Heparin-Independent and Heparin-Dependent Anti-CXCL4 Antibodies Have a Reciprocal Expression in a Systemic Sclerosis Patients’ Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. IFN-α and TNF-a Determination in Plasma and pDC Cultures
2.3. ELISA for Anti-CXCL4 Autoantibodies and Heparin-Dependent Antibodies in Plasma
2.4. Isolation of pDCs and Their Stimulation
2.5. Measurement of CXCL4 in Plasma of SSc Patients
2.6. Statistical Analyses
3. Results
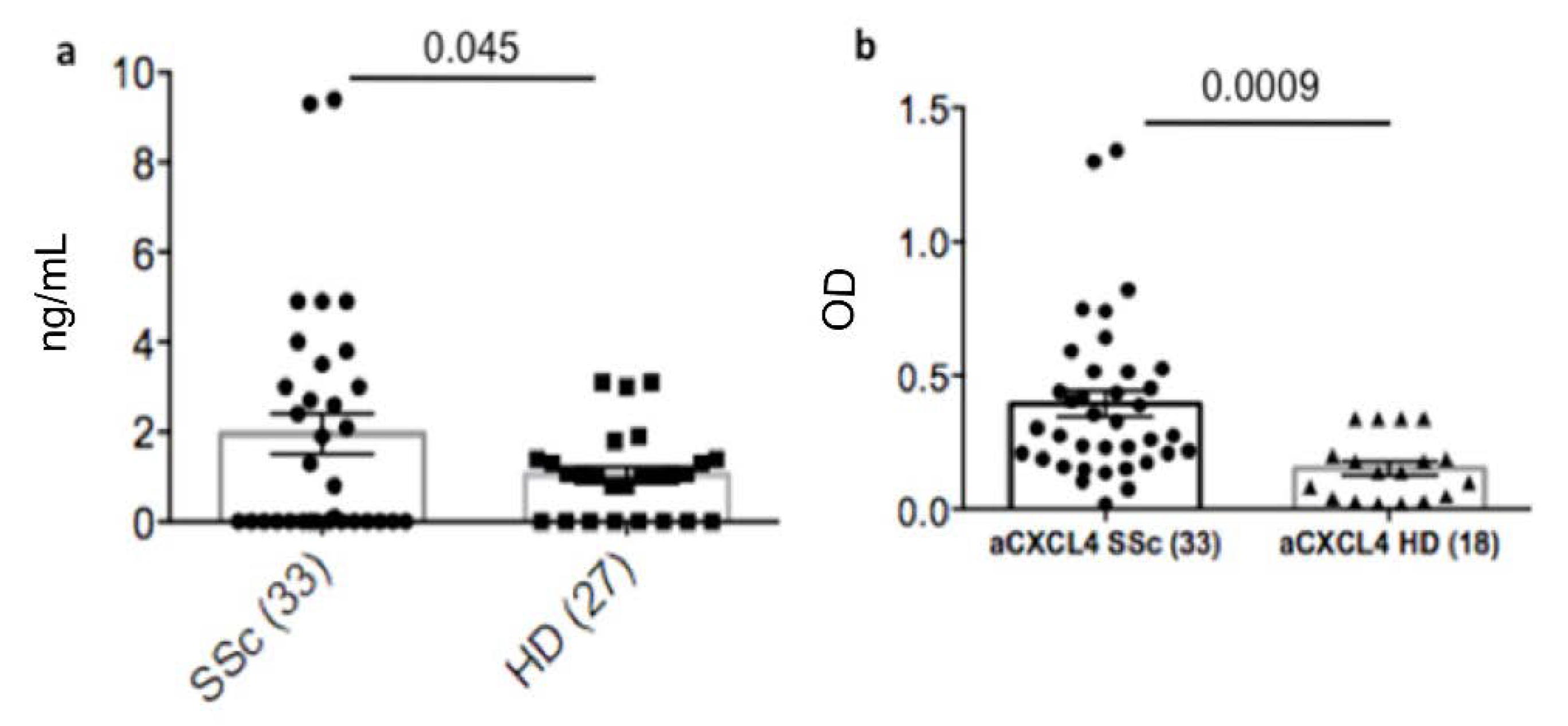
3.1. Plasma Heparin-Dependent Antibodies Are Present in SSc Patients
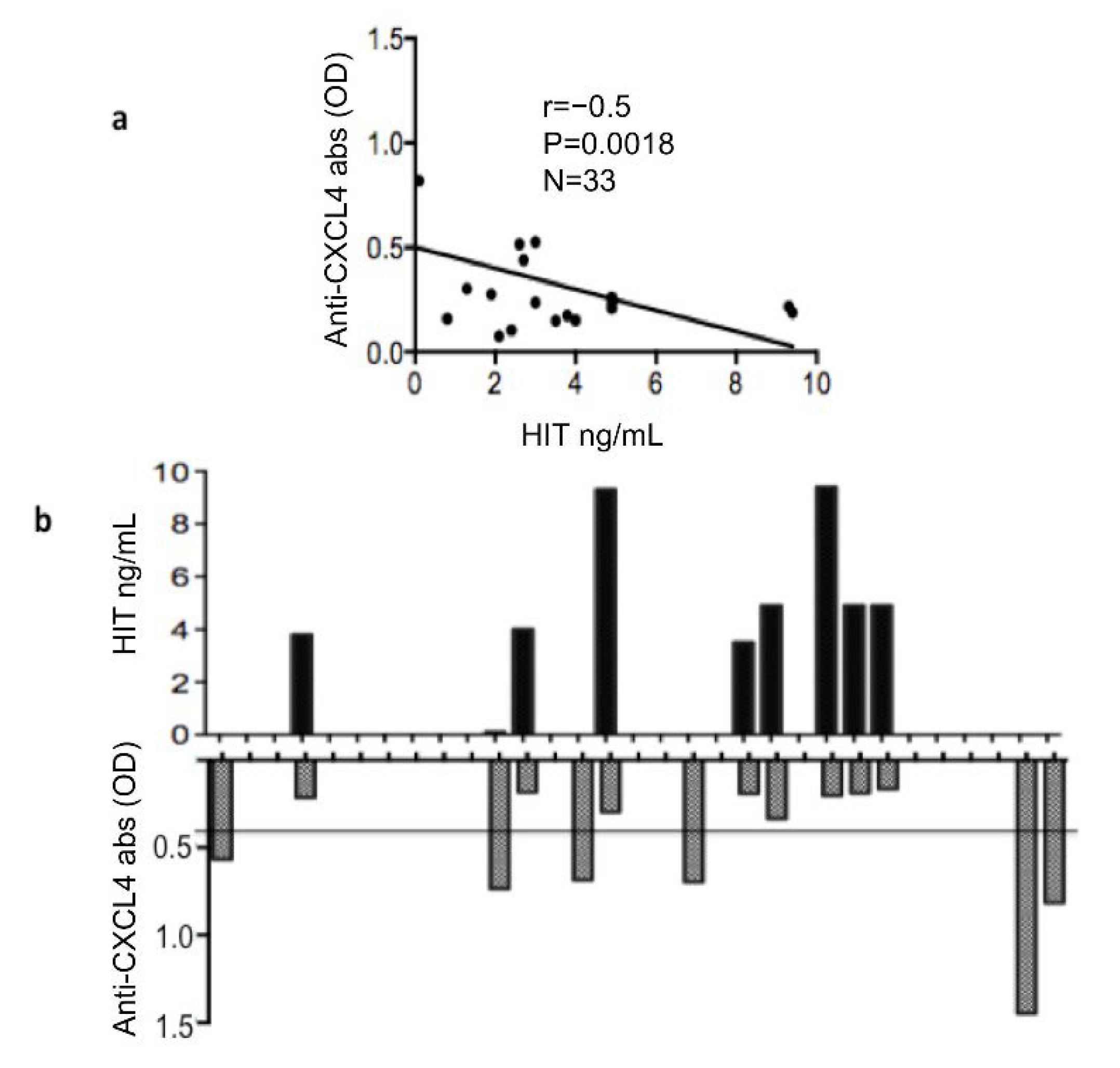
3.2. Plasma Heparin-Dependent Antibodies Inversely Correlate with Heparin-Independent Antibodies to CXCL4
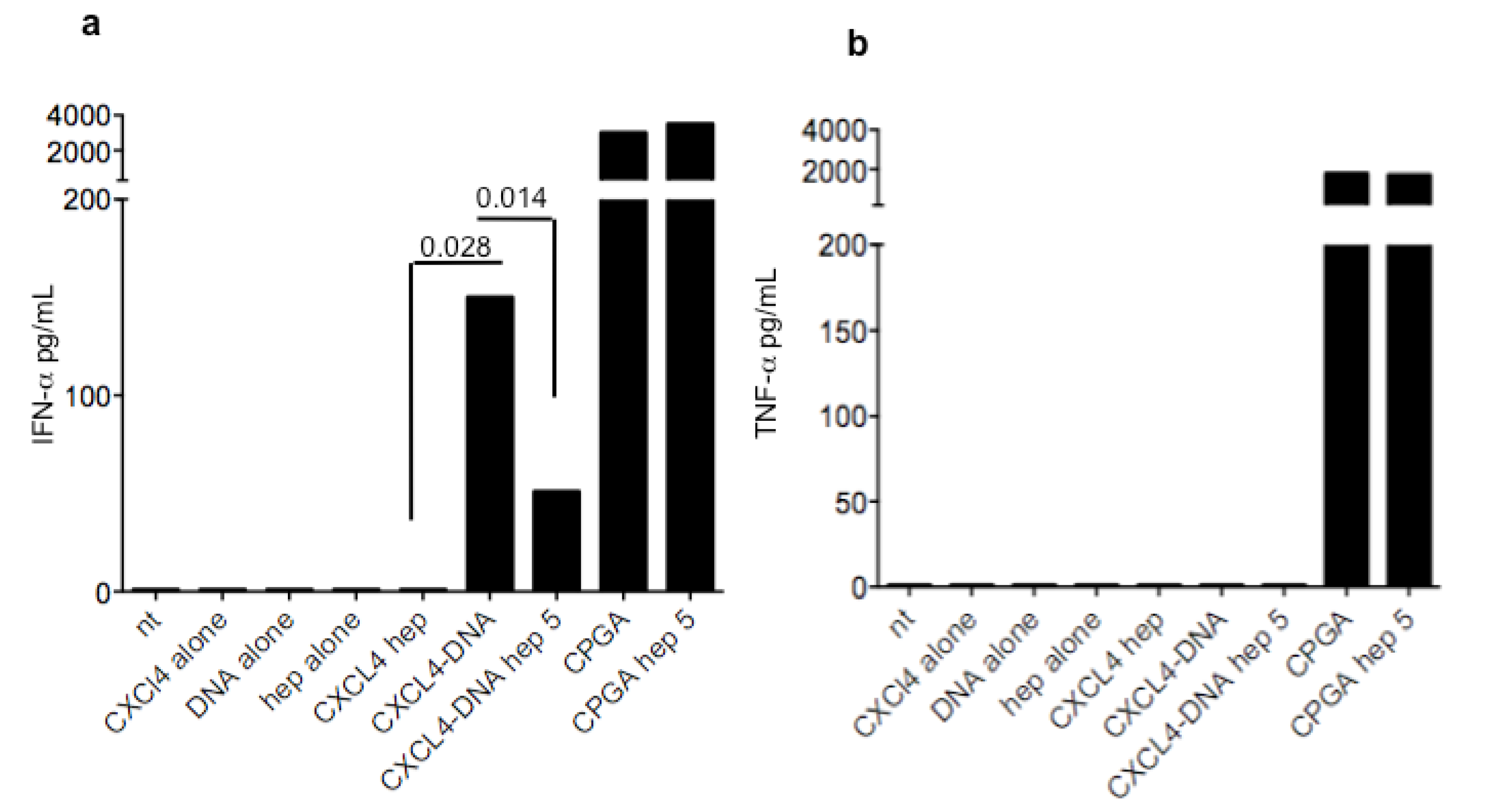
3.3. Heparin Blocks the Stimulatory Ability of CXCL4-DNA Complexes on IFN-α Production by pDCs
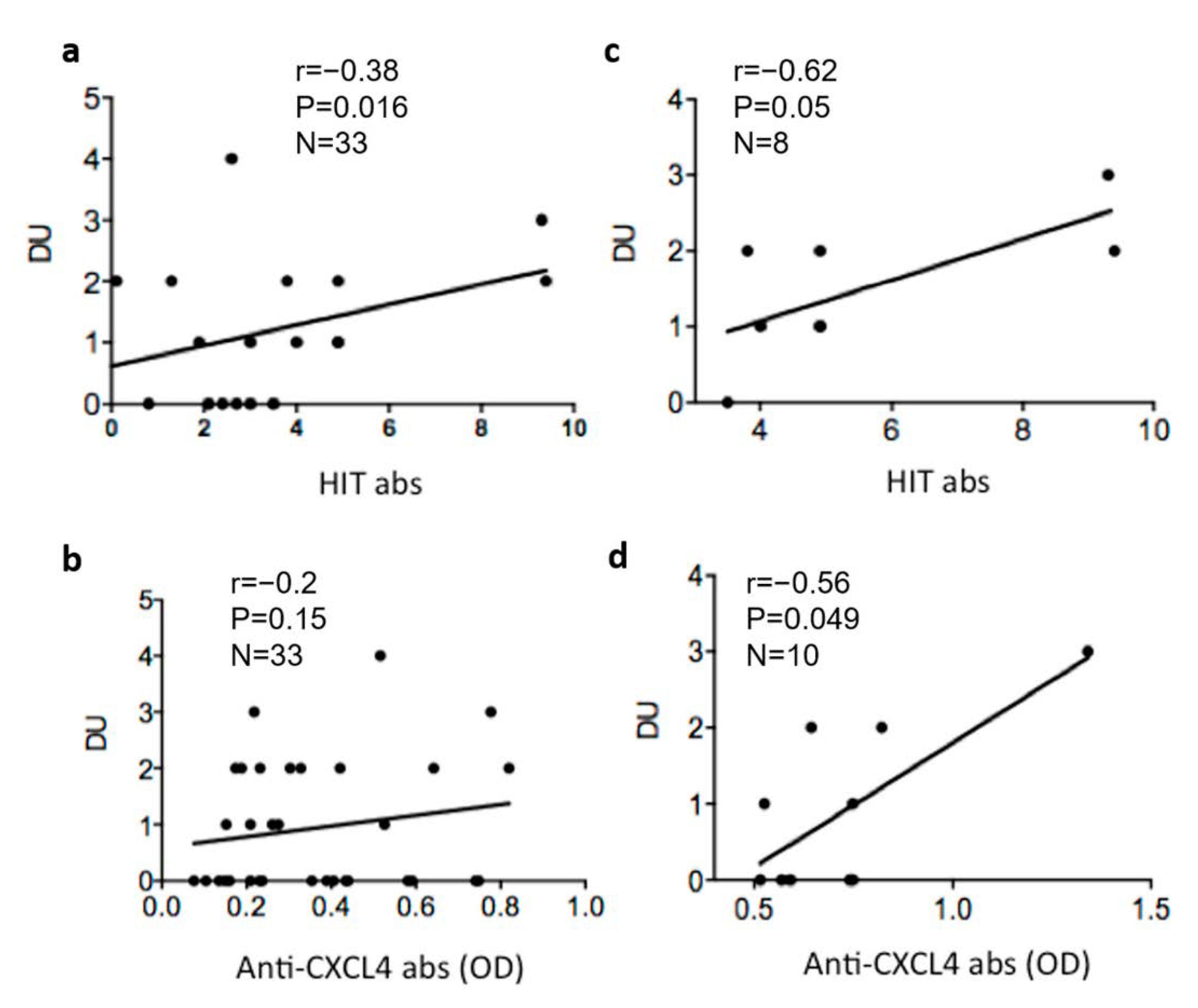
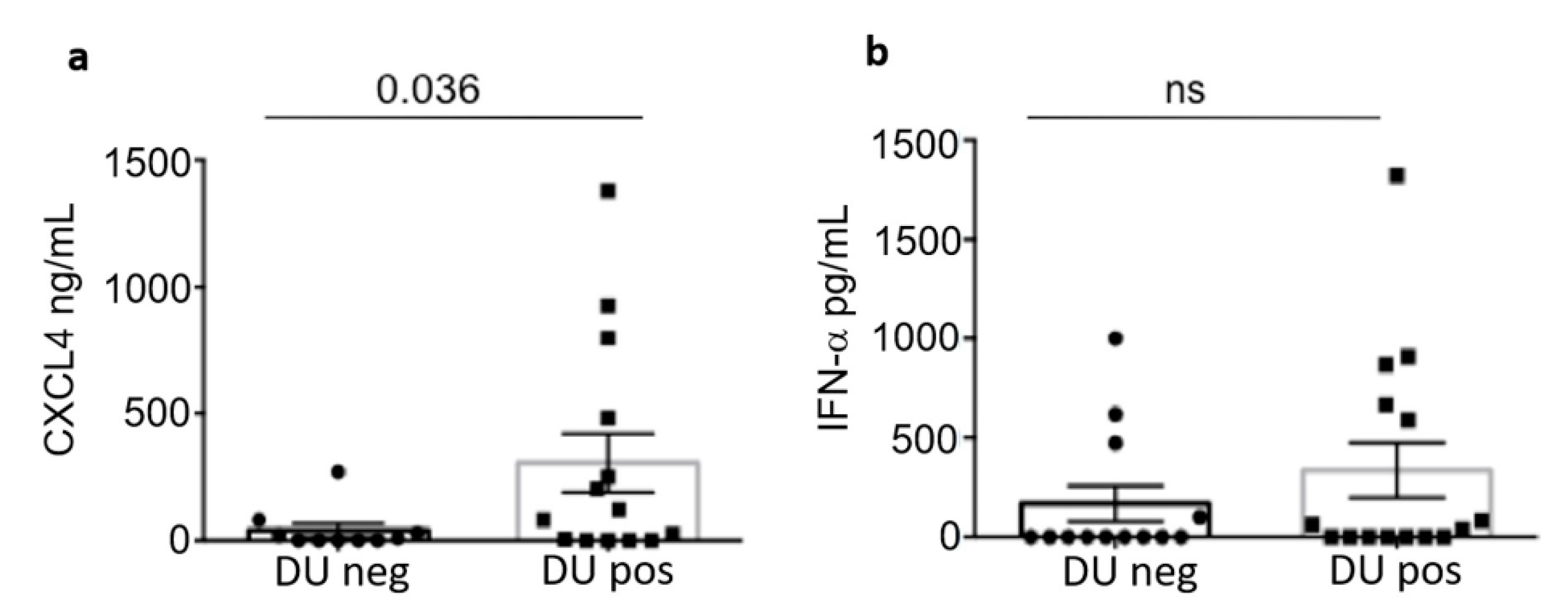
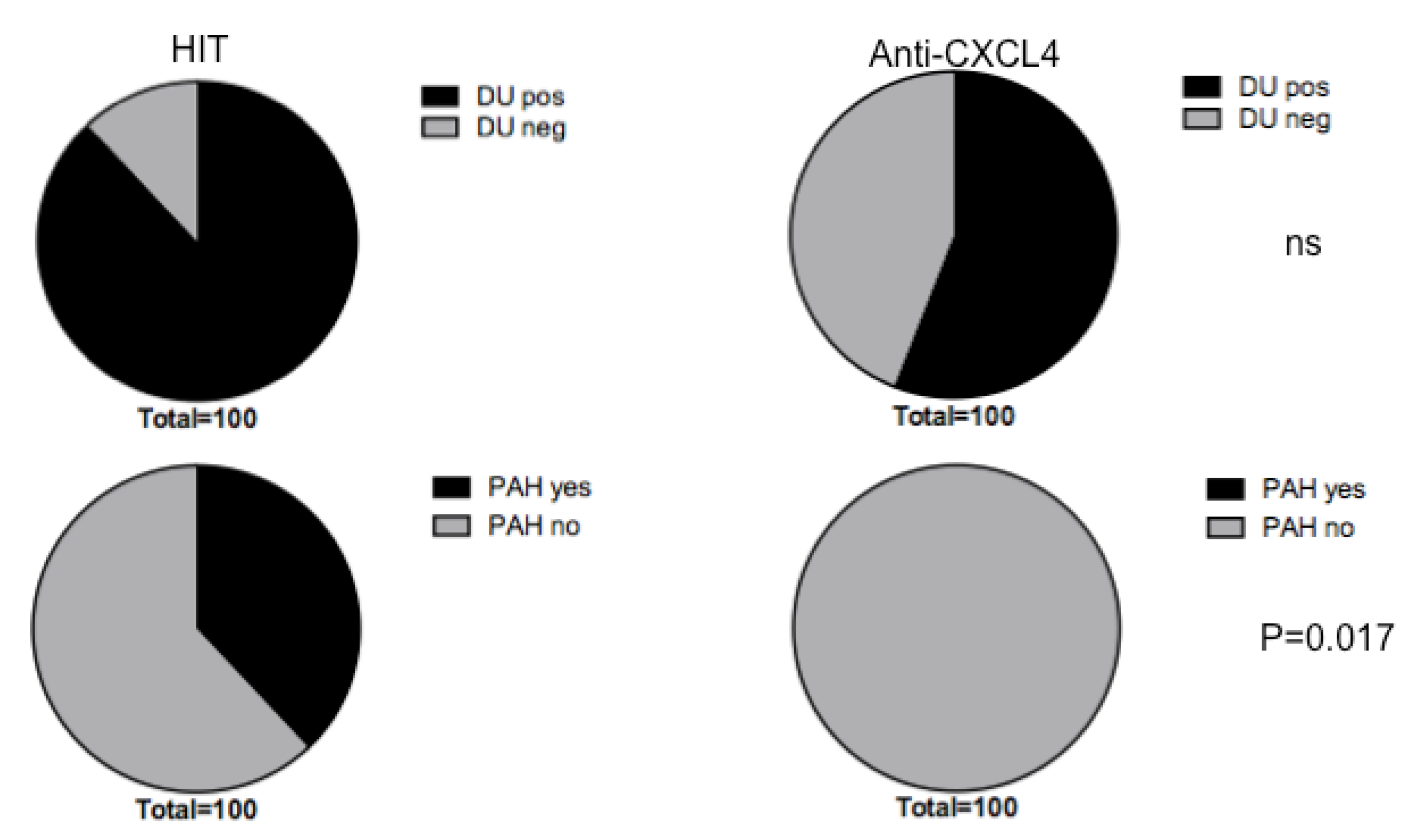
3.4. Heparin-Dependent (HIT) Antibodies Correlate with Digital Ulcers
