Spatial Variation in Environmental Impacts of Sugarcane Expansion in Brazil
Abstract
1. Introduction
2. Materials and Methods
2.1. Land Use Change Dynamics
2.2. LUC-Related CO2 Emissions
- CO2LUC = LUC-related CO2 emissions from sugarcane expansion, t CO2 ha−1year−1;
- Ct1 = Carbon stock in land use prior to conversion, t ha−1;
- Ct2 = Carbon stock in sugarcane land use after conversion, t ha−1;
- T = Amortization period, years;
- 44/12 = Conversion factor to convert C to CO2.
2.2.1. Biomass Carbon Stocks
2.2.2. Soil Organic Carbon Stocks
- SOCx = Soil organic carbon stock for land under land use/cover type x, t C ha−1;
- SOCref = The reference carbon stock, t C ha−1;
- FLU = Stock change factor for land use system x, unitless;
- FMG = Stock change factor for management regime for land use/cover x, unitless;
- FI = Stock change factor for input of organic matter for land use/cover x, unitless.
2.3. Mean Species Abundance
2.4. Soil Erosion
- A = Soil loss for sugarcane, t ha−1 h−1 year−1;
- R = Rainfall-runoff erosivity factor, MJ mm ha−1year−1;
- K = Soil erodibility factor t ha h ha−1 MJ−1 mm−1
- L = Slope-length factor, unitless;
- S = Slope steepness factor, unitless;
- C = Cover management factor, unitless;
- P = Conservation support practice factor, unitless.
2.5. Water Shortage
2.6. Environmental Performance Index
3. Results
3.1. Land Use Change Dynamics
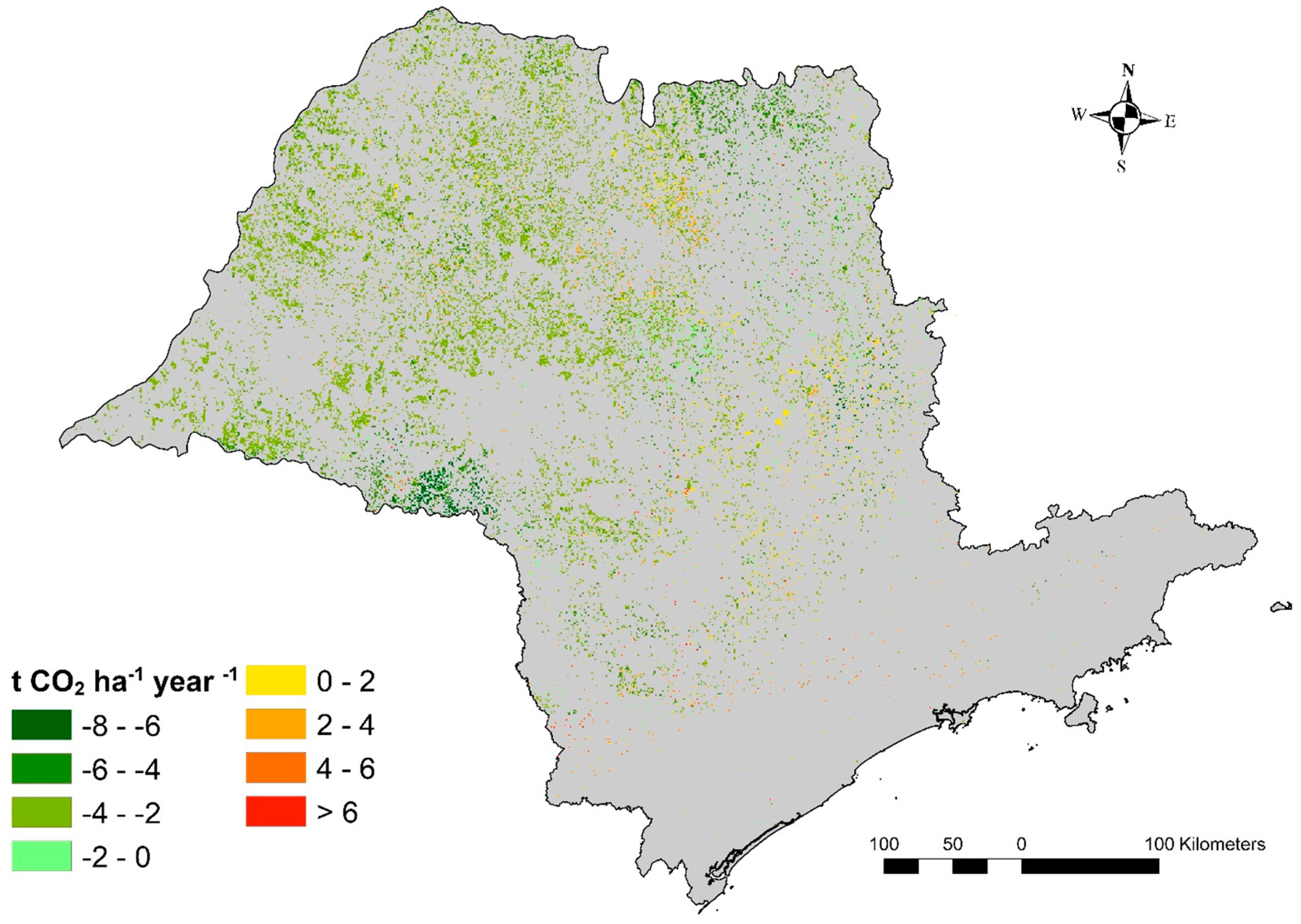
3.2. LUC-Related CO2 Emissions
3.3. Mean Species Abundance
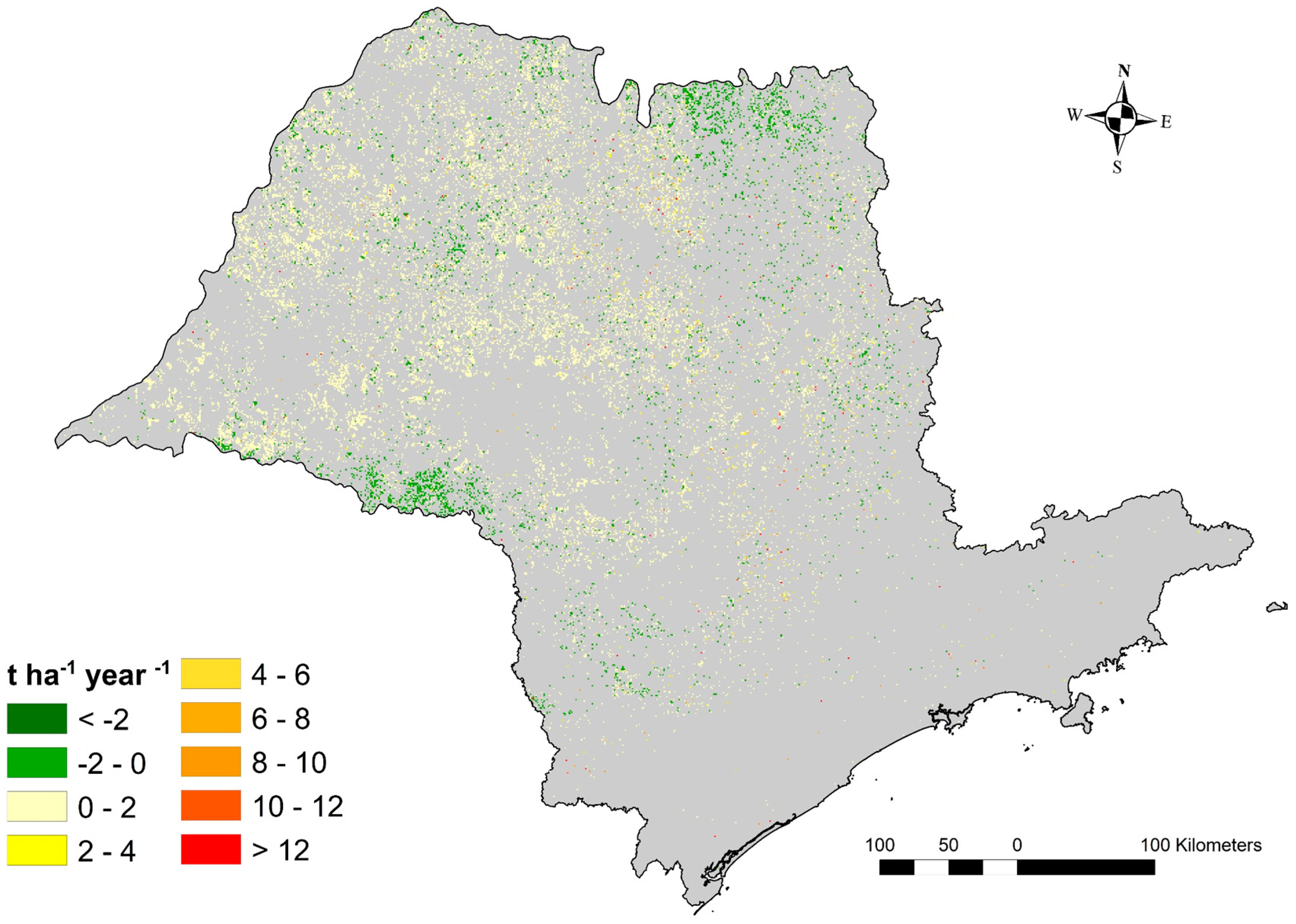
3.4. Soil Erosion
