Specific Autoantibodies and Microvascular Damage Progression Assessed by Nailfold Videocapillaroscopy in Systemic Sclerosis: Are There Peculiar Associations? An Update
Abstract
1. Introduction
2. Methods
3. Results
3.1. Evidence of the Association between SSc-Specific Autoantibodies and Microvascular Damage Detected by NVC in Clinical Studies
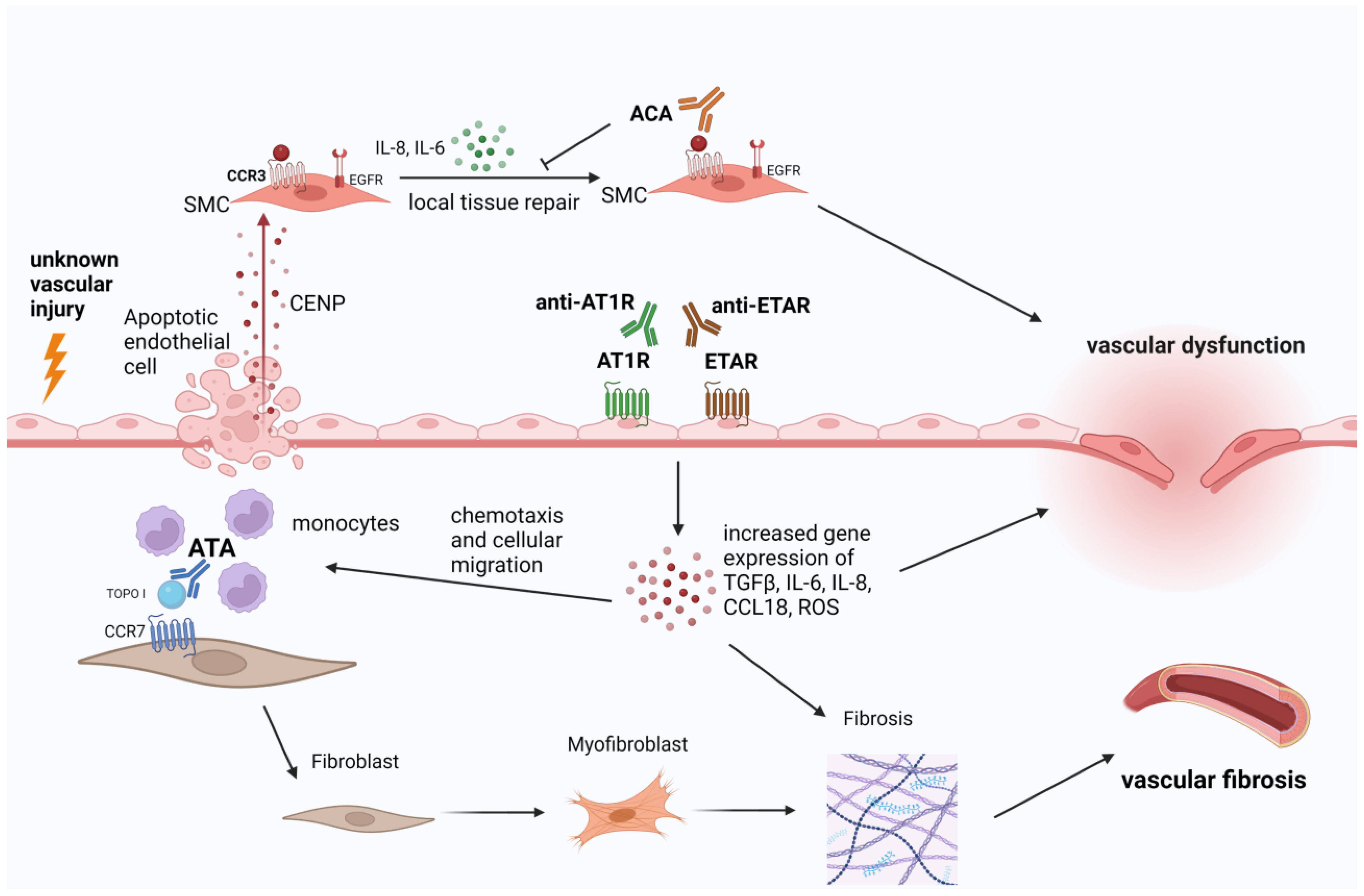
3.2. Evidence of Microvascular Damage Mediated by SSc-Specific Autoantibodies in Pre-Clinical Studies and Translational Research
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allanore, Y.; Simms, R.; Distler, O.; Trojanowska, M.; Pope, J.; Denton, C.P.; Varga, J. Systemic sclerosis. Nat. Rev. Dis. Prim. 2015, 1, 15002. [Google Scholar] [CrossRef] [PubMed]
- Denton, C.P.; Khanna, D. Systemic sclerosis. Lancet 2017, 390, 1685–1699. [Google Scholar] [CrossRef] [PubMed]
- Perelas, A.; Silver, R.M.; Arrossi, A.V.; Highland, K.B. Systemic sclerosis-associated interstitial lung disease. Lancet Respir. Med. 2020, 8, 304–320. [Google Scholar] [CrossRef] [PubMed]
- Hysa, E.; Cutolo, C.A.; Gotelli, E.; Paolino, S.; Cimmino, M.A.; Pacini, G.; Pizzorni, C.; Sulli, A.; Smith, V.; Cutolo, M. Ocular microvascular damage in autoimmune rheumatic diseases: The pathophysiological role of the immune system. Autoimmun. Rev. 2021, 20, 102796. [Google Scholar] [CrossRef] [PubMed]
- Bruni, C.; Ross, L. Cardiac involvement in systemic sclerosis: Getting to the heart of the matter. Best Pract. Res. Clin. Rheumatol. 2021, 35, 101668. [Google Scholar] [CrossRef]
- Hughes, M.; Allanore, Y.; Chung, L.; Pauling, J.D.; Denton, C.P.; Matucci-Cerinic, M. Raynaud phenomenon and digital ulcers in systemic sclerosis. Nat. Rev. Rheumatol. 2020, 16, 208–221. [Google Scholar] [CrossRef]
- Nihtyanova, S.I.; Denton, C.P. Autoantibodies as predictive tools in systemic sclerosis. Nat. Rev. Rheumatol. 2010, 6, 112–116. [Google Scholar] [CrossRef]
- Smith, V.; Herrick, A.L.; Ingegnoli, F.; Damjanov, N.; De Angelis, R.; Denton, C.P.; Distler, O.; Espejo, K.; Foeldvari, I.; Frech, T.; et al. Standardisation of nailfold capillaroscopy for the assessment of patients with Raynaud’s phenomenon and systemic sclerosis. Autoimmun. Rev. 2020, 19, 102458. [Google Scholar] [CrossRef]
- Cutolo, M.; Smith, V. Detection of microvascular changes in systemic sclerosis and other rheumatic diseases. Nat. Rev. Rheumatol. 2021, 17, 665–677. [Google Scholar] [CrossRef]
- van den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Medsger, T.A.; Carreira, P.E.; et al. 2013 classification criteria for systemic sclerosis: An American college of rheumatology/European league against rheumatism collaborative initiative. Ann. Rheum. Dis. 2013, 72, 1747–1755. [Google Scholar] [CrossRef] [PubMed]
- Koenig, M.; Joyal, F.; Fritzler, M.J.; Roussin, A.; Abrahamowicz, M.; Boire, G.; Goulet, J.R.; Rich, E.; Grodzicky, T.; Raymond, Y.; et al. Autoantibodies and microvascular damage are independent predictive factors for the progression of Raynaud’s phenomenon to systemic sclerosis: A twenty-year prospective study of 586 patients, with validation of proposed criteria for early systemic sclerosis. Arthritis Rheumatol. 2008, 58, 3902–3912. [Google Scholar] [CrossRef] [PubMed]
- Mahler, M.; Hudson, M.; Bentow, C.; Roup, F.; Beretta, L.; Simeón, C.P.; Guillén-Del-Castillo, A.; Casas, S.; Fritzler, M.J. Autoantibodies to stratify systemic sclerosis patients into clinically actionable subsets. Autoimmun. Rev. 2020, 19, 102583. [Google Scholar] [CrossRef] [PubMed]
