Topological Properties of Crystallographic Structure of Molecules
Abstract
1. Introduction
2. Research Aim
3. Applications of Topological Indices
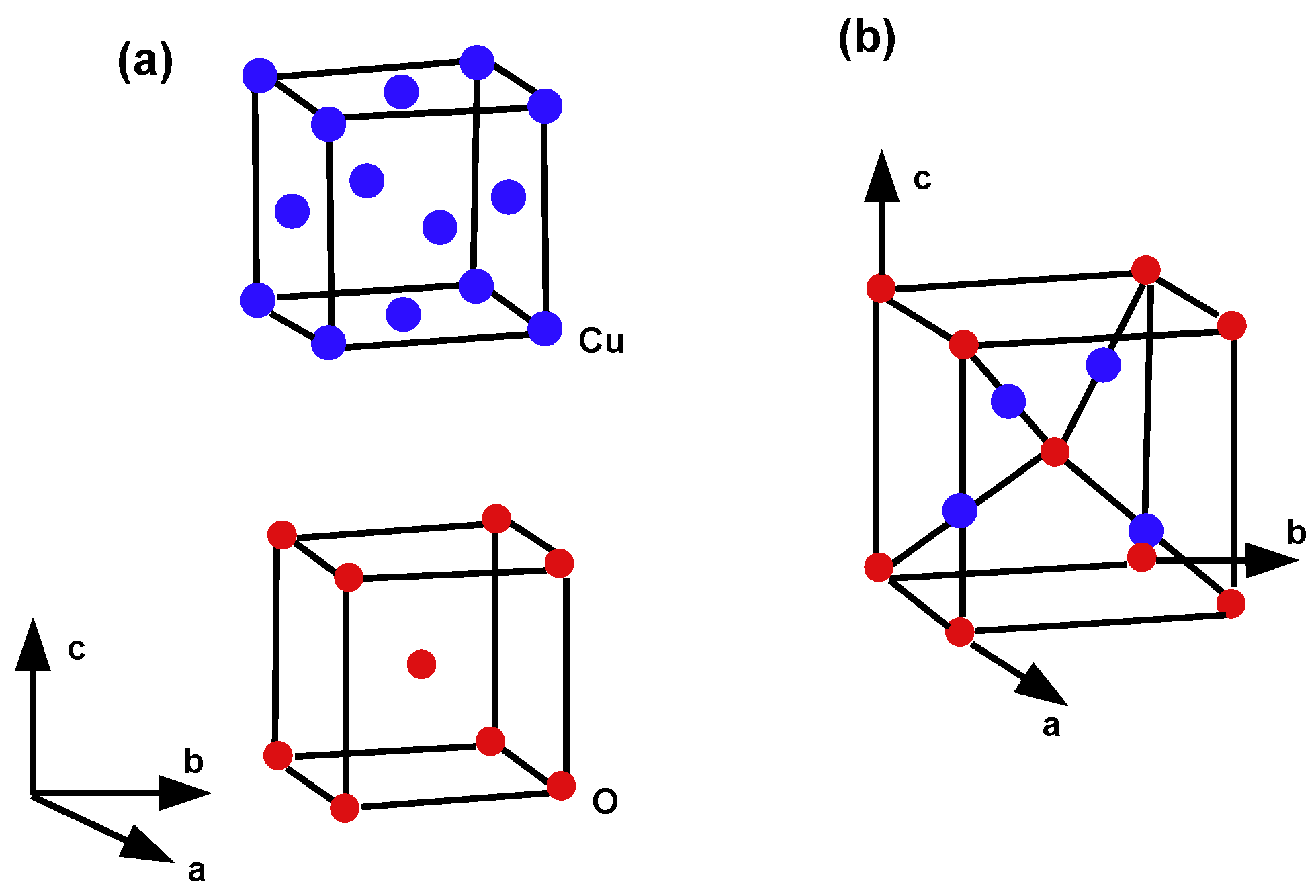
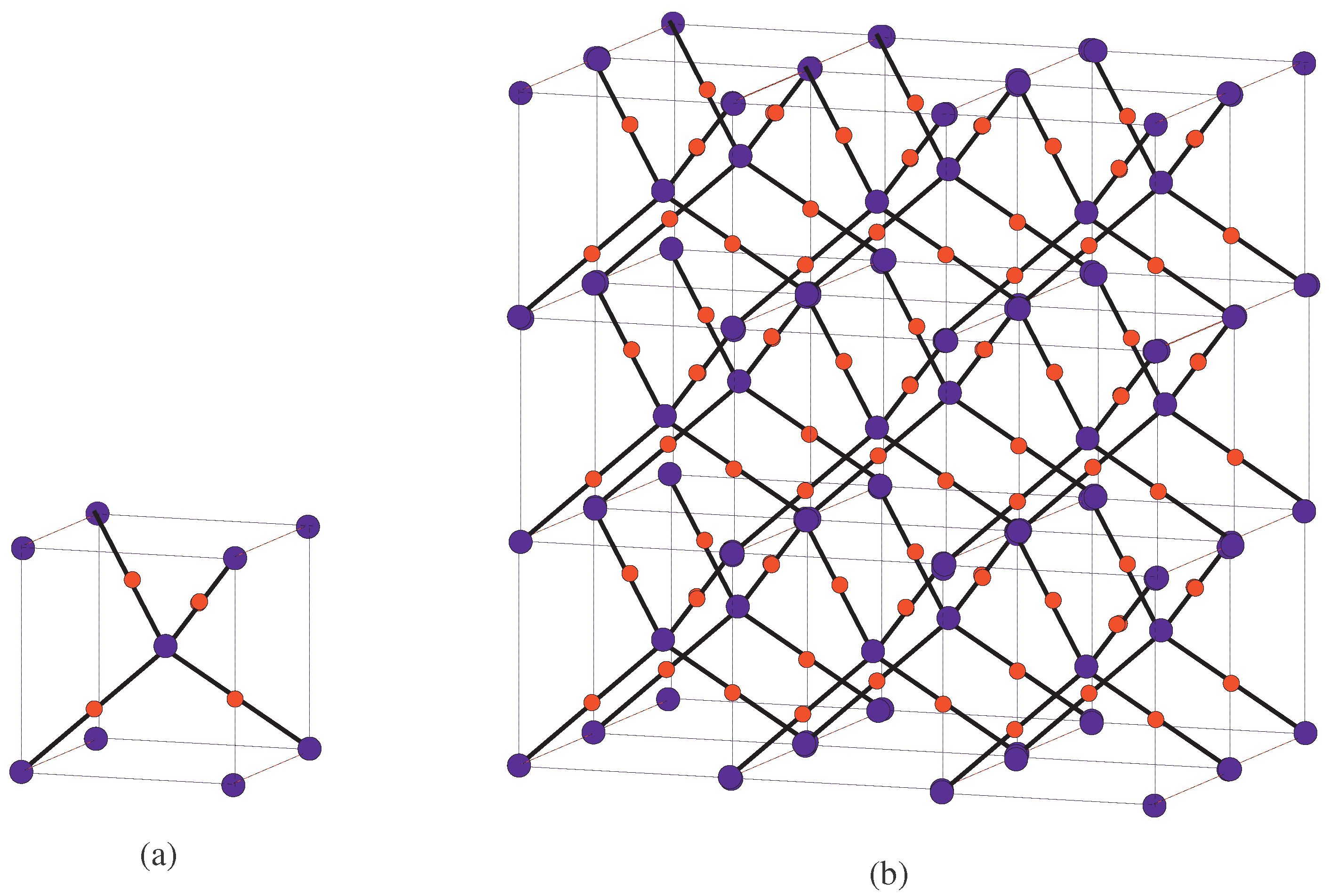
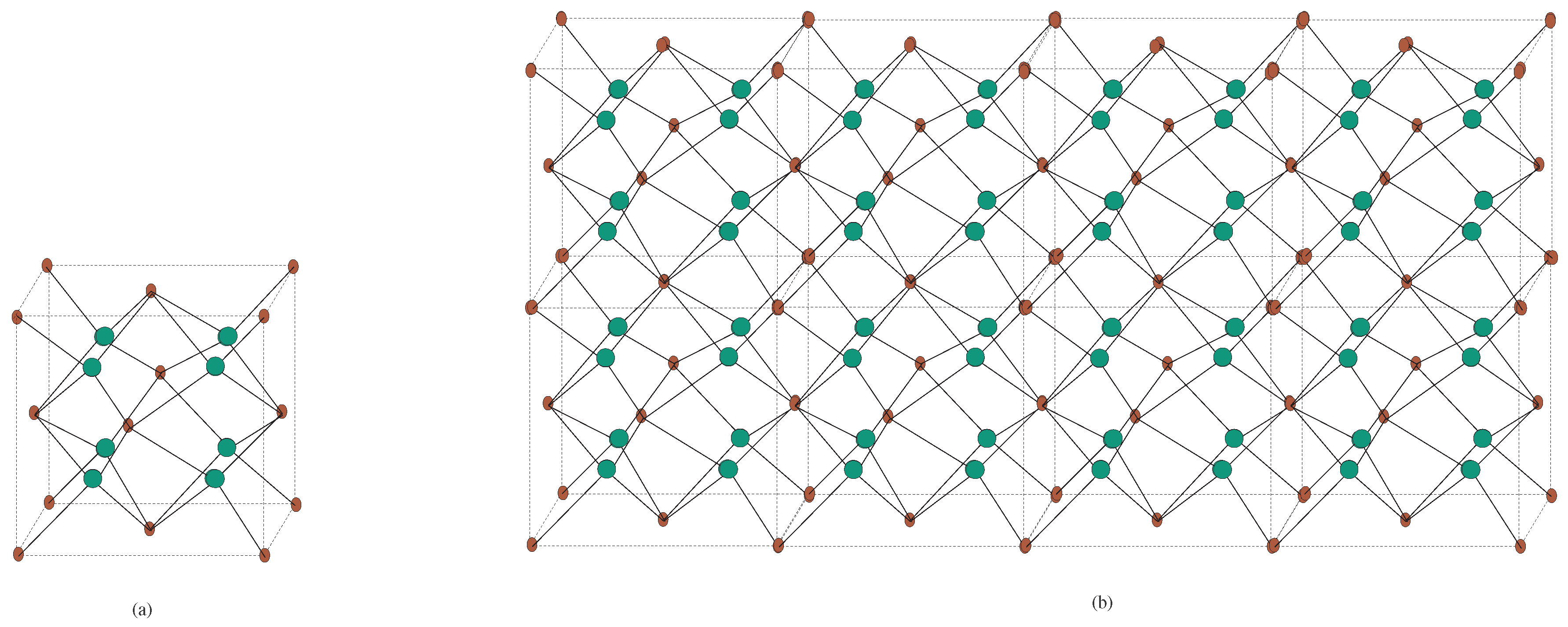
4. Crystallographic Structure of the Molecule
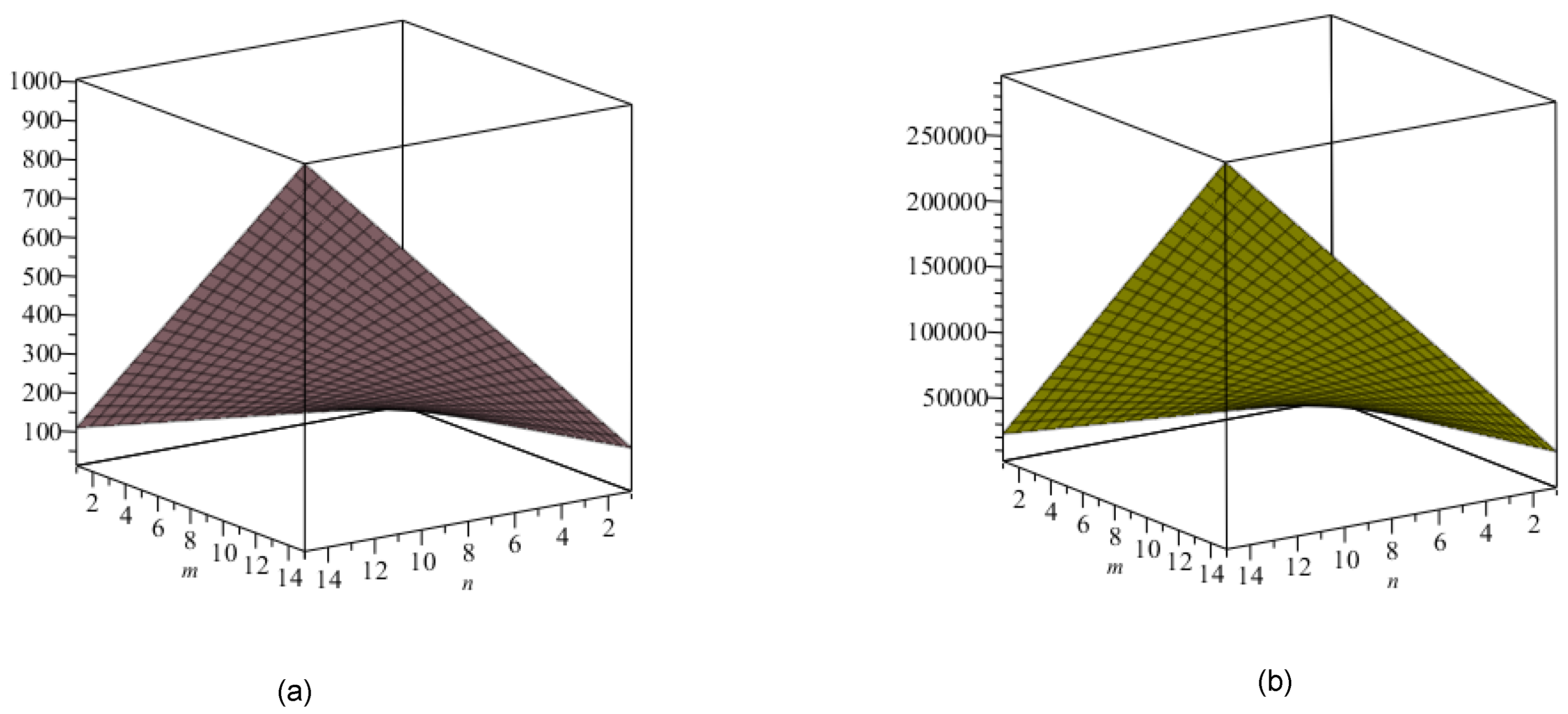
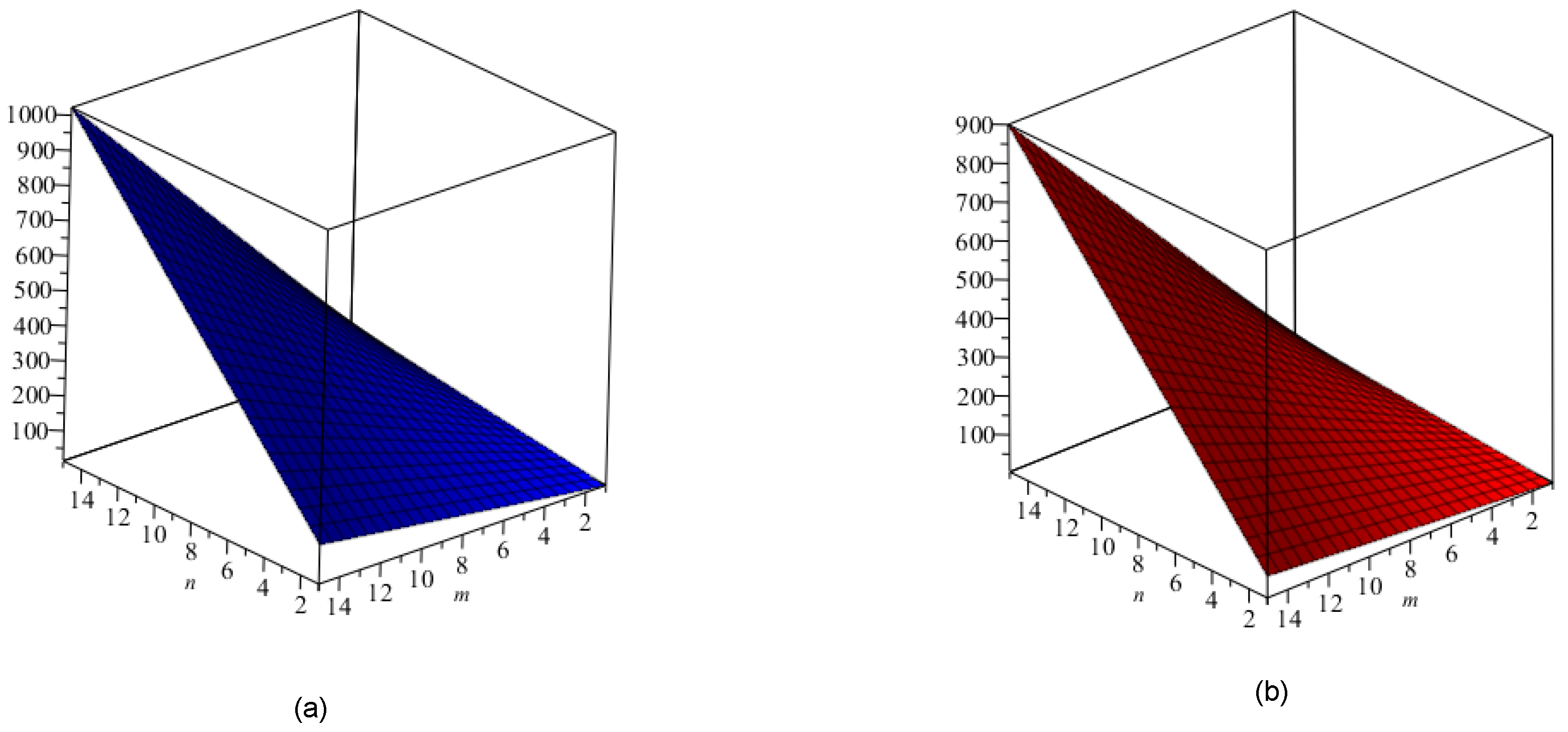
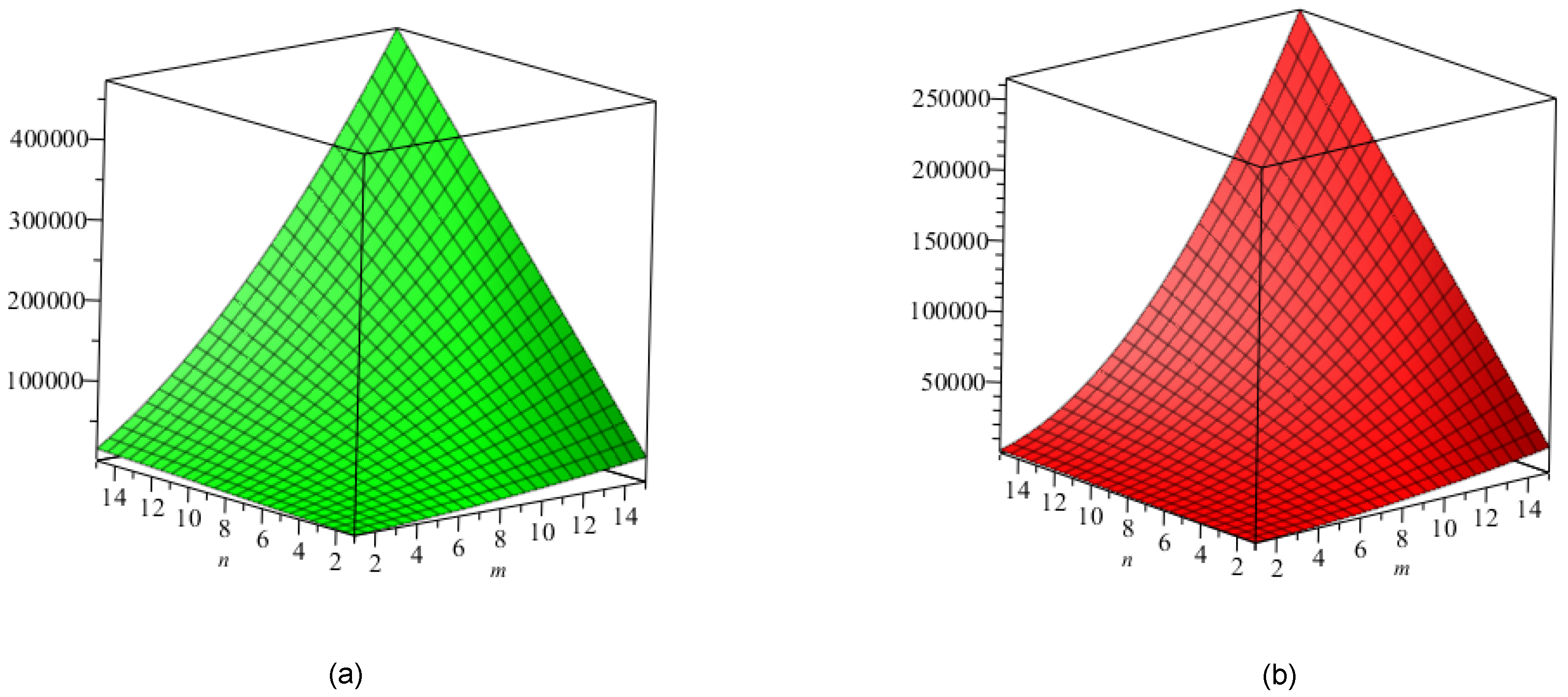
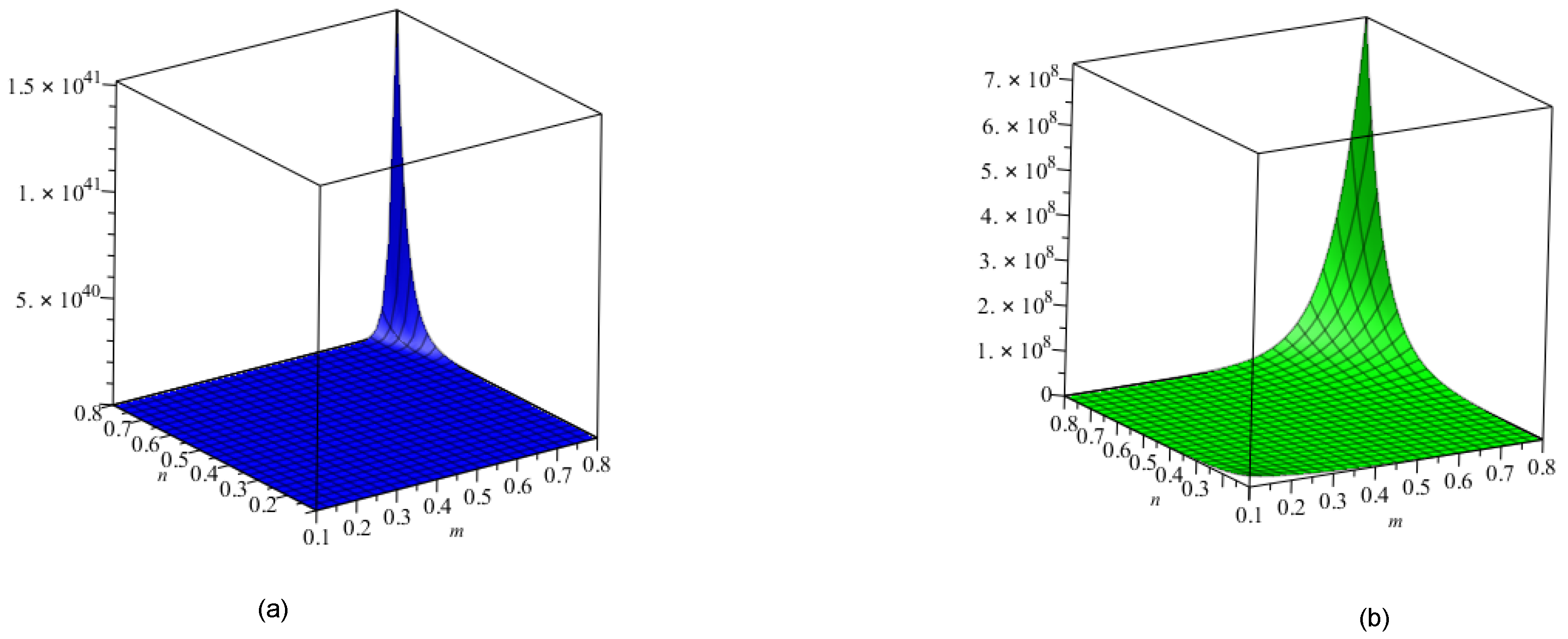
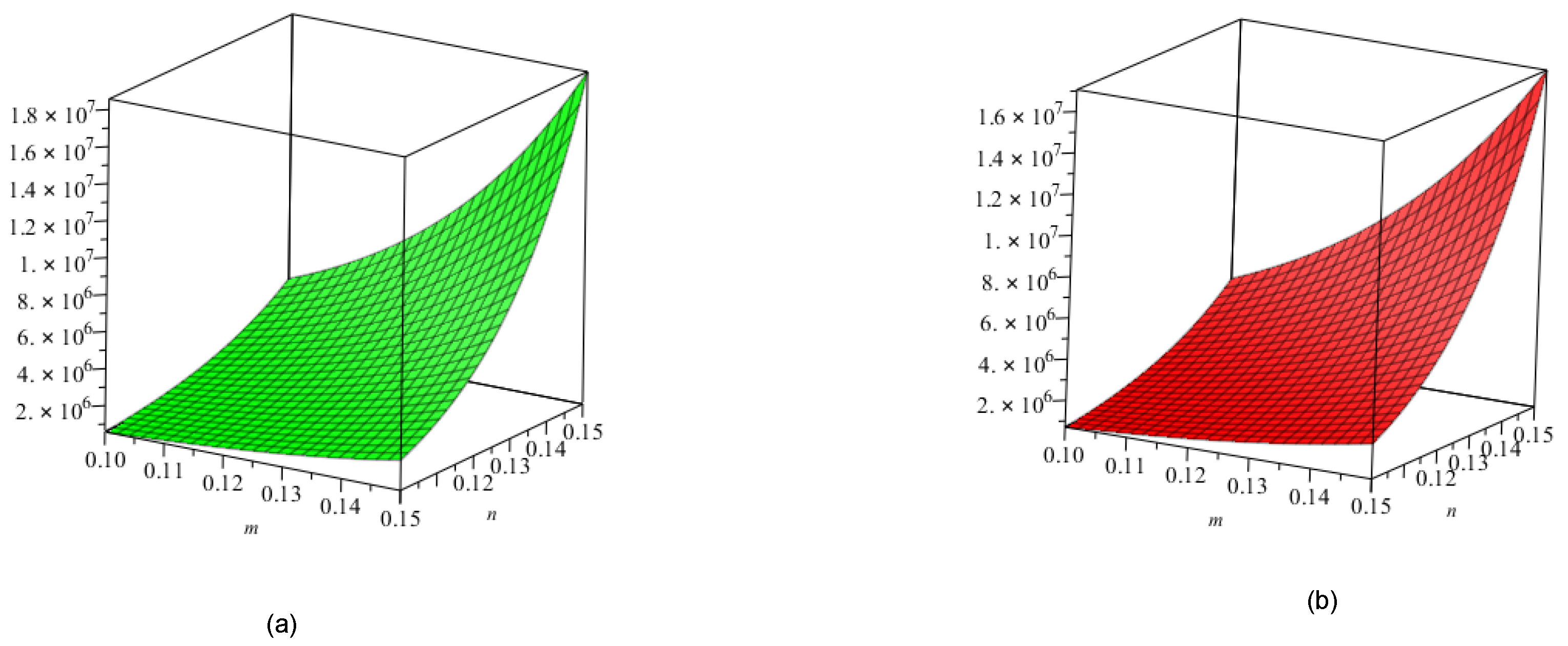
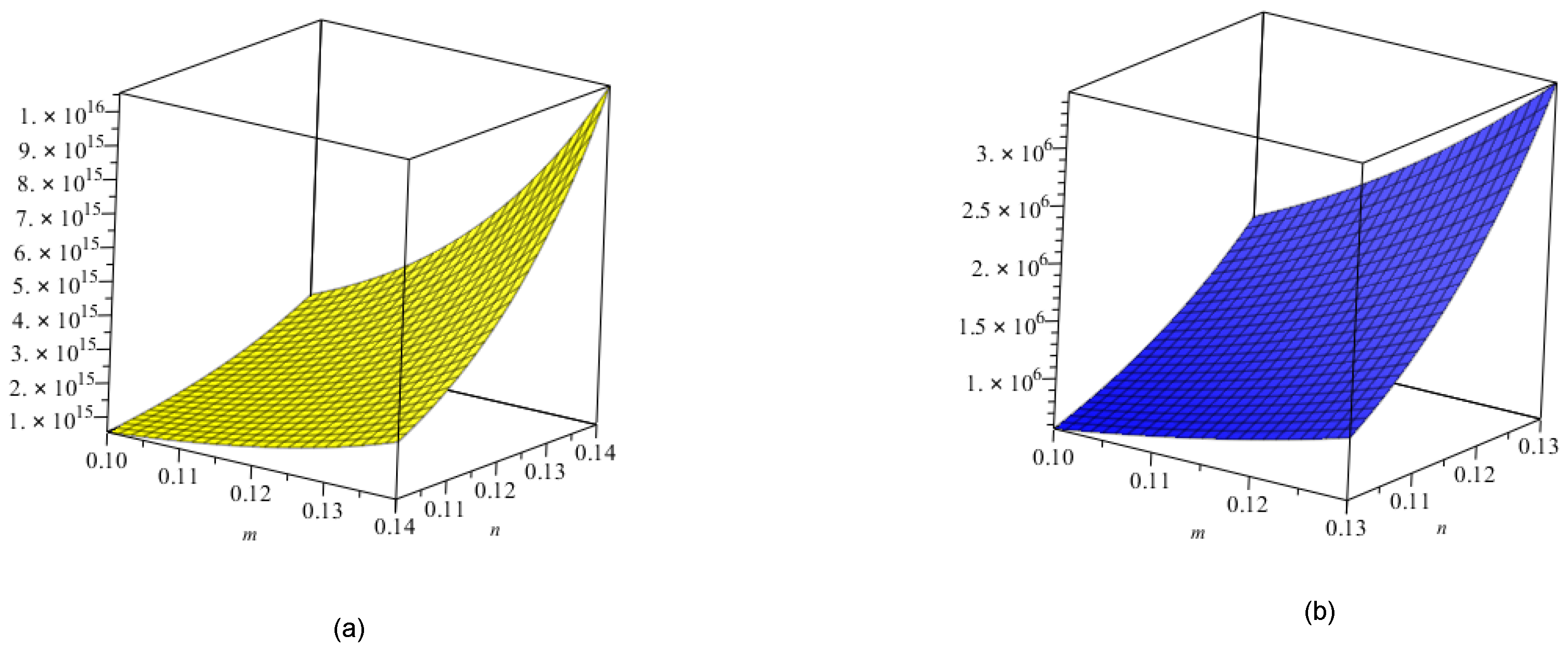
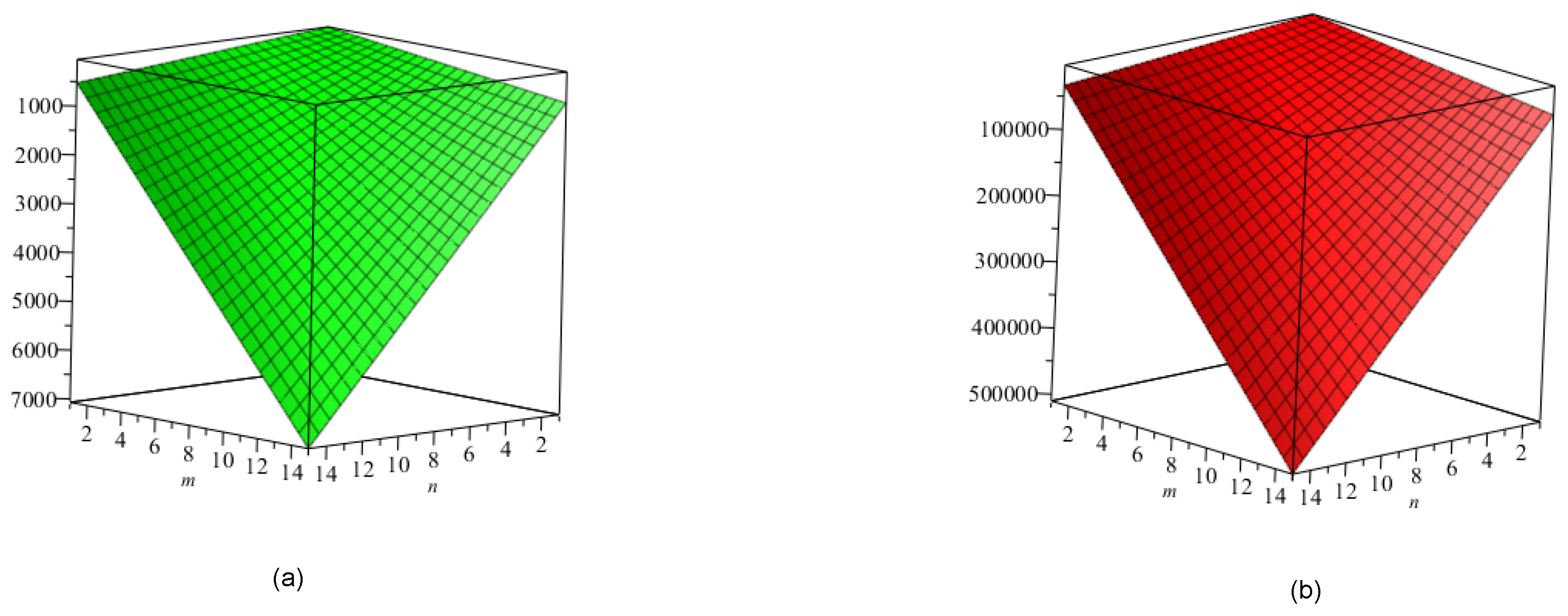
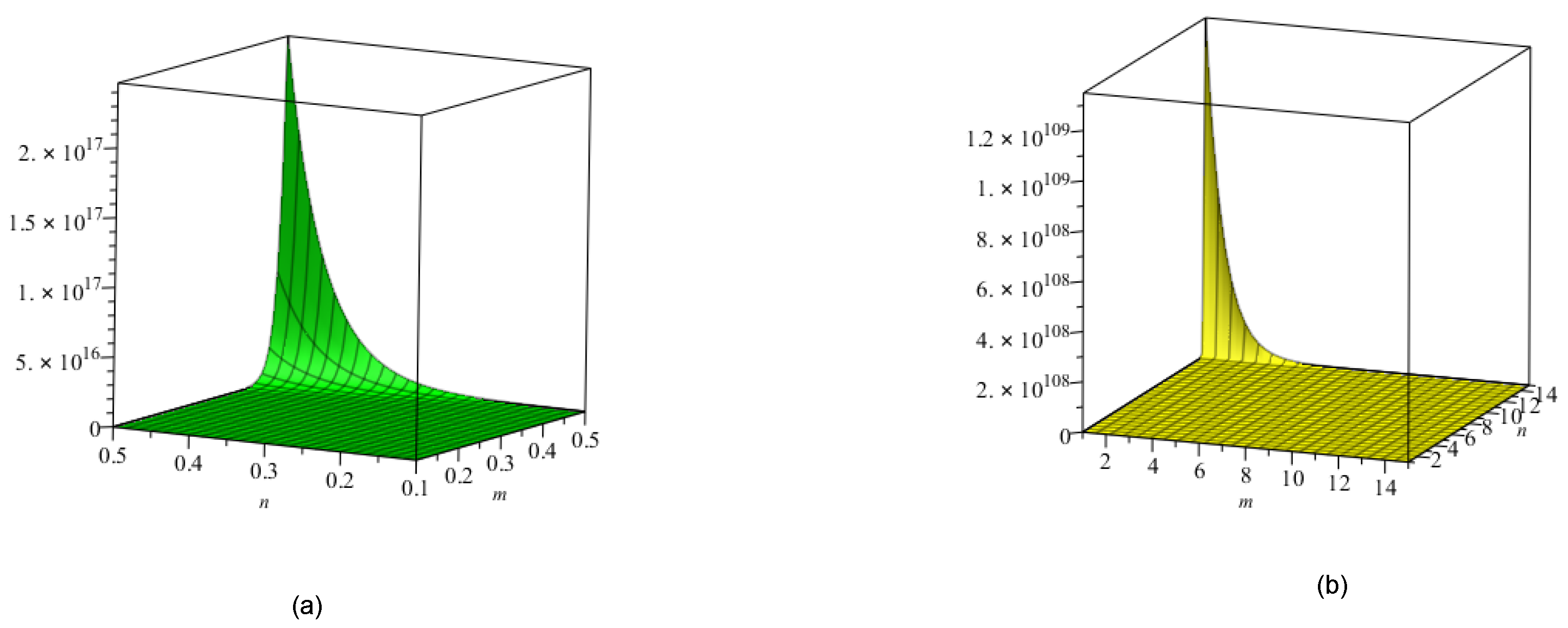
5. Crystal Structure of Titanium Difluoride
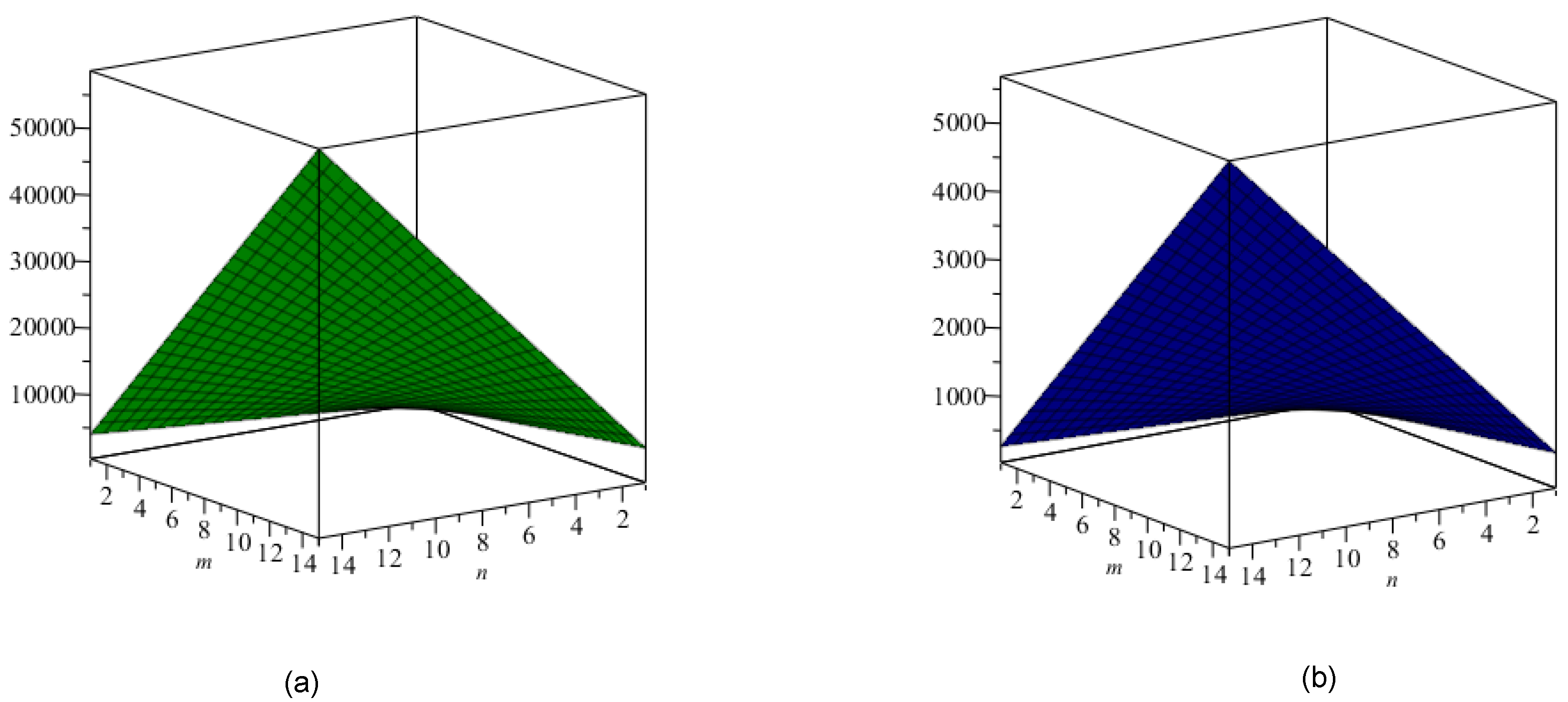
6. Discussion
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Farahani, M.R. Computing fourth atom bond connectivity index of V-phenylenic nanotubes and nanotori. Acta Chim. Slov. 2013, 60, 429–432. [Google Scholar] [PubMed]