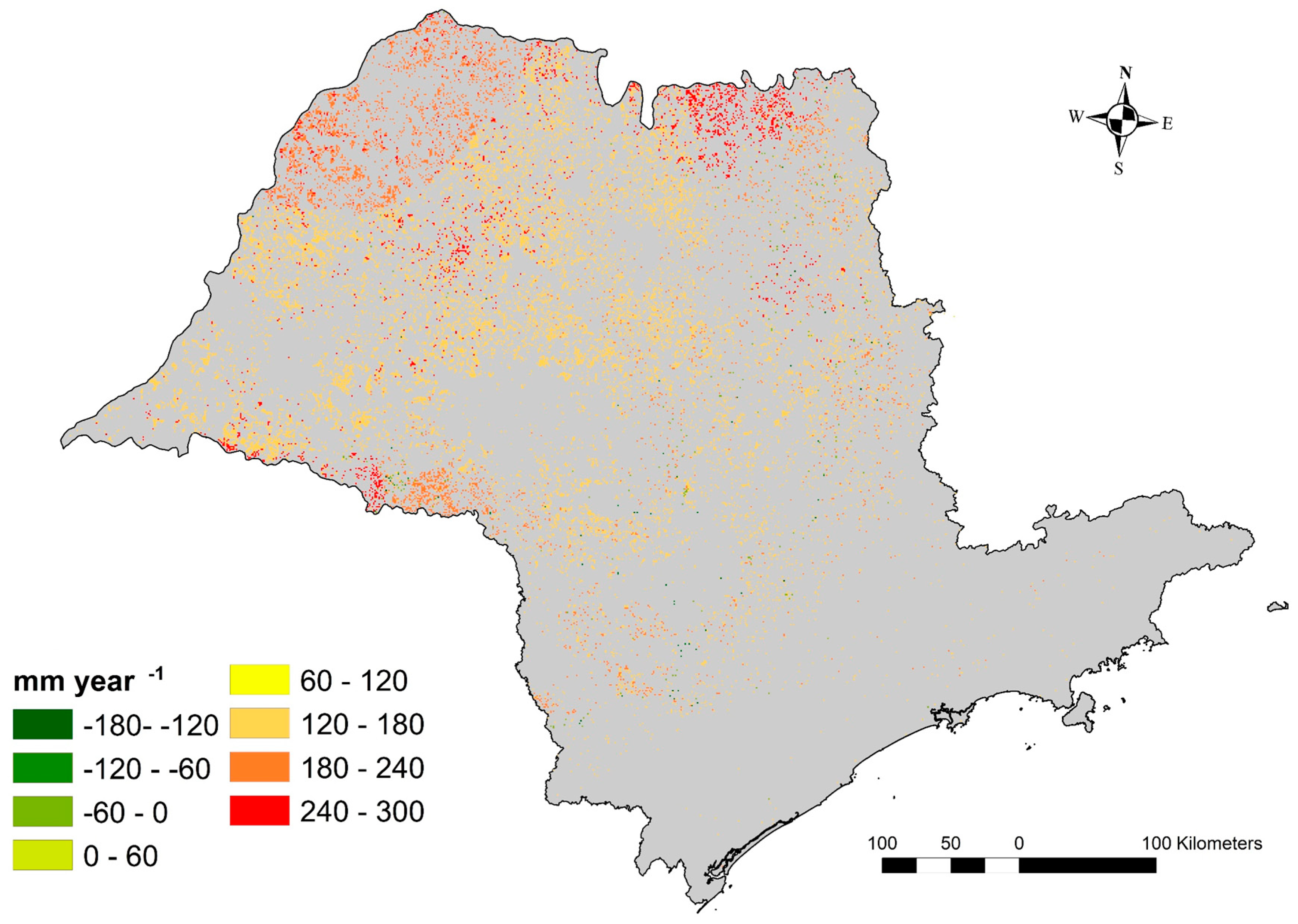
3.5. Water Shortage
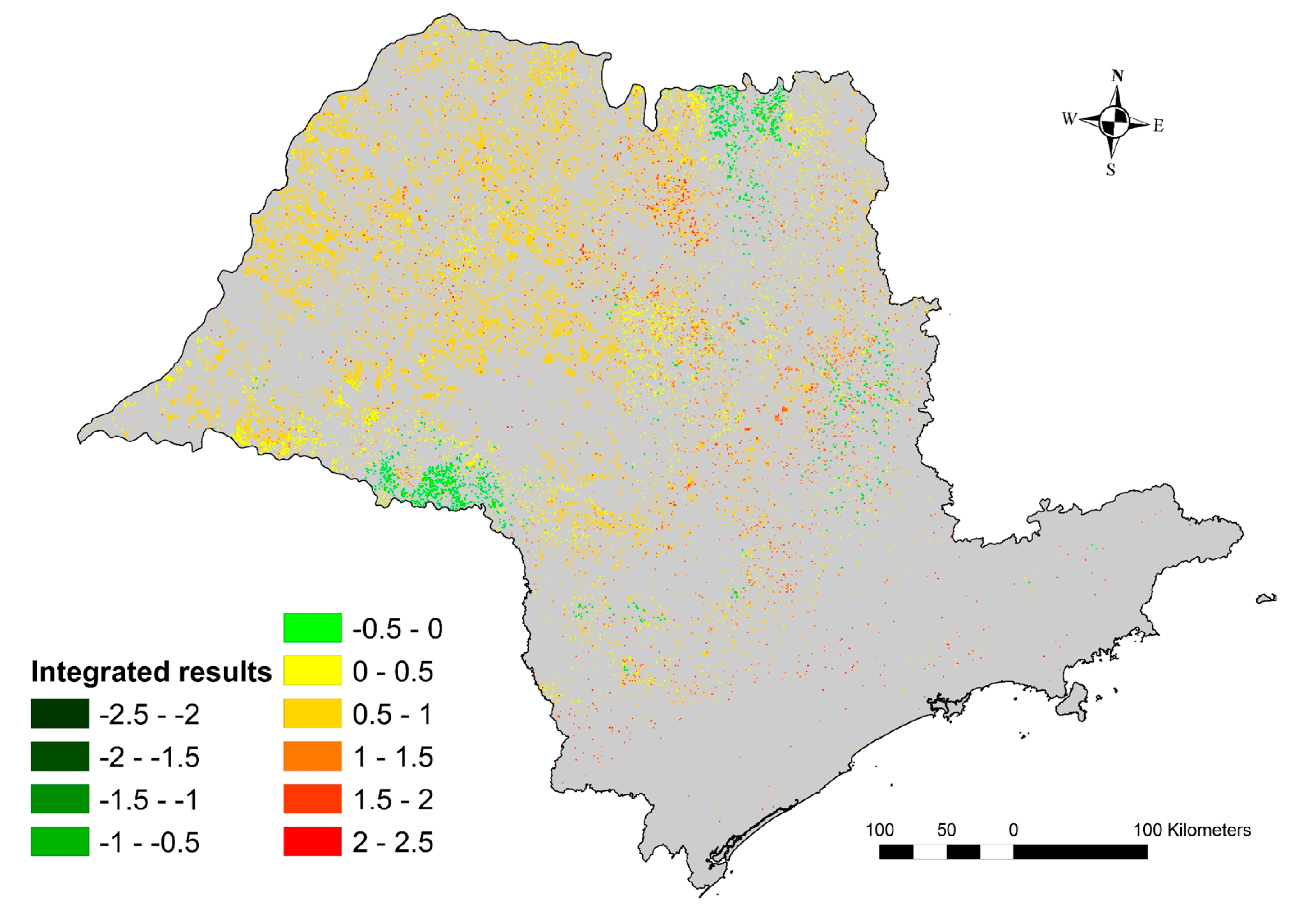
3.6. Environmental Performance Index
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rogelj, J.; Shindell, D.; Jiang, K.; Fifita, S.; Forster, P.; Ginzburg, V.; Handa, C.; Kheshgi, H.; Kobayashi, S.; Kriegler, E. Mitigation Pathways Compatible with 1.5 C in the Context of Sustainable Development. An IPCC Special Report on the Impacts og Global Warming of 1.5 C above Pre-Industrials Levels and Related Global Greenhouse Gas Emission Pathaways, in the Context of Strengthening the Global Response to the Threat of Climate Change. Sustainable Development, and Efforts to Erradicate Poverty, Geneva. 2018. Available online: https://pure.iiasa.ac.at/id/eprint/15515/ (accessed on 30 March 2019).
