Chiral Proportions of Nepheline Originating from Low-Viscosity Alkaline Melts. A Pilot Study
Abstract
:1. Introduction
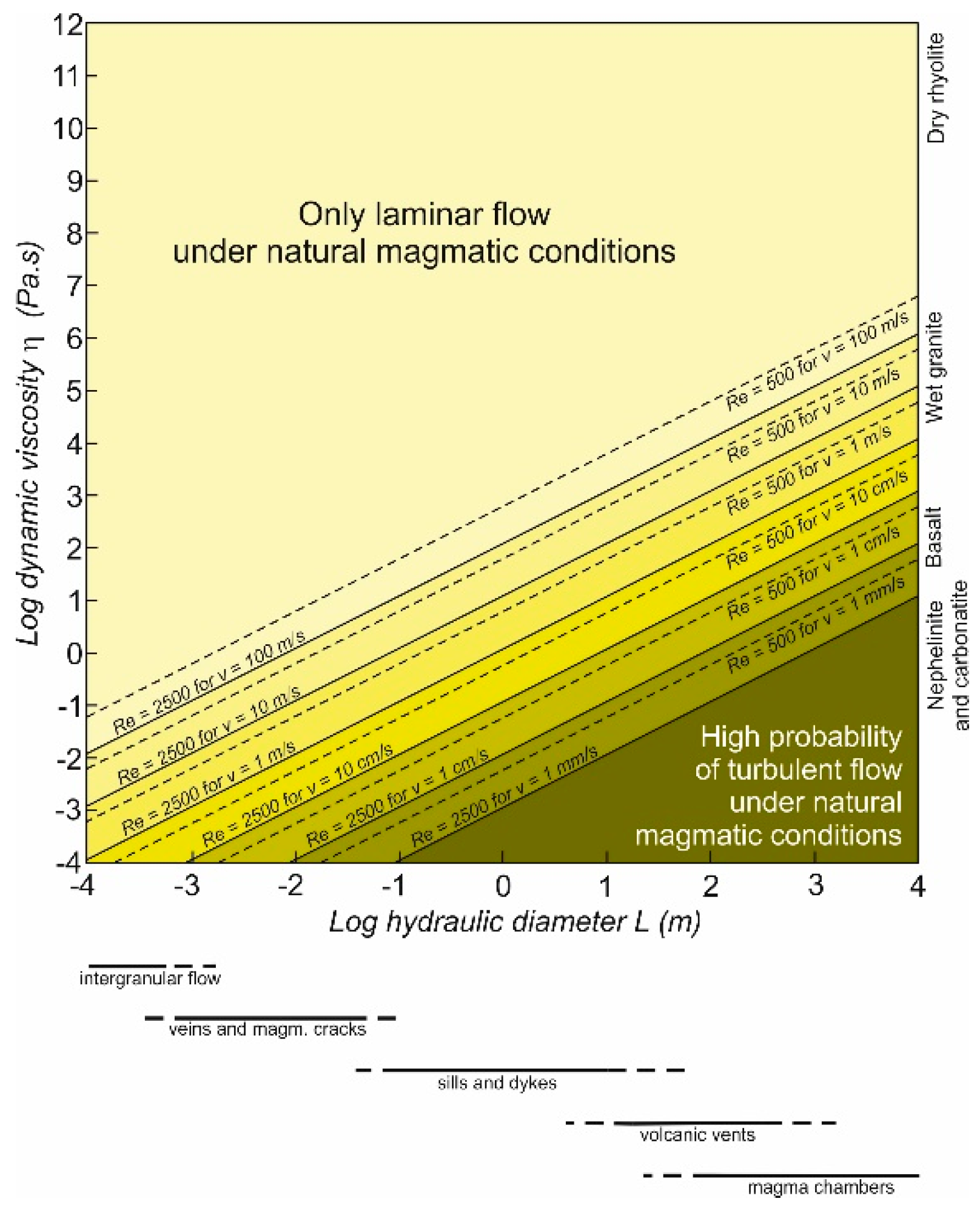
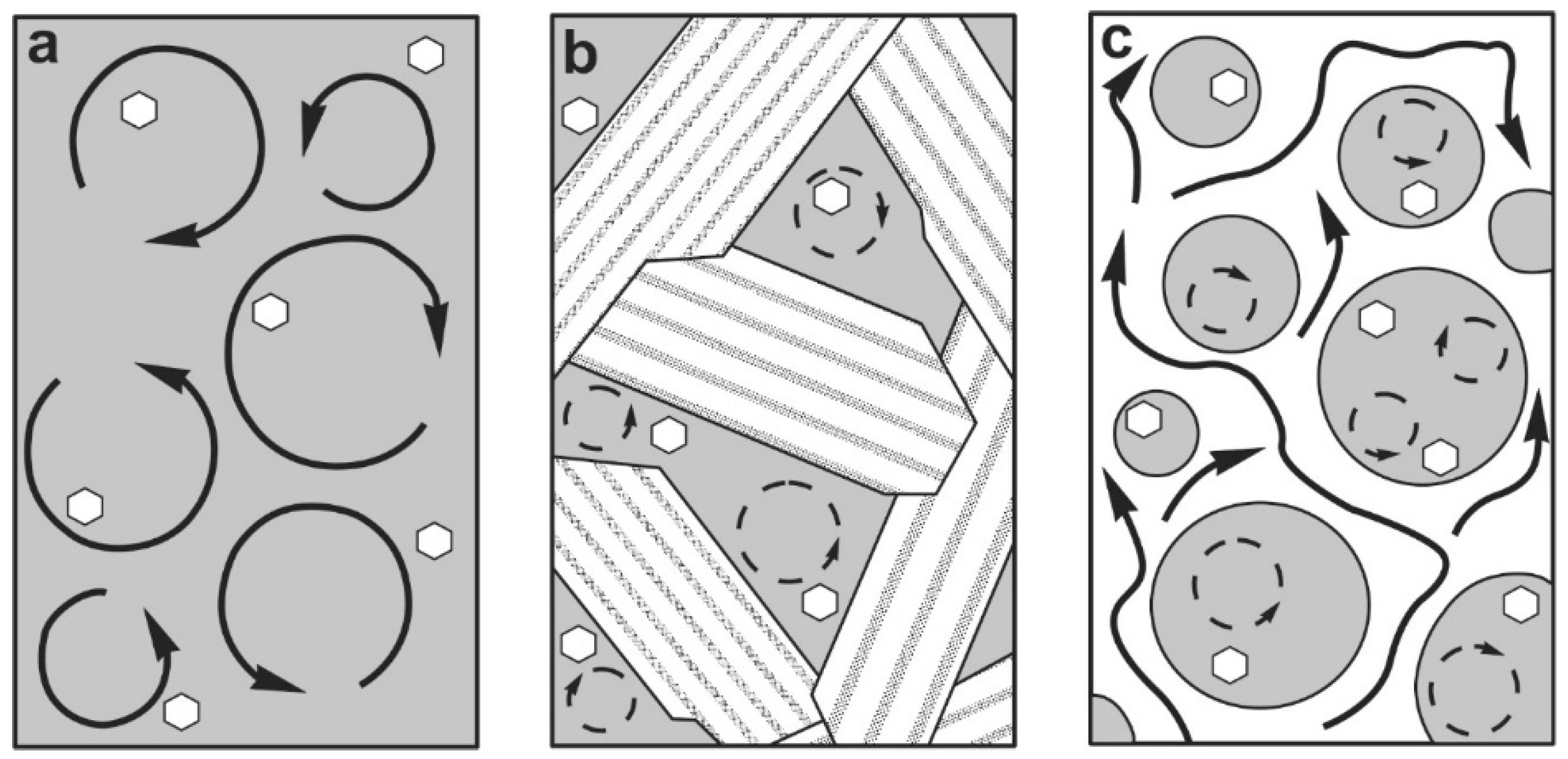
2. Viscosities, Ascent Rates and Flow Patterns of Magma under Natural Conditions
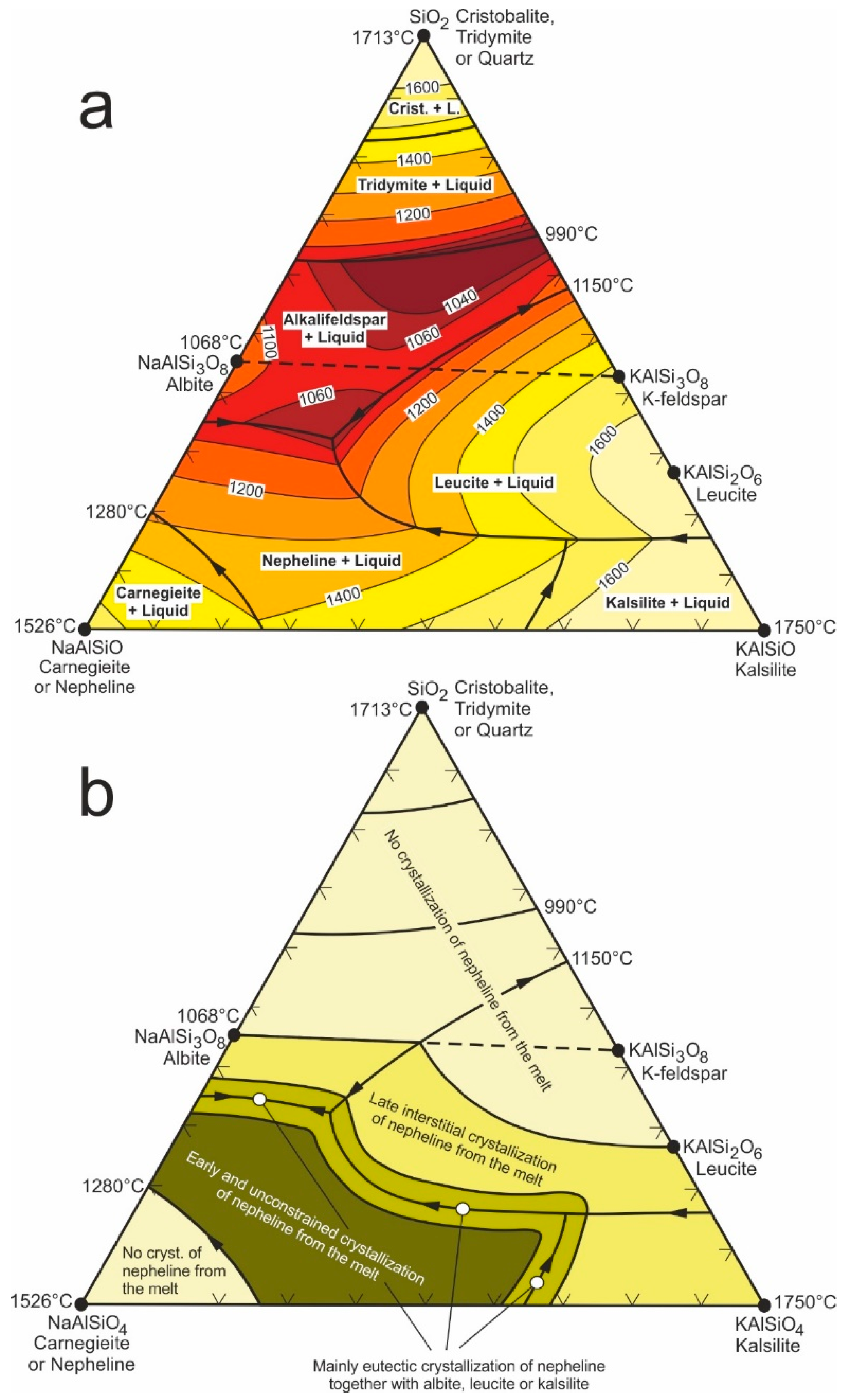
3. Occurrence, Etching Behavior and Chiral Proportions of Nepheline in Alkaline Igneous Rocks
4. Conclusions and Outlook
- Not all the nepheline crystals exhibit chiral etch figures on such section planes. Up to more than 50% of the crystals with suitable orientation in a thin section do not develop chiral etch figures when they are treated with diluted hydrofluoric acid (1% HF aqu. at 20 °C).
- Twinning occurs in magmatic nepheline but is not ubiquitous. In the investigated samples, most nepheline with chiral etch figures is not twinned.
- The investigated samples do not exhibit a strong chiral excess of nepheline. Counting statistics of three of four evaluated samples are well compatible with chiral parity; only one sample shows a slight chiral excess (41.67% L-type vs. 58.33% D-type) but at a rather low level of significance (p-value = 0.20) because of the paucity of countable crystals (15 vs. 21, respectively).
- Enantiomer separation by liquid chromatographic interaction between infiltrating molecular solutions and nepheline with chiral excess has not yet been tested in the laboratory.