- Lande, R.; Palazzo, R.; Mennella, A.; Pietraforte, I.; Cadar, M.; Stefanantoni, K.; Conrad, C.; Riccieri, V.; Frasca, L. New Autoantibody Specificities in Systemic Sclerosis and Very Early Systemic Sclerosis. Antibodies 2021, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Elhai, M.; Sritharan, N.; Boubaya, M.; Balbir-Gurman, A.; Siegert, E.; Hachulla, E.; de Vries-Bouwstra, J.; Riemekasten, G.; Distler, J.H.W.; Rosato, E.; et al. Stratification in systemic sclerosis according to autoantibody status versus skin involvement: A study of the prospective EUSTAR cohort. Lancet Rheumatol. 2022, 4, e785–e794. [Google Scholar] [CrossRef]
- Pashnina, I.A.; Krivolapova, I.M.; Fedotkina, T.V.; Ryabkova, V.A.; Chereshneva, M.V.; Churilov, L.P.; Chereshnev, V.A. Antinuclear Autoantibodies in Health: Autoimmunity Is Not a Synonym of Autoimmune Disease. Antibodies 2021, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Tang, S.; Zhu, D.; Ding, Y.; Qiao, J. Classical Disease-Specific Autoantibodies in Systemic Sclerosis: Clinical Features, Gene Susceptibility, and Disease Stratification. Front. Med. 2020, 7, 587773. [Google Scholar] [CrossRef]
- Cavazzana, I.; Vojinovic, T.; Airo’, P.; Fredi, M.; Ceribelli, A.; Pedretti, E.; Lazzaroni, M.G.; Garrafa, E.; Franceschini, F. Systemic Sclerosis-Specific Antibodies: Novel and Classical Biomarkers. Clin. Rev. Allergy Immunol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Chandran, G.; Smith, M.; Ahern, M.J.; Roberts-Thomson, P.J. A study of scleroderma in South Australia: Prevalence, subset characteristics and nailfold capillaroscopy. Aust. N. Z. J. Med. 1995, 25, 688–694. [Google Scholar] [CrossRef]
- Cutolo, M.; Pizzorni, C.; Tuccio, M.; Burroni, A.; Craviotto, C.; Basso, M.; Seriolo, B.; Sulli, A. Nailfold videocapillaroscopic patterns and serum autoantibodies in systemic sclerosis. Rheumatology 2004, 43, 719–726. [Google Scholar] [CrossRef]
- Sulli, A.; Ruaro, B.; Smith, V.; Pizzorni, C.; Zampogna, G.; Gallo, M.; Cutolo, M. Progression of nailfold microvascular damage and antinuclear antibody pattern in systemic sclerosis. J. Rheumatol. 2013, 40, 634–639. [Google Scholar] [CrossRef]
- Pizzorni, C.; Giampetruzzi, A.R.; Mondino, C.; Facchiano, A.; Abeni, D.; Paolino, S.; Ruaro, B.; Smith, V.; Sulli, A.; Cutolo, M. Nailfold capillaroscopic parameters and skin telangiectasia patterns in patients with systemic sclerosis. Microvasc. Res. 2017, 111, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Tieu, J.; Hakendorf, P.; Woodman, R.J.; Patterson, K.; Walker, J.; Roberts-Thomson, P. The role of nailfold capillary dropout on mortality in systemic sclerosis. Intern. Med. J. 2018, 48, 517–523. [Google Scholar] [CrossRef]
- Lambova, S.N.; Kurteva, E.K.; Dzhambazova, S.S.; Vasilev, G.H.; Kyurkchiev, D.S.; Geneva-Popova, M.G. Capillaroscopy and Immunological Profile in Systemic Sclerosis. Life 2022, 12, 498. [Google Scholar] [CrossRef] [PubMed]
- Caramaschi, P.; Canestrini, S.; Martinelli, N.; Volpe, A.; Pieropan, S.; Ferrari, M.; Bambara, L.M.; Carletto, A.; Biasi, D. Scleroderma patients nailfold videocapillaroscopic patterns are associated with disease subset and disease severity. Rheumatology 2007, 46, 1566–1569. [Google Scholar] [CrossRef]
