Scale Issue for Organic and Inorganic Carbon Exports to Oceans: Case Study in the Sub-Tropical Thukela River Basin, South Africa
Abstract
1. Introduction
2. Materials and Methods
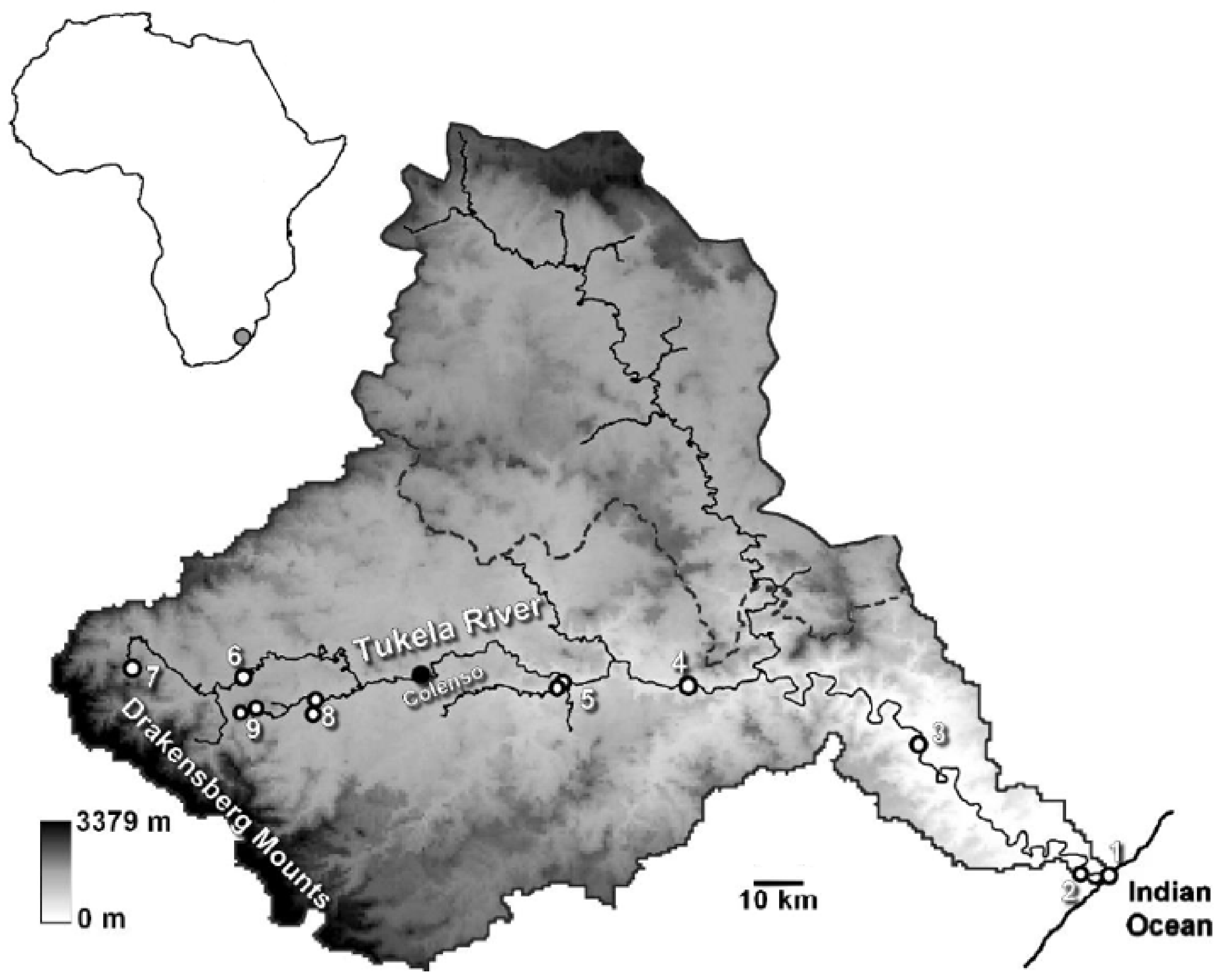
2.1. Study Site
2.2. Experimental Setup
2.3. Sampling Procedure
2.4. Characterization of DOM and POM
3. Results
3.1. Variability in Runoff and Chemical Elements
3.2. Variations in C Concentrations in Runoff and Sediments
3.3. Spatial Scale Issue on Organic and Inorganic C Fluxes
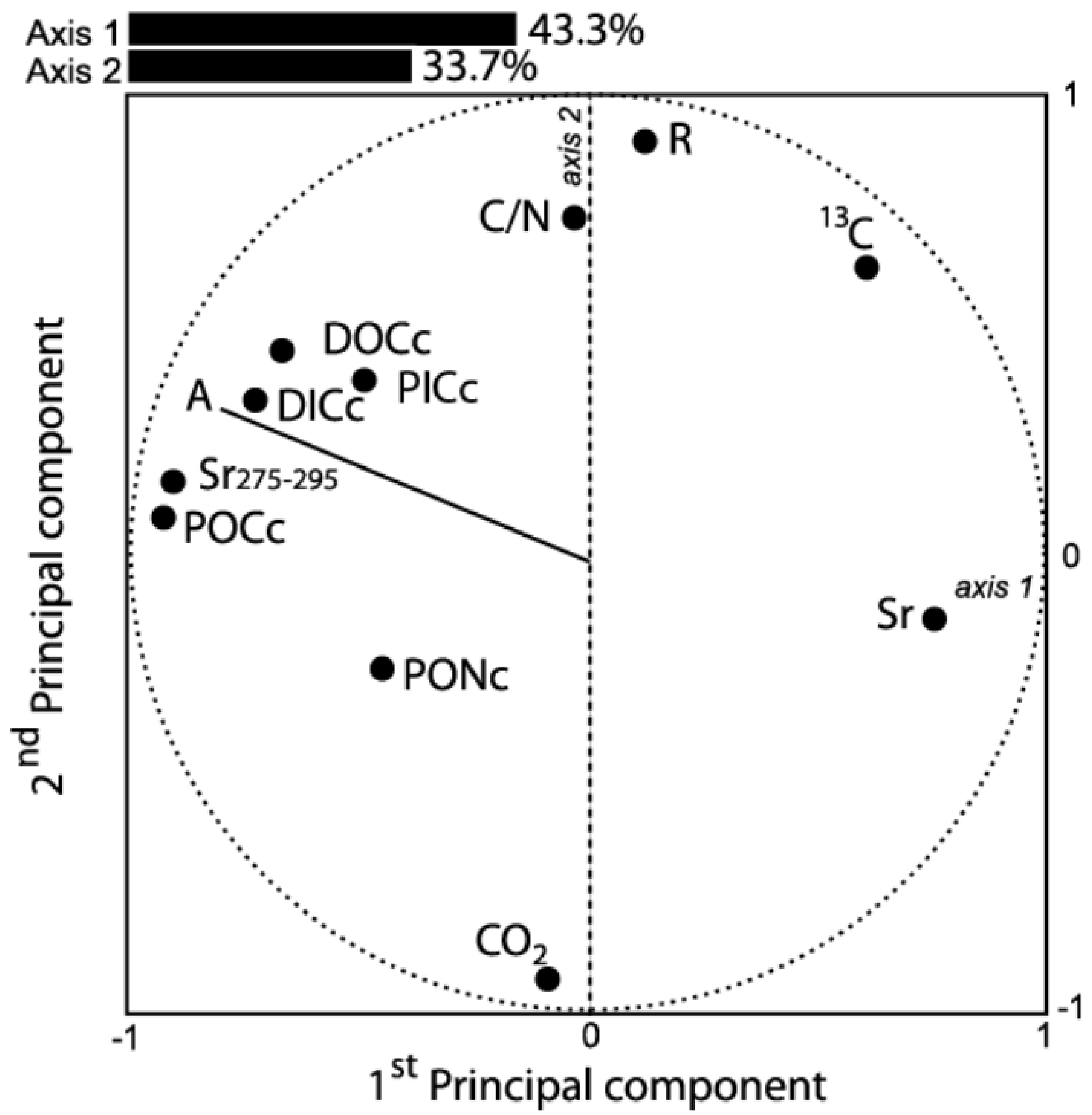
3.4. Factors Controlling POM and DOM Fluxes and Quality
4. Discussion
4.1. On the Fluxes and Sources of Water from Headwater to the Ocean
4.2. On the Link between Runoff and POM and DOM Content and Quality
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wen, H.; Sullivan, P.L.; Billings, S.A.; Ajami, H.; Cueva, A.; Flores, A.; Hirmas, D.R.; Koop, A.N.; Murenbeeld, K.; Zhang, X.; et al. From soils to streams: Connecting terrestrial carbon transformation, chemical weathering, and solute export across hydrological regimes. Water Resour. Res. 2022, 58, e2022WR032314. [Google Scholar] [CrossRef]
- Chaplot, V.; Mutema, M. Sources and main controls of dissolved organic and inorganic carbon in river basins: A worldwide meta-analysis. J. Hydrol. 2021, 603, 126941. [Google Scholar] [CrossRef]
- Müller-Nedebock, D.; Chaplot, V. Soil carbon losses by sheet erosion: A potentially critical contribution to the global carbon cycle. Earth Surf. Process. Landf. 2015, 40, 1803–1813. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Melack, J.M. Transport of organic carbon in the world’s rivers. Tellus 1981, 33, 172–187. [Google Scholar]
- Bianchi, T.S.; Filley, T.; Dria, K.; Hatcher, P.G. Temporal variability in sources of dissolved organic carbon in the lower Mississippi river. Geochim. Cosmochim. Acta 2004, 68, 959–967. [Google Scholar]
- Martoura, R.F.C.; Woodward, E.M.S. Conservative behavior of riverine dissolved organic carbon in the Severn estuary: Chemical and geochemical implications. Geochim. Cosmochim. Acta 1983, 47, 1293–1309. [Google Scholar] [CrossRef]
