Assessing Stormwater Nutrient and Heavy Metal Plant Uptake in an Experimental Bioretention Pond
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
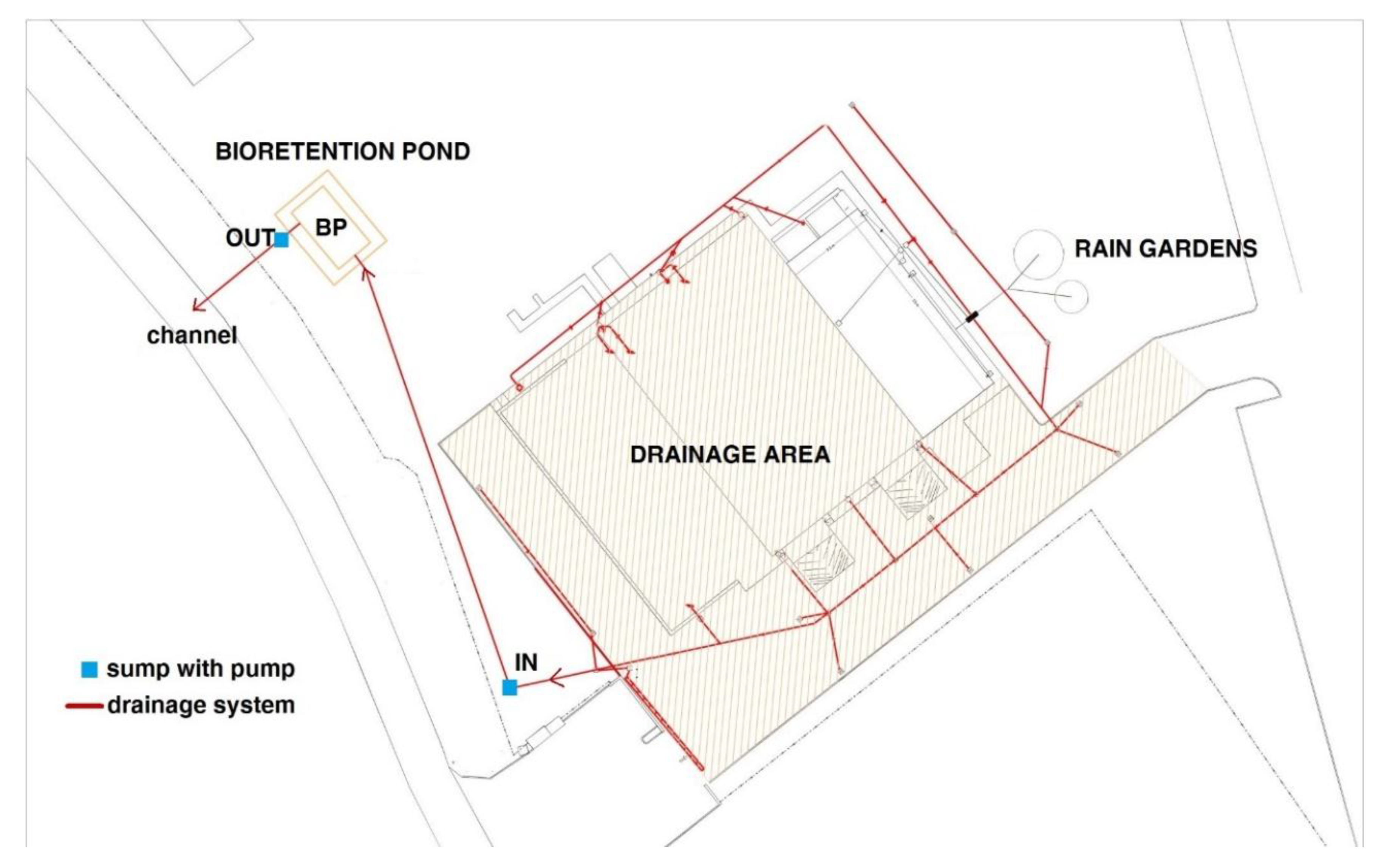
3.1. Hydrological Behaviour of the BP
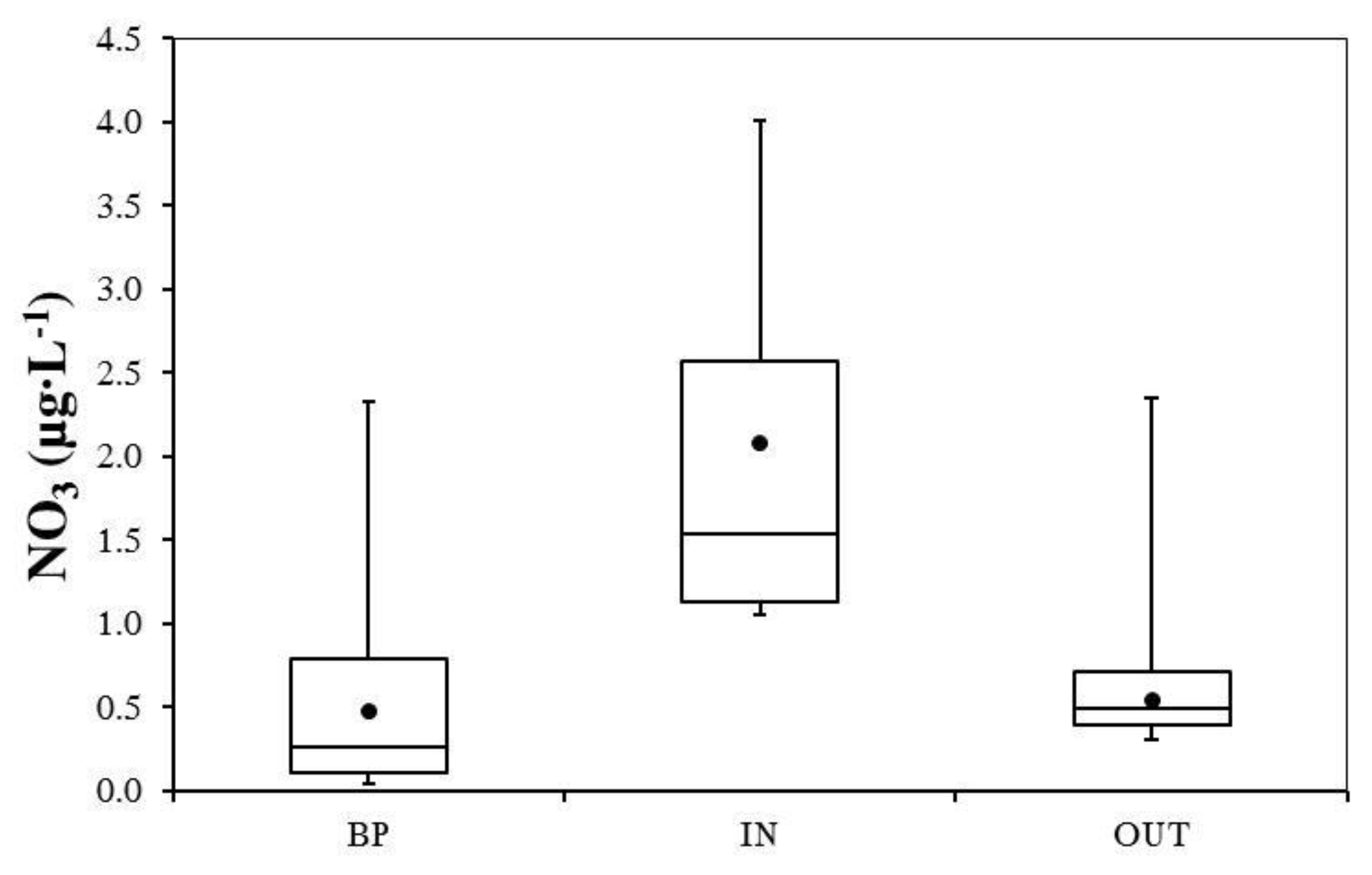
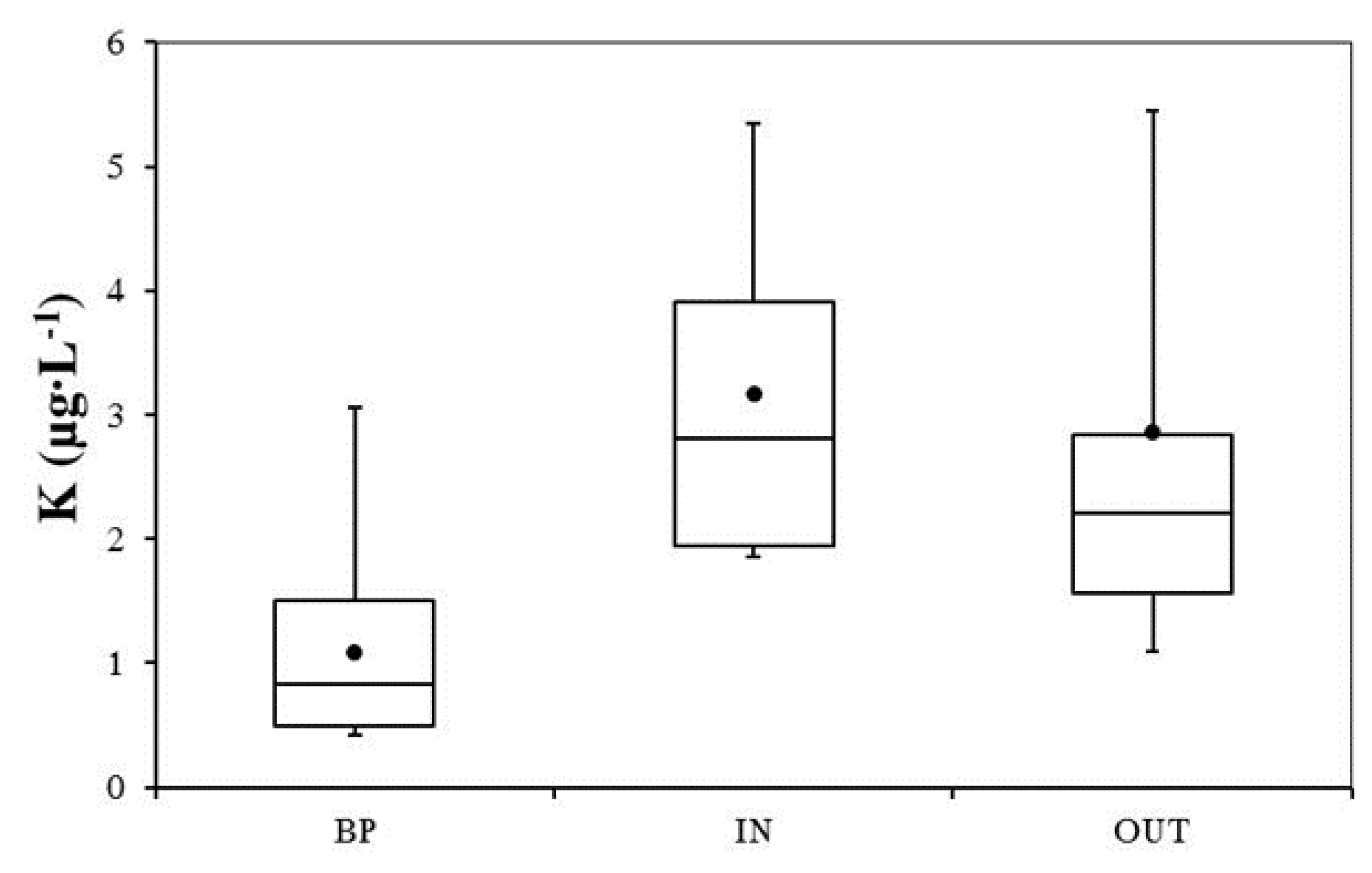
3.2. Nutrient and Heavy Metal Concentration in Stormwater
3.3. Plant Growth and Heavy Metal Accumulation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jennings, D.B.; Jarnagin, S.T. Changes in anthropogenic impervious surfaces, precipitation and daily streamflow discharge: A historical perspective in a mid-Atlantic subwatershed. Landscape Ecol. 2002, 17, 471–489. [Google Scholar] [CrossRef]
- Dietz, M.E.; Clausen, J.C. Stormwater runoff and export changes with development in a traditional and low impact subdivision. J. Environ. Manag. 2008, 87, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Deletic, A.; Maksumovic, C.T. Evaluation of water quality factors in storm runoff from paved areas. J. Environ. Eng. 1998, 124, 869–879. [Google Scholar] [CrossRef]
- US EPA. Handbook—Urban Runoff Pollution Prevention and Control Planning; EPA 625-R-93-004; U.S. Environmental Protection Agency: Washington, DC, USA, 1993.
- Bronstert, A.; Niehoff, D.; Bürger, G. Effects of climate and land-use change on storm runoff generation: Present knowledge and modelling capabilities. Hydrol. Process. 2002, 16, 509–529. [Google Scholar] [CrossRef]
- Sofia, G.; Roder, G.; Dalla Fontana, G.; Tarolli, P. Flood dynamics in urbanised landscapes: 100 years of climate and humans’ interaction. Sci. Rep. 2017, 7, 40527. [Google Scholar] [CrossRef] [PubMed]
- D’Arcy, B.; Frost, A. The role of best management practices in alleviating water quality problems associated with diffuse pollution. Sci. Total Environ. 2001, 265, 359–367. [Google Scholar] [CrossRef]
- Lloyd, S.D. Water Sensitive Urban Design in the Australian Context; Technical Report No. 01/7; Cooperative Research Centre for Catchment Hydrology: Melbourne, Australia, 2001. [Google Scholar]
- Prince George’s County. Bioretention Manual; PGC: Landover, MD, USA, 2007. [Google Scholar]
- Woods-Ballard, B.; Kellagher, R.; Martin, P.; Jefferies, C.; Bray, R.; Shaffer, P. The SUDS Manual; CIRIA C697: London, UK, 2007. [Google Scholar]
- Field, R.; Tafuri, A.N.; Muthukrishnan, S.; Acquisto, B.A.; Selvakumar, A. (Eds.) The Use of Best Management Practices (BMPs) in Urban Watersheds; DEStech Publications Inc.: Lancaster, PA, USA, 2006. [Google Scholar]
- Fletcher, T.D.; Shuster, W.; Hunt, W.F.; Ashley, R.; Butler, D.; Arthur, S.; Trowsdale, S.; Barraud, S.; Semadeni-Davies, A.; Bertrand-Krajewski, J.; et al. SUDS, LID, BMPs, WSUD and more–The evolution and application of terminology surrounding urban drainage. Urban Water J. 2015, 12, 525–542. [Google Scholar] [CrossRef]