- De Santis, M.; Ceribelli, A.; Cavaciocchi, F.; Crotti, C.; Massarotti, M.; Belloli, L.; Marasini, B.; Isailovic, N.; Generali, E.; Selmi, C. Nailfold videocapillaroscopy and serum VEGF levels in scleroderma are associated with internal organ involvement. Autoimmun. Highlights 2016, 7, 5. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fichel, F.; Baudot, N.; Gaitz, J.P.; Trad, S.; Barbe, C.; Francès, C.; Senet, P. Systemic sclerosis with normal or nonspecific nailfold capillaroscopy. Dermatology 2014, 228, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Ghizzoni, C.; Sebastiani, M.; Manfredi, A.; Campomori, F.; Colaci, M.; Giuggioli, D.; Ferri, C. Prevalence and evolution of scleroderma pattern at nailfold videocapillaroscopy in systemic sclerosis patients: Clinical and prognostic implications. Microvasc. Res. 2015, 99, 92–95. [Google Scholar] [CrossRef]
- Markusse, I.M.; Meijs, J.; de Boer, B.; Bakker, J.A.; Schippers, H.P.C.; Schouffoer, A.A.; Marsan, N.A.; Kroft, L.J.M.; Ninaber, M.K.; Huizinga, T.W.J.; et al. Predicting cardiopulmonary involvement in patients with systemic sclerosis: Complementary value of nailfold videocapillaroscopy patterns and disease-specific autoantibodies. Rheumatology 2017, 56, 1081–1088. [Google Scholar] [CrossRef][Green Version]
- van Leeuwen, N.M.; Ciaffi, J.; Schoones, J.W.; Huizinga, T.W.J.; de Vries-Bouwstra, J.K. Contribution of Sex and Autoantibodies to Microangiopathy Assessed by Nailfold Videocapillaroscopy in Systemic Sclerosis: A Systematic Review of the Literature. Arthritis Care Res. 2021, 73, 722–731. [Google Scholar] [CrossRef]
- Ingegnoli, F.; Ardoino, I.; Boracchi, P.; Cutolo, M.; Airò, P.; Ananieva, L.P.; Ancuta, C.; Andrade, L.E.; Becvar, R.; Benenati, A.; et al. Nailfold capillaroscopy in systemic sclerosis: Data from the EULAR scleroderma trials and research (EUSTAR) database. Microvasc. Res. 2013, 89, 122–128. [Google Scholar] [CrossRef]
- Robitaille, G.; Christin, M.S.; Clément, I.; Senécal, J.L.; Raymond, Y. Nuclear autoantigen CENP-B transactivation of the epidermal growth factor receptor via chemokine receptor 3 in vascular smooth muscle cells. Arthritis Rheumatol. 2009, 60, 2805–2816. [Google Scholar] [CrossRef] [PubMed]
- Robitaille, G.; Hénault, J.; Christin, M.S.; Senécal, J.L.; Raymond, Y. The nuclear autoantigen CENP-B displays cytokine-like activities toward vascular smooth muscle cells. Arthritis Rheumatol. 2007, 56, 3814–3826. [Google Scholar] [CrossRef]
- Hénault, J.; Robitaille, G.; Senécal, J.L.; Raymond, Y. DNA topoisomerase I binding to fibroblasts induces monocyte adhesion and activation in the presence of anti-topoisomerase I autoantibodies from systemic sclerosis patients. Arthritis Rheumatol. 2006, 54, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Arcand, J.; Robitaille, G.; Koenig, M.; Senécal, J.L.; Raymond, Y. The autoantigen DNA topoisomerase I interacts with chemokine receptor 7 and exerts cytokine-like effects on dermal fibroblasts. Arthritis Rheumatol. 2012, 64, 826–834. [Google Scholar] [CrossRef]
- Arcand, J.; Robitaille, G.; Koenig, M.; Senécal, J.L.; Raymond, Y. Heparin inhibits the interaction of DNA topoisomerase I/anti-topoisomerase I immune complexes with heparan sulfate on dermal fibroblasts. Arthritis Rheumatol. 2012, 64, 1632–1641. [Google Scholar] [CrossRef]
- Corallo, C.; Cheleschi, S.; Cutolo, M.; Soldano, S.; Fioravanti, A.; Volpi, N.; Franci, D.; Nuti, R.; Giordano, N. Antibodies against specific extractable nuclear antigens (ENAs) as diagnostic and prognostic tools and inducers of a profibrotic phenotype in cultured human skin fibroblasts: Are they functional? Arthritis Res. 2019, 21, 152. [Google Scholar] [CrossRef]