- Van Heemst, J.D.; Megens, L.; Hatcher, P.G.; de Leeuw, J.W. Nature, origin and average age of estuarine ultrafiltered dissolved organic matter as determined by molecular and carbon isotope characterization. Org. Geochem. 2000, 31, 847–857. [Google Scholar] [CrossRef]
- Liu, S.; Wang, P.; Huang, Q.; Yu, J.; Pozdniakov, S.P.; Kazak, E.S. Seasonal and spatial variations in riverine DOC exports in permafrost-dominated Arctic river basins. J. Hydrol. 2022, 612, 128060. [Google Scholar] [CrossRef]
- Raymond, P.A.; Bauer, J.E. DOC cycling in a temperate estuary: A mass balance approach using natural 14C and 13C isotopes. Limnol. Oceanogr. 2001, 46, 655–667. [Google Scholar] [CrossRef]
- Mayorga, E.; Aufdenkampe, A.K.; Masiello, C.A.; Krusche, A.V.; Hedges, J.I.; Quay, P.D.; Brown, T.A. Young organic matter as a source of carbon dioxide outgassing from Amazonian rivers. Nature 2005, 436, 538–541. [Google Scholar] [CrossRef]
- Ishikawa, N.F.; Butman, D.; Raymond, P.A. Radiocarbon age of different photoreactive fractions of freshwater dissolved organic matter. Org. Geochem. 2019, 135, 11–15. [Google Scholar] [CrossRef]
- Sharp, E.L.; Parsons, S.A.; Jefferson, B. Seasonal variations in natural organic matter and its impact on coagulation in water treatment. Sci. Total Environ. 2006, 363, 183–194. [Google Scholar] [CrossRef]
- Monahan, E.C.; Dam, H.G. Bubbles: An estimate of their role in the global oceanic flux of carbon. J. Geophys. Res. Ocean. 2001, 106, 9377–9383. [Google Scholar] [CrossRef]
- Spencer, R.G.; Aiken, G.R.; Wickland, K.P.; Striegl, R.G.; Hernes, P.J. Seasonal and spatial variability in dissolved organic matter quantity and composition from the Yukon River basin, Alaska. Glob. Biogeochem. Cycles 2008, 22. [Google Scholar] [CrossRef]
- Andersson, J.C.M.; Zehnder, A.J.B.; Jewitt, G.P.W.; Yang, H. Water availability, demand and reliability of in situ water harvesting in smallholder rain-fed agriculture in the Thukela River Basin, South Africa. Hydrol. Earth Syst. Sci. 2009, 13, 2329–2347. [Google Scholar] [CrossRef]
- Mutema, M.; Chivenge, P.; Nivet, F.; Rabouille, C.; Thieu, V.; Chaplot, V. Changes in carbon and nutrient fluxes from headwaters to ocean in a mountainous temperate to subtropical basin. Earth Surf. Process. Landf. 2017, 42, 2038. [Google Scholar] [CrossRef]