- Prince George’s County. Low-Impact Development Design Strategies: An Integrated Design Approach; MD Department of Environmental Resources: Largo, MD, USA, 2000. [Google Scholar]
- US EPA. National Management Measures to Control Nonpoint Source Pollution from Urban Areas; EPA 841-B-05-004; U.S. Environmental Protection Agency: Washington, DC, USA, 2005. [Google Scholar]
- Coutts, A.M.; Tapper, N.J.; Beringer, J.; Loughnan, M.; Demuzere, M. Watering our cities: The capacity for Water Sensitive Urban Design to support urban cooling and improve human thermal comfort in the Australian context. Prog. Phys. Geogr. 2013, 37, 2–28. [Google Scholar] [CrossRef]
- Melbourne Water. WSUD Engineering Procedures: Stormwater; CSIRO Publishing: Melbourne, Australia, 2005. [Google Scholar]
- CIRIA. Sustainable Urban Drainage Systems: Design Manual for Scotland and Northern Ireland; CIRIA C521; Construction Industry Research and Information Association: London, UK, 2000. [Google Scholar]
- Dietz, M.E. Low impact development practices: A review of current research and recommendations for future directions. Water Air Soil Pollut. 2007, 186, 351–363. [Google Scholar] [CrossRef]
- Chang, N.B. Hydrological connections between low-impact development, watershed best management practices, and sustainable development. J. Hydrol. Eng. 2010, 15, 384–385. [Google Scholar] [CrossRef]
- Roy-Poirier, A.; Champagne, P.; Filion, Y. Review of bioretention system research and design: Past, present, and future. J. Environ. Eng. 2010, 136, 878–889. [Google Scholar] [CrossRef]
- Bortolini, L.; Semenzato, P. Low impact development techniques for urban sustainable design: A rain garden case study. Acta Hortic. 2010, 881, 327–330. [Google Scholar] [CrossRef]
- Brears, R.C. Blue-Green Infrastructure in Managing Urban Water Resources. In Blue and Green Cities; Palgrave Macmillan Ed.: London, UK, 2018; pp. 43–61. [Google Scholar]
- DeBusk, K.M.; Hunt, W.F.; Line, D.E. Bioretention outflow: Does it mimic nonurban watershed shallow interflow? J. Hydrol. Eng. 2010, 16, 274–279. [Google Scholar] [CrossRef]
- Dietz, M.E.; Clausen, J.C. A field evaluation of rain garden flow and pollutant treatment. Water Air Soil Pollut. 2005, 167, 123–138. [Google Scholar] [CrossRef]
- Hunt, W.F.; Jarrett, A.R.; Smith, J.T.; Sharkey, J.L. Evaluating bioretention hydrology and nutrient removal at three field sites in North Carolina. J. Irrig. Drain. Eng. 2006, 132, 600–608. [Google Scholar] [CrossRef]
- Muthanna, T.M.; Viklander, M.; Blecken, G.T.; Thorolfsson, S.T. Snowmelt pollutant removal in bioretention areas. Water Res. 2007, 41, 4061–4072. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.P. Field performance of bioretention: Hydrology impacts. J. Hydrol. Eng. 2008, 13, 90–95. [Google Scholar] [CrossRef]