- Raschi, E.; Privitera, D.; Bodio, C.; Lonati, P.A.; Borghi, M.O.; Ingegnoli, F.; Meroni, P.L.; Chighizola, C.B. Scleroderma-specific autoantibodies embedded in immune complexes mediate endothelial damage: An early event in the pathogenesis of systemic sclerosis. Arthritis Res. 2020, 22, 265. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, C.; Renzoni, E.; Sestini, P.; Pantelidis, P.; Lagan, A.; Bunn, C.; McHugh, N.; Welsh, K.I.; Du Bois, R.M.; Denton, C.P.; et al. Endothelin axis polymorphisms in patients with scleroderma. Arthritis Rheumatol. 2006, 54, 3034–3042. [Google Scholar] [CrossRef] [PubMed]
- Svegliati, S.; Amico, D.; Spadoni, T.; Fischetti, C.; Finke, D.; Moroncini, G.; Paolini, C.; Tonnini, C.; Grieco, A.; Rovinelli, M.; et al. Agonistic Anti-PDGF Receptor Autoantibodies from Patients with Systemic Sclerosis Impact Human Pulmonary Artery Smooth Muscle Cells Function In Vitro. Front. Immunol. 2017, 8, 75. [Google Scholar] [CrossRef]
- Hénault, J.; Tremblay, M.; Clément, I.; Raymond, Y.; Senécal, J.L. Direct binding of anti-DNA topoisomerase I autoantibodies to the cell surface of fibroblasts in patients with systemic sclerosis. Arthritis Rheumatol. 2004, 50, 3265–3274. [Google Scholar] [CrossRef]
- Pizzorni, C.; Ferrari, G.; Schenone, C.; Hysa, E.; Carmisciano, L.; Gotelli, E.; Pacini, G.; Sulli, A.; Paolino, S.; Smith, V.; et al. Capillaroscopic analysis of the microvascular status in mixed versus undifferentiated connective tissue disease. Microvasc. Res. 2022, 142, 104367. [Google Scholar] [CrossRef]
- Cattelan, F.; Hysa, E.; Gotelli, E.; Pizzorni, C.; Bica, P.F.; Grosso, M.; Barisione, E.; Paolino, S.; Carmisciano, L.; Sulli, A.; et al. Microvascular capillaroscopic abnormalities and occurrence of antinuclear autoantibodies in patients with sarcoidosis. Rheumatol. Int. 2022, 42, 2199–2210. [Google Scholar] [CrossRef]
- Hysa, E.; Pizzorni, C.; Sammori, S.; Gotelli, E.; Pogna, A.; Cere, A.; Schenone, C.; Gerli, V.; Paolino, S.; Sulli, A.; et al. Capillaroscopic Findings in Autoimmune Connective Tissue Diseases: Results from 20 Years of Experience in a Training Referral Center [abstract]. Arthritis Rheumatol. 2022, 74 (Suppl. 9). [Google Scholar]
- Cutolo, M.; Soldano, S.; Smith, V. Pathophysiology of systemic sclerosis: Current understanding and new insights. Expert Rev. Clin. Immunol. 2019, 15, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Mostmans, Y.; Cutolo, M.; Giddelo, C.; Decuman, S.; Melsens, K.; Declercq, H.; Vandecasteele, E.; De Keyser, F.; Distler, O.; Gutermuth, J.; et al. The role of endothelial cells in the vasculopathy of systemic sclerosis: A systematic review. Autoimmun. Rev. 2017, 16, 774–786. [Google Scholar] [CrossRef] [PubMed]
- Magna, M.; Pisetsky, D.S. The Alarmin Properties of DNA and DNA-associated Nuclear Proteins. Clin. Ther. 2016, 38, 1029–1041. [Google Scholar] [CrossRef]
- Brown, M.; O’Reilly, S. The immunopathogenesis of fibrosis in systemic sclerosis. Clin. Exp. Immunol. 2019, 195, 310–321. [Google Scholar] [CrossRef]
- Walker, U.A.; Tyndall, A.; Czirják, L.; Denton, C.; Farge-Bancel, D.; Kowal-Bielecka, O.; Müller-Ladner, U.; Bocelli-Tyndall, C.; Matucci-Cerinic, M. Clinical risk assessment of organ manifestations in systemic sclerosis: A report from the EULAR Scleroderma Trials And Research group database. Ann. Rheum. Dis. 2007, 66, 754–763. [Google Scholar] [CrossRef]