- Schulze, R. South African Atlas of Agro Hydrology and Climatology; TT82/96; Water Research Commission, Republic of South Africa: Pretoria, South Africa, 1997. [Google Scholar]
- Weishaar, J.L.; Aiken, G.R.; Bergamaschi, B.A.; Fram, M.S.; Fujii, R.; Mopper, K. Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ. Sci. Technol. 2003, 37, 4702–4708. [Google Scholar] [CrossRef]
- Aiken, G.R.; Hsu-Kim, H.; Ryan, J.N. Influence of dissolved organic matter on the environmental fate of metals, nanoparticles and colloids. Environ. Sci. Technol. 2011, 45, 3196. [Google Scholar] [CrossRef]
- Fang, C.; Moncrieff, J.B. The dependence of soil CO2 efflux on temperature. Soil Biol. Biochem. 2001, 33, 155–165. [Google Scholar] [CrossRef]
- Sickman, J.O.; DiGiorgio, C.L.; Davisson, M.L.; Lucero, D.M.; Bergamaschi, B. Identifying sources of dissolved organic carbon in agriculturally dominated rivers using radiocarbon age dating: Sacramento–San Joaquin River Basin, California. Biogeochemistry 2010, 99, 79–96. [Google Scholar] [CrossRef]
- Van de Giesen, N.C.; Stomph, T.J.; de Ridder, N. Scale effect of Hortonian overland flow and rainfall-runoff dynamics in a West African catena landscape. Hydrol. Process. 2000, 14, 165–175. [Google Scholar] [CrossRef]
- Joel, A.; Messing, I.; Seguel, O.; Casanova, M. Measurement of surface water runoff from plots of two different sizes. Hydrol. Process. 2002, 16, 1467–1478. [Google Scholar] [CrossRef]
- Asadzadeh, F.; Gorji, M.; Vaezi, A.; Sokouti, R.; Shorafa, M. Scale effect on runoff from field plots under natural rainfall. Am.-Eur. J. Agric. Environ. Sci. 2012, 12, 1148–1152. [Google Scholar]
- Thomaz, E.L.; Vestena, L.R. Measurement of runoff and soil loss from two differently sized plots in a subtropical environment (Brazil). Earth Surf. Process. Landf. 2012, 37, 363–373. [Google Scholar] [CrossRef]
- Mutema, M.; Chaplot, V.; Jewitt, G.; Chivenge, P.; Blöschl, G. Annual water, sediment, nutrient, and organic carbon fluxes in river basins: A global meta-analysis as a function of scale. Water Resour. Res. 2015, 51, 8949–8972. [Google Scholar] [CrossRef]
- Cammeraat, E.L.H. Scale dependent thresholds in hydrological and erosion response of a semi-arid catchment in southeast Spain. Agric. Ecosyst. Environ. 2004, 104, 317–332. [Google Scholar] [CrossRef]