- Imran, M.; Ali, M.A.; Ahmad, S.; Siddiqui, M.K.; Baig, A.Q. Topological sharacterization of the symmetrical structure of bismuth tri-iodide. Symmetry 2018, 10, 201. [Google Scholar] [CrossRef]
- Gao, W.; Siddiqui, M.K. Molecular descriptors of nanotube, oxide, silicate, and triangulene networks. J. Chem. 2017, 2017, 6540754. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, C.; Lin, W.; Chen, Q.; Guo, X.; Qian, Y. Quantitative Structure-Property Relationship (QSPR) Modeling of Drug-Loaded Polymeric Micelles via Genetic Function Approximation. PLoS ONE 2015, 10, 0119575. [Google Scholar] [CrossRef] [PubMed]
- Hayat, S.; Malik, M.A.; Imran, M. Computing topological indices of honeycomb derived networks. Roman. J. Inf. Sci. Technol. 2015, 18, 144–165. [Google Scholar]
- Bie, R.J.; Siddiqui, M.K.; Razavi, R.; Taherkhani, M.; Najaf, M. Possibility of C38 and Si19Ge19 nanocages in anode of metal ion batteries: computational examination. Acta Chim. Slov. 2018, 65, 303–311. [Google Scholar] [CrossRef]
- Holgate, S.A. Understanding Solid State Physics; CRC Press: Boca Raton, FL, USA, 2009; pp. 177–178. ISBN 1420012320. [Google Scholar]
- Van, Z.B. Section 2.3: Energy Bands. Principles of Semiconductor Devices. Ph.D Thesis, Electrical, Computer, Energy Engineering Department, University of Colorado, Boulder, CO, USA, 13 March 2017. [Google Scholar]
- Siddiqui, M.K.; Imran, M.; Ahmad, A. On Zagreb indices, Zagreb polynomials of some nanostar dendrimers. Appl. Math. Comput. 2016, 280, 132–139. [Google Scholar] [CrossRef]
- Siddiqui, M.K.; Naeem, M.; Rahman, N.A.; Imran, M. Computing topological indicesof certain networks. J. Optoelectron. Adv. Mater. 2016, 18, 884–892. [Google Scholar]