- Nepheline bearing rocks are not (yet) known from early Archean terrains. The eldest well-documented nepheline occurrences are about 1 Ga younger than the earliest evidence of life. At the present state of knowledge, this time gap is an obstacle for the validity of the outlined liquid chromatographic hypothesis with nepheline as a stationary phase.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bailey, J.; Chrysostomou, A.; Hough, J.H.; Gledhill, T.M.; McCall, A.; Clark, S.; Ménard, F.; Tamura, M. Circular polarization in star-forming region: Implications for biomolecular homochirality. Science 1998, 281, 672–674. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J. Astronomical sources of circularly polarized light and the origin of homochirality. Orig. Life Evol. Biosph. 2000, 31, 167–183. [Google Scholar] [CrossRef]
- Modica, P.; Meinert, C.; de Marcellus, P.; Nahon, L.; Meierhenrich, U.J.; le Sergeant d’Hendecourt, L. Enantiomeric excesses induced in amino acids by ultraviolet circularly polarized light irradiation of extraterrestrial ice analogs: A possible source of asymmetry for prebiotic chemistry. Astrophys. J. 2014, 787, 1–11. [Google Scholar] [CrossRef]
- Bibring, J.-P.; Rosenbauer, H.; Boenhardt, H.; Ulamec, S.; Biele, J.; Espinasse, S.; Feuerbacher, B.; Gaudon, P.; Hemmerich, B.; Kletzkine, P.; et al. The Rosetta Lander (“Philae”) investigations. Space Sci. Rev. 2007, 128, 205–220. [Google Scholar] [CrossRef]
- Goesmann, F.; Rosenbauer, H.; Roll, R.; Szopa, C.; Raulin, F.; Sternberg, R.; Israel, G.; Meierhenrich, U.; Thiemann, W.; Munoz Caro, G.M. COSAC, the cometary sampling and composition experiment on Philae. Space Sci. Rev. 2007, 128, 257–280. [Google Scholar] [CrossRef]
- Goesmann, F.; Rosenbauer, H.; Bredehöft, J.H.; Cabane, M.; Ehrenfreund, P.; Gautier, Th.; Giri, Ch.; Krüger, H.; leRoy, L.; MacDermott, A.J.; et al. Organic compounds on comet 67P/Churyumov-Gerasimenko revealed by COSAC mass spectrometry. Science 2015, 349. [Google Scholar] [CrossRef] [PubMed]
- Meierhenrich, U. Amino Acids and the Asymmetry of Life; Springer: Berlin/Heidelberg, Germany, 2008; Volume XII, p. 241. ISBN 978-3-540-76885-2. [Google Scholar]
- Meierhenrich, U. Comets and Their Origin. The Tool to Decipher a Comet; Wiley-VCH: Weinheim, Germany, 2015; Volume XXX, p. 352. ISBN 978-3-527-41281-5. [Google Scholar]
- Hejl, E. Are fission tracks in enantiomorphic minerals a key to the emergence of homochirality? J. Mineral. Geochem. 2017, 194, 97–106. [Google Scholar] [CrossRef]
- Morris, R.V.; Vaniman, D.T.; Blake, D.F.; Gellert, R.; Chipera, S.J.; Rampe, E.B.; Ming, D.W.; Morrison, S.M.; Downs, R.T.; Treiman, A.H.; et al. Silicic volcanism on Mars evidenced by tridymite in high-SiO2 sedimentary rock at Gale crater. Proc. Natl. Acad. Sci. USA 2016, 113, 7071–7076. [Google Scholar] [CrossRef] [PubMed]
- Bannister, F.A. A chemical, optical, and X-ray study of nepheline and kaliophilite. Mineral. Mag. 1931, 22, 569–608. [Google Scholar] [CrossRef]
