Abstract
In this study, both trivalent and hexavalent forms of chromium were examined in urban and agricultural Mediterranean soils. Chromium partitioning in different soil fractions was studied. Pot experiments included contamination of soil samples using Cr solutions, as well as a further study regarding Cr distribution in naturally contaminated soils. The soils were subjected to quantitative determination of both the available and total Cr concentration, as well as Cu and Zn, which were naturally present in the soil samples. Metal concentrations in the soil fractions were quantified after the application of the BCR fractional extraction method. The numbers of both trivalent and hexavalent Cr ions in each extract were determined. Considerable discrepancies were noticed regarding the Cr content of each soil fraction in both municipal and cultivated soils, indicating the possible origin of the pollution. The increasing impact of pollution is a significant parameter for the availability of chromium ions in both agricultural and urban soils. Increased pollution durations resulted in a significant increase in the non-available fraction of toxic Cr (VI), mainly in urban soil. Variations were also observed in the chromium species, as changes in soil parameters and in the conditions of the experiment seem to affect the conversion of the less harmful trivalent chromium to the toxic hexavalent chromium. In urban soils, the amount of toxic Cr (VI) bound to iron and manganese oxides exceeds 37.8%, while in agricultural soils, the amount of Cr (VI) associated with soil organic matter reaches 35%. Knowing the mechanisms and variables influencing Cr availability in agricultural and urban Mediterranean soils is desirable, as safe living in ecologically acceptable fields and producing safe goods in healthy soil systems are paramount goals.
1. Introduction
In the past few years, chromium pollution in soil has become an important environmental threat [1]. Cr occurs in soils as a metallic element with valence 0, as well as in a reduced form or in an oxidized form, i.e., with valences (III) and (VI), respectively [2,3]. Metallic Cr is rarely found in soil systems because it is not a very reactive element [4]. However, the predominant abundant Cr form is the hexavalent form, owing its existence to a variety of anthropogenic activities [5]. The trivalent form of Cr is less soluble and therefore less available as it is often adsorbed by the solid phase of the soil, mainly when strong negative charges are present [6]. Trivalent chromium is a necessary nutrition trace element, wholly nontoxic and relatively immobile as it precipitates as Cr(OH)3 when pH ranges from 4 to 8, making it difficult to be absorbed [7]. It may improve the effectiveness of insulin and receptor binding, minimizing the risk of diabetes. Abundant Cr (ΙΙΙ) over the acceptable limit may cause long-term toxicity and carcinogenicity [8]. Hexavalent Cr ions are extremely dangerous for the soil environment, for animals, and, of course, for humans, as they pose the risk of many diseases, especially cancer [9]. Hexavalent Cr’s presence in both urban and cultivated soils requires study and continuous monitoring [10], as it can lead to a reduction in production and a deterioration in the quality of the products produced. Chromate and bichromate ions are the most common forms in which hexavalent chromium occurs in soil systems [11]. The reduction of Cr (VI) to Cr (III) often requires low soil pH and the absence of oxidative conditions. The transfer of electrons from Fe (II), sulfides, soil organic matter, or microbial processes occurs [6]. The main known reaction is as follows:
CrO42− + 3Fe2+ +4H2O → Cr(OH)3 + 3Fe3+ + 5OH−
The oxidation state of soil chromium is strongly regulated by its reaction, i.e., soil pH value and redox potential. Specifically, when the soil pH is below 6.5, Cr is present mainly in the form of the anions HCrO4− and Cr2O72− [3]. In the case of a slightly alkaline soil pH, only CrO42− anions can be detected. These anions can easily be absorbed by plants in contaminated soil environments [12]. Khan et al., investigating the global soil burden of heavy metals and potential toxic elements, concluded that Cr occurs ionically in one of the abovementioned forms in both cultivated and municipal soils [13]. Χu et al. studied the ongoing conversion of trivalent to hexavalent Cr, as environmental factors generate redox conditions that amplify and accelerate continuous transformations from one state to the other [5].
Wan et al. demonstrated that chromium, among other metals, is a common potential toxic element in agricultural soils that contributes to soil pollution over time [14]. Although its origin is often geochemical, diverse anthropogenic activities contribute to its accumulation, raising concerns for the environment and the healthy growth of living beings [15]. In agricultural soils in China, Li et al. performed a survey from 1989 to 2016 to depict the accumulation status of Cr and concluded that the application of superphosphate fertilizers may act as a significant source of Cr [16].
In recent decades, urban soil pollution has appeared as a distinct area of research of particular interest [17]. Urban soils, including the processes of their creation and ongoing modification, as well as their distinguished vertical and horizontal spatial distributions, have drawn increased attention. Binner et al., after a systematic review of municipal soils in 29 European countries, found that the metals that most commonly exceed national safety thresholds are chromium, copper, nickel, lead, and zinc [18].
As there are insufficient published data in both urban and agricultural Mediterranean soils regarding Cr speciation, this study aimed to conduct a preliminary investigation to (1) examine the factors influencing the availability of trivalent and hexavalent chromium ions in the different soil types, (2) identify the soil fractions where Cr ions are confined, and (3) explore the possibility of appropriate management of the more toxic hexavalent form of Cr in aging or recently contaminated soils.
2. Materials and Methods
2.1. Soil Sampling, Soil Aging, and Pot Experiment
An investigation was established in the Soil Science Laboratory of the Aristotle University of Thessaloniki. The soil samples originated from four sites, two agricultural samples from the Central Greece region [19] and two from the urban area of Thessaloniki [20]. These samples were selected since they contained high enough levels of chromium to meet the purpose of the present study. Each soil sample constituted a single composite homogenized sample, consisting of six sub-samples located on the perimeter of an ideal circle about two meters in diameter. However, two of the soil samples (S1 and S3) were taken two years before the initiation of the study to represent soils with “pollution aging”. The samples were stored (for 2 years) in paper boxes to prevent additional “fresh” contamination. Moreover, they were kept in room temperature (20–22 °C) and humidity (30% w/w) conditions to avoid anaerobic processes, preventing soil redox alterations and consequently the conversion between two forms of chromium.
2.2. Soil Analyses
The soil samples were transferred to the Soil Science Laboratory for air drying and the proper pretreatment for further soil and chemical analyses. Soil analysis, as explained by Page (1982), was conducted [21] in three replicates (n = 3) each. As a result, the Bouyoucos process was employed to measure soil texture, and the soil acidity value was identified using a soil–water mix at a 1:2.5 ratio. To identify the value of the electrical conductivity (EC), a soil–water saturation paste was mixed in an identical ratio, i.e., 1:1. Nutrient contents were identified using the procedures outlined in [19,22]. Total nitrogen was determined using the Kjehldal method, soluble phosphorus was determined using the Olsen method following extraction with sodium bicarbonate solution (pH = 8.5), and exchangeable potassium concentrations were measured via a flame photometer (Sherwood 410) upon extraction with 1M CH3COONH4. Organic carbon was measured in order to quantify soil organic matter (OM) via the Walkley–Black technique.