- Stander, E.K.; Borst, M.; O’Connor, T.P.; Rowe, A.A. The effects of rain garden size on hydrological performance. In Proceedings of the IECA Northeast Chapter Conference and Trade Show, Hartford, CT, USA, 27–29 October 2009. [Google Scholar]
- Guo, J.C.Y. Cap-orifice as a flow regulator for rain garden design. J. Irrig. Drain. Eng. 2012, 138, 198–202. [Google Scholar] [CrossRef]
- Sidek, L.M.; Muha, N.E.; Noor, N.A.M.; Basri, H. Constructed rain garden systems for stormwater quality control under tropical climates. IOP Conf. Ser. Earth Environ. Sci. 2013, 16, 012020. [Google Scholar] [CrossRef]
- Yergeau, S.E.; Obropta, C.C. Preliminary field evaluation of soil compaction in rain gardens. J. Environ. Eng. 2013, 139, 625–634. [Google Scholar] [CrossRef]
- Turk, R.L.; Kraus, H.T.; Bilderback, T.E.; Hunt, W.F.; Fonteno, W.C. Rain garden filter bed substrates affect stormwater nutrient remediation. HortScience 2014, 49, 645–652. [Google Scholar]
- Richards, P.J.; Farrell, C.; Tom, M.; Williams, N.S.; Fletcher, T.D. Vegetable raingardens can produce food and reduce stormwater runoff. Urban For. Urban Green. 2015, 14, 646–654. [Google Scholar] [CrossRef]
- Mehring, A.S.; Hatt, B.E.; Kraikittikun, D.; Orelo, B.D.; Rippy, M.A.; Grant, S.B.; Gonzales, J.P.; Jiang, S.C.; Ambrose, R.F.; Levin, L.A. Soil invertebrates in Australian rain gardens and their potential roles in storage and processing of nitrogen. Ecol. Eng. 2016, 97, 138–143. [Google Scholar] [CrossRef]
- Tang, S.; Luo, W.; Jia, Z.; Liu, W.; Li, S.; Wu, Y. Evaluating retention capacity of infiltration rain gardens and their potential effect on urban stormwater management in the Sub-humid Loess Region of China. Water Res. Manag. 2016, 30, 983–1000. [Google Scholar] [CrossRef]
- Ishimatsu, K.; Ito, K.; Mitani, Y.; Tanaka, Y.; Sugahara, T.; Naka, Y. Use of rain gardens for stormwater management in urban design and planning. Landsc. Ecol. Eng. 2017, 13, 205–212. [Google Scholar] [CrossRef]
- Bortolini, L.; Zanin, G. The experimental and educational rain gardens of the Agripolis Campus (north-east Italy): Preliminary results on hydrological and plant behavior. Acta Hortic. 2017, 1189, 531–536. [Google Scholar] [CrossRef]
- Bortolini, L.; Zanin, G. Hydrological behaviour of rain gardens and plant suitability: A study in the Veneto plain (north-eastern Italy) conditions. Urban For. Urban Green 2018, 34, 121–133. [Google Scholar] [CrossRef]
- Headley, T.R.; Tanner, C.C. Applications of Floating Wetlands for Enhanced Stormwater Treatment: A Review; Auckland Regional Council, Technical Publication: Auckland, New Zealand, 2006. [Google Scholar]
