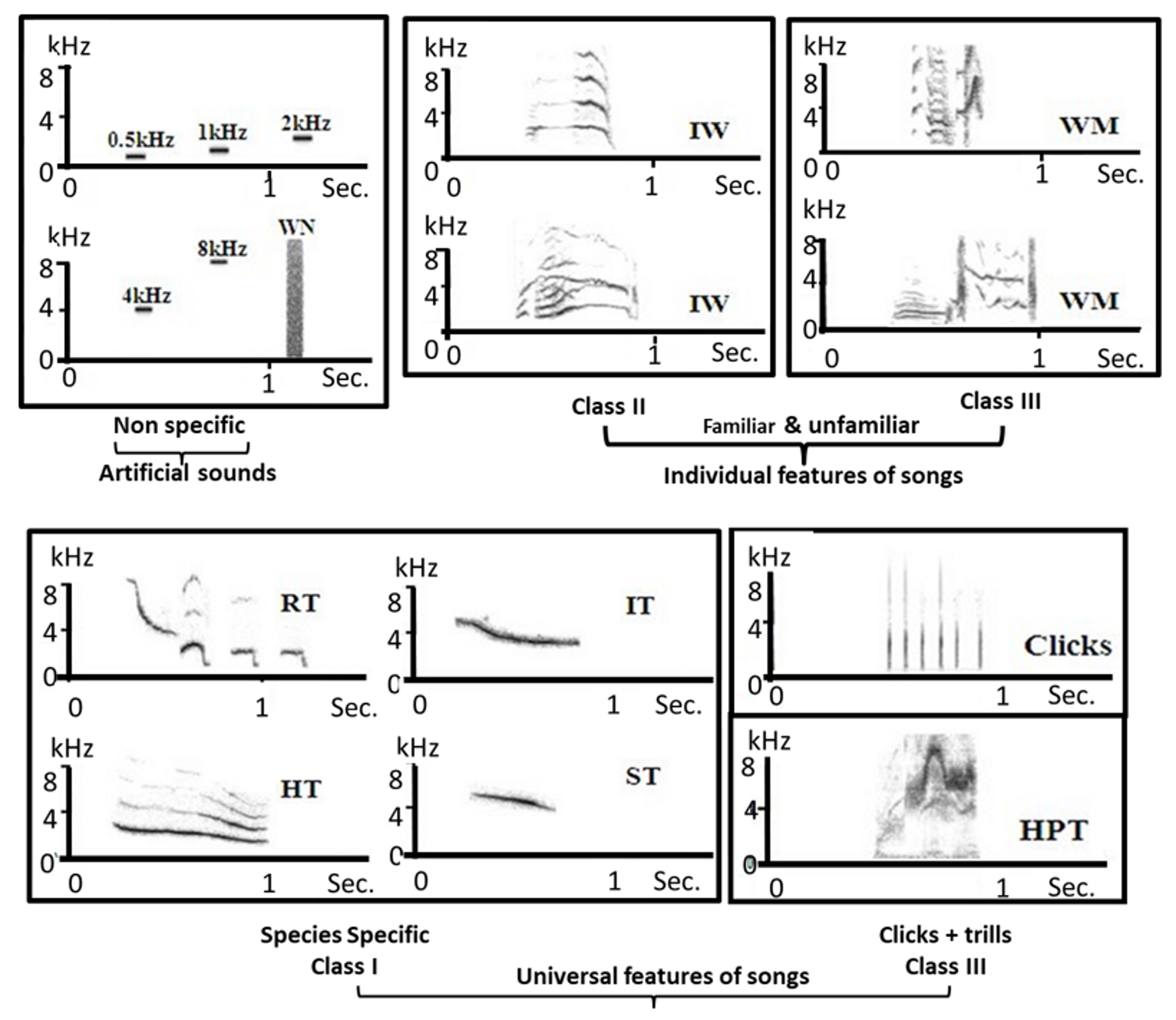
4.2.3. Auditory Stimuli
4.2.4. Statistical Analyses
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Broca, P. Remarques sur le siège de la faculté du langage articulé suivies d’une observation d’aphémie. Bull. Soc. Anat. 1861, 6, 398–407. [Google Scholar]
- Rogers, L.J.; Andrew, R.J. Comparative Vertebrate Lateralization; Cambridge University Press: Cambridge, UK, 2002; ISBN 0-521-78161-2. [Google Scholar]
- Mc Neilage, P.F.; Rogers, L.J.; Vallortigara, G. Origins of left and right Brain. Sci. Am. 2009, 301, 60–67. [Google Scholar] [CrossRef]
- Vallortigara, G.; Chiandetti, C.; Sovrano, V.A. Brain asymmetry (animal). Adv. Rev. 2011, 2, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.; Vallortigara, G. When and why did brains break symmetry? Symmetry 2015, 7, 2181–2194. [Google Scholar] [CrossRef]
- Taglialatela, J.P. Functional and structural asymmetries for auditory perception and vocal production in nonhuman primates. Spec. Top. Primatol. 2007, 5, 120–145. [Google Scholar]
- Ocklenburg, S.; Ströckens, F.; Güntürkün, O. Lateralisation of conspecific vocalization in non-human vertebrates. Laterality 2013, 18, 1–31. [Google Scholar] [CrossRef]
- George, I. Hemispheric asymmetry of songbirds. In The Two Halves of the Brain: Information Processing in the Cerebral Hemispheres; MIT Press Scholarship Online; Hugdahl, K., Westerhausen, R., Eds.; MIT Press: Cambridge, MA, USA, 2010; pp. 91–120. [Google Scholar] [CrossRef]
- Konerding, W.S.; Zimmermann, E.; Bleich, E.; Hedrich, H.J.; Scheumann, M. The head turn paradigm to assess auditory laterality in cats: Influence of ear position and repeated sound presentation. PeerJ 2017, 5, e3925. [Google Scholar] [CrossRef]
- Böye, M.; Güntürkün, O.; Vauclair, J. Right ear advantage for conspecific calls in adults and subadults, but not infants, California sea lions (Zalophus californianus): Hemispheric specialization for communication? Eur. J. Neurosci. 2005, 21, 1727–1732. [Google Scholar] [CrossRef]
- Ehret, G. Left hemisphere advantage in the mouse brain for recognizing ultrasonic communications calls. Nature 1987, 325, 249–251. [Google Scholar] [CrossRef]
- Palleroni, A.; Hauser, M. Experience-dependant plasticity for auditory processing in a raptor. Science 2003, 299, 1195. [Google Scholar] [CrossRef]
- Siniscalchi, M.; Laddago, S.; Quaranta, A. Auditory lateralization of conspecific and heterospecific vocalizations in cats. Laterality 2016, 21, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Hauser, M.D.; Andersson, K. Left hemisphere dominance for processing vocalizations in adult, but no infant, rhesus monkeys: Field experiments. Proc. Natl. Acad. Sci. USA 1994, 91, 3946–3948. [Google Scholar] [CrossRef] [PubMed]