- Buerger, M.J.; Klein, G.E.; Donnay, G. Determination of the crystal structure of Nepheline. Am. Mineral. 1954, 39, 805–818. [Google Scholar]
- Baumhauer, H. Ueber den Nephelin. Zeitschrift für Kristallographie—Cryst. Mater. 1882, 6, 209–216. (In German) [Google Scholar]
- Traube, H. Beiträge zur Kenntnis des Nephelins und des Davyns. N. Jb. Min. Geol. Palaeont. 1895, IX, 466–479. (In German) [Google Scholar]
- Dmitrienko, V.E.; Ishida, K.; Kirfel, A.; Ovchinnikova, E.N. Polarization anisotropy of X-ray atomic factors and ‘forbidden’ resonant reflections. Acta Crystallogr. A 2005, 61, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Kojima, T.; Takata, Y.; Chainani, A.; Lovesey, S.W.; Knight, K.S.; Takeuchi, T.; Oura, M.; Senba, Y.; Ohashi, H.; Shin, S. Determination of structural chirality of berlinite and quartz using resonant X-ray diffraction with circularly polarized X-rays. Phys. Rev. B 2010, 81, 144104. [Google Scholar] [CrossRef]
- Heritsch, H. Die Verteilung von Rechts- und Linksquarzen in Schriftgraniten [in german]. Tschermaks Miner. Petrogr. Mitt. 1953, 3, 115–125. [Google Scholar] [CrossRef]
- Palache, C.; Bermann, G.B.; Frondel, C. Relative frequencies of left and right quartz. In The System of Mineralogy; Frondel, C., Ed.; Wiley: New York, NY, USA, 1962; p. 17. [Google Scholar]
- Frondel, C. Characters of quartz fibers. Am. Mineral. 1978, 63, 17–27. [Google Scholar]
- Kondepudi, D.K.; Kaufmann, R.J.; Singh, N. Chiral symmetry breaking in sodium chlorate crystallization. Science 1990, 250, 275–276. [Google Scholar] [CrossRef] [PubMed]
- Kondepudi, D.K.; Asakura, K. Chiral autocatalysis, spontaneous symmetry breaking, and stochastic behavior. Acc. Chem. Res. 2001, 34, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Lesher, Ch.E.; Spera, F.J. Thermodynamic and transport properties of silicate melts and magma. In The Encyclopedia of Volcanoes, 2nd ed.; Sigurdson, H., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 114–141. [Google Scholar]
- Hess, P.C. Polymer model of silicate melts. Geochim. Cosmochim. Acta 1971, 35, 289–306. [Google Scholar] [CrossRef]
- Hess, P.C. Polymerization model for silicate melts. In Physics of Magmatic Properties; Hargraves, R.B., Ed.; Princeton University Press: Princeton, NJ, USA, 1980; pp. 3–48. [Google Scholar]
- Charles, R.J. The origin of immiscibility in silicate solutions. Phys. Chem. Glasses 1967, 10, 169–178. [Google Scholar]
- Jones, A.R.; Winter, R.; Greaves, G.N.; Smith, I.H. MAS NMR study of soda-lime-silicate glasses with variable degree of polymerisation. J. Non Cryst. Solids 2001, 293–295, 87–92. [Google Scholar] [CrossRef]
- Lee, S.K.; Cody, G.D.; Fei, Y.; Mysen, B.O. Nature of polymerization and properties of silicate melts and glasses at high pressures. Geochim. Cosmochim. Acta 2004, 68, 4189–4200. [Google Scholar] [CrossRef]
- Scarfe, C.M.; Mysen, B.O.; Virgo, D. Pressure dependence of the viscosity of silicate melts. In Magmatic Processes: Physicochemical Principles; Mysen, B.O., Ed.; Special Publication no.1; Geochemical Society: Boston, MA, USA, 1987; pp. 59–67. [Google Scholar]