- Ge, Z.; Feng, C.; Wang, X.; Zhang, J. Seasonal applicability of three vegetation constructed floating treatment wetlands for nutrient removal and harvesting strategy in urban stormwater retention ponds. Int. Biodeter. Biodegrad. 2016, 112, 80–87. [Google Scholar] [CrossRef]
- De Stefani, G.; Tocchetto, D.; Salvato, M.; Borin, M. Performance of a floating treatment wetland for in-stream water amelioration in NE Italy. Hydrobiologia 2011, 674, 157–167. [Google Scholar] [CrossRef]
- Mietto, A.; Borin, M.; Salvato, M.; Ronco, P.; Tadiello, N. Tech-IA floating system introduced in urban wastewater treatment plants in the Veneto region—Italy. Water Sci. Technol. 2013, 68, 1144–1150. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration. Guidelines for Computing Crop Water Requirements; FAO Irrigation and Drainage Paper n. 56; Food and Agriculture Organisation of the United Nations: Rome, Italy, 1998. [Google Scholar]
- Zancan, S.; Cesco, S.; Ghisi, R. Effect of UV-B radiation on iron content and distribution in maize plants. Environ. Exp. Bot. 2006, 55, 266–272. [Google Scholar] [CrossRef]
- Göbel, P.; Dierkers, C.; Coldewey, W.G. Storm water runoff concentration matrix for urban areas. J. Contam. Hydrol. 2007, 91, 26–42. [Google Scholar] [CrossRef] [PubMed]
- Zgheib, S.; Moilleron, R.; Chebbo, G. Priority pollutants in urban stormwater: Part 1—Case of separate storm sewers. Water Res. 2012, 46, 6683–6692. [Google Scholar] [CrossRef] [PubMed]
- Gnecco, I.; Beretta, C.; Lanza, L.G.; La Barbera, P. Storm water pollution in the urban environment of Genoa, Italy. Atmos. Res. 2005, 77, 60–73. [Google Scholar] [CrossRef]
- Tanner, C.C.; Headley, T.R. Components of floating emergent macrophyte treatment wetlands influencing removal of stormwater pollutants. Ecol. Eng. 2011, 37, 474–486. [Google Scholar] [CrossRef]
- Van De Moortel, A.M.K.; Meers, E.; De Pauw, N.; Tack, F.M.G. Effects of vegetation, season and temperature on the removal of pollutants in experimental floating treatment wetlands. Water Air Soil Pollut. 2010, 212, 281–297. [Google Scholar] [CrossRef]
- Borne, K.E.; Fassman, E.A.; Tanner, C.C. Floating treatment wetland retrofit to improve stormwater pond performance for suspended solids, copper and zinc. Ecol. Eng. 2013, 54, 173–182. [Google Scholar] [CrossRef]
- Matthews, D.J.; Moran, B.M.; Otte, M.L. Screening the wetland plant species Alisma plantago-aquatica, Carex rostrata and Phalaris arundinacea for innate tolerance to zinc and comparison with Eriophorum angustifolium and Festuca rubra Merlin. Environ. Pollut. 2005, 134, 343–351. [Google Scholar] [CrossRef]
- Reeves, R.D.; Baker, A.J.M. Phytoremediation of Toxic Metals: Using Plants to Clean up the Environment; Raskin, I., Ensley, B.D., Eds.; John Wiley and Sons Inc.: New York, NY, USA, 2000. [Google Scholar]