- Hauser, M.D.; Agnetta, B.; Perez, C. Orienting asymmetries in rhesus monkeys: The effect on time-domain changes on acoustic perception. Anim. Behav. 1998, 56, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Szymanska, J.; Trojan, M.; Jakucinska, A.; Wejchert, K.; Kapusta, M.; Sikorska, J. Brain functional asymmetry of chimpanzees (Pan troglodytes): The example of auditory laterality. Pol. Psychol. Bull. 2017, 48, 87–92. [Google Scholar] [CrossRef]
- Heffner, H.E.; Heffner, R.S. Temporal lobe lesions and perception of species-specific vocalizations by macaques. Science 1984, 236, 75–76. [Google Scholar] [CrossRef]
- Heffner, H.E.; Heffner, R.S. Effect of unilateral and bilateral auditory cortex lesions on the discrimination of vocalizations by Japanese macaques. J. Neurophysiol. 1986, 56, 683–701. [Google Scholar] [CrossRef] [PubMed]
- Okanoya, K.; Ikebuchi, M.; Uno, H.; Watanabe, S. Left-side dominance for song discrimination in Bengalese finches (Lonchura striata var. domestica). Anim. Cogn. 2001, 4, 241–245. [Google Scholar] [CrossRef]
- Ghazanfar, A.A.; Smith-Rohrberg, D.; Hauser, M.D. The Role of Temporal Cues in Rhesus Monkey Vocal Recognition: Orienting Asymmetries to Reversed Calls. Brain Behav. Evol. 2001, 58, 163–172. [Google Scholar] [CrossRef]
- Cynx, J.; Williams, H.; Nottebohm, F. Hemispheric differences in avian song discrimination. Proc. Natl. Acad. Sci. USA 1992, 89, 1372–1375. [Google Scholar] [CrossRef]
- George, I.; Vernier, B.; Richard, J.-P.; Hausberger, M.; Cousillas, H. Hemispheric specialization in the primary auditory area of awake and anesthetized starlings. Behav. Neurosci. 2004, 118, 597–610. [Google Scholar] [CrossRef]
- George, I.; Cousillas, H.; Richard, J.-P.; Hausberger, M. State-dependent hemispheric specialization in the songbird brain. J. Comp. Neurol. 2005, 18, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Gil-da-Costa, R.; Hauser, M.D. Vervet monkeys and humans show brain asymmetries for processing conspecific vocalizations, but with opposite patterns of laterality. Proc. R. Soc. B 2006, 273, 2313–2318. [Google Scholar] [CrossRef] [PubMed]
- Lemasson, A.; Koda, H.; Kato, A.; Oyakawa, C.; Blois-Heulin, C.; Masataka, N. Influence of sound specificity and familiarity on Japanese macaques’ (Macaca fuscata) auditory laterality. Behav. Brain Res. 2010, 208, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Arve, E.; Hugdahl, K. Attentional effect in dichotic listening. Brain and language 1995, 49, 189–201. [Google Scholar]
- Locke, J.L.; Snow, C. Social influences on vocal learning in human and non-human primates. In Social Influences on Vocal Development; Snowdon, C.T., Hausberger, M., Eds.; Cambridge University Press: Cambridge, UK, 1997; pp. 274–292. [Google Scholar]
- Watkins, J.A.S. Lateralization of Auditory Learning and Processing in the Domestic Chick (Gallus gallus domesticus). Ph.D. Thesis, University of Sussex, Sussex, UK, 1999. [Google Scholar]