3.5. Heparin-Dependent Anti-CXCL4 (HIT) Antibodies Are Linked to Pulmonary Artherial Hypertension (PAH)
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gabrielli, A.; Avvedimento, E.V.; Krieg, T. Scleroderma. N. Engl. J. Med. 2009, 360, 1989–2003. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.Y.; Lagares, D.; Tager, A.M.; Kapoor, M. Fibrosis: A lethal component of systemic sclerosis. Nat. Rev. Rheumatol. 2014, 10, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Van Bon, L.; Affandi, A.J.; Broen, J.; Christmann, R.B.; Marijnissen, R.J.; Stawski, L.; Farina, G.A.; Stifano, G.; Mathes, A.L.; Cossu, M.; et al. Proteome-wide analysis and CXCL4 as a biomarker in systemic sclerosis. N. Engl. J. Med. 2014, 370, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Ah Kioon, M.D.; Tripodo, C.; Fernandez, D.; Kirou, K.A.; Spiera, R.F.; Crow, M.K.; Gordon, J.K.; Barrat, F.J. Plasmacytoid dendritic cells promote systemic sclerosis with a key role for TLR8. Sci. Transl. Med. 2018, 423, eaam8458. [Google Scholar] [CrossRef] [PubMed]
- Lande, R.; Lee, E.Y.; Palazzo, R.; Marinari, B.; Pietraforte, I.; Santos, G.S.; Mattenberger, Y.; Spadaro, F.; Stefanantoni, K.; Iannace, N.; et al. CXCL4 assembles DNA into liquid crystalline complexes to amplify TLR9-mediated interferon-alpha production in systemic sclerosis. Nat. Commun. 2019, 10, 1731–1744. [Google Scholar] [CrossRef]
- Frasca, L.; Lande, R. Toll-like receptors in mediating pathogenesis in systemic sclerosis review. Clin. Exp. Immunol. 2020, 201, 14–24. [Google Scholar] [CrossRef]
- Kim, D.; Anders, P.; Santer, D.; Patole, P.; Schwartz, S.M. Induction of interferon-alpha by scleroderma sera containing autoantibodies to topoisomerase I: Association of higher interferon-alpha activity with lung fibrosis. Arthritis Rheum. 2008, 58, 2163–2173. [Google Scholar] [CrossRef]
- Eloranta, M.L.; Franck-Larsson, K.; Lövgren, T.; Kalamajski, S.; Rönnblom, A.; Rubin, K.; Alm, G.V.; Rönnblom, L. Type I interferon system activation and association with disease manifestations in systemic sclerosis. Ann. Rheum. Dis. 2010, 69, 1396–1402. [Google Scholar] [CrossRef]