| Beginning of the Experiment | End of the Experiment | Beginning of the Experiment | End of the Experiment | |||||
|---|---|---|---|---|---|---|---|---|
| Substrate | Substrate | |||||||
| No | Yes | Sig ^ | No | Yes | Sig ^ | |||
| Alisma parviflora (ALSSU) | Mentha aquatic (MENAQ) | |||||||
| AGPO | 5.37 ± 1.81 | 2.96 | 6.78 | * | 4.66 ± 2.12 | 12.8 | 38.6 | * |
| BGPO | 0.801 ± 0.22 | 2.50 | 4.52 | * | 0.633 ± 0.22 | 23 | 47.1 | * |
| WP | 6.17 ± 1.51 | 5.46 | 11.3 | * | 5.29 ± 2.21 | 35.8 | 85.7 | * |
| Bacopa caroliniana (BAOCA) | Oenanthe javanica ‘Flamingo’ (OENJA) | |||||||
| AGPO | 0.257 ± 0.22 | - | 0.288 | 3.13 ± 0.37 | - | 6.32 | ||
| BGPO | 1.36 ± 0.32 | - | 0.260 | 0.290 ± 0.35 | - | 11.4 | ||
| WP | 1.62 ± 0.53 | - | 0.548 | 3.42 ± 0.36 | - | 17.7 | ||
| Canna indica (CNNIN) | Phalaris arundinacea ‘Picta’ (PHAAP) | |||||||
| AGPO | 1.49 ± 0.57 | 3.05 ± 0.12 | 0.80 | 3.68 | * | |||
| BGPO | 1.11 ± 0.21 | 1.75 ± 0.14 | 1.32 | 8.77 | *** | |||
| WP | 2.60 ± 0.66 | 4.80 ± 0.23 | 2.12 | 12.4 | ** | |||
| Caltha palustris (CTAPA) | Sagittaria sagittifolia (SAGSA) | |||||||
| AGPO | 4.79 ± 1.00 | 5.77 | 11.41 | ns | 0.501 ± 0.21 | - | 0.297 | |
| BGPO | 9.67 ± 3.51 | 13.4 | 31.4 | * | 0.440 ± 0.15 | - | 0.548 | |
| WP | 14.5 ± 3.83 | 19.1 | 42.8 | * | 0.941 ± 0.43 | - | 0.844 | |
| Iris ‘Black Gamecock’ (IRISS) | Typha laxmannii (TYHLX) | |||||||
| AGPO | 0.91 ± 0.19 | 2.34 | 3.65 | ns | 1.26 ± 0.35 | 4.13 | 11.8 | * |
| BGPO | 2.91 ± 0.96 | 9.38 | 14.8 | ns | 1.93 ± 1.20 | 2.07 | 11.7 | * |
| WP | 3.82 ± 1.14 | 11.7 | 18.4 | ns | 3.19 ± 1.55 | 6.2 | 23.5 | ** |
| Lysimachia punctata ‘Alexander’ (LYSPU) | ||||||||
| AGPO | 1.94 ± 1.57 | 8.24 | 11.3 | ns | ||||
| BGPO | 1.05 ± 0.94 | 7.18 | 10.9 | ns | ||||
| WP | 2.99 ± 0.63 | 15.4 | 22.2 | ns | ||||
| Beginning of the Experiment | End of the First Year | End of the Second Year | |||||
|---|---|---|---|---|---|---|---|
| Substrate | Substrate | ||||||
| No | Yes | Sig ^ | No | Yes | Sig ^ | ||
| A.parviflora (ALSSU) | |||||||
| Stem number | 1.00 ± 0.0 | 1.13 | 1.13 | ns | 2.27 | 1.75 | ns |
| Plant height (cm) | 22.4 ± 4.87 | 24 | 20.2 | ns | 11.6 | 13.4 | ns |
| Leaf number | 4.75 ± 1.86 | 6.1 | 7.2 | ns | 20.3 | 17.7 | ns |
| Root length (cm) | 3.92 ± 1.16 | 41.3 | 46.4 | ns | 70.4 | 63.2 | ns |
| RVR (1–9 scale) | 1.5 | 1.67 | ns | 4.36 | 2.75 | ns | |
| AVR (1–9 scale) | 2.08 | 1.75 | ns | 3 | 2.42 | ns | |
| Mortality (%) | 8.33 | 0.0 | ns | ||||
| B. caroliniana (BAOCA) | |||||||
| Stem number | 5.96 ± 1.46 | 3.75 | 3.92 | ns | - | 5.25 | |
| Plant height (cm) | 15.7 ± 4.6 | 8.08 | 8.92 | ns | - | 6.63 | |
| Root length (cm) | 6.50 ± 1.93 | 25.0 | 15.3 | ** | - | 16.1 | |
| Bud number | 7.00 | 13.3 | *** | ||||
| RVR (1–9 scale) | 2.08 | 2.58 | ns | - | 1.38 | ||
| AVR (1–9 scale) | 2.58 | 5.10 | ** | - | 1.38 | ||