- Mitri, G.M.; Lucas, M.; Fertig, N.; Steen, V.D.; Medsger, T.A., Jr. A comparison between anti-Th/To- and anticentromere antibody-positive systemic sclerosis patients with limited cutaneous involvement. Arthritis Rheumatol. 2003, 48, 203–209. [Google Scholar] [CrossRef]
- Cappelli, S.; Randone, S.B.; Camiciottoli, G.; De Paulis, A.; Guiducci, S.; Matucci-Cerinic, M. Interstitial lung disease in systemic sclerosis: Where do we stand? Eur. Respir. Rev. 2015, 24, 411–419. [Google Scholar] [CrossRef]
- Radić, M.; Kaliterna, D.M.; Ljutić, D. The level of anti-topoisomerase I antibodies highly correlates with metacarpophalangeal and proximal interphalangeal joints flexion contractures in patients with systemic sclerosis. Clin. Exp. Rheumatol. 2006, 24, 407–412. [Google Scholar] [PubMed]
- Senécal, J.L.; Hoa, S.; Yang, R.; Koenig, M. Pathogenic roles of autoantibodies in systemic sclerosis: Current understandings in pathogenesis. J. Scleroderma Relat. Disord. 2020, 5, 103–129. [Google Scholar] [CrossRef] [PubMed]
- Riemekasten, G.; Philippe, A.; Näther, M.; Slowinski, T.; Müller, D.N.; Heidecke, H.; Matucci-Cerinic, M.; Czirják, L.; Lukitsch, I.; Becker, M.; et al. Involvement of functional autoantibodies against vascular receptors in systemic sclerosis. Ann. Rheum. Dis. 2011, 70, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.; Scirè, C.A.; Talarico, R.; Airo, P.; Alexander, T.; Allanore, Y.; Bruni, C.; Codullo, V.; Dalm, V.; De Vries-Bouwstra, J.; et al. Systemic sclerosis: State of the art on clinical practice guidelines. RMD Open 2018, 4, e000782. [Google Scholar] [CrossRef] [PubMed]
- Kowal-Bielecka, O.; Fransen, J.; Avouac, J.; Becker, M.; Kulak, A.; Allanore, Y.; Distler, O.; Clements, P.; Cutolo, M.; Czirjak, L.; et al. Update of EULAR recommendations for the treatment of systemic sclerosis. Ann. Rheum. Dis. 2017, 76, 1327–1339. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, A.C.; Pizzorni, C.; Ruaro, B.; Paolino, S.; Sulli, A.; Smith, V.; Cutolo, M. Effects of Longterm Treatment with Bosentan and Iloprost on Nailfold Absolute Capillary Number, Fingertip Blood Perfusion, and Clinical Status in Systemic Sclerosis. J. Rheumatol. 2016, 43, 2033–2041. [Google Scholar] [CrossRef] [PubMed]
- Soldano, S.; Paolino, S.; Pizzorni, C.; Trombetta, A.C.; Montagna, P.; Brizzolara, R.; Corallo, C.; Giordano, N.; Sulli, A.; Cutolo, M. Dual endothelin receptor antagonists contrast the effects induced by endothelin-1 on cultured human microvascular endothelial cells. Clin. Exp. Rheumatol. 2017, 35, 484–493. [Google Scholar]
- Corallo, C.; Cutolo, M.; Kahaleh, B.; Pecetti, G.; Montella, A.; Chirico, C.; Soldano, S.; Nuti, R.; Giordano, N. Bosentan and macitentan prevent the endothelial-to-mesenchymal transition (EndoMT) in systemic sclerosis: In vitro study. Arthritis Res. Ther. 2016, 18, 228. [Google Scholar] [CrossRef]
- Cabral-Marques, O.; Riemekasten, G. Vascular hypothesis revisited: Role of stimulating antibodies against angiotensin and endothelin receptors in the pathogenesis of systemic sclerosis. Autoimmun. Rev. 2016, 15, 690–694. [Google Scholar] [CrossRef]
- Avouac, J.; Lepri, G.; Smith, V.; Toniolo, E.; Hurabielle, C.; Vallet, A.; Amrouche, F.; Kahan, A.; Cutolo, M.; Allanore, Y. Sequential nailfold videocapillaroscopy examinations have responsiveness to detect organ progression in systemic sclerosis. Semin. Arthritis Rheum. 2017, 47, 86–94. [Google Scholar] [CrossRef]