- Dawson, J.B.; Pinkerton, H.; Norton, G.E.; Pyle, D.M. Physicochemical properties of alkali carbonatite lavas. Data from the 1988 eruption of Oldoinyo Lengai, Tanzania. Geology 1990, 18, 260–263. [Google Scholar] [CrossRef]
- Shaw, H.R. The fracture mechanisms of magma transport from the mantle to the surface. In Physics of Magmatic Processes; Hargraves, R.B., Ed.; Princeton University Press: Princeton, NJ, USA, 1980; pp. 201–264. [Google Scholar]
- Giordano, D.; Russel, J.K.; Dingwell, D.B. Viscosity of magmatic liquids: A model. Earth Planet. Sci. Lett. 2008, 271, 123–134. [Google Scholar] [CrossRef]
- Stasiuk, M.V.; Jaupart, C. Lava flow shapes and dimensions as reflections of magma system conditions. J. Volcanol. Geotherm. Res. 1997, 78, 31–50. [Google Scholar] [CrossRef]
- Schairer, J.F.; Bowen, N.L. Preliminary report on equilibrium-relations between feldspathoids, alkali-feldspars, and silica. Trans. Am. Geophys. Union 1935, 16, 325–328. [Google Scholar] [CrossRef]
- Schairer, J.F. The alkali-feldspar joint in the system NaAlSiO4-KAlSiO4-SiO2. J. Geol. 1950, 58, 512–517. [Google Scholar] [CrossRef]
- Hamilton, D.L.; MacKenzie, W.S. Phase equilibrium studies in the system NaAlSiO4 (nepheline)-KAlSiO4 (kalsilite). Mineral. Mag. 1965, 34, 214–231. [Google Scholar] [CrossRef]
- Winter, J.D. An Introduction to Igneous and Metamorphic Petrology; Prentice Hall: Upper Saddle River, NJ, USA, 2001; p. 699. ISBN 978-0132403429. [Google Scholar]
- Le Bas, M.J. Carbonatite-Nephelinite Volcanism; An African Case History; John Wiley: London, UK, 1977; p. 362. ISBN 978-0471994227. [Google Scholar]
- Johnson, R.L. The Shawa and Dorowa carbonatite complexes, Rhodesia. In Carbonatites; Tuttle, O.F., Gittins, J., Eds.; John Wiley: New York, NJ, USA, 1966; pp. 205–224. [Google Scholar]
- Garson, M.S. Carbonatites in Southern Malawi; Ministry of Natural Resources Geological Survey Department: Lilongwe, Malawi, 1965.
- Garson, M.S. The Tundulu carbonatite ring complex in southern Nyasaland. Mem. Nyasaland Geol. Surv. 1962, 2, 248. [Google Scholar]
- Kresten, P.; Troll, V.R. The Alnö Carbonatite Complex, Central Sweden; Springer: Berlin/Heidelberg, Germany, 2018; Volume XXXI, p. 196. ISBN 978-3-319-9022-1. [Google Scholar]
- Nkoumbou, C.; Déruelle, B.; Velde, D. Petrology of Mt. Etinde nephelinite series. J. Petrol. 1995, 36, 373–395. [Google Scholar] [CrossRef]
- Woolley, A.R. Alkaline Rocks and Carbonatites of the World. Part 3: Africa; London Geological Society: London, UK, 2001; p. 372. ISBN 1-86239-083-5. [Google Scholar]
- Paslick, C.; Halliday, A.N.; James, D.; Dawson, J.B. Enrichment of the continental lithosphere by OIB melts: Isotopic evidence from the volcanic province of northern Tanzania. Earth Planet. Sci. Lett. 1995, 130, 109–126. [Google Scholar] [CrossRef]
- Dawson, J.B. Sodium carbonatite intrusions from Oldoinyo Lengai, Tanzania: Implications for carbonatite complex genesis. In Carbonatites Genesis and Evolution; Bell, K., Ed.; Unwin Hyman: London, UK, 1989; pp. 255–277. [Google Scholar]