- Mayor, A.G.; Bautista, S.; Bellot, J. Scale-dependent variation in runoff and sediment yield in a semiarid Mediterranean catchment. J. Hydrol. 2011, 397, 128–135. [Google Scholar] [CrossRef]
- Le Bissonnais, Y.; Benkhadra, H.; Chaplot, V.; Fox, D.; King, D.; Daroussin, J. Crusting, runoff and sheet erosion on silty loamy soils at various scales and upscaling from m2 to small catchments. Soil Tillage Res. 1998, 46, 69–80. [Google Scholar] [CrossRef]
- Parsons, A.J.; Brazier, R.E.; Wainwright, J.; Powell, D.M. Scale relationships in hillslope runoff and erosion. Earth Surf. Process. Landf. 2006, 31, 1384–1393. [Google Scholar] [CrossRef]
- Doble, R.; Brunner, P.; McCallum, J.; Cook, P.G. An analysis of riverbank slope and unsaturated flow effects on bank storage. Groundwater 2012, 50, 77–86. [Google Scholar] [CrossRef]
- Eder, A.; Exner-Kittridge, M.; Strauss, P.; Blöschl, G. Re-suspension of bed sediment in a small stream–results from two flushing experiments. Hydrol. Earth Syst. Sci. 2014, 18, 1043–1052. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Y.; Yuan, D.; Cui, J.; Li, Y.; Yang, J.; Cao, M. Source and flux of anthropogenically enhanced dissolved inorganic carbon: A comparative study of urban and forest karst catchments in Southwest China. Sci. Total Environ. 2020, 725, 138255. [Google Scholar] [CrossRef] [PubMed]
- Coynel, A.; Seyler, P.; Etcheber, H.; Meybeck, M.; Orange, D. Spatial and seasonal dynamics of total suspended sediment and organic carbon species in the Congo River. Glob. Biogeochem. Cycles 2005, 19, GB4019. [Google Scholar] [CrossRef]
- Hammond, E.G.; Johnson, L.A.; Su, C.; Wang, T.; White, P.J. Soybean oil. In Bailey’s Industrial Oil and Fat Products, 6th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Spencer, R.G.M.; Butler, K.D.; Aiken, G.R. Dissolved organic carbon and chromophoric dissolved organic matter properties of rivers in the USA. J. Geophys. Res. 2012, 117, G03001. [Google Scholar] [CrossRef]
- Drake, T.; Wickland, K.; Spencer, R.; Striegl, R. Ancient low–molecular-weight organic acids in permafrost fuel rapid carbon dioxide production upon thaw. Proc. Natl. Acad. Sci. USA 2015, 112, 13946–13951. [Google Scholar] [CrossRef] [PubMed]