| Author, Year [Reference] | Population | Sample size (n) Sex (F: %) Age (M ± SD) Duration of Disease or Symptoms in Years, Average (Range) | Autoantibodies | Immunoassays | Results |
|---|---|---|---|---|---|
| Chandran, 1995 [18] | SSc patients, defined with diagnosis reported in the clinical files | n = 52 F: 83% 45 ± 10 NR | ACA, ATA, RNP | NR | ATA+ patients showed more severe nailfold changes (46% showed moderate capillary loss and 54% severe capillary loss) compared to ACA+ patients (39% having dilations and 21% giant capillaries, 34% moderate capillary loss and 6% severe capillary loss) and RNP+ patients. |
| Cutolo, 2004 [19] | SSc patients, classified with ACR 1980 criteria or LeRoy criteria | n = 241 F: 94.1% 57 ± 15 5 (1–13) | ATA, ACA | ANA: indirect immunofluorescence (IIF) on HEp-2 cells SSc-specific autoantibodies: ELISA | ATA+ more frequent in “Active” and “Late” patterns on NVC than in “Early”. |
| Koenig, 2008 [11] | RP at risk for developing SSc | n = 784 F: 82.75% age: 39.6 ± 13 RP duration (years): 3 (1–7) | ACA, anti-Th/To ATA ARA | ANA: indirect immunofluorescence (IIF) on HEp-2 cells SSc-specific autoantibodies: ELISA | ACA and anti-Th/To predicted enlarged capillaries (HR 6.64). ACA, anti-Th/To and ARA predicted capillary loss (HR 2.62) ACA predicted capillary teleangectasias (HR 3.1) |
| Sulli, 2013 [20] | SSc patients, classified with 1980 LeRoy criteria | n = 42 NR 47 ± 19 1 (IQR 3) | ACA, ATA | ANA: indirect immunofluorescence (IIF) on HEp-2 cells ACA and ATA: ELISA | ATA more often present in “late” pattern than in “early” and “active”. Non-significant associations between NVC patterns with other ANA patterns. |
| Ingegnoli, 2013 [30] | SSc patients classified according to the presence of clinical features, NVC patterns and autoantibodies | n = 2754 F: 87.15% age: 54.97 ± 13.6 disease duration: 7.62 ± 7.38 | ACA, ATA | ANA: indirect immunofluorescence (IIF) on HEp-2 cells SSc-specific autoantibodies: ELISA | ATA more often present in the” late” pattern than in “early” and “active” |
| Pizzorni, 2017 [21] | SSc patients, classified with ACR 2013 criteria | n = 33 F: 84.8% 59 ± 21 years Mean SSc duration 6.6 ± 5 years) | ACA, ATA | ANA: indirect immunofluorescence (IIF) on HEp-2 cells SSc-specific autoantibodies: ELISA | “Early” and “active” pattern more often present in ACA patients, late pattern more often present in ATA patients. |
| Tieu, 2018 [22] | SSc patients classified according to the presence of clinical features, NVC patterns and autoantibodies | n = 152 | ACA, ATA, RNP, RNAPIII | NR | ARA+ showed a higher grade of capillary damage compared with ACA and RNP+ (p < 0.001); ATA and ARA had a higher capillary dropout compared with ACA |
| Lambova, 2022 [23] | SSc patients, clinically diagnosed according to the extent of cutaneous involvement (limited vs. diffuse) | n = 19 51.56 ± 15.07 | 13 SSc-related autoantigens: ATA, CENP A, CENP B, RP11/RNAP-III, RP155/RNAP-III, fibrillarin, NOR-90, Th/To, PM-Scl100, PMScl75, Ku, PDGFR and Ro-52 | ANA: indirect immunofluorescence (IIF) on HEp-2 cells Line immunoblot assay for detection of SSc-specific autoantibodies | ATA: associated with a lower mean capillary density and with a a higher frequency of “active” and “late” patterns. ARA: associated with a higher mean capillary density. No active and late patterns. Only one patient with early pattern. |
