Reducing Nitrogen Fertilization in Olive Growing by the Use of Natural Chabazite-Zeolitite as Soil Improver
Abstract
1. Introduction
2. Materials and Methods
2.1. Zeolitite
2.2. Experimental Set-Up
- (1)
- CNT: 100% fertilizer N input and unamended soil (common practice);
- (2)
- ZEO: 50% fertilizer N input and addition of natural zeolitite as soil amendment (500 g added to each plant at planting phase in 2016–2017 at a depth of 30–40 cm).
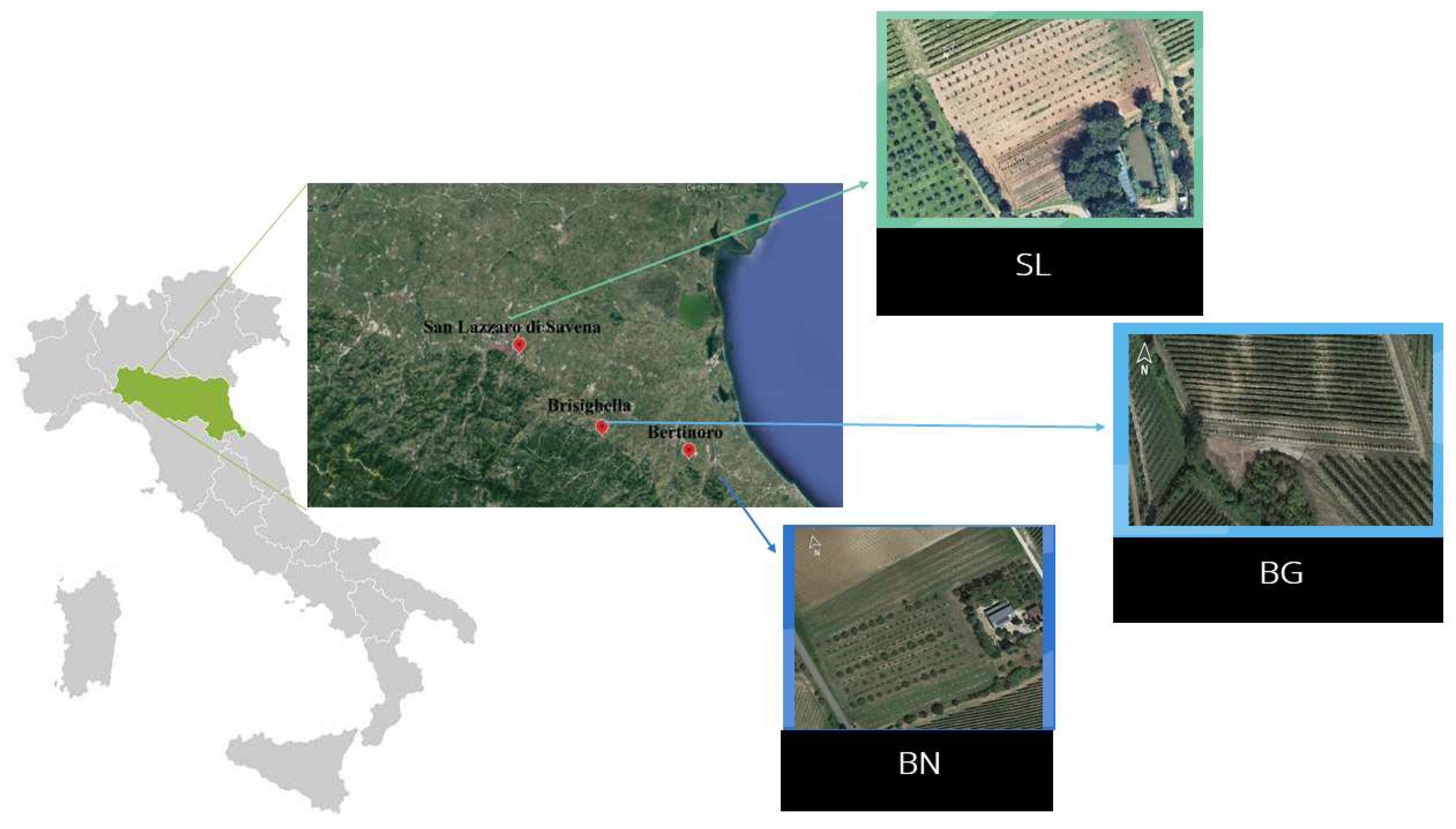
2.2.1. Site 1: San Lazzaro di Savena (SL)
2.2.2. Site 2: Brisighella (BG)
2.2.3. Site 3: Bertinoro (BN)
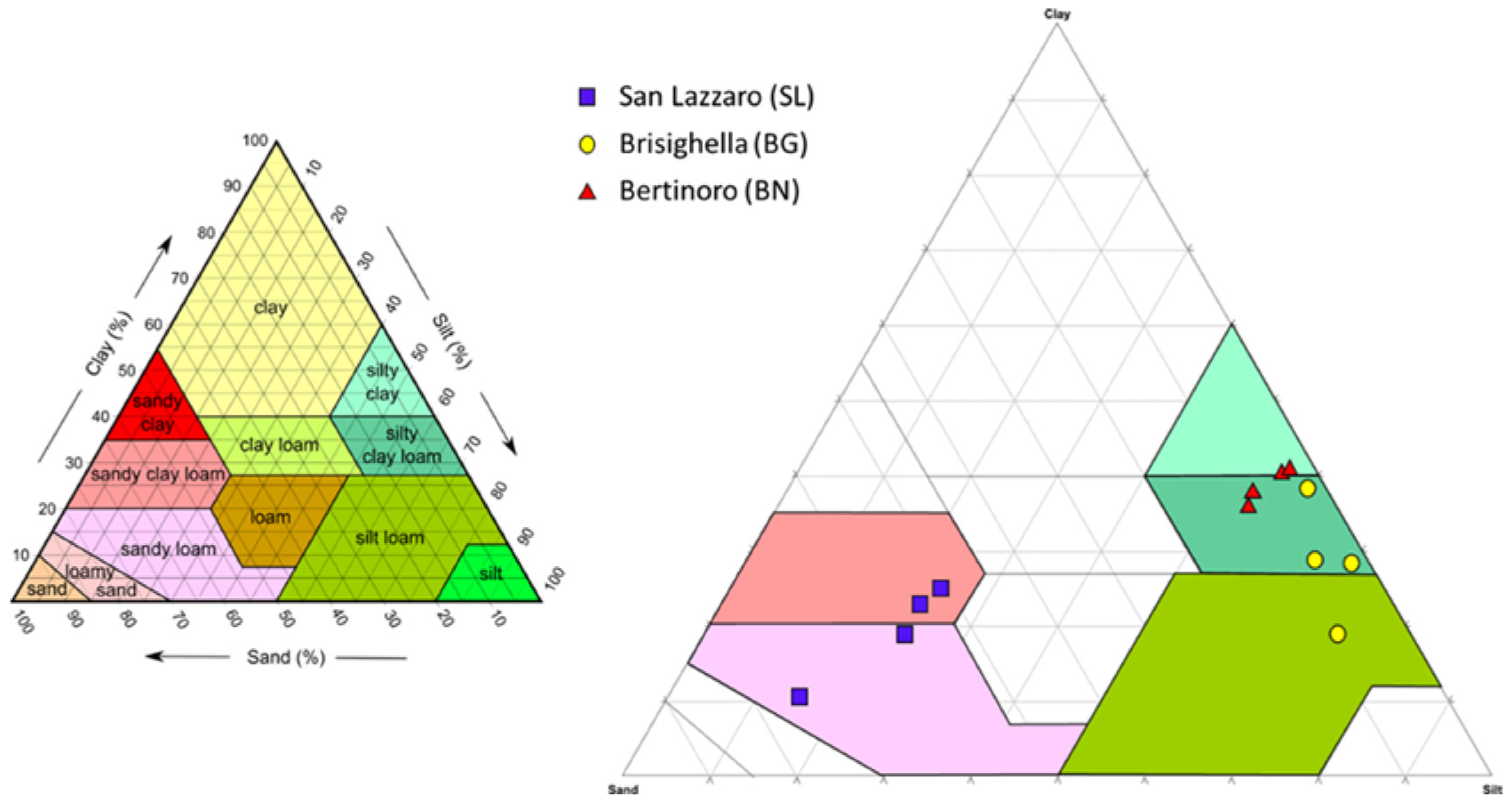