| Survival (%) | 100 | 100 | ns | 0.0 | 66.7 | *** | |
| C. indica (CNNIN) | |||||||
| Stem number | 1.00 ± 0.0 | 1.17 | 1.67 | ns | |||
| Plant height (cm) | 27.9 ± 6.96 | 21.2 | 24.3 | ns | |||
| Leaf number | 4.17 ± 1.19 | 6.67 | 8.83 | ns | |||
| Root length (cm) | 6.33 ± 1.03 | 67.5 | 48.8 | * | |||
| RVR (1–9 scale) | 2.00 | 3.67 | * | ||||
| AVR (1–9 scale) | 2.50 | 3.50 | ns | ||||
| Survival (%) | 100 | 100 | ns | 0.0 | 0.0 | ns | |
| C. palustris (CTAPA) | |||||||
| Stem number | 3.10 ± 1.41 | 2.33 | 3.58 | ** | 4.50 | 7.75 | ns |
| Plant height (cm) | 14.2 ± 1.56 | 4.58 | 11.0 | *** | 30.5 | 65.5 | * |
| Leaf number | 4.2 ± 1.92 | 2.50 | 6.58 | *** | 10.2 | 16.9 | ns |
| Root length (cm) | 11.7 ± 1.10 | 28.7 | 20.7 | *** | 50.9 | 68.6 | * |
| RVR (1–9 scale) | 3.50 | 4.50 | * | 4.40 | 7.75 | ns | |
| AVR (1–9 scale) | 1.92 | 4.08 | ** | 3.20 | 5.25 | * | |
| Survival (%) | 100 | 100 | ns | 83.3 | 100 | * | |
| Beginning of the Experiment | End of the First Year | End of the Second Year | |||||
|---|---|---|---|---|---|---|---|
| Substrate | Substrate | ||||||
| No | Yes | Sig ^ | No | Yes | Sig ^ | ||
| Iris ‘Black Gamecock’ (IRISS) | |||||||
| Stem number | 1.08 ± 0.28 | 1.92 | 1.67 | ns | 2.30 | 2.42 | *** |
| Plant height (cm) | 29.1 ± 4.02 | 13.0 | 18.3 | * | 32.6 | 36.3 | ** |
| Leaf number | 4.25 ± 1.54 | 11.8 | 11.1 | ns | 15.3 | 16.7 | ns |
| Root length (cm) | 5.25 ± 0.89 | 23.1 | 29.3 | ** | 33.7 | 38.0 | ns |
| RVR (1–9 scale) | 2.33 | 5.25 | *** | 2.30 | 2.75 | * | |
| AVR (1–9 scale) | 1.92 | 2.17 | ns | 2.83 | 2.58 | ** | |
| Survival (%) | 100 | 100 | ns | 100 | 100 | ns | |
| L. punctata ‘Alexander’ (LYSPU) | |||||||
| Stem number | 1.79 ± 0.72 | 2.17 | 3.75 | * | 4.00 | 7.83 | * |
| Plant height (cm) | 33.7 ± 6.05 | 7.33 | 8.17 | ns | 23.3 | 26.3 | ns |
| Root length (cm) | 4.67 ± 1.23 | 24.8 | 21.5 | ns | 48.8 | 52.5 | ns |
| New shoot number | 0.83 | 1.83 | ns | ||||
| RVR (1–9 scale) | 2.58 | 3.08 | ns | 3.50 | 4.58 | ns | |
| AVR (1–9 scale) | 2.42 | 3.33 | ns | 3.00 | 4.75 | * | |
| Survival (%) | 100 | 100 | ns | 100 | 100 | ns | |
| Mentha aquatica (MENAQ) | |||||||
| Stem number | 6.54 ± 2.08 | 8.42 | 9.00 | ns | 5.67 | 23.8 | * |
| Plant height (cm) | 24.7 ± 3.62 | 36.0 | 39.5 | ns | 38.0 | 64.3 | *** |
| Root length (cm) | 6.31 ± 4.25 | 30.4 | 37.7 | * | 79.4 | 100.8 | ** |
| New shoot number | 0.83 | 3.50 | ** | ||||
| RVR (1–9 scale) | 7.75 | 8.17 | ns | 5.78 | 8.33 | * | |
| AVR (1–9 scale) | 3.25 | 5.08 | ** | 4.11 | 7.67 | * | |
| Survival (%) | 100 | 100 | ns | 75 | 100 | ** | |
| Oenanthe javanica ‘Flamingo’ (OENJA) | |||||||
| Stem number | 5.88 ± 1.08 | 7.5 | 16.1 | *** | - | 24.8 | |
| Plant height (cm) | 29.4 ± 4.05 | 8.58 | 10.2 | * | - | 25.6 | |