- Pohl, P. Central auditory processing. V: Ear advantages acoustic stimuli in Baboons. Brain Lang. 1983, 20, 44–53. [Google Scholar] [CrossRef]
- Leliveld, L.M.C.; Scheumann, M.; Zimmermann, E. Effects of caller characteristics on auditory laterality in an early primate (Microcebus murinus). PLoS ONE 2010, 5, e9031. [Google Scholar] [CrossRef]
- Basile, M.; Lemasson, A.; Blois-Heulin, C. Social and Emotional Values of Sounds Influence Human (Homo sapiens) and Non-human Primate (Cercopithecus campbelli) Auditory Laterality. PLoS ONE 2009, 4, e6295. [Google Scholar] [CrossRef]
- Xue, F.; Fang, G.; Yang, P.; Zhao, E.; Brauth, S.E.; Tang, Y. The biological significance of acoustic stimuli determines ear preference in the music frog. J. Exp. Biol. 2015, 218, 740–747. [Google Scholar] [CrossRef]
- Scheumann, M.; Zimmermann, E. Sex-specific asymmetries I communication sound perception are not related to hand preference in an early primate. BMC Biol. 2008, 6, 3. [Google Scholar] [CrossRef]
- Reinholz-Trojan, A.; Włodarczyk, E.; Trojan, M.; Kulcz Nski, A.; Stefá Nska, J. Hemispheric specialization in domestic dogs Canis familiaris for processing different types of acoustic stimuli. Behav. Proc. 2012, 91, 202–205. [Google Scholar] [CrossRef]
- Andrew, R.J.; Watkins, J.A.S. Evidence for cerebral lateralization from senses other than vision. In Comparative Vertebrate Lateralization; Rogers, L.J., Andrew, R.J., Eds.; Cambridge University Press: Cambridge, UK, 2002; pp. 365–382. ISBN 0-521-78161-2. [Google Scholar]
- Chanvallon, S.; Blois-Heulin, C.; de Latour, P.R.; Lemasson, A. Spontaneous approaches of divers by free-ranging orcas (Orcinus orca): Age- and sex-differences in exploratory behaviours and visual laterality. Sci. Rep. 2017, 7, 10922. [Google Scholar] [CrossRef] [PubMed]
- Vallortigara, G.; Rogers, L.J.; Bisazza, A. Possible evolutionary origins of cognitive brain lateralization. Brain Res. Rev. 1999, 30, 164–175. [Google Scholar] [CrossRef]
- Karino, G.; George, I.; Loison, L.; Heyraud, C.; De Groof, G.; Hausberger, M.; Cousillas, H. Anesthesia and brain sensory processing: Impact on neuronal responses in a female songbird. Sci. Rep. 2016, 6, 39143. [Google Scholar] [CrossRef] [PubMed]
- Rochais, C.; Sébilleau, M.; Menoret, M.; Oger, M.; Henry, S.; Hausberger, M.; Cousillas, H. Attentional state and brain processes: State-dependent lateralization of EEG profiles in horses. Sci. Rep. 2018, 8, 10153. [Google Scholar] [CrossRef] [PubMed]
- De Groof, G.; Poirier, C.; George, I.; Hausberger, M.; Van der Linden, A. Functional changes between seasons in the male songbird auditory forebrain. Front. Behav. Neurosci. 2013, 7, 196. [Google Scholar] [CrossRef] [PubMed]
- Lemasson, A.; Zuberbühler, K.; Hausberger, M. Socially meaningful vocal plasticity in Campbell’s monkeys. J. Comp. Psychol. 2005, 119, 220–229. [Google Scholar] [CrossRef]