Available and pseudo-total Cr concentrations were determined after extraction via Diethylenetriamine Pentaacetic Acid solution and Aqua Regia (ISO/DIS 11466) mixture, respectively. The bioavailable metal portion was identified as the proportion of the DTPA-extractable concentrations to the total concentration, i.e., the Aqua Regia-extractable concentrations. This was stated as a proportion of the plant’s total concentration, irrespective of the overall amount in the soil. The BCR fractionation method, an adoption and modification of the step-by-step extraction method stated by Tessier et al., was also used in order to obtain the Cr concentrations as follows: (1) the concentration available to plants was determined using 0.11 M CH3COOH solution; (2) the concentration used to form Fe and Mn oxides, or the reducible portion, was detected using 0.5 M hydroxylammonium chloride (pH 1.5), (3) the proportion that formed soil organic carbon was found via 8.8 M H2O2 (pH = 2) and 1 M CH3COONH4, and (4) the amount of unavailable Cr was determined via a HCl-HNO3 mixture [23].
Cr, Mn, Fe, Cu, and Pb quantifications were accomplished via Atomic Absorption Spectrometry (AAS) with flame (Shimadzu 6300) and graphite furnace (Shimadzu 7000) equipment. Furthermore, a CRM 141R standard soil was used. For Cr (III) and total Cr (trivalent and hexavalent), the accuracy ranged from 8.7 to 10.2%. Cr speciation was conducted by employing a voltammetry method, as follows: the simultaneous determination of pseudo-total Cr (III) and Cr (VI) was accomplished using the methodology developed by Calvo-Pérez et al., which is applicable when the cation concentration is higher than 10 mg L−1 [24]. For Cr (III) concentrations lower than 10 mg L−1, the analytical methodology detailed by Chatzitheodorou et al. was followed [25]. Finally, the available Cr concentrations were calculated following the method applied by Jorge et al. [26].
2.3. Statistical Analysis
The relative weighting method was used to calculate the ratio between the mathematical prediction variables and the values obtained from the multiple correlation equations [27]. In order to evaluate potential differences between trivalent and hexavalent Cr fractions in BCR extraction schemes or their bioavailable forms extracted with DTPA, a two-way analysis of variance (ANOVA) was applied. The factors considered were aging and different land use types (agricultural vs. urban soil), and the protected LSD test was used for mean comparisons at p ≤ 0.05. For all statistical analyses, Statgraphics software was used (STATGRAPHICS, Centurion XVI, version 16.1.11, StatPoint Technologies, Inc., Warrenton, VA, USA).
3. Results
3.1. Physicochemical Features of Soil Samples
The physicochemical properties of the soil samples used in this study are presented in Table 1.

Table 1.
Values of physicochemical properties, nutrition parameters, and trace element contents of soil samples.
We can note the similar properties of the soils, although the agricultural soils are alkaline while the urban soils are slightly acidic [6]. The researchers Mónok et al. demonstrated that soil samples from urban and non-urban areas can be significantly distinguished by their physicochemical properties, primarily through soil reactions, i.e., soil pH [28]. In urban soil, the pH value tends to increase, mainly due to the materials deposited on the native minerals and rocks. However, there are two categories of urban soils: green soils, i.e., those that are found in beds and cultivated, so in addition to receiving cultural treatments, their soil properties are also affected by plants, and urban soils that remain uncultivated for a long time without any chemical and physical intervention. Green urban soils tend to have lower pH values than uncultivated soils. In the present study, the first two soils (S1 and S2) are agricultural and have slightly alkaline pH values, while the next two soils (S3 and S4) are green urban soils, for which there has been an intervention by the urban greening service of the Municipality of Thessaloniki, as described in previous studies [29]. The high values of EC in the urban soils are likely due to the fertilizers used for urban gardening, providing increased amounts of salt, as suggested by Geisseler and Scow (2014) in their research [30]. The discrepancies in the organic matter percentages between the two categories of soils seem to follow the expected trends for agricultural and urban soils. Bekier et al., studying the characteristics of urban soils, observed that their OM values are significantly lower than those of agricultural and continuously cultivated soils [31]. Regarding the percentage of clay content, the soil samples differ significantly, resulting in their different textures. Consequently, both soil samples have a sandy clay loam texture, depending on soil taxonomic grading following soil fragment identification using the Bouyoucos method [21]. The first urban soil (S3) has a significantly large amount of clay, which is characteristic of urban soils [32].
3.2. Nutrition Parameters and Trace Element Content of Soil Samples
Useful information regarding the nutrient content of soils (N, P, K) and available trace element (Cu, Zn, Fe, and Mn) concentrations can be obtained from Table 1. Researchers Penn and Camberato described the chemical processes that take place and the effect of soil pH on the availability of soil phosphorus to plants. In this study, the soils were selected based on their similar nutrient values, and even though the two urban soil samples are acidic, the Olsen-extractable phosphorus concentrations do not appear to differ [33].
The trace element availability quotient (α = CDTPA/CAqRe) was higher in acidic soils than in alkaline soils for each of the four trace elements studied. In other words, the trace elements’ mobility and possible availability from the soil to plants are higher than in more alkaline soils, as was also found in a study by Rolka and Wyszkowski [34]. Furthermore, Neina thoroughly studied, in a review article, all the potential effects of soil pH on several physical, chemical, and biochemical processes, inversely affecting the mobility and available forms of metal ions in soil systems [35].
3.3. Cr (III) and Cr (VI) Determination Using Voltammetry Techniques
Heavy metals are usually determined using analytical procedures such as spectroscopy, atomic absorption spectrophotometry, and electroanalysis [25]. Nowadays, scientific concern involves original and fast analytical methodologies and analytical techniques for heavy metal analysis, mainly relying on voltammetry. Voltammetry represents a very famous electroanalytical technique owing to its popularity, simplicity, speedy analysis, usefulness for in situ analysis, specificity, and low detection limits. In the present study, the quantification of trivalent and hexavalent Cr was carried out using voltammetry, meaning the determination of metal ions was fast and cost-effective.