| Root length (cm) | 6.17 ± 2.04 | 20.8 | 35.8 | *** | - | 57.9 | |
| New shoot number | 1.92 | 9.92 | *** | ||||
| RVR (1–9 scale) | 2.33 | 7.58 | *** | - | 6.67 | ||
| AVR (1–9 scale) | 2.42 | 4.92 | ** | - | 3.92 | ||
| Survival (%) | 100 | 100 | ns | 0.0 | 100 | *** | |
| Beginning of the Experiment | End of the First Year | End of the Second Year | |||||
|---|---|---|---|---|---|---|---|
| Substrate | Substrate | ||||||
| No | Yes | Sig ^ | No | Yes | Sig ^ | ||
| Phalaris arundinacea ‘Picta’ (PHAAP) | |||||||
| Stem number | 1.54 ± 0.59 | 5.00 | 7.08 | * | 2.50 | 9.92 | * |
| Plant height (cm) | 45.8 ± 13.9 | 21.7 | 21.1 | ns | 14.8 | 31.4 | * |
| Root length (cm) | 6.00 ± 2.17 | 37.5 | 35.8 | ns | 20.2 | 38.8 | * |
| RVR (1–9 scale) | 4.83 | 5.92 | * | 2.00 | 3.75 | * | |
| AVR (1–9 scale) | 2.75 | 4.67 | ** | 1.33 | 3.92 | * | |
| Survival (%) | 100 | 100 | ns | 50 | 100 | *** | |
| Sagittaria sagittifolia (SAGSA) | |||||||
| Stem number | 1.00 ± 0.0 | 1.40 | 1.00 | ns | - | 1.09 | |
| Plant heigh (cm) | 12.3 ± 2.07 | 13.2 | 15.6 | * | - | 14.1 | |
| Leaf number | 3.91 ± 2.66 | 5.13 | 7.14 | * | - | 16.6 | |
| Root length (cm) | 7.00 ± 3.30 | 32.0 | 33.1 | ns | - | 42.6 | |
| RVR (1–9 scale) | 2.70 | 2.58 | ns | - | 1.64 | ||
| AVR (1–9 scale) | 2.42 | 3.33 | * | - | 1.55 | ||
| Survival (%) | 83.3 | 100 | * | 0.0 | 91.7 | *** | |
| Typha laxmannii (TYHLX) | |||||||
| Stem number | 1.13 ± 0.34 | 1.75 | 2.92 | * | 2.14 | 4.82 | * |
| Plant height (cm) | 44.3 ± 3.68 | 14.8 | 35.2 | ns | 46.3 | 62.6 | * |
| Leaf number | 5.83 ± 1.63 | 7.17 | 13.58 | *** | 16.1 | 37.2 | * |
| Root length (cm) | 3.44 ± 2.22 | 12.3 | 14.9 | ns | 33.6 | 45.5 | ns |
| RVR (1–9 scale) | 1.25 | 2.50 | ** | 2.14 | 4.36 | * | |
| AVR (1–9 scale) | 1.92 | 3.17 | ** | 1.57 | 4.00 | ** | |
| Survival (%) | 100 | 100 | ns | 58.3 | 91.7 | ** | |
| Heavy Metal Concentration (µg g−1 Dry Matter) | Heavy Metal Content (µg Plant−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| Cd | Cu | Pb | Zn | Cd | Cu | Pb | Zn | |
| Alisma parviflora (ALSSU) | ||||||||
| AGPO | 0.454 ± 0.338 | 51.5 ± 19.9 | 1.55 ± 1.80 | 309 ± 108 | 2.09 ± 0.61 | 288 ± 94 | 6.2 ± 5.4 | 1739 ± 568 |
| BGPO | 0.490 ± 0.270 | 97.6 ± 38.6 | 9.97 ± 6.04 | 413 ±130 | 1.56 ± 0.34 | 359 ± 160 | 30.6 ± 5.9 | 1553 ± 745 |
| WP | 3.66 ± 0.28 | 647 ± 244 | 36.8 ± 2.8 | 3292 ± 1053 | ||||
| Caltha palustris (CTAPA) | ||||||||
| AGPO | 0.076 ± 0.026 | 29.6 ± 3.1 | 0.60 ± 0.01 | 105 ± 28 | 0.83 ± 0.20 | 339 ± 103 | 6.8 ± 1.7 | 1163 ± 220 |
| BGPO | 0.330 ± 0.049 | 70.2 ± 4.5 | 2.54 ± 0.61 | 248 ± 24 | 10.32 ± 1.18 | 2199 ± 58 | 80.0 ± 20.5 | 7761 ± 373 |
| WP | 11.15 ± 1.00 | 2538 ± 54 | 86.9 ± 19.2 | 8924 ± 562 | ||||
| Iris ‘Black Gamecock’ (IRISS) | ||||||||
| AGPO | 0.269 ± 0.119 | 14.0 ± 1.6 | 0.37 ± 0.14 | 102 ± 14 | 0.93 ± 0.21 | 51 ± 8 | 1.4 ± 0.6 | 372 ± 99 |