- Lemasson, A.; Glas, L.; Barbu, S.; Lacroix, A.; Guilloux, M.; Remeuf, K.; Koda, H. Youngsters do not pay attention to conversational rules: Is this so for nonhuman primates? Sci. Rep. 2011, 1, 22. [Google Scholar] [CrossRef]
- Hausberger, M.; Foraste, M.; Richard-Yris, M.-A.; Nygren, C. Differential response of female starlings to shared and nonshared song types. Etologia 1997, 5, 31–38. [Google Scholar]
- George, I.; Richard, J.-P.; Cousillas, H.; Hausberger, M. No need to Talk, I Know You: Familiarity Influences Early Multisensory Integration in a Songbird’s Brain. Front. Behav. Neurosci. 2011, 4, 193. [Google Scholar] [CrossRef]
- Hubel, D.H.; Henson, C.O.; Rupert, A.; Galambos, R. “Attention” Units in the Auditory Cortex. Science 1959, 129, 1279–1280. [Google Scholar] [CrossRef]
- Henry, L.; Bourguet, C.; Coulon, M.; Aubry, C.; Hausberger, M. Sharing mates and nestboxes is associated with female ‘friendship’ in European starlings Sturnus vulgaris. J. Comp. Psychol. 2013, 157, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lemasson, A.; Hausberger, M. Patterns of vocal sharing and social dynamics in a Campbell’s monkeys. J. Comp. Psychol. 2004, 118, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Lemasson, A.; Blois-Heulin, C.; Jubin, R.; Hausberger, M. Female social relationships in a captive group of Campbell’s monkeys (Cercopithecus campbelli campbelli). Am. J. Prim. 2006, 68, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Lemasson, A.; Boutin, A.; Boivin, S.; Blois-Heulin, C.; Hausberger, M. Horse (Equus caballus) whinnies: A source of social information. Anim. Cogn. 2009, 12, 693–704. [Google Scholar] [CrossRef]
- Rogers, L.J. Evolution of hemispheric specialization: Advantages and disadvantages. Brain Lang. 2000, 73, 236–253. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, W.D.; Fernandez Carriba, S. Laterality of communicative behaviours in Non-human Primates: A critical analysis. In Comparative Vertebrate Lateralization; Rogers, L.J., Andrew, R.J., Eds.; Cambridge University Press: Cambridge, UK, 2002; pp. 445–479. ISBN 0-521-78161-2. [Google Scholar]
- Ouattara, K.; Lemasson, A.; Zuberbühler, K. The alarm calls system of female Campbell’s monkeys. Anim. Behav. 2009, 78, 35–44. [Google Scholar] [CrossRef]
- Lemasson, A.; Gandon, E.; Hausberger, M. Attention to elders’ voice in non-human primates. Biol. Lett. 2010. [Google Scholar] [CrossRef]
- Basile, M.; Boivin, S.; Boutin, A.; Blois-Heulin, C.; Hausberger, M.; Lemasson, A. Socially dependent auditory laterality in domestic horses Equus caballus. Anim. Cogn. 2009, 12, 611–619. [Google Scholar] [CrossRef]
- Hauber, M.E.; Cassey, P.; Woolley, S.M.; Theunissen, F.E. Neurophysiological response selectivity for conspecific songs over synthetic sounds in the auditory forebrain of non-singing female songbirds. J. Comp. Physiol. A 2007, 193, 765–774. [Google Scholar] [CrossRef]