Bobrowski et al. declare that there are two main categories of instrumental procedures available for Cr (VI) and Cr (III) trace determination: (1) Valence-specific methods, such as molecular absorption, that allow for the direct determination of Cr (VI) in the presence of Cr (III). These methods can also include either voltammetry or spectrophotometry. These techniques are predicated on the distinct complex-forming capacities of Cr (III) and Cr (VI). (2) Analytical methodologies that are not valence-specific only enable the measurement of total chromium concentration, while valence-specific analytical methodologies enable the direct determination of Cr (VI) in the presence of Cr (III) and vice versa [36]. These analytical methodologies enable chromium speciation in complex environmental matrices. This is followed by a non-specific measurement of the separated form, which is typically performed using inductively coupled plasma–atomic emission spectrometry (ICP AES), flame or electrothermal atomic absorption spectrometry (AAS, ET AAS), inductively coupled plasma–atomic mass spectrometry (ICP MS), or sometimes X-ray fluorescence spectroscopy (XFS) and neutron activation analysis (NAA). In contrast, Hue et al. found that the working electrode utilized in voltammetry, mainly via the AdSV technique, was another issue worth considering. In the past, most analytical methodologies involved hanging mercury drop electrodes (HMDEs) or static mercury drop electrodes (SMDEs). The main drawback in this context is the use of toxic mercury, although these techniques offer excellent reproducibility and analytical sensitivity. Studies on the use of bismuth film electrodes (BiFEs) and mercury film electrodes (MFEs) on glassy carbon disk surfaces have enhanced environmentally friendly BiFEs and made them less expensive and simpler to manufacture [37].
Nowadays, developments in the field of electroanalysis toward the detection of chromium utilize diverse electrode platforms, such as solid or screen-printed electrodes (SPEs) and various functional materials. Hilali et al. have extensively investigated the applications of developed electrochemical sensors and biosensors over the last five years as effective devices for chromium detection [38].
3.4. Cr Speciation and Fractions in the Soil Samples of the Study
3.4.1. Cr (ΙΙΙ) and Cr (VI) Available and Pseudo-Total Concentrations
The concentrations of Cr (III) and Cr (VI) were assessed through two distinct extraction methods: the pseudo-total concentrations were obtained via Aqua Regia extraction and the bioavailable concentrations were determined by DTPA extraction. Figure 1 illustrates these concentrations for the studied soils. Specifically, the availability rates of Cr (III) in agricultural soil samples S1 and S2 were found to be 0.197% and 0.257%, respectively, while for Cr (VI) they were 0.085% and 0.104%. In urban soil samples S3 and S4, the Cr (III) availability rates were 0.116% and 0.140%, respectively, and for Cr (VI) they were 0.079% and 0.107%. Across all samples, Cr (VI) consistently showed lower availability rates compared with Cr (III).
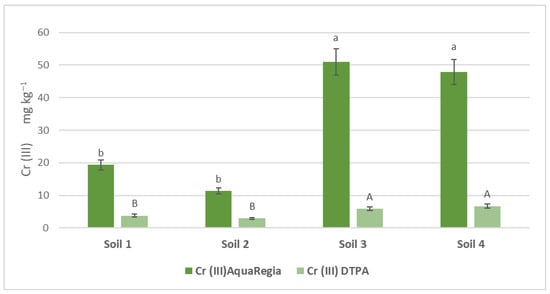
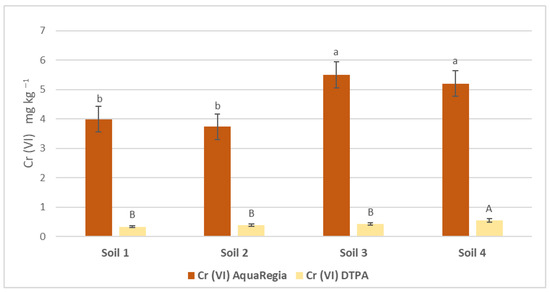
Figure 1.
Available and pseudo-total soil Cr (ΙΙΙ) and Cr (VI) concentrations in the soil samples. Comparisons were calculated using the LSD test (p < 0.05) and are presented with lowercase characters. Identical letters imply no significant difference.
Lowercase characters indicate significant differences between treatments for DTPA-extractable Cr (III) and Cr (VI) amounts, while uppercase characters indicate significant differences for Aqua Regia Cr (III) and Cr (VI) amounts. Error bars indicate the standard deviation (p < 0.05).
In agricultural soils, the pseudo-total concentrations of chromium were notably high, but the availability of Cr (VI), which is more toxic, remained low. This suggests that agricultural products can be safe despite having high total chromium levels. Similarly, urban soils exhibited high total chromium concentrations, but the availability of both Cr (III) and Cr (VI) was significantly reduced. This study’s findings align with previous research by Park et al., who also observed variability in pseudo-total chromium values in urban soils depending on their proximity to pollution sources and the development of pollution [39]. The origins of chromium in soil are often geochemical but can also be attributed to anthropogenic activities, including fertilizer usage, vehicular emissions, and fossil fuel combustion [18,40].
The reduced availability of hexavalent Cr ions compared to trivalent Cr ions in both municipal and cultivated soils may be due to several factors, mainly the reduction of Cr to a lower valence form, as well as adsorption by soil components that it may be complexed with, such as organic matter or clay minerals [41]. A study by Shi et al. further supports the idea that acidic soil pH decreases Cr (VI) availability, a trend that was also observed in the present study. Thus, even in soils heavily contaminated by urban activities, aged and acidic conditions significantly reduce the availability of toxic Cr (VI) [42]. Although Cr (III) tends to bind to soil clay, precipitate, or form complex compounds, thereby reducing its availability, the authors of [43] concluded in their study that Cr’s chemical behavior is responsible for the frequent conversion of hexavalent ions to trivalent ions, especially when conditions favor chromium reduction within the soil system. Therefore, the lower availability of one form and the frequent conversion of one form to the other are expected, as climatic conditions as well as soil constituents can determine this shift.
3.4.2. Fractionation of Cr (III) and Cr (VI) Using the BCR Sequential Extraction Method and Relationships Between Their Fractions and Bioavailable Forms Extracted with DTPA
The chromium (III) contents in various soil fractions were identified via BCR, as shown in Figure 2. This method enables a detailed analysis of metal distribution across different soil fractions after successive extractions. The first fraction (BCR1) represents the water-soluble and exchangeable metal concentration that plants can take up [29,44]. Trivalent chromium was abundant in the soluble soil fraction, reaching the following percentages for soil samples S1, S2, S3, and S4, respectively: 15.7%, 13.9%, 10.9%, and 11.6%. These percentages were significantly higher than those for Cr (VI), which were 11.5%, 10.8%, 8.7%, and 5.8% for the same samples.