| BGPO | 0.120 ± 0.026 | 16.9 ± 2.5 | 0.63 ± 0.56 | 59 ± 10 | 1.74 ± 0.59 | 254 ± 100 | 10.3 ± 10.1 | 902 ± 395 |
| WP | 2.67 ± 0.40 | 304 ± 107 | 11.7 ± 10.7 | 1274 ± 480 | ||||
| Lysimachia punctata ‘Alexander’ (LYSPU) | ||||||||
| AGPO | 0.168 ± 0.113 | 20.4 ± 6.1 | 1.27 ± 0.99 | 94 ± 16 | 1.01 ± 0.38 | 206 ± 86 | 10.1 ± 2.2 | 1060 ± 662 |
| BGPO | 0.229 ± 0.160 | 51.9 ± 3.1 | 2.87 ± 0.74 | 182 ± 38 | 2.48 ± 2.45 | 562 ± 556 | 30.8 ± 31.0 | 1959 ± 1964 |
| WP | 3.49 ± 2.13 | 768 ± 585 | 40.9 ± 33.1 | 3019 ± 2066 | ||||
| Mentha aquatica (MENAQ) | ||||||||
| AGPO | 0.060 ± 0.026 | 12.6 ± 1.3 | 0.21 ± 0.07 | 44 ± 11 | 2.58 ± 2.22 | 495 ± 254 | 8.5 ± 6.0 | 1787 ± 1219 |
| BGPO | 0.154 ± 0.106 | 41.3 ± 7.6 | 2.15 ± 0.40 | 133 ± 18 | 7.03 ± 4.54 | 2023 ± 1042 | 104.1 ± 48.9 | 6193 ± 1898 |
| WP | 9.61 ± 4.89 | 2518 ± 1285 | 112.6 ± 53.8 | 7980 ± 2958 | ||||
| Oenanthe javanica ‘Flamingo’ (OENJA) | ||||||||
| AGPO | 0.121 ± 0.027 | 31.0 ± 1.1 | 0.44 ± 0.18 | 193 ± 10 | 0.79 ± 0.40 | 197 ± 75 | 3.0 ± 2.3 | 1208 ± 386 |
| BGPO | 0.481 ± 0.133 | 57.4 ± 4.9 | 2.34 ± 0.75 | 339 ± 57 | 4.97 ± 1.86 | 637 ± 304 | 25.9 ± 17.0 | 3649 ± 1541 |
| WP | 5.76 ± 1.66 | 834 ± 240 | 28.9 ± 15.6 | 4857 ± 1196 | ||||
| Phalaris arundinacea ‘Picta’ (PHAAP) | ||||||||
| AGPO | 0.150 ± 0.105 | 21.6 ± 2.4 | 0.51 ±0.15 | 220 ± 18 | 0.55 ± 0.38 | 79 ± 5 | 1.9 ± 0.5 | 807 ± 43 |
| BGPO | 0.077 ± 0.051 | 61.7 ± 7.1 | 1.66 ± 0.12 | 270 ± 44 | 0.67 ± 0.42 | 540 ± 51 | 14.6 ± 1.1 | 2357 ± 325 |
| WP | 1.22 ± 0.59 | 619 ± 56 | 16.5 ± 0.8 | 3164 ± 346 | ||||
| Typha laxmannii (TYHLX) | ||||||||
| AGPO | 0.075 ± 0.052 | 13.9 ± 1.0 | 0.27 ± 0.12 | 62 ± 7 | 0.92 ± 0.72 | 164 ± 25 | 3.2 ± 1.5 | 741 ± 156 |
| BGPO | 0.146 ± 0.006 | 48.1 ± 8.9 | 3.27 ± 1.14 | 190 ± 35 | 1.71 ± 0.33 | 571 ±186 | 39.8 ± 20.2 | 2194 ± 343 |
| WP | 2.63 ± 0.75 | 735 ± 208 | 42.9 ± 21.6 | 2935 ± 496 | ||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanin, G.; Bortolini, L.; Borin, M. Assessing Stormwater Nutrient and Heavy Metal Plant Uptake in an Experimental Bioretention Pond. Land 2018, 7, 150. https://doi.org/10.3390/land7040150
Zanin G, Bortolini L, Borin M. Assessing Stormwater Nutrient and Heavy Metal Plant Uptake in an Experimental Bioretention Pond. Land. 2018; 7(4):150. https://doi.org/10.3390/land7040150
Chicago/Turabian StyleZanin, Giampaolo, Lucia Bortolini, and Maurizio Borin. 2018. "Assessing Stormwater Nutrient and Heavy Metal Plant Uptake in an Experimental Bioretention Pond" Land 7, no. 4: 150. https://doi.org/10.3390/land7040150
APA StyleZanin, G., Bortolini, L., & Borin, M. (2018). Assessing Stormwater Nutrient and Heavy Metal Plant Uptake in an Experimental Bioretention Pond. Land, 7(4), 150. https://doi.org/10.3390/land7040150