- Vallortigara, G.; Andrew, R.J. Laterality of response by chicks to change in a model partner. Anim. Behav. 1991, 41, 187–194. [Google Scholar] [CrossRef]
- Deng, C.; Rogers, L.J. Social recognition and approach in the chick: Laterality and effect of visual experience. Anim. Behav. 2002, 63, 697–706. [Google Scholar] [CrossRef]
- Zucca, P.; Sovrano, V.A. Animal lateralization and social recognition: Quails use their visual hemifield when approaching a companion and their right visual hemi-field when approaching a stranger. Cortex 2008, 44, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Peirce, J.W.; Leigh, A.E.; Kendrick, K.M. Configurational coding, familiarity and the right hemisphere advantage for face recognition in sheep. Neuropsychologia 2000, 38, 475–483. [Google Scholar] [CrossRef]
- Siniscalchi, M.; Quaranta, A.; Rogers, L.J. Hemispheric specialization in dogs for processing different acoustic stimuli. PLoS ONE 2008, 3, e3349. [Google Scholar] [CrossRef] [PubMed]
- Siniscalchi, M.; d’Ingeo, S.; Minunno, M.; Quaranta, A. Communication in dogs. Animals 2018, 8, 131. [Google Scholar] [CrossRef]
- Baciadonna, L.; Nawroth, C.; Briefer, E.F.; McElligott, A.G. Perceptual lateralization of vocal stimuli in goats. Curr. Zool 2018, 3, 1–8. [Google Scholar] [CrossRef]
- Lang, P.J.; Greenwald, M.K.; Bradley, M.M.; Hamm, A.O. Looking at pictures: Affective, facial, visceral, and behavioral reactions. Psychophysiology 1993, 30, 261–273. [Google Scholar] [CrossRef]
- Andics, A.; Gácsi, M.; Faragó, T.; Kis, A.; Miklósi, A. Report voice-sensitive regions in the dog and human brain are revealed by comparative fMRI. Curr. Biol. 2014, 24, 574–578. [Google Scholar] [CrossRef]
- Ratcliffe, V.F.; Reby, D. Orienting asymmetries in dogs’ responses to different communicatory components of human speech. Curr. Biol. 2014, 24, 2908–2912. [Google Scholar] [CrossRef]
- Andrew, R.J. The differential roles of right and left sides of the brain in memory formation. Behav. Brain Res. 1999, 98, 289–295. [Google Scholar] [CrossRef]
- Vallortigara, G.; Andrew, R.J. Differential involvement of right and left hemisphere in individual recognition in the domestic chick. Behav. Proc. 1994, 33, 41–57. [Google Scholar] [CrossRef]
- Austin, N.P.; Rogers, L.J. Asymmetry of flight and escape turning responses in horses. Laterality 2007, 12, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Blois-Heulin, C.; Crevel, M.; Böye, M.; Lemasson, A. Visual laterality in dolphins: Importance of the familiarity of stimuli. BMC Neurosci. 2012, 13, 2–8. [Google Scholar] [CrossRef] [PubMed]
- De Boyer Des Roches, A.; Richard-Yris, M.A.; Henry, S.; Ezzaouïab, M.; Hausberger, M. Laterality and emotions: Visual laterality in the domestic horse (Equus caballus) differs with objects’ emotional value. Physiol. Behav. 2008, 94, 487–490. [Google Scholar] [CrossRef]