- Brkic, Z.; van Bon, L.; Cossu, M.; van Helden-Meeuwsen, C.G.; Vonk, M.C.; Knaapen, H.; van den Berg, W.; Dalm, V.A.; Van Daele, P.L.; Severino, A.; et al. The interferon type I signature is present in systemic sclerosis before overt fibrosis and might contribute to its pathogenesis through high BAFF gene expression and high collagen synthesis. Ann. Rheum. Dis. 2016, 75, 1567–1573. [Google Scholar] [CrossRef]
- Wu, M.; Assassi, S. The role of type 1 interferon in systemic sclerosis. Front. Immunol. 2013, 4, 266. [Google Scholar] [CrossRef]
- Lande, R.; Mennella, A.; Palazzo, R.; Pietraforte, I.; Stefanantoni, K.; Iannace, N.; Butera, A.; Boirivant, M.; Pica, R.; Conrad, C.; et al. Anti-CXCL4 antibody reactivity is present in Systemic Sclerosis (SSc) and correlates with the SSc Type I Interferon signature. Int. J. Mol. Sci. 2020, 21, 5102. [Google Scholar] [CrossRef]
- Lande, R.; Palazzo, R.; Mennella, A.; Pietraforte, I.; Cadar, M.; Stefanantoni, K.; Conrad, C.; Riccieri, V.; Frasca, L. New Autoantibody Specificities in Systemic Sclerosis and Very Early Systemic Sclerosis. Antibodies 2021, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Arepally, G.M.; Cines, D.B. Pathogenesis of heparin-induced thrombocytopenia. Transl. Res. 2020, 225, 131–140. [Google Scholar] [CrossRef]
- Vandercappellen, J.; Van Damme, J.; Struyf, S. The role of the CXC chemokines platelet factor-4 (CXCL4/PF-4) and its variant (CXCL4L1/PF-4var) in inflammation, angiogenesis and cancer. Cytokine Growth Factor Rev. 2011, 22, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Tanaka, Y.; Okazaki, Y.; Kaburaki, J.; Ikeda, Y.; Kuwana, M. Heparin-dependent and -independent anti-platelet factor 4 autoantibodies in patients with systemic lupus erythematosus. Rheumatology 2012, 51, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, G.A.; Franchini, S.; Rovere-Querini, P.; Sabbadini, M.G.; Manfredi, A.A.; Maugeri, N. The role of platelets in the pathogenesis of systemic sclerosis. Front. Immunol. 2012, 3, 160. [Google Scholar] [CrossRef] [PubMed]