- Randić, M. On characterization of molecular branching. J. Am. Chem. Soc. 1975, 97, 6609–6615. [Google Scholar] [CrossRef]
- Bollobás, B.; Erdös, P. Graphs of extremal weights. Ars Comb. 1998, 50, 225–233. [Google Scholar] [CrossRef]
- Amic, D.; Beslo, D.; Lucic, B.; Nikolic, S.; Trinajstić, N. The vertex-connectivity index revisited. J. Chem. Inf. Comput. Sci. 1998, 38, 819–822. [Google Scholar] [CrossRef]
- Shao, Z.; Siddiqui, M.K.; Muhammad, M.H. Computing zagreb indices and zagreb polynomials for symmetrical nanotubes. Symmetry 2018, 10, 244. [Google Scholar] [CrossRef]
- Li, X.; Gutman, I. Mathematical Aspects of Randić type Molecular Structure Descriptors; Mathematical Chemistry Monographs No. 1: Kragujevac, Serbia, 2006; Volume 330. [Google Scholar]
- Estrada, E.; Torres, L.; Rodríguez, L.; Gutman, I. An atom-bond connectivity inde. Modelling the enthalpy of formation of alkanes. Indian J. Chem. 1998, 37A, 849–855. [Google Scholar]
- Vukičević, D.; Furtula, B. Topological index based on the ratios of geometrical and arithmetical means of end-vertex degrees of edges. J. Math. Chem. 2009, 46, 1369–1376. [Google Scholar]
- Gutman, I.; Trinajstć, N. Graph theory and molecular orbitals, Total π-electron energy of alternant hydrocarbons. Chem. Phys. Lett. 1972, 17, 535–538. [Google Scholar] [CrossRef]
- Gutman, I.; Das, K.C. The first Zagreb index 30 years after. MATCH Commun. Math. Comput. Chem. 2004, 50, 83–92. [Google Scholar]
- Ghorbani, A.; Hosseinzadeh, M.A. Computing ABC4 index of nanostar dendrimers. Optoelectron. Adv. Mater.-Rapid Commun. 2010, 4, 1419–1422. [Google Scholar]
- Graovac, A.; Ghorbani, M.; Hosseinzadeh, M.A. Computing fifth geometric–arithmetic index for nanostar dendrimers. J. Math. Nanosci. 2011, 1, 33–42. [Google Scholar]