- Klaudius, J.; Keller, J. Peralkaline silicate lavas at Oldoinyo Lengai, Tanzania. Lithos 2001, 91, 173–190. [Google Scholar] [CrossRef]
- Maarten de Moor, J.; Fischer, T.P.; King, P.L.; Botcharnikov, R.E.; Hervig, R.L.; Hilton, D.R.; Barry, P.H.; Mangasini, F.; Ramirez, C. Volatile-rich silicate melts from Oldoinyo Lengai volcano (Tanzania): Implications for carbonatite genesis and eruptive behavior. Earth Planet. Sci. Lett. 2013, 361, 379–390. [Google Scholar] [CrossRef]
- Bühn, B.; Trumbull, R.B. Comparison of petrogenetic signatures between mantle-derived alkali silicate intrusives with and without associated carbonatite, Namibia. Lithos 2003, 66, 201–221. [Google Scholar] [CrossRef]
- Cox, K.G.; Bell, J.D.; Pankhurst, R.J. Interpretation of Igneous Rocks; George Allen & Unwin: London, UK, 1979; p. 450. [Google Scholar]
- Baumhauer, H. Ueber die Krystallisation des Nephelin. Zeitschrift für Kristallographie—Cryst. Mater. 1891, 18, 611–618, in German. [Google Scholar]
- Hejl, E. First observation of etched uranium fission tracks in nepheline by Hermann Traube (1895)? Mitt. Österr. Miner. Ges. 2017, 162, 83–90. [Google Scholar]
- Blichert-Toft, J.; Arndt, N.T.; Ludden, J.N. Precambrian alkaline magmatism. Lithos 1996, 37, 97–111. [Google Scholar] [CrossRef]
- Ohtomo, Y.; Kakegawa, T.; Ishida, A.; Nagase, T.; Rosing, M.T. Evidence for biogenic graphite in early Archean Isua metasedimentary rocks. Nat. Geosci. 2014, 7, 25–28. [Google Scholar] [CrossRef]
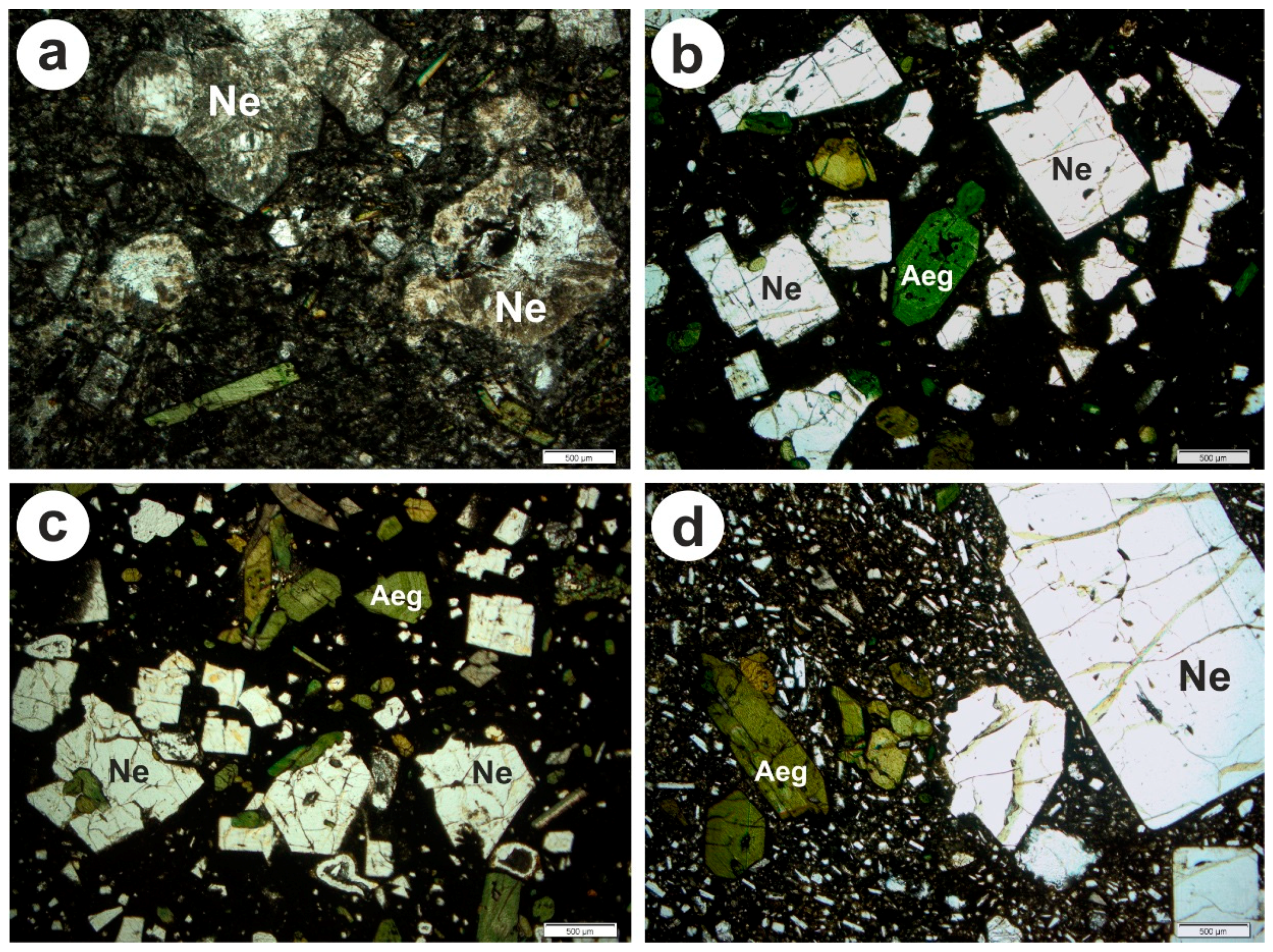
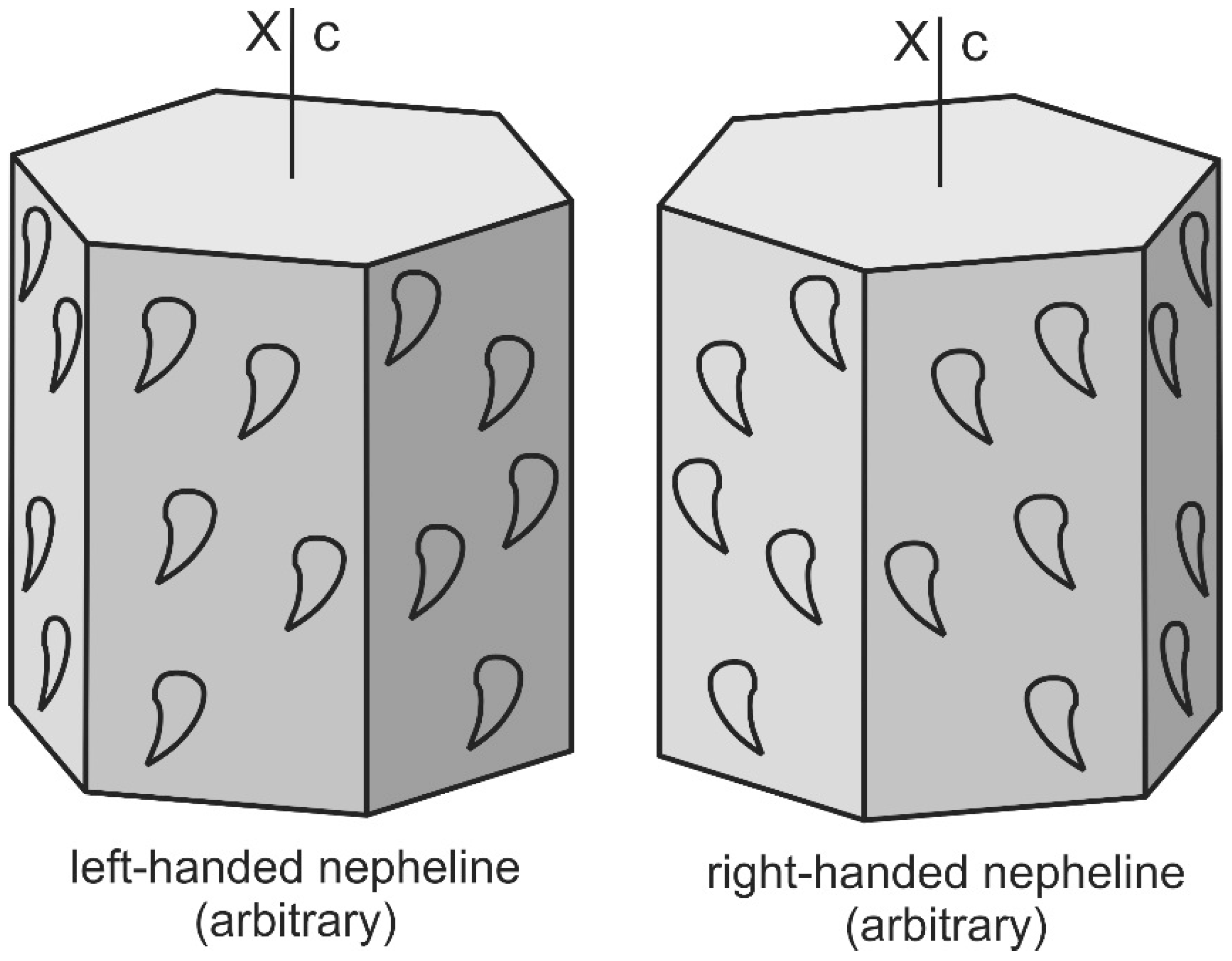
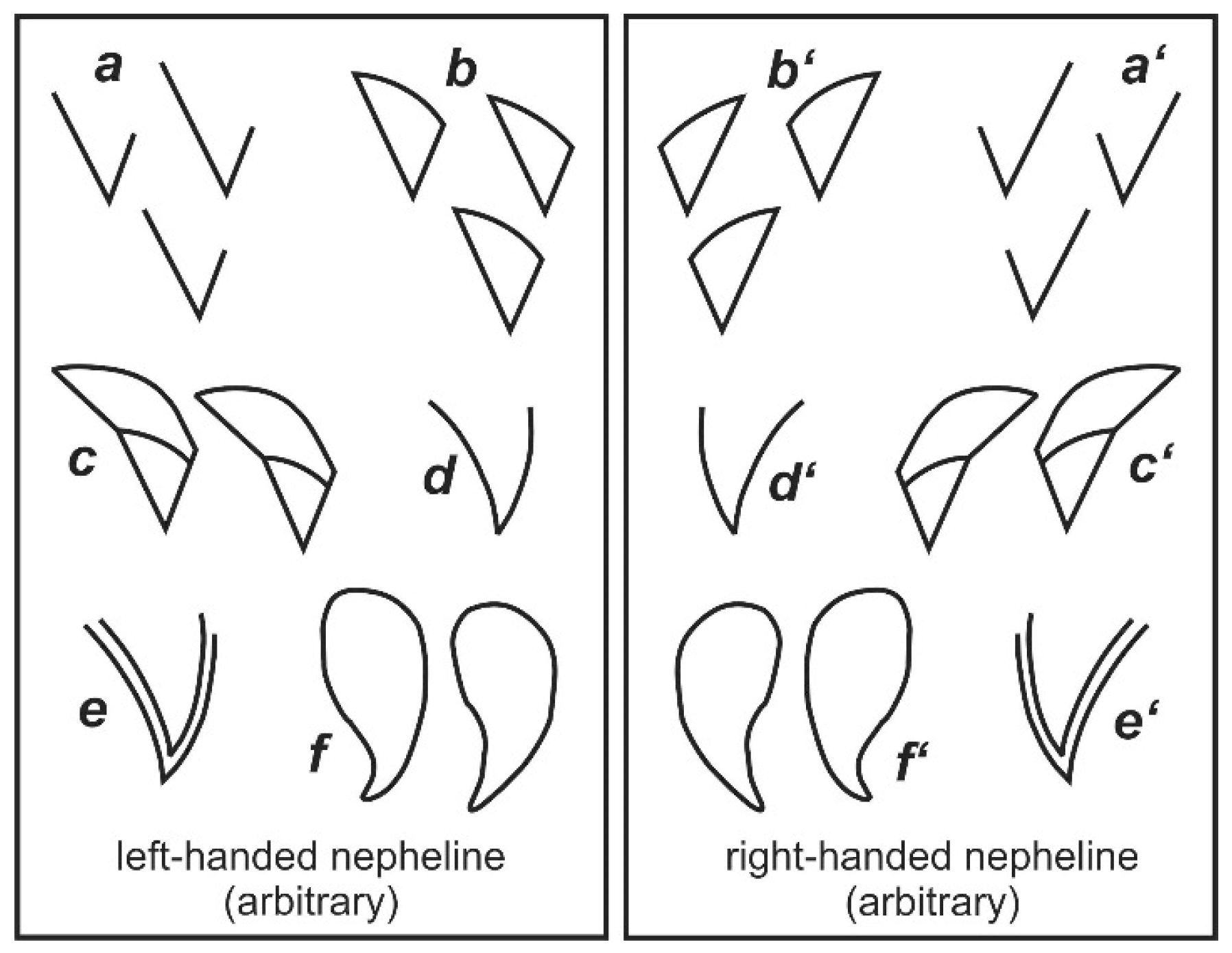
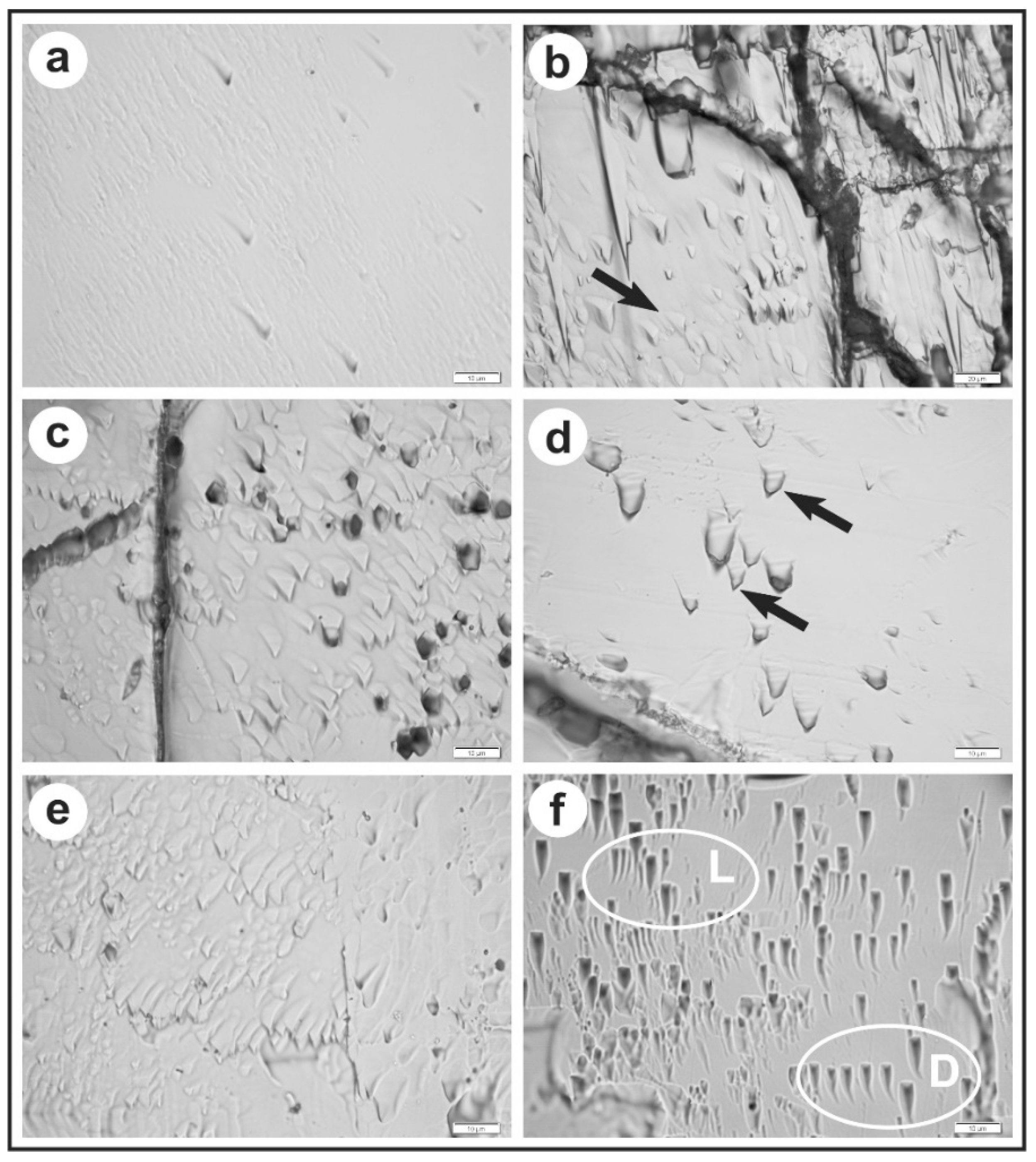
| No. | Sample Code | Rock Type | Location | Coordinates | Ref. |
|---|---|---|---|---|---|
| 1 | BM.1953, 133 (22) | Phonolite | Homa Montain, Kenya | 0°23′ S; 34°30′ E | [37] |
| 2 | BM.1965, P19 (6) | Phonolitic nephelinite | Dorowa compl., Zimbabwe | 19°04′ S; 31°45′ E | [38] |
| 3 | BM.1968, P37 (401) | Phonolite | Namangali, Malawi | 15°49′ S; 35°35′ E | [39] |
| 4 | BM.1980, P31 (2) | Aegirine biotite phonol. | Tundulu complex, Malawi | 15°32′ S; 35°48′ E | [40] |
| 5 | BM.1980, P31 (24) | Nephelinite | Tundulu complex, Malawi | 15°32′ S; 35°48′ E | [40] |
| 6 | BM.1980, P31 (27) | Phonolite | Tundulu complex, Malawi | 15°32′ S; 35°48′ E | [40] |
| 7 | BM.1981, P14 (440) | Nepheline carbonatite | Alnö, Sweden | 62°24′N; 17°28′ E | [41] |
| 8 | BM.1981, P3 | Phonolitic nephelinite | Limbe (Victoria), Cameroon | 4°04°N; 9°08′ E | [42,43] |
| 9 | BM.1995, P6 (40) | Phonolitic nephelinite | Kerimasi, Tanzania | 2°52′ S; 35°57′ E | [44] |
| 10 | BM.1995, P6 (43) | Phonolitic nephelinite | Kerimasi, Tanzania | 2°52′ S; 35°57′ E | [44] |
| 11 | BM.2004, P12 (28) | Phonolitic nephelinite | Oldoinyo Lengai, Tanzania | 2°46′ S; 35°55′ E | [45,46,47] |
| 12 | BM.2004, P12 (75) | Phonolitic nephelinite | Oldoinyo Lengai, Tanzania | 2°46′ S; 35°55′ E | [45,46,47] |