- Melsens, K.F.; De Keyser, S.; Decuman, Y.; Piette, E.; Vandecasteele, E.; Smith, V. Disease activity indices in systemic sclerosis: A systematic literature review. Clin. Exp. Rheumatol. 2016, 34 (Suppl. 100), 186–192. [Google Scholar]
- Lande, R.; Gregorio, J.; Facchinetti, V.; Chatterjee, B.; Wang, Y.H.; Homey, B.; Cao, W.; Wang, Y.H.; Su, B.; Nestle, F.O.; et al. Plamacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 2007, 449, 564–569. [Google Scholar] [CrossRef]
- Murdaca, G.; Spano’, F.; Contatore, M.; Guastalla, A.; Puppo, F. Potential use of TNF-α inhibitors in systemic sclerosis. Immunotherapy 2014, 6, 283–289. [Google Scholar] [CrossRef]
- Sachais, B.S.; Litvinov, R.I.; Yarovoi, S.V.; Rauova, L.; Hinds, J.L.; Rux, A.H.; Arepally, G.M.; Poncz, M.; Cuker, A.; Weisel, J.W.; et al. Dynamic antibody-binding properties in the pathogenesis of HIT. Blood 2012, 120, 1137–1142. [Google Scholar] [CrossRef]
- Minet, V.; Dogné, J.M.; Mullier, F. Functional Assays in the Diagnosis of Heparin-Induced Thrombocytopenia: A Review. Molecules 2017, 22, 617. [Google Scholar] [CrossRef] [PubMed]
- Von Hundelshausen, P.; Petersen, F.; Brandt, E. Platelet-derived chemokines in vascular biology. Thromb. Haemost. 2007, 97, 704–713. [Google Scholar] [CrossRef] [PubMed]

| Clinical and Demographic Characteristics of Patients and Controls 1 | SSc (33) | HD (27) |
|---|---|---|
| Age, mean (range): years | 50 (32–61) | 40 (29–55) |
| Sex (M/F): | 0/33 | 3/24 |
| SSc Form (limited/diffuse) | 1/32 | N/A |
| DU | 55% | N/A |
| Lung fibrosis (%) | 33% | N/A |
| PAH | 25% | N/A |
| ATA | 70% | N/A |
| ACA | 5% | N/A |
| aRNAP3 positivity | 2% | N/A |
| Raynaud’s phenomenon | 94% | N/A |
| DMARDs | 99% | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palazzo, R.; Stefanantoni, K.; Cadar, M.; Butera, A.; Riccieri, V.; Lande, R.; Frasca, L. Heparin-Independent and Heparin-Dependent Anti-CXCL4 Antibodies Have a Reciprocal Expression in a Systemic Sclerosis Patients’ Cohort. Antibodies 2022, 11, 77. https://doi.org/10.3390/antib11040077
Palazzo R, Stefanantoni K, Cadar M, Butera A, Riccieri V, Lande R, Frasca L. Heparin-Independent and Heparin-Dependent Anti-CXCL4 Antibodies Have a Reciprocal Expression in a Systemic Sclerosis Patients’ Cohort. Antibodies. 2022; 11(4):77. https://doi.org/10.3390/antib11040077
Chicago/Turabian StylePalazzo, Raffaella, Katia Stefanantoni, Marius Cadar, Alessia Butera, Valeria Riccieri, Roberto Lande, and Loredana Frasca. 2022. "Heparin-Independent and Heparin-Dependent Anti-CXCL4 Antibodies Have a Reciprocal Expression in a Systemic Sclerosis Patients’ Cohort" Antibodies 11, no. 4: 77. https://doi.org/10.3390/antib11040077
APA StylePalazzo, R., Stefanantoni, K., Cadar, M., Butera, A., Riccieri, V., Lande, R., & Frasca, L. (2022). Heparin-Independent and Heparin-Dependent Anti-CXCL4 Antibodies Have a Reciprocal Expression in a Systemic Sclerosis Patients’ Cohort. Antibodies, 11(4), 77. https://doi.org/10.3390/antib11040077