- Gao, W.; Siddiqui, M.K.; Naeem, M.; Rehman, N.A. Topological Characterization of Carbon Graphite and Crystal Cubic Carbon Structures. Molecules 2017, 22, 1496. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Wu, P.; Zhang, X.; Dimitrov, D.; Liu, J. On the maximum ABC index of graphs with prescribed size and without pendent vertices. IEEE Access 2018, 6, 27604–27616. [Google Scholar] [CrossRef]
- Shao, Z.; Wu, P.; Gao, Y.; Gutman, I.; Zhang, X. On the maximum ABC index of graphs without pendent vertices. Appl. Math. Comput. 2017, 315, 298–312. [Google Scholar] [CrossRef]
- Imran, M.; Siddiqui, M.K.; Naeem, M.; Iqbal, M.A. On Topological Properties of Symmetric Chemical Structures. Symmetry 2018, 10, 173. [Google Scholar] [CrossRef]
- Gao, W.; Siddiqui, M.K.; Imran, M.; Jamil, M.K.; Farahani, M.R. Forgotten Topological Index of Chemical Structure in Drugs. Saudi Pharm. J. 2016, 24, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Sun, C.; Song, S.; Xue, D. Polymorphic crystallization of Cu2O compound. CrystEngComm 2014, 16, 52–57. [Google Scholar] [CrossRef]
- Yuhas, B.D.; Yang, P. Nanowire-Based All-Oxide Solar Cells. J. Am. Chem. Soc. 2009, 131, 3756–3761. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, J.; Peng, Q.; Wang, X.; Li, Y. Nearly Monodisperse Cu2O and CuO Nanospheres: Preparation and Applications for Sensitive Gas Sensors. Chem. Mater. 2006, 18, 867–871. [Google Scholar] [CrossRef]
- Cotton, F.A.; Wilkinson, G.; Murillo, C.A.; Bochmann, M. Advanced Inorganic Chemistry; John Wiley and Sons: Hoboken, NJ, USA, 1999. [Google Scholar]

| Frequency | Set of Edges | |
|---|---|---|
| Frequency | Set of Edges | |
|---|---|---|
| Frequency | Set of Edges | |
|---|---|---|
| 8 | ||
| Frequency | Set of Edges | |
|---|---|---|
| 8 | ||
| 16 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.-B.; Siddiqui, M.K.; Zahid, M.A.; Naeem, M.; Baig, A.Q. Topological Properties of Crystallographic Structure of Molecules. Symmetry 2018, 10, 265. https://doi.org/10.3390/sym10070265
Liu J-B, Siddiqui MK, Zahid MA, Naeem M, Baig AQ. Topological Properties of Crystallographic Structure of Molecules. Symmetry. 2018; 10(7):265. https://doi.org/10.3390/sym10070265
Chicago/Turabian StyleLiu, Jia-Bao, Muhammad Kamran Siddiqui, Manzoor Ahmad Zahid, Muhammad Naeem, and Abdul Qudair Baig. 2018. "Topological Properties of Crystallographic Structure of Molecules" Symmetry 10, no. 7: 265. https://doi.org/10.3390/sym10070265
APA StyleLiu, J.-B., Siddiqui, M. K., Zahid, M. A., Naeem, M., & Baig, A. Q. (2018). Topological Properties of Crystallographic Structure of Molecules. Symmetry, 10(7), 265. https://doi.org/10.3390/sym10070265