- IEA. World Energy Outlook 2018; IEA: Paris, France, 2018. [Google Scholar]
- Souza, G.M.; Ballester, M.V.R.; de Brito Cruz, C.H.; Chum, H.; Dale, B.; Dale, V.H.; Fernandes, E.C.M.; Foust, T.; Karp, A.; Lynd, L.; et al. The role of bioenergy in a climate-changing world. Environ. Dev. 2017, 23, 57–64. [Google Scholar]
- Renewable Fuel Association. RFA Industry Statistics. Available online: https://ethanolrfa.org/resources/industry/statistics/#1454098996479-8715d404-e546 (accessed on 16 October 2018).
- REN21. Renewables 2018 Global Status Report; UN Environment: Paris, France, 2018; Available online: http://www.ren21.net/wp-content/uploads/2018/06/17-8652_GSR2018_FullReport_web_final_.pdf (accessed on 10 June 2019).
- de Dias, M.O.S.; Maciel Filho, R.; Mantelatto, P.E.; Cavalett, O.; Rossell, C.E.V.; Bonomi, A.; Leal, M.R.L.V. Sugarcane processing for ethanol and sugar in Brazil. Environ. Dev. 2015, 15, 35–51. [Google Scholar] [CrossRef]
- UNICA. Sugarcane Planted Area, UNICADATA. 2018. Available online: http://www.unicadata.com.br/historico-de-area-ibge.php?idMn=33&tipoHistorico=5&acao=visualizar&idTabela=1972&produto=Planted+area&anoIni=2006&anoFim=2016&estado=RS%252CSC%252CPR%252CSP%252CRJ%252CMG%252CES%252CMS%252CMT%252CGO%252CDF%252CBA%252CSE%252CAL%252CPE%252CPB%252CRN%252CCE%252CPI%252 (accessed on 16 October 2018).
- de Bordonal, R.O.; Carvalho, J.L.N.; Lal, R.; de Figueiredo, E.B.; de Oliveira, B.G.; La Scala, N. Sustainability of sugarcane production in Brazil. A review. Agron. Sustain. Dev. 2018, 38, 13. [Google Scholar]
- Filoso, S.; do Carmo, J.B.; Mardegan, S.F.; Lins, S.R.M.; Gomes, T.F.; Martinelli, L.A. Reassessing the environmental impacts of sugarcane ethanol production in Brazil to help meet sustainability goals. Renew. Sustain. Energy Rev. 2015, 52, 1847–1856. [Google Scholar] [CrossRef]
- Walter, A.; Dolzan, P.; Quilodrán, O.; de Oliveira, J.G.; da Silva, C.; Piacente, F.; Segerstedt, A. Sustainability assessment of bio-ethanol production in Brazil considering land use change, GHG emissions and socio-economic aspects. Energy Policy 2011, 39, 5703–5716. [Google Scholar]
- Turetta, A.P.D.; Kuyper, T.; Malheiros, T.F.; da Coutinho, H.L.C. A framework proposal for sustainability assessment of sugarcane in Brazil. Land Use Policy 2017, 68, 597–603. [Google Scholar]
- de Bordonal, R.O.; Lal, R.; Ronquim, C.C.; de Figueiredo, E.B.; Carvalho, J.L.N.; Maldonado, W.; Milori, D.M.B.P.; La Scala, N. Changes in quantity and quality of soil carbon due to the land-use conversion to sugarcane (Saccharum officinarum) plantation in southern Brazil. Agric. Ecosyst. Environ. 2017, 240, 54–65. [Google Scholar]
- de Oliveira Bordonal, R.; Lal, R.; Alves Aguiar, D.; de Figueiredo, E.B.; Ito Perillo, L.; Adami, M.; Theodor Rudorff, B.F.; La Scala, N. Greenhouse gas balance from cultivation and direct land use change of recently established sugarcane (Saccharum officinarum) plantation in south-central Brazil. Renew. Sustain. Energy Rev. 2015, 52, 547–556. [Google Scholar]
- Egeskog, A.; Freitas, F.; Berndes, G.; Sparovek, G.; Wirsenius, S. Greenhouse gas balances and land use changes associated with the planned expansion (to 2020) of the sugarcane ethanol industry in Sao Paulo, Brazil. Biomass Bioenergy 2014, 63, 280–290. [Google Scholar]
- da Oliveira, D.M.S.; Paustian, K.; Davies, C.A.; Cherubin, M.R.; Franco, A.L.C.; Cerri, C.C.; Cerri, C.E.P. Soil carbon changes in areas undergoing expansion of sugarcane into pastures in south-central Brazil. Agric. Ecosyst. Environ. 2016, 228, 38–48. [Google Scholar]
- Franco, A.L.C.; Cherubin, M.R.; Pavinato, P.S.; Cerri, C.E.P.; Six, J.; Davies, C.A.; Cerri, C.C. Soil carbon, nitrogen and phosphorus changes under sugarcane expansion in Brazil. Sci. Total Environ. 2015, 515–516, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Mello, F.F.C.; Cerri, C.E.P.; Davies, C.A.; Holbrook, N.M.; Paustian, K.; Maia, S.M.F.; Galdos, M.V.; Bernoux, M.; Cerri, C.C. Payback time for soil carbon and sugar-cane ethanol. Nat. Clim. Chang. 2014, 4, 605–609. [Google Scholar] [CrossRef]
- Assad, E.D.; Pinto, H.S.; Martins, S.C.; Groppo, J.D.; Salgado, P.R.; Evangelista, B.; Vasconcellos, E.; Sano, E.E.; Pavão, E.; Luna, R. Changes in soil carbon stocks in Brazil due to land use: Paired site comparisons and a regional pasture soil survey. Biogeosciences 2013, 10, 6141–6160. [Google Scholar] [CrossRef]
- Alkimim, A.; Clarke, K.C. Land use change and the carbon debt for sugarcane ethanol production in Brazil. Land Use Policy 2018, 72, 65–73. [Google Scholar] [CrossRef]
- Silva-Olaya, A.M.; Davies, C.A.; Cerri, C.E.P.; Allen, D.J.; Mello, F.F.C.; Cerri, C.C. Quantifying above and belowground biomass carbon inputs for sugar-cane production in Brazil. Soil Res. 2017, 55, 640–648. [Google Scholar] [CrossRef]