| 13 | KF 85 | Nepheline syenite | Kalkfeld, Namibia | 20°48′ S; 16°07′ E | [48] |
| 14 | KF 88 | Nepheline syenite | Kalkfeld, Namibia | 20°48′ S; 16°07′ E | [48] |
| No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Major elements (wt%) | ||||||||||||
| SiO2 | 52.04 | 45.71 | 53.82 | 51.45 | 34.99 | 46.87 | 18.75 | 45.25 | 47.65 | 46.49 | 48.32 | 45.67 |
| TiO2 | 0.52 | 0.65 | 0.75 | 0.42 | 3.10 | 1.22 | 1.31 | 1.11 | 1.21 | 1.48 | 1.06 | 1.02 |
| Al2O3 | 19.95 | 16.03 | 19.89 | 19.06 | 10.32 | 19.24 | 8.89 | 19.05 | 15.92 | 17.51 | 17.17 | 17.05 |
| Fe2O3 | 6.28 | 7.46 | 3.81 | 5.71 | 11.05 | 5.93 | 9.17 | 6.74 | 9.68 | 8.82 | 7.52 | 7.27 |
| MnO | 0.30 | 0.15 | 0.19 | 0.44 | 0.19 | 0.21 | 0.30 | 0.37 | 0.28 | 0.24 | 0.22 | 0.21 |
| MgO | 0.33 | 3.60 | 0.88 | 0.16 | 14.72 | 1.29 | 1.30 | 1.23 | 1.07 | 1.63 | 0.42 | 0.42 |
| CaO | 1.97 | 7.64 | 2.16 | 1.63 | 12.18 | 3.82 | 33.46 | 6.32 | 8.07 | 6.93 | 3.97 | 5.26 |
| Na2O | 6.40 | 11.55 | 9.96 | 7.51 | 3.00 | 8.68 | 2.95 | 8.10 | 8.54 | 9.29 | 10.05 | 9.51 |
| K2O | 7.19 | 1.62 | 5.72 | 7.97 | 3.21 | 7.43 | 1.56 | 6.09 | 5.31 | 4.99 | 5.88 | 5.71 |
| P2O5 | 0.08 | 1.21 | 0.20 | 0.17 | 1.12 | 0.25 | 1.89 | 0.16 | 0.34 | 0.46 | 0.14 | 0.61 |
| SO3 | 0.12 | 0.44 | 0.25 | 0.10 | 0.39 | 0.11 | 0.21 | 0.29 | 0.05 | 0.11 | 0.22 | 0.17 |
| F | 0.17 | 0.20 | 0.20 | 0.22 | 0.43 | 0.31 | 0.18 | 0.32 | 0.18 | 0.21 | 0.27 | 1.25 |
| L.O.I. | 4.77 | 3.64 | 1.66 | 4.35 | 4.83 | 3.70 | 18.91 | 3.51 | 1.16 | 1.26 | 4.04 | 5.06 |
| Total | 100.12 | 99.90 | 99.49 | 99.19 | 99.53 | 99.06 | 98.88 | 98.54 | 99.46 | 99.42 | 99.28 | 99.21 |
| Trace elements (ppm) | ||||||||||||
| Ba | 2272 | 980 | 705 | 286 | 2986 | 1942 | 1055 | 3370 | 1760 | 1333 | 1716 | 1923 |
| Ce | 248 | 39 | 273 | 375 | 232 | 185 | 1284 | 212 | 270 | 246 | 249 | 115 |
| Cl | 626 | 146 | 1739 | 90 | 173 | 886 | 71 | 2619 | 231 | 1184 | 1491 | 319 |
| Co | 5 | 20 | 8 | 4 | 57 | 8 | 17 | 8 | 9 | 15 | 9 | 8 |
| Cr | 8 | 32 | 28 | 8 | 384 | 28 | 26 | 14 | 22 | 70 | 10 | 3 |
| Ga | 40 | 20 | 25 | 42 | 13 | 26 | 10 | 26 | 32 | 28 | 30 | 33 |
| La | 122 | 20 | 169 | 167 | 123 | 112 | 647 | 175 | 161 | 143 | 156 | 98 |
| Nb | 278 | 55 | 193 | 749 | 99 | 202 | 528 | 343 | 190 | 156 | 159 | 132 |
| Nd | 41 | 11 | 51 | bdl. | 84 | 22 | 525 | 4 | 69 | 83 | 65 | 15 |
| Ni | 9 | 26 | 13 | 10 | 399 | 20 | 13 | 9 | 13 | 14 | 8 | 8 |
| Pb | 37 | 24 | 20 | 30 | 5 | 6 | bdl. | bdl. | 29 | 22 | 25 | 8 |
| Rb | 186 | 36 | 165 | 222 | 104 | 160 | 14 | 192 | 131 | 95 | 125 | 97 |
| Sr | 777 | 837 | 511 | 871 | 2267 | 2847 | 6205 | 6184 | 1539 | 1660 | 2201 | 1862 |
| Th | 89 | bdl. | 33 | 107 | 5 | 9 | 38 | bdl. | 29 | 26 | 28 | 7 |
| U | 13 | bdl. | bdl. | 47 | bdl. | 15 | bdl. | 10 | 5 | bdl. | 7 | 8 |
| V | 87 | 101 | 42 | 31 | 258 | 91 | 241 | 242 | 189 | 146 | 179 | 172 |
| W | 11 | 16 | 17 | 21 | 7 | 19 | 12 | 17 | 14 | 11 | 19 | 13 |
| Y | 29 | 12 | 30 | 45 | 25 | 23 | 59 | 26 | 33 | 36 | 31 | 45 |
| Zn | 246 | 114 | 153 | 338 | 101 | 145 | 91 | 210 | 203 | 166 | 187 | 198 |