| Caramaschi, 2007 [24] | SSc patients, classified with ACR 1980 criteria | n = 103 F: 88.3% 54.3 ± 13.6 7 (1–46) | ACA, ATA | Anticentromere antibodies (ACA) were tested by indirect immunofluorescence on HEp-2 cells; anti-Scl70 antibodies were determined by ELISA | Non-significant associations between ACA, ATA and “early”, “active” and “late” scleroderma patterns. |
| Fichel, 2014 [26] | SSc patients, classified with 1980 LeRoy criteria | n = 88 NR 54.9 ±16.1 Duration of RP: 17.2 ± 14.8 | ACA, ATA | ANA: indirect immunofluorescence (IIF) on HEp-2 cells SSc-specific autoantibodies: ELISA | Non-significant associations between autoantibodies and NVC patterns (defined as normal or SSc pattern). |
| Ghizzoni, 2015 [27] | SSc patients, classified with ACR 2013 criteria | n = 275 F: 90.1% 54.9 ± 14.2 Disease duration (months): 36.9 ± 65.5 | ACA, ATA | ANA: indirect immunofluorescence (IIF) on HEp-2 cells SSc-specific autoantibodies: ELISA | Non-significant associations between autoantibodies and NVC patterns (defined as normal or SSc pattern). |
| De Santis, 2016 [25] | SSc patients, classified with ACR 2013 criteria | n = 44 F: 95.4% 66 (34–80) 9 (1–16) | ACA, ATA | ACA and ATA: ELISA | Non-significant associations between autoantibodies and “early”, “active” and “late” scleroderma patterns on NVC |
| Markusse, 2017 [28] | SSc patients, classified with ACR 2013 criteria or LeRoy criteria | n = 287 F: 82% 57 ± 14 3 (0.6–9) | ACA, ATA, RNAPIII, RNP, U3 RNP, Pm/Scl, Th/To, Ku | ANA: indirect immunofluorescence (IIF) on HEp-2 cells fluorescence ELISA | Non-significant associations between autoantibodies and “early”, “active” and “late” scleroderma patterns on NVC |
| Author, Year [Reference] | Sample Size (n) Classification Criteria Sex (F: %) Age, Average (Range) Duration of Disease or Symptoms in Years, Average (Range) | Autoantibodies | Immunoassays | Substrate | Results |
|---|---|---|---|---|---|
| Svegliati, 2017 [39] | n: 11 ACR/EULAR 2013 F: 81.9% Age: 56 (43–73) Disease duration: 7 (2–21) | Agonist Anti-PDGFRα | Fluorescence microscopy, FACS analysis | Human pulmonary artery smooth muscle cells | Anti-PDGFRα increased ROS production, NOX4 and mTORC1 expression in HPASMC inducing them to a synthetic state. |
| Raschi, 2020 [37] | n: 12 ACR/EULAR 2013 F: 100% Age: 47 (31–55) Disease duration: 30 (26–33) | ACA, ATA, ARA, anti-Th/To | IIF, chemiluminescent immunoassays, flow-cytometry analysis | Endothelial cells and fibroblasts | SSc-Ab-ICs induce a pro-inflammatory and pro-fibrotic endothelial cells phenotype. ACA-ICs, anti-Th/To-ICs: ↑ ICAM-1 All SSc-ICs but anti-Th/To-ICs augmented IL-8 levels ATA-ICs and anti-Th/To-ICs: ↑ ET-1 All SSc-ICs but ARA-ICs: ↑ TGF-Beta1 ATA-ICs and ACA-ICs: ↑ TLR-2, TLR-3 and TLR-4 on endothelial cells whereas anti-Th/To-ICs ↑ TLR9. Fibroblasts stimulated with with SSc-IC: ↑ TGF-beta1, alfa-SMA. Specifically, ATA-IC and ACA-IC: ↑colα1 and ACA-ICs: ↑ IL-6 |
| Fonseca, 2006 [38] | n: 205 ACR 1980 criteria F, age and disease duration not reported | ACA, ATA, ARA | NR | NR | ARA+ patients showed a higher frequency of polymorphisms for EDNRA compared with ARA- and HCs (p < 0.05) |
| Corallo, 2019 [36] | n: 20 ACR 2013 criteria F: 80% Age: NR Disease duration: 10 (2–15) | ACA, ATA | IIF | Dermal fibroblasts | Pro-fibrotic activation in the human dermal fibroblasts through an hyper-expression of pro-fibrotic genes (increased mRNA of ACTA2, COL1A1 and TAGLN) and upregulated protein synthesis of α-SMA, Col-1 and SM22 |
| Robitaille, 2009 [31] | ACR 1980 criteria Other data NR | ACA | ELISA | PASMCs (human pulmonary artery smooth muscle cells | Extracellular CENP-B autoantigens bind to human pulmonary artery smooth muscle cells (SMCs) stimulating their migration and their production of IL-6 and IL-8. |