- Newbold, T.; Hudson, L.N.; Hill, S.L.L.; Contu, S.; Lysenko, I.; Senior, R.A.; Börger, L.; Bennett, D.J.; Choimes, A.; Collen, B.; et al. Global effects of land use on local terrestrial biodiversity. Nature 2015, 520, 45. [Google Scholar] [CrossRef] [PubMed]
- Lapola, D.M.; Schaldach, R.; Alcamo, J.; Bondeau, A.; Koch, J.; Koelking, C.; Priess, J.A. Indirect land-use changes can overcome carbon savings from biofuels in Brazil. Proc. Natl. Acad. Sci. USA 2010, 107, 3388–3393. [Google Scholar] [CrossRef]
- Carvalho, F.M.V.; De Marco, P.; Ferreira, L.G. The Cerrado into-pieces: Habitat fragmentation as a function of landscape use in the savannas of central Brazil. Biol. Conserv. 2009, 142, 1392–1403. [Google Scholar] [CrossRef]
- Gasparatos, A.; Stromberg, P.; Takeuchi, K. Biofuels, ecosystem services and human wellbeing: Putting biofuels in the ecosystem services narrative. Agric. Ecosyst. Environ. 2011, 142, 111–128. [Google Scholar] [CrossRef]
- Ribeiro, M.C.; Martensen, A.C.; Metzger, J.P.; Tabarelli, M.; Scarano, F.; Fortin, M.-J. The Brazilian Atlantic Forest: A Shrinking Biodiversity Hotspot. In BT—Biodiversity Hotspots: Distribution and Protection of Conservation Priority Areas; Zachos, F.E., Habel, J.C., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 405–434. ISBN 978-3-642-20992-5. [Google Scholar]
- Dalle Laste, K.C.; Durigan, G.; Andersen, A.N. Biodiversity responses to land-use and restoration in a global biodiversity hotspot: Ant communities in Brazilian Cerrado. Austral Ecol. 2019, 44, 313–326. [Google Scholar] [CrossRef]
- de Moraes, M.C.P.; de Mello, K.; Toppa, R.H. Protected areas and agricultural expansion: Biodiversity conservation versus economic growth in the Southeast of Brazil. J. Environ. Manag. 2017, 188, 73–84. [Google Scholar]
- Oliveira, D.M.S.; Cherubin, M.R.; Franco, A.L.C.; Santos, A.S.; Gelain, J.G.; Dias, N.M.S.; Diniz, T.R.; Almeida, A.N.; Feigl, B.J.; Davies, C.A.; et al. Is the expansion of sugarcane over pasturelands a sustainable strategy for Brazil’s bioenergy industry? Renew. Sustain. Energy Rev. 2019, 102, 346–355. [Google Scholar]
- Martinelli, L.A.; Filoso, S. Expansion of sugarcane ethanol production in Brazil: Environmental and social challenges. Ecol. Appl. 2008, 18, 885–898. [Google Scholar]
- Hunke, P.; Mueller, E.N.; Schröder, B.; Zeilhofer, P. The Brazilian Cerrado: Assessment of water and soil degradation in catchments under intensive agricultural use. Ecohydrology 2015, 8, 1154–1180. [Google Scholar]
- Youlton, C.; Wendland, E.; Anache, J.A.A.; Poblete-Echeverría, C.; Dabney, S. Changes in erosion and runoff due to replacement of pasture land with sugarcane crops. Sustainability 2016, 8, 685. [Google Scholar]
- Cherubin, M.R.; Franco, A.L.C.; Cerri, C.E.P.; da Oliveira, D.M.S.; Davies, C.A.; Cerri, C.C. Sugarcane expansion in Brazilian tropical soils—Effects of land use change on soil chemical attributes. Agric. Ecosyst. Environ. 2015, 211, 173–184. [Google Scholar]
- Cherubin, M.R.; Karlen, D.L.; Franco, A.L.C.; Cerri, C.E.P.; Tormena, C.A.; Cerri, C.C. A Soil Management Assessment Framework (SMAF) evaluation of Brazilian sugarcane expansion on soil quality. Soil Sci. Soc. Am. J. 2016, 80, 215–226. [Google Scholar]
- Watkins, D.W.; de Moraes, M.M.G.A.; Asbjornsen, H.; Mayer, A.S.; Licata, J.; Lopez, J.G.; Pypker, T.G.; Molina, V.G.; Marques, G.F.; Carneiro, A.C.G.; et al. Bioenergy Development Policy and Practice Must Recognize Potential Hydrologic Impacts: Lessons from the Americas. Environ. Manag. 2015, 56, 1295–1314. [Google Scholar]
- Fachinelli, N.P.; Pereira, A.O. Impacts of sugarcane ethanol production in the Paranaiba basin water resources. Biomass Bioenergy 2015, 83, 8–16. [Google Scholar]
- Spera, S.A.; Galford, G.L.; Coe, M.T.; Macedo, M.N.; Mustard, J.F. Land-use change affects water recycling in Brazil’s last agricultural frontier. Glob. Chang. Biol. 2016, 22, 3405–3413. [Google Scholar]
- Hernandes, T.A.D.; Bufon, V.B.; Seabra, J.E.A. Water footprint of biofuels in Brazil: Assessing regional differences. Biofuels Bioprod. Biorefin. 2013, 8, 241–252. [Google Scholar] [CrossRef]
- Georgescu, M.; Lobell, D.B.; Field, C.B.; Mahalov, A. Simulated hydroclimatic impacts of projected Brazilian sugarcane expansion. Geophys. Res. Lett. 2013, 40, 972–977. [Google Scholar] [CrossRef]
- UNICA. Ethanol Production Data, UNICADATA. 2020. Available online: http://www.unicadata.com.br/historico-de-producao-e-moagem.php?idMn=31&tipoHistorico=2&acao=visualizar&idTabela=1985&produto=etanol_total&safraIni=1980%252F1981&safraFim=2017%252F2018&estado=RS%252CSC%252CPR%252CSP%252CRJ%252CMG%252CES%252CMS%252CMT%252CG (accessed on 21 September 2018).