- Larose, C.; Rogers, L.J.; Richard, M.A.; Hausberger, M. Laterality of horses associated with emotionality in novel situations. Laterality 2006, 11, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.V.; Proops, L.; Grounds, K.; Wathan, J.; McComb, K. Functionally relevant responses to human facial expressions of emotion in the domestic horse (Equus caballus). Biol. Lett. 2016, 12, 20150907. [Google Scholar] [CrossRef]
- Zimmerman, P.H.; Buijs, S.A.F.; Bolhuis, J.E.; Keeling, L.J. Behaviour of domestic fowl in anticipation of positive and negative stimuli. Anim. Behav. 2011, 81, 569–577. [Google Scholar] [CrossRef]
- Davidson, R.J. Emotion and affective style: Hemispheric substrates. Psychol. Sci. 1992, 3, 39–43. [Google Scholar] [CrossRef]
- Quaranta, A.; Siniscalchi, M.; Vallortigara, G. Asymetric tail-wagging responses by dogs to different emotive stimuli. Curr. Biol. 2007, 117, 199–201. [Google Scholar] [CrossRef]
- Siniscalchi, M.; Lusito, R.; Vallortigara, G.; Quaranta, A. Seeing left- or right-asymmetric tail wagging produces different emotional responses in dogs. Curr. Biol. 2013, 23, 2279–2282. [Google Scholar] [CrossRef]
- Armony, J.L.; Dolan, R.J. Modulation of spatial attention by fear-conditioned stimuli: An event-related fMRI study. Neuropsychologia 2002, 40, 817–826. [Google Scholar] [CrossRef]
- Fox, E.; Russo, R.; Dutton, K. Attentional bias for threat: Evidence for delayed disengagement from emotional faces. Cogn. Emot. 2002, 16, 355–379. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.V.; Proops, L.; Grounds, K.; Wathan, J.; Scott, S.K.; McComb, K. Domestic horses (Equus caballus) discriminate between negative and positive human nonverbal vocalizations. Sci. Rep. 2018, 8, 13052. [Google Scholar] [CrossRef] [PubMed]
- Welp, T.; Rushen, J.; Kramer, D.L.; Festa-Bianchet, M.; de Passillé, A.M.B. Vigilance as a measure of fear in dairy cattle. Appl. Anim. Behav. Sci. 2004, 87, 1–13. [Google Scholar] [CrossRef]
- Balconi, M.; Vanutelli, M.E. Vocal and visual stimulation, congruence and lateralization affect brain oscillations in interspecies emotional positive and negative interactions. J. Soc. Neuro. 2016, 11, 297–310. [Google Scholar] [CrossRef]
- Proops, L.; Mc Comb, K. Cross-modal individual recognition in domestic horses (Equus caballus) extends to familiar humans. Proc. R. Soc. B 2012, 279, 3131–3138. [Google Scholar] [CrossRef] [PubMed]
- Karakas, S.; Erzengin, Ö.U.; Başar, E. A new strategy involving multiple cognitive paradigms demonstrates that ERP components are determined by the superposition of oscillatory responses. Clin. Neurophysiol. 2000, 111, 1719–1732. [Google Scholar] [CrossRef]
- Syka, J.; Kuta, D.; Popelal, J. Responses to species-specific vocalizations in the auditory cortex of awake and anesthetized guinea pigs. Hear. Res. 2005, 206, 177–184. [Google Scholar] [CrossRef]