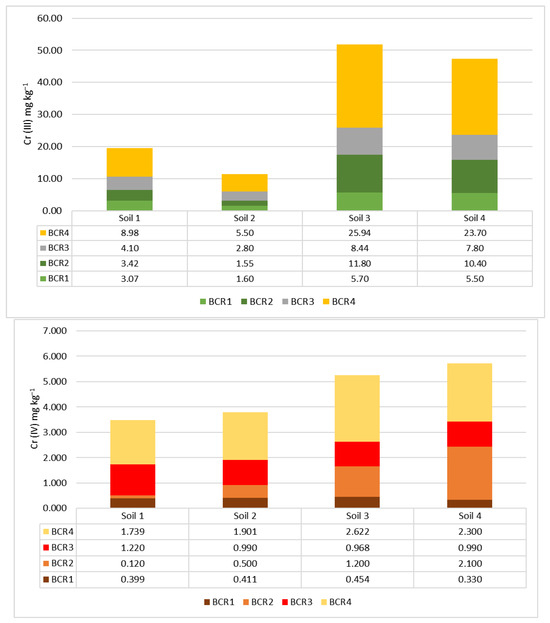
Figure 2.
Cr (ΙΙΙ) and Cr (VI) concentrations in soil fractions after the BCR method.
In the soil samples representing the older pollution, the difference in availability between Cr (III) and Cr (VI) was 4.2%, indicating a reduced risk of producing Cr-laden products. The availability of chromium ions can also change when bound to iron and manganese oxides (BCR2) or controlled by soil organic matter (BCR3). In urban soils (S3 and S4), the Cr (III) concentrations were 23% and 22% in BCR2 and 16% and 17% in BCR3. For Cr (VI), the concentrations were 23% and 38% in BCR2 and 19% and 17% in BCR3. This suggests that Cr ions in urban soils are more closely associated with iron and manganese oxides than organic matter. On the contrary, in agricultural soils (S1 and S2), the Cr (III) concentrations were 21% and 25% in BCR3 and 18% and 14% in BCR2. For Cr (VI), the concentrations in BCR3 were 35% and 26%, while in BCR2, they were 4% and 13%. This indicates that soil organic matter plays a significant role in managing Cr (VI) in agricultural soils. Lastly, the fourth fraction (BCR4), which represents the residual and non-bioavailable metal fraction, contained the highest percentages of Cr (III) and Cr (VI) in all soil samples. This fraction does not readily release metals, reducing the risk of soil contamination.
The backward stepwise selection method identified which BCR extraction fractions significantly contributed to the overall variability of bioavailable trivalent or hexavalent Cr extracted with DTPA. For Cr (III), the prediction model was based solely on the amounts of Cr extracted in the first step of the sequential extraction procedure (BCR1), while for Cr (VI), a multiple regression model was produced based on the second and fourth BCR extraction steps:
Cr (III) DTPA = 1.34 + 0.87 * Cr (III) BCR1, r2 = 0.93, p ≤ 0.001
Cr (VI) DTPA = 0.40 + 0.12 * Cr (VI) BCR2—0.04 * Cr (VI) BCR4, r2 = 0.97, p ≤ 0.001
The relative weight analysis of Equation (2) revealed that approximately 80% of the total variability in DTPA-extracted Cr (VI) can be positively attributed to the Cr (VI) extracted in the BCR2 step, while the negative impact of the Cr (VI) extracted in the BCR4 step accounts for 20.4%. For Cr (III) in Equation (1), the regression model shows a positive coefficient for Cr (III) in the BCR1 step, indicating that the presence of Cr (III) in this easily extractable fraction strongly correlates with its extraction by DTPA. The BCR1 step typically targets exchangeable and weakly bound fractions, including those associated with carbonates, which are the least tightly bound and more readily available for extraction. Hexavalent chromium often exhibits low bioavailability in natural soils, despite its solubility in pure systems, due to various processes within the soil environment, most notably the reduction of Cr (VI) to Cr (III). Cr (VI) is known to be thermodynamically unstable in many soil conditions and can be reduced to Cr (III) by soil organic matter (SOM), Fe2+ and Mn2+ minerals, and microbial activity, and it is immobilized through adsorption and precipitation [6].
In contrast, the positive influence of Cr (VI) in the BCR2 step on the Cr (VI) extracted by DTPA indicates that the Cr species associated with the reducible phases targeted in the BCR2 step are more accessible for DTPA extraction. This suggests that these Cr forms are less tightly bound and more labile and bioavailable, aligning with the types of species that DTPA is designed to extract. Moreover, the negative influence of Cr (VI) in the BCR4 step on the amount of Cr (VI) extracted by DTPA indicates that the Cr in the BCR4 step is in a more stable, less bioavailable form that DTPA cannot efficiently extract.
The BCR sequential extraction method provides a comprehensive overview of metal distribution in soils, revealing that Cr (III) is more readily available in the BCR1 fraction than Cr (VI). This aligns with findings by Shaheen et al., who noted similar trends for other elements. The higher availability of Cr (III) compared to Cr (VI) under current climatic conditions suggests a lower risk of Cr-laden products in the studied soils [45]. It is well known that Cr (VI) has low bioavailability in soils. This is mainly due to its accelerated reduction to Cr (III)ρ, subsequent adsorption onto mineral surfaces, precipitation of insoluble hydroxides, and complexation with organic matter [6]. Soil pH and redox conditions largely regulate these processes. The residual fraction (BCR4) contained the highest percentages of Cr (III) and Cr (VI), suggesting that these metals are largely immobilized in both municipal and cultivated soils. This finding confirms the conclusions of Osakwe and Cao et al., who found that the metal concentration in the fourth fraction of the sequential extraction method is essentially trapped as it remains immobile, reducing environmental risks [46,47].
3.4.3. Evaluating Differences in Cr (III) and Cr (VI) Fractions in BCR Extraction Schemes or DTPA Based on Aging or Different Land Use Parameters
Municipal soils showed a higher proportion of Cr ions in the fraction associated with iron and manganese oxides (BCR2) compared to the organic matter fraction (BCR3). This distribution pattern aligns with the findings of Scharenbroch et al., who linked metal accumulation in urban soils to anthropogenic activities [48]. It has been sufficiently verified, in a study by Yang et al., that in soils in which the pollution rate is increased or climatic factors have changed, it is possible to alter the stability and thus the availability of Cr forms [49].
In cultivated soils, the higher concentration of Cr (III) and Cr (VI) formed along with the organic carbon fraction (BCR3) indicates that soil organic matter significantly influences Cr availability. This is supported by a study by Chen et al., which highlighted the role of organic matter in trace element distribution. In the present study, when comparing aged and more recent soil samples, it was found that in agricultural soils, long-term pollution led to a 30.5% reduction in Cr (III) availability and a 22.7% reduction in Cr (VI) availability. For urban soils, long-term pollution resulted in a 20.9% reduction in Cr (III) availability and a more pronounced 35.3% reduction in Cr (VI) availability [50].