- MME. Programa RenovaBIO. 2017. Available online: http://www.mme.gov.br/web/guest/secretarias/petroleo-gas-natural-e-combustiveis-renovaveis/programas/renovabio/principal (accessed on 20 November 2019).
- van der Hilst, F.; Verstegen, J.A.; Woltjer, G.; Smeets, E.M.W.; Faaij, A.P.C. Mapping land use changes resulting from biofuel production and the effect of mitigation measures. GCB Bioenergy 2018, 10, 804–824. [Google Scholar] [CrossRef]
- IPCC. 2006 IPCC Guidelines for National Greenhouse Gas Inventories; Institute for Global Environmental Strategies: Kanagawa, Japan, 2006; Available online: https://www.ipcc-nggip.iges.or.jp/public/2006gl/ (accessed on 15 January 2018).
- Alkemade, R.; van Oorschot, M.; Miles, L.; Nellemann, C.; Bakkenes, M.; ten Brink, B. GLOBIO3: A Framework to Investigate Options for Reducing Global Terrestrial Biodiversity Loss. Ecosystems 2009, 12, 374–390. [Google Scholar] [CrossRef]
- Renard, K.G.; Foster, G.R.; Weesies, G.A.; McCool, D.K.; Yoder, D.C. Predicting Soil Erosion by Water: A Guide to Conservation Planning with the Revised Universal Soil Loss Equation (RUSLE); United States Department of Agriculture: Washington, DC, USA, 1997; Volume 703. [Google Scholar]
- Brouwer, C.; Heibloem, M. Irrigation water management: Irrigation water needs. Train. Man. 1986, 3, 1–102. [Google Scholar]
- TUDelft. Land Cover Maps from 2003 up to 2015 for São Paulo State–Brazil. 2016. Available online: http://be-basic.grs.tudelft.nl/maps/316#more (accessed on 26 May 2016).
- FAO and IIASA. Crop Suitability Index (Value) for Intermediate Input Level Rain-Fed Sugarcane. 2012. Available online: http://gaez.fao.org/Main.html# (accessed on 13 July 2018).
- FAO and IIASA. Crop Suitability Index (Value) for Intermediate Input Level Rain-Fed Alfalfa. 2012. Available online: http://gaez.fao.org/Main.html# (accessed on 13 July 2018).
- Avitabile, V.; Herold, M.; Heuvelink, G.B.M.; Lewis, S.L.; Phillips, O.L.; Asner, G.P.; Armston, J.; Ashton, P.S.; Banin, L.; Bayol, N.; et al. An integrated pan-tropical biomass map using multiple reference datasets. Glob. Chang. Biol. 2016, 22, 1406–1420. [Google Scholar] [CrossRef] [PubMed]
- IPBES. Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; IPBES: Bonn, Germany, 2019. [Google Scholar]
- Trisurat, Y.; Alkemade, R.; Verburg, P.H. Projecting Land-Use Change and Its Consequences for Biodiversity in Northern Thailand. Environ. Manag. 2010, 45, 626–639. [Google Scholar] [CrossRef]
- Petz, K.; Alkemade, R.; Bakkenes, M.; Schulp, C.J.E.; van der Velde, M.; Leemans, R. Mapping and modelling trade-offs and synergies between grazing intensity and ecosystem services in rangelands using global-scale datasets and models. Glob. Environ. Chang. 2014, 29, 223–234. [Google Scholar]
- van Rooij, W. Manual for Biodiversity Modelling on a National Scale Using GLOBIO3 and CLUE Methodology to Calculate Current and Future Status of Biodiversity; MNP: Bilthoven, The Netherlands, 2008. [Google Scholar]
- Smith, P.; House, J.I.; Bustamante, M.; Sobocká, J.; Harper, R.; Pan, G.; West, P.C.; Clark, J.M.; Adhya, T.; Rumpel, C.; et al. Global change pressures on soils from land use and management. Glob. Chang. Biol. 2016, 22, 1008–1028. [Google Scholar] [CrossRef]
- de Souza, G.S.; de Souza, Z.M.; da Silva, R.B.; Barbosa, R.S.; Araújo, F.S. Effects of traffic control on the soil physical quality and the cultivation of sugarcane. Rev. Bras. Ciênc. Solo 2014, 38, 135–146. [Google Scholar] [CrossRef]
- Wischmeier, W.H.; Smith, D.D. Predicting Rainfall Erosion Losses—A Guide to Conservation Planning; USDA, Science and Education Administration: Hyattsville, MD, USA, 1978. [Google Scholar]
- de Medeiros, G.O.R.; Giarolla, A.; Sampaio, G.; de Marinho, M.A. Estimates of Annual Soil Loss Rates in the State of São Paulo, Brazil. Rev. Bras. Ciênc. Solo 2016, 40. [Google Scholar] [CrossRef]
- Laflen, J.M.; Moldenhauer, W.C. The USLE story. Spec. Publ. WASWC 2003, 1, 1–54. [Google Scholar]
- Panagos, P.; Meusburger, K.; Ballabio, C.; Borrelli, P.; Alewell, C. Soil erodibility in Europe: A high-resolution dataset based on LUCAS. Sci. Total Environ. 2014, 479, 189–200. [Google Scholar] [CrossRef]
- Panagos, P.; Borrelli, P.; Meusburger, K.; van der Zanden, E.H.; Poesen, J.; Alewell, C. Modelling the effect of support practices (P-factor) on the reduction of soil erosion by water at European scale. Environ. Sci. Policy 2015, 51, 23–34. [Google Scholar] [CrossRef]
- Scanlon, B.R.; Reedy, R.C.; Stonestrom, D.A.; Prudic, D.E.; Dennehy, K.F. Impact of land use and land cover change on groundwater recharge and quality in the southwestern US. Glob. Chang. Biol. 2005, 11, 1577–1593. [Google Scholar] [CrossRef]
- Edwards, R.; O’Connell, A.; Padella, M.; Mulligan, D.; Giuntoli, J.; Agostini, A.; Koeble, R.; Moro, A.; Marelli, L. Definition of input data to assess GHG default emissions from biofuels in EU legislation. JRC Sci. Policy Rep. EUR 2016, 11, 1577–1593. [Google Scholar]
- European Parlament. Directive (EU) 2018/2001 of the European parliament and of the council of 11 December 2018 on the promotion of the use of energy from renewable sources. Off. J. Eur. Union 2018, 328, 1–128. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32018L2001&from=EN (accessed on 20 February 2019).