| Zr | 985 | 168 | 882 | 4512 | 287 | 815 | 312 | 875 | 587 | 408 | 494 | 516 |
| No. | Remarks | Counting Statistics | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Indistinct | Twins | L-Type | D-Type | Total | |||||||
| n | % | n | % | n | % | n | % | n | % | ||
| 1 | fine-grained matrix with few altered phenocrysts of nepheline and sanidine | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- |
| 2 | fine-grained matrix with some small nepheline phenocrysts; no etch figures | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- |
| 3 | sanidine phenocrysts (>5 mm) in fine-grained matrix; no neph. phenocrysts | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- |
| 4 | altered phenocrysts of sanidine and aegirine in a fine-grained matrix | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- |
| 5 | few large nepheline phenocrysts (>5 mm) in a cryptocrystalline matrix | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- |
| 6 | cryptocryst. matrix with altered pheno-crysts of leucite and few nepheline | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- |
| 7 | medium-grained fabric of carbonate, aegirine, and altered nepheline | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- |
| 8 | dark cryptocrystalline matrix with leucite, nosean, and few nepheline | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- |
| 9 | many fresh nepheline (>1 mm) and some aegirine in cryptocryst. matrix | 53 | 44.20 | 13 | 10.80 | 28 | 23.30 | 26 | 21.70 | 120 | 100 |
| 10 | many fresh nepheline (>1 mm) and some aegirine in cryptocryst. matrix | 37 | 46.25 | 7 | 8.75 | 15 | 18.75 | 21 | 26.25 | 80 | 100 |
| 11 | fresh and big nepheline (1–7 mm) and some aegirine in fine-grained matrix | 23 | 46.00 | 6 | 12.00 | 11 | 22.00 | 10 | 20.00 | 50 | 100 |
| 12 | slightly alterd nepheline (1–5 mm) and aegirine in a dark cryptocryst. matrix | 25 | 50.00 | 5 | 10.00 | 9 | 18.00 | 11 | 22.00 | 50 | 100 |
| 13 | porphyric K-feldspar in a groundmass of medium-grained feldspar and biotite | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- |
| 14 | equigranular fabric of perthitic feldspar with few interstitial nepheline | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hejl, E.; Finger, F. Chiral Proportions of Nepheline Originating from Low-Viscosity Alkaline Melts. A Pilot Study. Symmetry 2018, 10, 410. https://doi.org/10.3390/sym10090410
Hejl E, Finger F. Chiral Proportions of Nepheline Originating from Low-Viscosity Alkaline Melts. A Pilot Study. Symmetry. 2018; 10(9):410. https://doi.org/10.3390/sym10090410
Chicago/Turabian StyleHejl, Ewald, and Friedrich Finger. 2018. "Chiral Proportions of Nepheline Originating from Low-Viscosity Alkaline Melts. A Pilot Study" Symmetry 10, no. 9: 410. https://doi.org/10.3390/sym10090410
APA StyleHejl, E., & Finger, F. (2018). Chiral Proportions of Nepheline Originating from Low-Viscosity Alkaline Melts. A Pilot Study. Symmetry, 10(9), 410. https://doi.org/10.3390/sym10090410