- Huetz, C.; Philibert, B.; Edeline, J.M. A spike-timing code for discriminating conspecific vocalizations in the thalamocortical system of anesthetized and awake guinea pigs. J. Neurosci. 2009, 29, 334–350. [Google Scholar] [CrossRef]
- Ishii, R.; Canuet, L.; Ishihara, T.; Aoki, Y.; Ikeda, S.; Hata, M.; Katsimichas, T.; Gunji, A.; Takahashi, H.; Nakahachi, T.; et al. Frontal midline theta rhythm and gamma power changes during focused attention on mental calculation: An MEG beam former analysis. Front. Hum. Neurosci. 2014, 8, 1–10. [Google Scholar] [CrossRef]
- Andics, A.; Gábor, A.; Gácsi, M.; Faragó, T.; Szabó, D.; Miklósi, Á. Neural mechanisms for lexical processing in dogs. Science 2016, 353, 1030–1032. [Google Scholar] [CrossRef] [PubMed]
- Bach, J.-P.; Lüpke, M.; Dziallas, P.; Wefstaedt, P.; Uppenkamp, S.; Seifert, H.; Nolte, I. Auditory functional magnetic resonance imaging in dogs—Normalization and group analysis and the processing of pitch in the canine auditory pathways. BMC Vet. Res. 2016, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Borod, J.C.; Koff, E.; Caron, H.S. Right hemispheric specialization for the expression and appreciation of emotion; a focus on face. In Cognitive Processes in the Right Hemisphere; Perecman, E., Ed.; Academic Press: New York, NY, USA, 1983. [Google Scholar]
- Tucker, D.M. Lateral brain function, emotion and conceptualization. Psychol. Bull. 1981, 89, 19–46. [Google Scholar] [CrossRef] [PubMed]
- Siberman, E.K.; Weingarten, H. Hemispheric lateralization of function related to emotion. Brain Cogn. 1986, 5, 322–353. [Google Scholar] [CrossRef]
- Sackeim, H.; Gur, R.C. Lateral asymmetry in intensity of emotional expression. Neuropsychologia 1978, 16, 473–481. [Google Scholar] [CrossRef]
- Cousillas, H.; George, I.; Alcaix, S.; Henry, L.; Richard, J.P.; Hausberger, M. Seasonal female brain plasticity in processing social vs. sexual vocal signals. Eur. J. Neurosci. 2013, 37, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Dawson, A. Plasma gonadal steroid levels in wild starlings (Sturnus vulgaris) during the annual cycle and in relation to the stages of breeding. Gen. Comp. Endocrinol. 1983, 49, 286–294. [Google Scholar] [CrossRef]
- Ball, G.F.; Wingfield, J.C. Changes in plasma levels of luteinizing hormone and sex steroid hormones in relation to multiple-broodedness and nest-site density in male starlings. Physiol. Zool. 1987, 60, 191–199. [Google Scholar] [CrossRef]
- De Ridder, E.; Pinxten, R.; Mees, V.; Eens, M. Short- and longterm effects of male-like concentrations of testosterone on female European starlings (Sturnus vulgaris). Auk 2002, 119, 487–497. [Google Scholar] [CrossRef]
- George, I.; Cousillas, H.; Richard, J.-P.; Hausberger, M. A new extensive approach to single-unit responses using multisite recording electrodes: Application to the songbird brain. J. Neurosci. Methods 2003, 125, 65–71. [Google Scholar] [CrossRef]