The results of the ANOVA align with the above findings, also showing significant differences due to land use, likely attributable to the varying soil properties between urban and agricultural areas. For Cr (III), urban soils exhibited higher concentrations in all steps of the BCR extraction scheme, as well as in the DTPA-extracted fractions. On the contrary, Cr (VI) displayed higher concentrations in the urban soils only in the second and fourth steps of the BCR extraction, as well as in the DTPA-extracted fractions. Conversely, there were no significant differences in the exchangeable and weakly bound fractions of the BCR1 step or the oxidizable bound fractions (BCR3).
The results related to the aging factor are illustrated in Figure 3 and Figure 4. For Cr (III), soils aged via pollution, regardless of land use, exhibited significantly higher concentrations in all steps of the BCR extraction scheme (Figure 3). However, no significant differences were observed in Cr (III) extracted with DTPA (Figure 5). In contrast, for Cr (VI), the aging factor only led to a significant increase in the fourth step of the BCR extraction scheme. Conversely, Cr (VI) in the second step of the BCR extraction and the Cr (VI) extracted with DTPA showed a significant decrease due to aging. There were no significant differences in the first and third steps of the BCR extraction scheme concerning the aging factor (Figure 4).
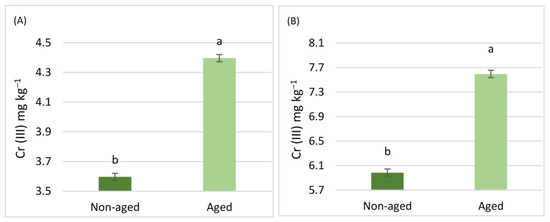
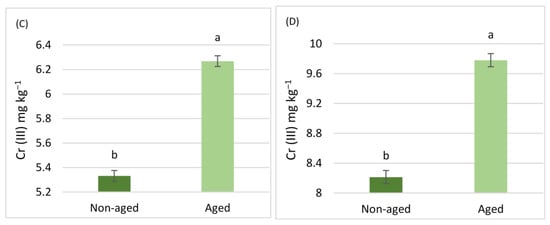
Figure 3.
Comparisons between the mean concentrations of Cr (III) in the soils for the four steps of the BCR extraction scheme [(A) BCR1, (B) BCR2, (C) BCR3, (D) BCR4)]. Differences were observed due to the aging factor. Comparisons were calculated using the LSD test (p < 0.05) and are presented with lowercase characters.
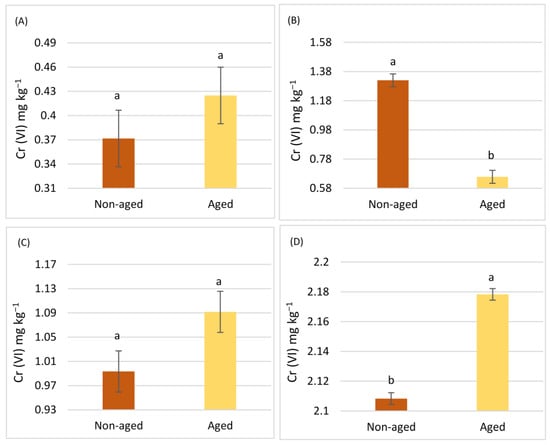
Figure 4.
Comparisons between the mean concentrations of Cr (VI) in soils for the four steps of the BCR extraction scheme [(A) BCR1, (B) BCR2, (C) BCR3, (D) BCR4)]. Differences were observed due to the aging factor. Comparisons were calculated using the LSD test (p < 0.05) and are presented with lowercase characters. Identical letters imply no significant difference.
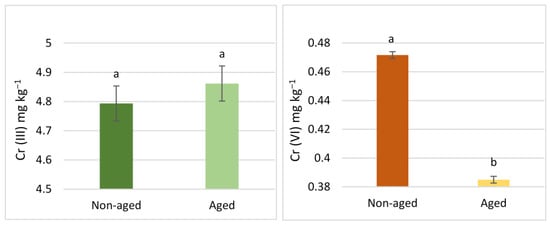
Figure 5.
Comparisons between the mean concentrations of Cr (III) and Cr (VI), extracted with DTPA. Differences were observed due to the aging factor. Comparisons were accomplished using the LSD test (p < 0.05) and are presented using lowercase characters. Identical letters imply no significant difference.
The interaction between the two factors in the ANOVA resulted in interesting outcomes, providing insights into how aging affects Cr levels depending on the type of land use (Figure 6, Figure 7 and Figure 8). For DTPA extraction, aging produced opposite effects for different forms of Cr: it reduced the concentration of Cr (III) in urban soil while increasing it in agricultural soil. On the contrary, for Cr (VI), the aging parameter resulted in lower concentrations in agricultural and urban soils (Figure 8). However, the decrease was more pronounced in urban soil, as indicated by the steeper slope line in the corresponding interaction plot.
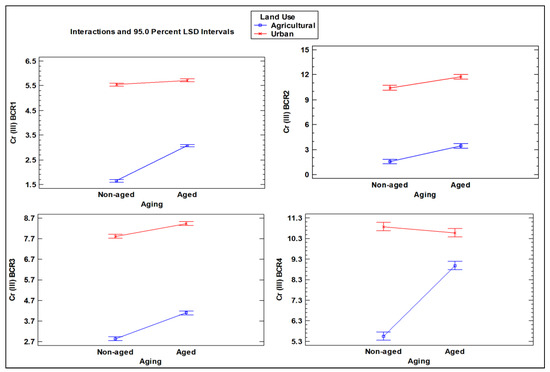
Figure 6.
Interaction plot of Cr (III) concentrations in aged and non-aged soils (mg kg–1) for the four steps of the BCR extraction scheme, as influenced by different land use types (agricultural vs. urban soil).
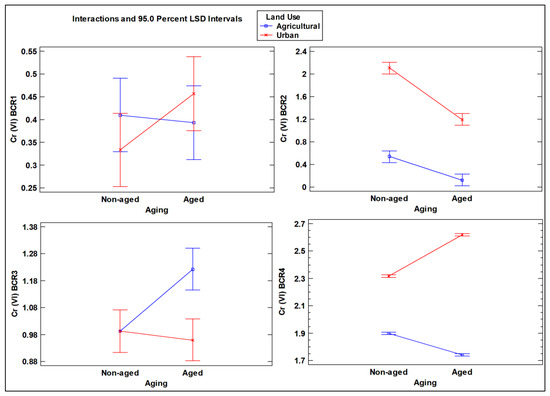
Figure 7.