- Chaplot, V.; Ribolzi, O. Hydrograph separation to improve understanding of dissolved organic carbon dynamics in headwater catchments. Hydrol. Process. 2014, 28, 5354–5366. [Google Scholar] [CrossRef]
- Keene, W.C.; Galloway, J.N. Organic acidity in precipitation of North America. Atmos. Environ. (1967) 1984, 18, 2491–2497. [Google Scholar] [CrossRef]
- Sedlak, D.L.; Hoigné, J. The role of copper and oxalate in the redox cycling of iron in atmospheric waters. Atmos. Environ. Part A Gen. Top. 1993, 27, 2173–2185. [Google Scholar] [CrossRef]
| Sampling Site | A (A%) | D | R | SC | Si | Na | Mn | Fe | Zn | Ca | Mg | K | HCO3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| km2 | km | L day−1 km−2 | g L−1 | mg L−1 | |||||||||
| Sub catchment (9) | 9.75 (0.03%) | 433.8 | 904 ± 180 | 0.14 ± 0.03 | 57.5 ± 8.6 | 1.6 ± 0.2 | 25.0 ± 4.0 | 365 ± 58 | 7.5 ± 1.2 | 5.5 ± 0.9 | 2.3 ± 0.4 | 0.8 ± 0.1 | 48.5 ± 5.8 |
| Catchment (5) | 7614 (26%) | 275.6 | 301 ± 62 | 0.08 ± 0.01 | 68.5 ± 6.9 | 2.5 ± 0.2 | 28.3 ± 3.0 | 633 ± 67 | 12.9 ± 1.4 | 10.5 ± 1.2 | 5.1 ± 0.6 | 1.9 ± 0.2 | 58.2 ± 4.6 |
| Large catchment (4) | 14,478 (50%) | 230.6 | 164 ± 12 | 0.02 ± 0.00 | 71.3 ± 5.7 | 1.8 ± 0.1 | 28.3 ± 2.4 | 305 ± 26 | 16.5 ± 1.4 | 17.5 ± 1.6 | 9.6 ± 0.9 | 3.7 ± 0.4 | 114.5 ± 7.3 |
| Sub-basin (3) | 27,845 (96%) | 7.1 | 191 ± 8 | 0.02 ± 0.00 | 78.3 ± 3.9 | 2.3 ± 0.1 | 23.2 ± 1.2 | 413 ± 22 | 23.7 ± 1.3 | 21.2 ± 1.2 | 12.0 ± 0.7 | 2.9 ± 0.2 | 120.4 ± 4.8 |
| Basin (2) | 28,937 (100%) | 0 | 192 ± 7 | 0.01 ± 0.00 | 85.2 ± 4.3 | 3.6 ± 0.2 | 29.7 ± 1.6 | 971 ± 52 | 34.0 ± 1.9 | 22.0 ± 1.2 | 10.0 ± 0.6 | 5.3 ± 0.3 | 155.6 ± 6.2 |
| Sampling Site | POCc | PONc | PICc | DOCc | DICc | C/N | 13C | CO2 | S275–295 | SR |
|---|---|---|---|---|---|---|---|---|---|---|
| % | mg L−1 | ‰ | mgC-CO2 m−2 | Nm−1 | ||||||
| Sub catchment (9) | 0.55 ± 0.12 | 0.09 ± 0.01 | 0.00 ± 0.00 | 9.68 ± 1.55 | 9.91 ± 1.59 | 6.33 ± 1.04± | −20.31 ± 3.45 | 1.21 ± 0.21 | 10.90 ± 1.96 | 1.06 ± 0.13 |
| Catchment (5) | 1.36 ± 0.24 | 0.84 ± 0.08 | 0.00 ± 0.00 | 17.43 ± 2.86 | 11.84 ± 1.26 | 4.46 ± 0.49 | −19.63 ± 2.22 | 1.29 ± 0.15 | 12.53 ± 1.50 | 1.20 ± 0.10 |
| Large catchment (4) | 1.66 ± 0.19 | 0.23 ± 0.02 | 0.12 ± 0.01 | 20.00 ± 1.71 | 24.21 ± 2.07 | 7.40 ± 0.65 | −17.53 ± 1.59 | 0.97 ± 0.09 | 8.29 ± 0.79 | 1.28 ± 0.08 |