- Capsius, B.; Leppelsack, H.J. Response patterns and their relationship to frequency analysis in auditory forebrain centers of a songbird. Hear. Res. 1999, 136, 91–99. [Google Scholar] [CrossRef]
- Cousillas, H.; Leppelsack, H.J.; Leppelsack, E.; Richard, J.P.; Mathelier, M.; Hausberger, M. Functional organization of the forebrain auditory centers of the European starling. A study based on natural sounds. Hear. Res. 2005, 207, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Leppelsack, H.J.; Vogt, M. Responses of auditory neurons in the forebrain of a songbird to stimulation with species-specific sounds. J. Comp. Physiol. 1976, 107, 263–274. [Google Scholar] [CrossRef]
- Hausberger, M. Social influences on song acquisition and sharing in the European starling (Sturnus vulgaris). In Social Influences on Vocal Development; Snowdon, C.T., Hausberger, M., Eds.; Cambridge University Press: Cambridge, UK, 1997; pp. 128–156. [Google Scholar]
- Verheyen, R.F. Breeding strategies of the starling. In Bird Problems in Agriculture; Wright, E.N., Inglis, I.R., Feare, C.J., Eds.; British Crop. Protection Council: Croydon, UK, 1980; pp. 69–82. [Google Scholar]
- Henry, L.; Hausberger, M.; Jenkins, P.F. The use of song repertoire changes with pairing status in male European starling. Bioacoustics 1994, 5, 261–266. [Google Scholar] [CrossRef]
- Amin, N.; Grace, J.A.; Theunissen, F.E. Neural response to bird’s own song and tutor song in the zebra finch field L and caudal mesopallium. J. Comp. Physiol. 2004, 190, 469–489. [Google Scholar] [CrossRef]
- Grace, J.A.; Amin, N.; Singh, N.C.; Theunissen, F.E. Selectivity for conspecific song in the zebra finch auditory forebrain. J. Neurophysiol. 2003, 89, 472–487. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hausberger, M.; Cousillas, H.; Meter, A.; Karino, G.; George, I.; Lemasson, A.; Blois-Heulin, C. A Crucial Role of Attention in Lateralisation of Sound Processing? Symmetry 2019, 11, 48. https://doi.org/10.3390/sym11010048
Hausberger M, Cousillas H, Meter A, Karino G, George I, Lemasson A, Blois-Heulin C. A Crucial Role of Attention in Lateralisation of Sound Processing? Symmetry. 2019; 11(1):48. https://doi.org/10.3390/sym11010048
Chicago/Turabian StyleHausberger, Martine, Hugo Cousillas, Anaïke Meter, Genta Karino, Isabelle George, Alban Lemasson, and Catherine Blois-Heulin. 2019. "A Crucial Role of Attention in Lateralisation of Sound Processing?" Symmetry 11, no. 1: 48. https://doi.org/10.3390/sym11010048
APA StyleHausberger, M., Cousillas, H., Meter, A., Karino, G., George, I., Lemasson, A., & Blois-Heulin, C. (2019). A Crucial Role of Attention in Lateralisation of Sound Processing? Symmetry, 11(1), 48. https://doi.org/10.3390/sym11010048