| Robitaille, 2007 [32] | ACR 1980 criteria Other data NR | CENP-A and CENP-B | IIF, ELISA | Human pulmonary artery SMCs, normal human lung fibroblasts (NHLFs) and human pulmonary artery ECs | CENP-B does not bind fibroblasts or endothelial cells CENP-B released from apoptotic ECs was found to bind to SMCs. |
| Arcand, 2012 [34] | NR | ATA | ELISA | HDFs | The autoantigen topo I stimulated fibroblast migration via a G(αi) protein-coupled receptor, acting physiologically as a danger signal for the immune system to facilitate repair. CCR7 was found to interact directly with topo I. |
| Arcand 2012 [35] | NR | ATA | ELISA | HDFs | Topo I binds specifically to heparan sulfate proteoglycans on fibroblast surfaces and that anti-topo I autoantibodies from SSc patients amplify topo I binding to HS chains. The accumulation of topo I on cell surfaces by anti-topo I autoantibodies could contribute to the initiation of an inflammatory cascade stimulating the fibrosis. |
| Henault, 2004 [40] | 99 SSc ACR 1980 criteria Age, sex and disease duration NR | AFA, ATA | ELISA + immunoblot | Fibroblast Endothelial cells Human pulmonary artery smooth muscle cells | AFAs in SSc are strongly correlated with ATA which themselves display AFA activity by reacting with determinants at the fibroblast surface. |
| Henault, 2006 [33] | 37 SSc ACR 1980 criteria | ATA | IIF ELISA Immunobloting | Fibroblasts Endothelial cells Smooth muscle cells | The autoantigen topo I was found to bind specifically to fibroblasts in a dose-dependent manner, being recognized by ATA of SSc patients. The binding of ATA stimulated the adhesion and activation of cocultured monocytes. Topo I released from apoptotic endothelial cells was also found to bind specifically to fibroblasts. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hysa, E.; Campitiello, R.; Sammorì, S.; Gotelli, E.; Cere, A.; Pesce, G.; Pizzorni, C.; Paolino, S.; Sulli, A.; Smith, V.; et al. Specific Autoantibodies and Microvascular Damage Progression Assessed by Nailfold Videocapillaroscopy in Systemic Sclerosis: Are There Peculiar Associations? An Update. Antibodies 2023, 12, 3. https://doi.org/10.3390/antib12010003
Hysa E, Campitiello R, Sammorì S, Gotelli E, Cere A, Pesce G, Pizzorni C, Paolino S, Sulli A, Smith V, et al. Specific Autoantibodies and Microvascular Damage Progression Assessed by Nailfold Videocapillaroscopy in Systemic Sclerosis: Are There Peculiar Associations? An Update. Antibodies. 2023; 12(1):3. https://doi.org/10.3390/antib12010003
Chicago/Turabian StyleHysa, Elvis, Rosanna Campitiello, Silvia Sammorì, Emanuele Gotelli, Andrea Cere, Giampaola Pesce, Carmen Pizzorni, Sabrina Paolino, Alberto Sulli, Vanessa Smith, and et al. 2023. "Specific Autoantibodies and Microvascular Damage Progression Assessed by Nailfold Videocapillaroscopy in Systemic Sclerosis: Are There Peculiar Associations? An Update" Antibodies 12, no. 1: 3. https://doi.org/10.3390/antib12010003
APA StyleHysa, E., Campitiello, R., Sammorì, S., Gotelli, E., Cere, A., Pesce, G., Pizzorni, C., Paolino, S., Sulli, A., Smith, V., & Cutolo, M. (2023). Specific Autoantibodies and Microvascular Damage Progression Assessed by Nailfold Videocapillaroscopy in Systemic Sclerosis: Are There Peculiar Associations? An Update. Antibodies, 12(1), 3. https://doi.org/10.3390/antib12010003