Interaction plot of Cr (VI) concentrations in aged and non-aged soils (mg kg–1) for the four steps of the BCR extraction scheme, as influenced by different land use types (agricultural vs. urban soil).
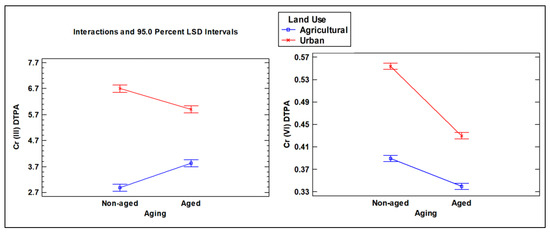
Figure 8.
Interaction plot for Cr (III) and Cr (VI) concentrations in aged and non-aged soils (mg kg−1) extracted with DTPA, as influenced by different land use types (agricultural vs. urban soil).
With regard to the BCR scheme, significant interactions were observed in all steps of the sequential extraction except for the BCR2 step for Cr (III), revealing that the aging parameter resulted in a higher impact on increasing the Cr (III) concentration in the agricultural soil (Figure 6). In contrast, for Cr (VI), the interactions between the two factors in the ANOVA showed significant results in the second, third, and fourth BCR steps, indicating a reduction in both agricultural and urban soils for step 2, while in the third step, the aging factor only produced an increase in the agricultural soil. On the contrary, the aging factor produced a significant increase in the non-available portion of Cr (VI) tightly bound to the mineral matrix of the soil (BCR4) (Figure 7).
Considering the above results, this study confirms that long-term pollution significantly impacts the availability of chromium ions and aligns with previous studies. Shi et al. investigated the impact of time (aging) on the forms of Cr (VI) in five different soils and discovered significant changes in its concentration distribution. Studying pollution aging is particularly crucial when examining and documenting the pollution load of an area [42]. Han et al., studying the effect of the residence time of metal contaminants in soils, concluded that it is possible to manipulate all forms of Cr availability, as it is possible to induce a change between its trivalent and hexavalent states [51]. Papadimou and Golia reached the same conclusion after comparing chronically polluted soils with recently polluted soils, noting that recently polluted soils, either due to recent fertilizer application or recent anthropogenic activities, show different availability patterns than soils with long-term metal loading [52].
In conclusion, pollution aging refers to the long-term physicochemical changes that chromium ions undergo after being deposited in the soil—e.g., redox transformations, adsorption/desorption, complexation, and incorporation into Fe/Mn minerals. These processes determine the availability (mobility and bioavailability) of the two main forms of chromium. However, differences in pH, organic matter, and biological activity determine whether Cr (III) will remain mobile or become immobilized in both urban and agricultural soils.
4. Conclusions
In both urban and cultivated soils, an investigation was conducted in order to study the distribution of trivalent and hexavalent Cr, as well as the parameters that can govern the mutual alternation between these forms. To this end, two agricultural and two urban soils were investigated, while one soil sample of each soil type was exposed to a persistent pollution load, i.e., they underwent pollution aging. It was observed that aging, i.e., the aged chromium contamination of soils, may be less hazardous, as there was a significantly lower concentration of available toxic Cr (VI) ions compared to Cr (III) ions, which are less hazardous to the environment and humans. Considerable variances were observed between the different soil types, i.e., agricultural and urban soils, regarding the distribution of trivalent and hexavalent chromium ion concentrations in the fractions after the step-by-step BCR method. In agricultural soils, a large proportion of Cr (VI) ions were formed along with the organic carbon fraction, whereas in urban soils, Cr (VI) was bound to the iron and manganese oxides. However, the largest fraction of chromium, including both types of ions, was captured and environmentally immobilized in the non-available portion (BCR4), i.e., the last portion obtained in the method, confirming that the risks from the presence of chromium in soils remain limited. Knowledge of the chemical behavior of chromium ions, including studying their fractional distribution in soils, is crucial, especially for Mediterranean soils. As environmental conditions change, mainly due to the climate crisis, a change from toxic hexavalent ions to nontoxic trivalent Cr, or vice versa, is likely to occur. Further studies need to be carried out on soils that have been flooded or affected by extreme changes due to climate change in the Mediterranean region to reduce, as far as possible, the risks posed by the presence of chromium in both agricultural cultivated soils and urban green soils within the urban fabric of Mediterranean cities.
Author Contributions
Conceptualization, E.E.G.; methodology, E.E.G., F.B., E.G., and S.G.; software, E.E.G., F.B., S.G.P., I.P., and D.A.; validation, E.E.G., F.B., E.G., S.G.P., I.P., and D.A.; formal analysis, E.E.G., F.B., E.G., S.G.P., I.P., and D.A.; investigation, E.E.G., F.B., E.G., and S.G.; resources, E.E.G.; data curation, E.E.G., F.B., E.G., and S.G.P.; writing—original draft preparation, E.E.G., F.B., S.G.P., I.P., and D.A.; writing—review and editing, E.E.G., F.B., S.G.P., I.P., and D.A.; visualization, E.E.G., F.B., S.G.P., I.P., and D.A.; supervision, E.E.G.; project administration, E.E.G.; funding acquisition, E.E.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data that supports the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors have no competing interests to declare that are relevant to the content of this article.