- Kennedy, C.M.; Hawthorne, P.L.; Sochi, K.; Miteva, D.A.; Baumgarten, L.; Uhlhorn, E.M.; Kiesecker, J.M. Biofuels Expansion and Environmental Quality in Brazil BT—Energy Sprawl Solutions: Balancing Global Development and Conservation; Kiesecker, J.M., Naugle, D.E., Eds.; Island Press/Center for Resource Economics: Washington, DC, USA, 2017; pp. 100–111. ISBN 978-1-61091-723-0. [Google Scholar]
- Merten, G.H.; Minella, J.P.G. The expansion of Brazilian agriculture: Soil erosion scenarios. Int. Soil Water Conserv. Res. 2013, 1, 37–48. [Google Scholar] [CrossRef]
- Bertoni, J.; Neto, F.L. Conservação do solo; Ícone: São Paulo, Brazil, 2005; ISBN 8527401436. [Google Scholar]
- Cherubin, M.R.; Karlen, D.L.; Franco, A.L.C.; Tormena, C.A.; Cerri, C.E.P.; Davies, C.A.; Cerri, C.C. Soil physical quality response to sugarcane expansion in Brazil. Geoderma 2016, 267, 156–168. [Google Scholar] [CrossRef]
- da Rocha, G.C. Conservação do solo e Cana-de-Açúcar: Aspectos Legais e Bibliométricos e uma Ferramenta de Determinação do Fator C; Universidade de São Paulo: São Paulo, Brazil, 2017. [Google Scholar]
- Cervi, W.R.; Lamparelli, R.A.C.; Seabra, J.E.A.; Junginger, M.; de Jong, S.; van der Hilst, F. Spatial modeling of techno-economic potential of biojet fuel production in Brazil. GCB Bioenergy 2020, 12, 136–157. [Google Scholar] [CrossRef]
- Wiedenfeld, B. Effects of irrigation water salinity and electrostatic water treatment for sugarcane production. Agric. Water Manag. 2008, 95, 85–88. [Google Scholar] [CrossRef]
- da Silva, V.D.; da Silva, B.B.; Albuquerque, W.G.; Borges, C.J.; de Sousa, I.F.; Neto, J.D. Crop coefficient, water requirements, yield and water use efficiency of sugarcane growth in Brazil. Agric. Water Manag. 2013, 128, 102–109. [Google Scholar]
- Taye, G.; Vanmaercke, M.; Poesen, J.; Van Wesemael, B.; Tesfaye, S.; Teka, D.; Nyssen, J.; Deckers, J.; Haregeweyn, N. Determining RUSLE P- and C-factors for stone bunds and trenches in rangeland and cropland, North Ethiopia. Land Degrad. Dev. 2018, 29, 812–824. [Google Scholar]
- Wicke, B.; Verweij, P.; Meijl, H.; Vuuren, D.P.; Faaij, A.P. Indirect land use change: Review of existing models and strategies for mitigation. Biofuels 2012, 3, 87–100. [Google Scholar]
- Verstegen, J.A.; van der Hilst, F.; Woltjer, G.; Karssenberg, D.; de Jong, S.M.; Faaij, A.P.C. What can and can’t we say about indirect land-use change in Brazil using an integrated economic–land-use change model? GCB Bioenergy 2016, 8, 561–578. [Google Scholar]
- de Miranda, S.D.C.; Bustamante, M.; Palace, M.; Hagen, S.; Keller, M.; Ferreira, L.G. Regional variations in biomass distribution in Brazilian savanna woodland. Biotropica 2014, 46, 125–138. [Google Scholar]
- Herrera, M.C. Cane, Sugar and the Environment; FAO: Cuba, Caribbean, 1999. [Google Scholar]
- UNICA. Sugarcane Production Data, UNICADATA. 2018. Available online: http://www.unicadata.com.br/historico-de-producao-e-moagem.php?idMn=31&tipoHistorico=2&acao=visualizar&idTabela=1985&produto=cana&safraIni=2003%2F2004&safraFim=2015%2F2016&estado=RS%2CSC%2CPR%2CSP%2CRJ%2CMG%2CES%2CMS%2CMT%2CGO%2CDF%2CBA%2CSE%2CAL%2CPE%2CP (accessed on 15 October 2018).
- Menandro, L.M.S.; Cantarella, H.; Franco, H.C.J.; Kölln, O.T.; Pimenta, M.T.B.; Sanches, G.M.; Rabelo, S.C.; Carvalho, J.L.N. Comprehensive assessment of sugarcane straw: Implications for biomass and bioenergy production. Biofuels Bioprod. Biorefining 2017, 11, 488–504. [Google Scholar]
- Zinn, Y.; Resck, D.V.; da Silva, J.E. Soil organic carbon as affected by afforestation with Eucalyptus and Pinus in the Cerrado region of Brazil. For. Ecol. Manag. 2002, 166, 285–294. [Google Scholar] [CrossRef]
- Macedo, I.C.; Seabra, J.E.A.; Silva, J.E.A.R. Green house gases emissions in the production and use of ethanol from sugarcane in Brazil: The 2005/2006 averages and a prediction for 2020. Biomass Bioenergy 2008, 32, 582–595. [Google Scholar]
- De Figueiredo, E.B.; La Scala, N. Greenhouse gas balance due to the conversion of sugarcane areas from burned to green harvest in Brazil. Agric. Ecosyst. Environ. 2011, 141, 77–85. [Google Scholar]
- de Freitas, P.L.; Landers, J.N. The Transformation of Agriculture in Brazil through Development and Adoption of Zero Tillage Conservation Agriculture. Int. Soil Water Conserv. Res. 2014, 2, 35–46. [Google Scholar] [CrossRef]
- da Silva, A.M. Rainfall erosivity map for Brazil. CATENA 2004, 57, 251–259. [Google Scholar] [CrossRef]
- IBGE. Mapa do Solos do Brail. 2001. Available online: https://mapas.ibge.gov.br/tematicos/solos (accessed on 3 June 2016).