| Sub-basin (3) | 3.74 ± 0.18 | 0.64 ± 0.03 | 0.27 ± 0.01 | 21.91 ± 1.17 | 32.70 ± 1.74 | 5.79 ± 0.32 | −21.89 ± 1.24 | 1.29 ± 0.07 | 5.77 ± 0.30 | 1.30 ± 0.05 |
| Basin (2) | 2.10 ± 0.08 | 0.34 ± 0.00 | 0.05 ± 0.00 | 15.59 ± 0.83 | 30.63 ± 1.63 | 6.26 ± 0.34 | −20.58 ± 1.10 | 1.21 ± 0.07 | 6.52 ± 0.39 | 1.29 ± 0.05 |
| Sampling Site | POCL | PICL | DOCL | DICL | CL | POCL | PICL | DOCL | DICL |
|---|---|---|---|---|---|---|---|---|---|
| g C day−1 km−2 | % | ||||||||
| Sub catchment (9) | 69.6 ± 15.3 | 0.00 ± 0.00 | 8.96 ± 1.26 | 8.7 ± 1.4 | 87.4 ± 14.0 | 79.7 ± 13.2 | 0.00 ± 0.00 | 10.0 ± 1.8 | 10.3 ± 1.9 |
| Catchment (5) | 32.8 ± 5.9 | 0.00 ± 0.00 | 3.56 ± 0.33 | 5.3 ± 0.5 | 41.6 ± 4.4 | 78.8 ± 8.0 | 0.00 ± 0.00 | 12.6 ± 1.5 | 8.6 ± 1.0 |
| Large catchment (4) | 5.5 ± 0.5 | 0.38 ± 0.03 | 3.97 ± 0.27 | 3.3 ± 0.3 | 13.2 ± 1.1 | 41.6 ± 3.6 | 3.00 ± 0.27 | 25.1 ± 2.3 | 30.3 ± 2.0 |
| Sub-basin (3) | 14.4 ± 0.7 | 1.04 ± 0.03 | 6.27 ± 0.27 | 4.2 ± 0.2 | 25.8 ± 1.4 | 55.5 ± 3.1 | 4.00 ± 0.22 | 16.3 ± 1.0 | 24.3 ± 1.5 |
| Basin (2) | 4.0 ± 0.1 | 0.11 ± 0.00 | 5.86 ± 0.10 | 3.0 ± 0.1 | 12.9 ± 0.3 | 31.0 ± 1.7 | 0.70 ± 0.04 | 23.0 ± 1.3 | 45.2 ± 2.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mutema, M.; Figlan, S.; Chaplot, V. Scale Issue for Organic and Inorganic Carbon Exports to Oceans: Case Study in the Sub-Tropical Thukela River Basin, South Africa. Land 2023, 12, 815. https://doi.org/10.3390/land12040815
Mutema M, Figlan S, Chaplot V. Scale Issue for Organic and Inorganic Carbon Exports to Oceans: Case Study in the Sub-Tropical Thukela River Basin, South Africa. Land. 2023; 12(4):815. https://doi.org/10.3390/land12040815
Chicago/Turabian StyleMutema, Macdex, Sandiswa Figlan, and Vincent Chaplot. 2023. "Scale Issue for Organic and Inorganic Carbon Exports to Oceans: Case Study in the Sub-Tropical Thukela River Basin, South Africa" Land 12, no. 4: 815. https://doi.org/10.3390/land12040815
APA StyleMutema, M., Figlan, S., & Chaplot, V. (2023). Scale Issue for Organic and Inorganic Carbon Exports to Oceans: Case Study in the Sub-Tropical Thukela River Basin, South Africa. Land, 12(4), 815. https://doi.org/10.3390/land12040815