References
- Sharma, P.; Singh, S.P.; Parakh, S.K.; Tong, Y.W. Health Hazards of Hexavalent Chromium (Cr (VI)) and Its Microbial Reduction. Bioengineered 2022, 13, 4923–4938. [Google Scholar] [CrossRef]
- Wang, A.; Dang, Z.; Wang, Y.; Fan, H.; Miao, S. Efficient Inorganic Stabilization Materials for Chromium and Arsenic Pollution in Water and Soil. Appl. Sci. 2025, 15, 7069. [Google Scholar] [CrossRef]
- Shahid, M.; Shamshad, S.; Rafiq, M.; Khalid, S.; Bibi, I.; Niazi, N.K.; Dumat, C.; Rashid, M.I. Chromium Speciation, Bioavailability, Uptake, Toxicity and Detoxification in Soil-Plant System: A Review. Chemosphere 2017, 178, 513–533. [Google Scholar] [CrossRef]
- Pandey, A.K.; Gautam, A.; Singh, A.K. Insight to Chromium Homeostasis for Combating Chromium Contamination of Soil: Phytoaccumulators-Based Approach. Environ. Pollut. 2023, 322, 121163. [Google Scholar] [CrossRef]
- Xu, S.; Yu, C.; Wang, Q.; Liao, J.; Liu, C.; Huang, L.; Liu, Q.; Wen, Z.; Feng, Y. Chromium Contamination and Health Risk Assessment of Soil and Agricultural Products in a Rural Area in Southern China. Toxics 2022, 11, 27. [Google Scholar] [CrossRef]
- Sparks, D.L. Environmental Soil Chemistry: An Overview. In Environmental Soil Chemistry; Elsevier: Amsterdam, The Netherlands, 2003; pp. 1–42. [Google Scholar]
- Kumar, V.; Sharma, A.; Kaur, P.; Singh Sidhu, G.P.; Bali, A.S.; Bhardwaj, R.; Thukral, A.K.; Cerda, A. Pollution Assessment of Heavy Metals in Soils of India and Ecological Risk Assessment: A State-of-the-Art. Chemosphere 2019, 216, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Tumolo, M.; Ancona, V.; De Paola, D.; Losacco, D.; Campanale, C.; Massarelli, C.; Uricchio, V.F. Chromium Pollution in European Water, Sources, Health Risk, and Remediation Strategies: An Overview. Int. J. Environ. Res. Public Health 2020, 17, 5438. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Overall Evaluations of Carcinogenicity: An Updating of IARC Monographs, Volumes 1 to 42; International Agency for Research on Cancer: Lyon, France, 1987; ISBN 9283214110. [Google Scholar]
- Chen, Z.; Chen, Y.; Liang, J.; Sun, Z.; Zhao, H.; Huang, Y. The Release and Migration of Cr in the Soil under Alternating Wet–Dry Conditions. Toxics 2024, 12, 140. [Google Scholar] [CrossRef]
- Li, Y.; Lin, J.; Wu, Y.; Jiang, S.; Huo, C.; Liu, T.; Yang, Y.; Ma, Y. Transformation of Exogenous Hexavalent Chromium in Soil: Factors and Modelling. J. Hazard. Mater. 2024, 480, 135799. [Google Scholar] [CrossRef] [PubMed]
- Wani, K.I.; Naeem, M.; Aftab, T. Chromium in Plant-Soil Nexus: Speciation, Uptake, Transport and Sustainable Remediation Techniques. Environ. Pollut. 2022, 315, 120350. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Naushad, M.; Lima, E.C.; Zhang, S.; Shaheen, S.M.; Rinklebe, J. Global Soil Pollution by Toxic Elements: Current Status and Future Perspectives on the Risk Assessment and Remediation Strategies—A Review. J. Hazard. Mater. 2021, 417, 126039. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Liu, J.; Zhuang, Z.; Wang, Q.; Li, H. Heavy Metals in Agricultural Soils: Sources, Influencing Factors, and Remediation Strategies. Toxics 2024, 12, 63. [Google Scholar] [CrossRef]
- Rashid, A.; Schutte, B.J.; Ulery, A.; Deyholos, M.K.; Sanogo, S.; Lehnhoff, E.A.; Beck, L. Heavy Metal Contamination in Agricultural Soil: Environmental Pollutants Affecting Crop Health. Agronomy 2023, 13, 1521. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Ma, J.; Liu, Q.; Shi, T.; Gong, Y.; Yang, S.; Wu, Y. Status of Chromium Accumulation in Agricultural Soils across China (1989–2016). Chemosphere 2020, 256, 127036. [Google Scholar] [CrossRef]
- Adewumi, A.J.; Ogundele, O.D. Hidden Hazards in Urban Soils: A Meta-Analysis Review of Global Heavy Metal Contamination (2010–2022), Sources and Its Ecological and Health Consequences. Sustain. Environ. 2024, 10, 2293239. [Google Scholar] [CrossRef]
- Binner, H.; Sullivan, T.; Jansen, M.A.K.; McNamara, M.E. Metals in Urban Soils of Europe: A Systematic Review. Sci. Total Environ. 2023, 854, 158734. [Google Scholar] [CrossRef]
- Golia, E.E.; Diakoloukas, V. Soil Parameters Affecting the Levels of Potentially Harmful Metals in Thessaly Area, Greece: A Robust Quadratic Regression Approach of Soil Pollution Prediction. Environ. Sci. Pollut. Res. 2022, 29, 29544–29561. [Google Scholar] [CrossRef]
- Golia, E.E. The Impact of Heavy Metal Contamination on Soil Quality and Plant Nutrition. Sustainable Management of Moderate Contaminated Agricultural and Urban Soils, Using Low Cost Materials and Promoting Circular Economy. Sustain. Chem. Pharm. 2023, 33, 101046. [Google Scholar] [CrossRef]
- Page, A.L. Methods of Soil Analysis Part 2, Chemical and Microbiological Properties, 2nd ed.; American Society of Agronomy: Madison, WI, USA, 1982. [Google Scholar]
- Golia, E.E.; Dimirkou, A.; Floras, S.A. Spatial Monitoring of Arsenic and Heavy Metals in the Almyros Area, Central Greece. Statistical Approach for Assessing the Sources of Contamination. Environ. Monit. Assess. 2015, 187, 399. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential Extraction Procedure for the Speciation of Particulate Trace Metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Calvo-Pérez, A.; Domínguez-Renedo, O.; Alonso-Lomillo, M.A.; Arcos-Martínez, M.J. Simultaneous Determination of Cr(III) and Cr(VI) by Differential Pulse Voltammetry Using Modified Screen—Printed Carbon Electrodes in Array Mode. Electroanalysis 2010, 22, 2924–2930. [Google Scholar] [CrossRef]
- Chatzitheodorou, E.; Economou, A.; Voulgaropoulos, A. Trace Determination of Chromium by Square-Wave Adsorptive Stripping Voltammetry on Bismuth Film Electrodes. Electroanalysis 2004, 16, 1745–1754. [Google Scholar] [CrossRef]