- da Silva, A.M.; Alvares, C.A. Levantamento de informações e estruturação de um banco dados sobre a erodibilidade de classes de solos no estado de São Paulo. Geociências 2007, 24, 33–41. [Google Scholar]
- Zhang, H.; Yang, Q.; Li, R.; Liu, Q.; Moore, D.; He, P.; Ritsema, C.J.; Geissen, V. Extension of a GIS procedure for calculating the RUSLE equation LS factor. Comput. Geosci. 2013, 52, 177–188. [Google Scholar]
- Moore, I.D.; Burch, G.J. Physical basis of the length-slope factor in the universal soil loss equation 1. Soil Sci. Soc. Am. J. 1986, 50, 1294–1298. [Google Scholar]
- Moore, I.D.; Wilson, J.P. Length-slope factors for the Revised Universal Soil Loss Equation: Simplified method of estimation. J. Soil Water Conserv. 1992, 47, 423–428. [Google Scholar]
- Hrabalikova, M.; Janeček, M. Comparison of different approaches to LS factor calculations based on a measured soil loss under simulated rainfall. Soil Water Res. 2017, 12, 69–77. [Google Scholar] [CrossRef]
- Weber, E.; Hasenack, H.; Ferreira, C.J.S. Adaptação do Modelo Digital de Elevação do SRTM para o Sistema de Referência Oficial Brasileiro e Recorte por Unidade da Federação; UFRGS Centro de Ecologia: Porto Alegre, Brazil, 2004; Available online: https://www.ufrgs.br/labgeo/index.php/50-dados-espaciais/262-modelos-digitais-de-elevacao-dos-estados-brasileiros-obtidos-a-partir-do-srtm-shuttle-radar-topography-mission (accessed on 19 October 2020).
- Silva, B.P.C.; Silva, M.L.N.; Batista, P.V.G.; Pontes, L.M.; Araújo, E.F.; Curi, N. Soil and water losses in eucalyptus plantation and natural forest and determination of the USLE factors at a pilot sub-basin in Rio Grande do Sul, Brazil. Ciênc. Agrotecnol. 2016, 40, 432–442. [Google Scholar]
- da Silva, M.A.; Silva, M.L.N.; Curi, N.; Oliveira, A.H.; Avanzi, J.C.; Norton, L.D. Water erosion risk prediction in eucalyptus plantations. Ciênc. Agrotecnol. 2014, 38, 160–172. [Google Scholar]
- Panagos, P.; Borrelli, P.; Meusburger, K.; Alewell, C.; Lugato, E.; Montanarella, L. Estimating the soil erosion cover-management factor at the European scale. Land Use Policy 2015, 48, 38–50. [Google Scholar]
- da Silva, A.M.; Casatti, L.; Alvares, C.A.; Leite, A.M.; Martinelli, L.A.; Durrant, S.F. Soil loss risk and habitat quality in streams of a meso-scale river basin. Sci. Agric. 2007, 64, 336–343. [Google Scholar] [CrossRef]
- Turc, L. Estimation of irrigation water requirements, potential evapotranspiration: A simple climatic formula evolved up to date. Ann. Agron 1961, 12, 13–49. [Google Scholar]
- Rudorff, B.F.T.; Aguiar, D.A.; Silva, W.F.; Sugawara, L.M.; Adami, M.; Moreira, M.A. Studies on the rapid expansion of sugarcane for ethanol production in São Paulo State (Brazil) using Landsat data. Remote Sens. 2010, 2, 1057–1076. [Google Scholar] [CrossRef]
- Jantalia, C.P.; Resck, D.V.S.; Alves, B.J.R.; Zotarelli, L.; Urquiaga, S.; Boddey, R.M. Tillage effect on C stocks of a clayey Oxisol under a soybean-based crop rotation in the Brazilian Cerrado region. Soil Tillage Res. 2007, 95, 97–109. [Google Scholar] [CrossRef]
- De Oliveira Ferreira Silva, C.; Lilla Manzione, R.; Albuquerque Filho, J.L. Large-scale spatial modeling of crop coefficient and biomass production in agroecosystems in Southeast Brazil. Horticulturae 2018, 4, 44. [Google Scholar] [CrossRef]
- INMET. Dados Historicos. 2018. Available online: http://www.inmet.gov.br/portal/index.php?r=bdmep/bdmep (accessed on 26 May 2016).
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Guidelines for computing crop water requirements-FAO Irrigation and drainage paper 56, FAO-Food and Agriculture Organisation of the United Nations, Rome (http://www. fao. org/docrep) ARPAV (2000), La caratterizzazione climatica della Regione Veneto, Quade. Geophysics 1998, 156, 178. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vera, I.; Wicke, B.; Hilst, F.v.d. Spatial Variation in Environmental Impacts of Sugarcane Expansion in Brazil. Land 2020, 9, 397. https://doi.org/10.3390/land9100397
Vera I, Wicke B, Hilst Fvd. Spatial Variation in Environmental Impacts of Sugarcane Expansion in Brazil. Land. 2020; 9(10):397. https://doi.org/10.3390/land9100397
Chicago/Turabian StyleVera, Ivan, Birka Wicke, and Floor van der Hilst. 2020. "Spatial Variation in Environmental Impacts of Sugarcane Expansion in Brazil" Land 9, no. 10: 397. https://doi.org/10.3390/land9100397
APA StyleVera, I., Wicke, B., & Hilst, F. v. d. (2020). Spatial Variation in Environmental Impacts of Sugarcane Expansion in Brazil. Land, 9(10), 397. https://doi.org/10.3390/land9100397