- Jorge, E.O.; Rocha, M.M.; Fonseca, I.T.E.; Neto, M.M.M. Studies on the Stripping Voltammetric Determination and Speciation of Chromium at a Rotating-Disc Bismuth Film Electrode. Talanta 2010, 81, 556–564. [Google Scholar] [CrossRef]
- Tonidandel, S.; LeBreton, J.M. RWA Web: A Free, Comprehensive, Web-Based, and User-Friendly Tool for Relative Weight Analyses. J. Bus. Psychol. 2015, 30, 207–216. [Google Scholar] [CrossRef]
- Mónok, D.; Kardos, L.; Pabar, S.A.; Kotroczó, Z.; Tóth, E.; Végvári, G. Comparison of Soil Properties in Urban and Non-urban Grasslands in Budapest Area. Soil Use Manag. 2021, 37, 790–801. [Google Scholar] [CrossRef]
- Papadimou, S.G.; Barbayiannis, Ν.; Golia, E.E. Preliminary Investigation of the Use of Silybum marianum (L.) Gaertn. as a Cd Accumulator in Contaminated Mediterranean Soils: The Relationships among Cadmium (Cd) Soil Fractions and Plant Cd Content. Euro-Mediterr. J. Environ. Integr. 2024, 9, 405–417. [Google Scholar] [CrossRef]
- Geisseler, D.; Scow, K.M. Long-Term Effects of Mineral Fertilizers on Soil Microorganisms—A Review. Soil Biol. Biochem. 2014, 75, 54–63. [Google Scholar] [CrossRef]
- Bekier, J.; Jamroz, E.; Walenczak-Bekier, K.; Uściła, M. Soil Organic Matter Composition in Urban Soils: A Study of Wrocław Agglomeration, SW Poland. Sustainability 2023, 15, 2277. [Google Scholar] [CrossRef]
- Guilland, C.; Maron, P.A.; Damas, O.; Ranjard, L. Biodiversity of Urban Soils for Sustainable Cities. Environ. Chem. Lett. 2018, 16, 1267–1282. [Google Scholar] [CrossRef]
- Penn, C.; Camberato, J. A Critical Review on Soil Chemical Processes That Control How Soil PH Affects Phosphorus Availability to Plants. Agriculture 2019, 9, 120. [Google Scholar] [CrossRef]
- Rolka, E.; Wyszkowski, M. Availability of Trace Elements in Soil with Simulated Cadmium, Lead and Zinc Pollution. Minerals 2021, 11, 879. [Google Scholar] [CrossRef]
- Neina, D. The Role of Soil PH in Plant Nutrition and Soil Remediation. Appl. Environ. Soil Sci. 2019, 2019, 5794869. [Google Scholar] [CrossRef]
- Bobrowski, A.; Mocak, J.; Dominik, J.; Pereira, H.; Bas, B.; Knap, W. Metrological Characteristics and Comparison of Analytical Methods for Determination of Chromium Trace in Water Samples. Acta Chim. Slov. 2004, 51, 77–93. [Google Scholar]
- Thị Hue, N.; Van Hop, N.; Thai Long, H.; Hai Phong, N.; Uyen, T.H.; Quoc Hung, L.; Nhi Phuong, N. Determination of Chromium in Natural Water by Adsorptive Stripping Voltammetry Using In Situ Bismuth Film Electrode. J. Environ. Public Health 2020, 2020, 1347836. [Google Scholar] [CrossRef] [PubMed]
- Hilali, N.; Mohammadi, H.; Amine, A.; Zine, N.; Errachid, A. Recent Advances in Electrochemical Monitoring of Chromium. Sensors 2020, 20, 5153. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-J.; Cheng, Z.; Yang, H.; Morris, E.E.; Sutherland, M.; McSpadden Gardener, B.B.; Grewal, P.S. Differences in Soil Chemical Properties with Distance to Roads and Age of Development in Urban Areas. Urban Ecosyst. 2010, 13, 483–497. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants; CRC Press: Boca Raton, FL, USA, 2010; ISBN 9780429192036. [Google Scholar]
- Choppala, G.; Kunhikrishnan, A.; Seshadri, B.; Park, J.H.; Bush, R.; Bolan, N. Comparative Sorption of Chromium Species as Influenced by PH, Surface Charge and Organic Matter Content in Contaminated Soils. J. Geochem. Explor. 2018, 184, 255–260. [Google Scholar] [CrossRef]
- Shi, J.; McGill, W.B.; Rutherford, P.M.; Whitcombe, T.W.; Zhang, W. Aging Shapes Cr(VI) Speciation in Five Different Soils. Sci. Total Environ. 2022, 804, 150066. [Google Scholar] [CrossRef]
- Ertani, A.; Mietto, A.; Borin, M.; Nardi, S. Chromium in Agricultural Soils and Crops: A Review. Water Air Soil Pollut. 2017, 228, 190. [Google Scholar] [CrossRef]
- Alloway, B.J. (Ed.) Heavy Metals in Soils. In Trace Metals and Metalloids in Soils and Their Bioavailability, 3rd ed.; Blackie Academic and Professional: London, UK, 2013. [Google Scholar]
- Shaheen, S.M.; Kwon, E.E.; Biswas, J.K.; Tack, F.M.G.; Ok, Y.S.; Rinklebe, J. Arsenic, Chromium, Molybdenum, and Selenium: Geochemical Fractions and Potential Mobilization in Riverine Soil Profiles Originating from Germany and Egypt. Chemosphere 2017, 180, 553–563. [Google Scholar] [CrossRef]
- Osakwe, S.A. Chemical Partitioning of Iron, Cadmium, Nickel and Chromium in Contaminated Soils of South-Eastern Nigeria. Chem. Speciat. Bioavailab. 2013, 25, 71–78. [Google Scholar] [CrossRef][Green Version]
- Cao, Q.; Zhao, J.; Ma, W.; Cui, D.; Zhang, X.; Liu, J.; Chen, H. Heavy Metals in Homestead Soil: Metal Fraction Contents, Bioaccessibility, and Risk Assessment. J. Hazard. Mater. 2024, 480, 135933. [Google Scholar] [CrossRef]
- Scharenbroch, B.C.; Trammell, T.L.; Paltseva, A.; Livesley, S.J.; Edmondson, J. Editorial: Urban Soil Formation, Properties, Classification, Management, and Function. Front. Ecol. Evol. 2022, 10, 987903. [Google Scholar] [CrossRef]
- Yang, J.; Guo, Q.; Li, L.; Wang, R.; Chen, Y.; Wang, X. Insights into the Evolution of Cr(VI) Species in Long-Term Hexavalent Chromium Contaminated Soil. Sci. Total Environ. 2023, 858, 160149. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, S.; Tao, Z.; Li, Y.; Magni, P.; Zhang, L.; Zheng, X.; Pan, K. The Importance of Organic Matter in Controlling the Metal Variability and Mobility in Seagrass Sediments. Environ. Pollut. 2025, 366, 125542. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.-S.; Park, J.H.; Ahn, J.S. Aging Effects on Fractionation and Speciation of Redox-Sensitive Metals in Artificially Contaminated Soil. Chemosphere 2021, 263, 127931. [Google Scholar] [CrossRef] [PubMed]
- Papadimou, S.G.; Golia, E.E. Green and Sustainable Practices for an Energy Plant Cultivation on Naturally Contaminated versus Spiked Soils. The Impact of Ageing Soil Pollution in the Circular Economy Framework. Environ. Res. 2024, 246, 118130. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).