2.3. Textural Analysis
2.4. Chemical Analyses
2.5. Vegetative Measurements
2.6. Statistical Analysis
3. Results and Discussion
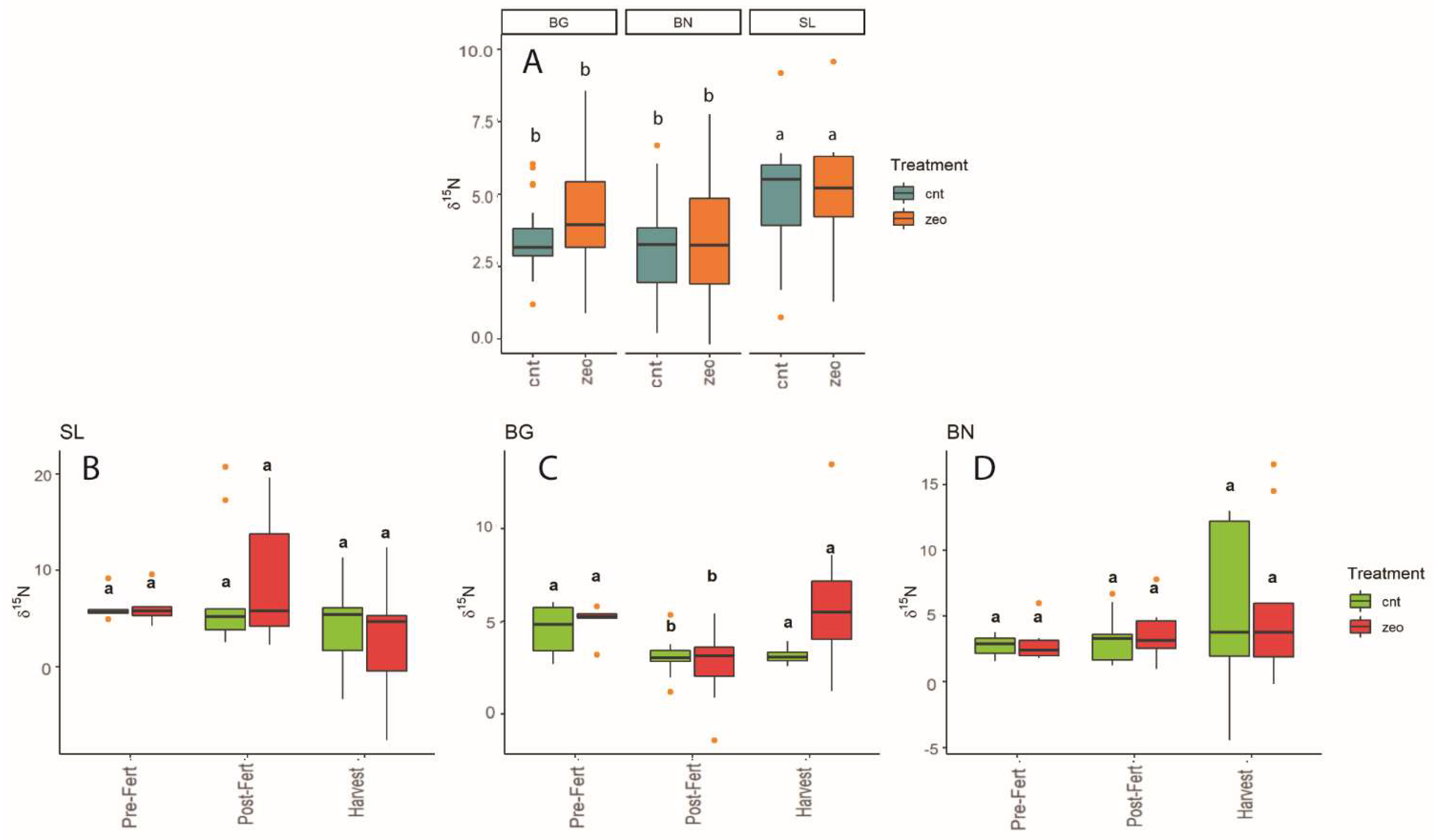
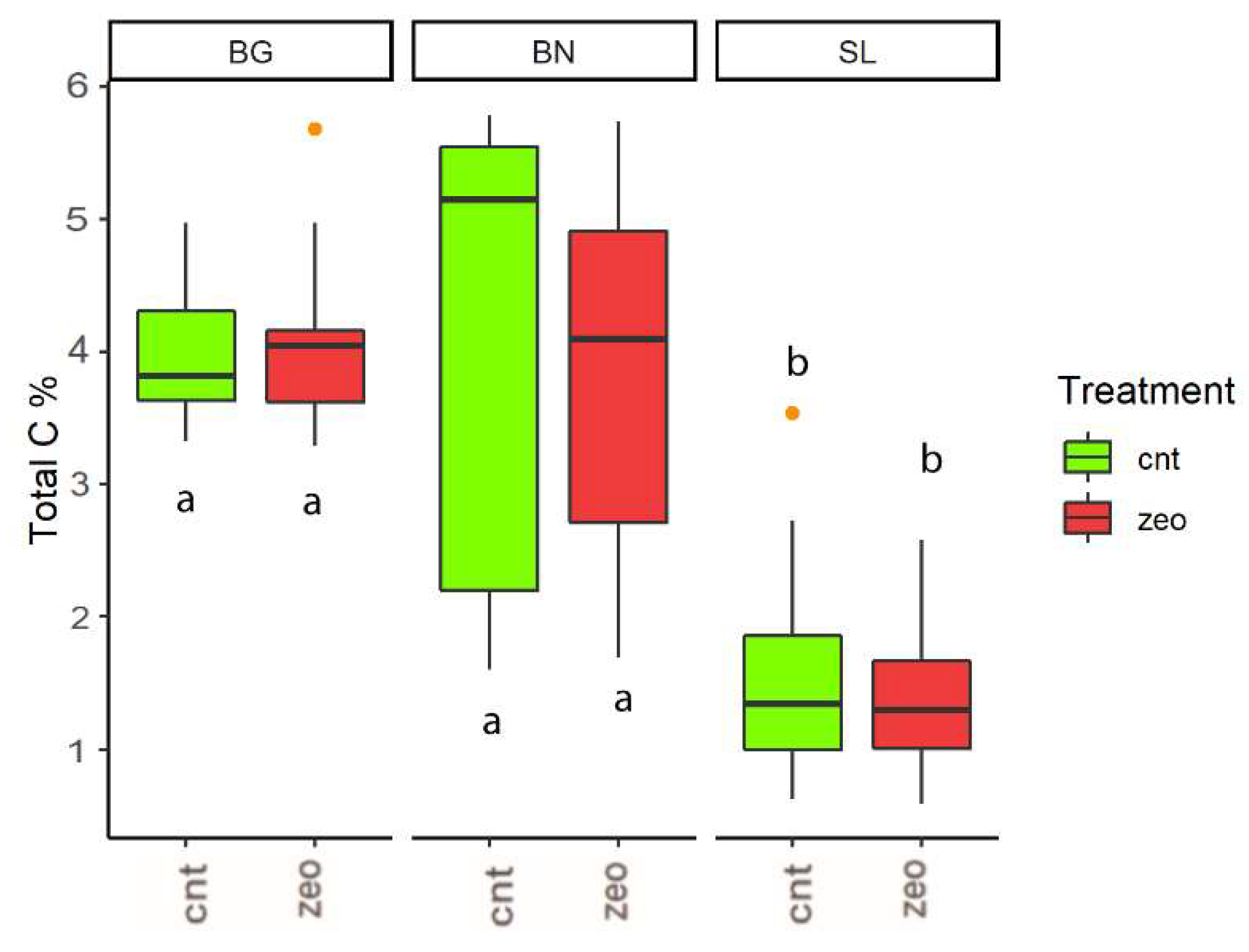
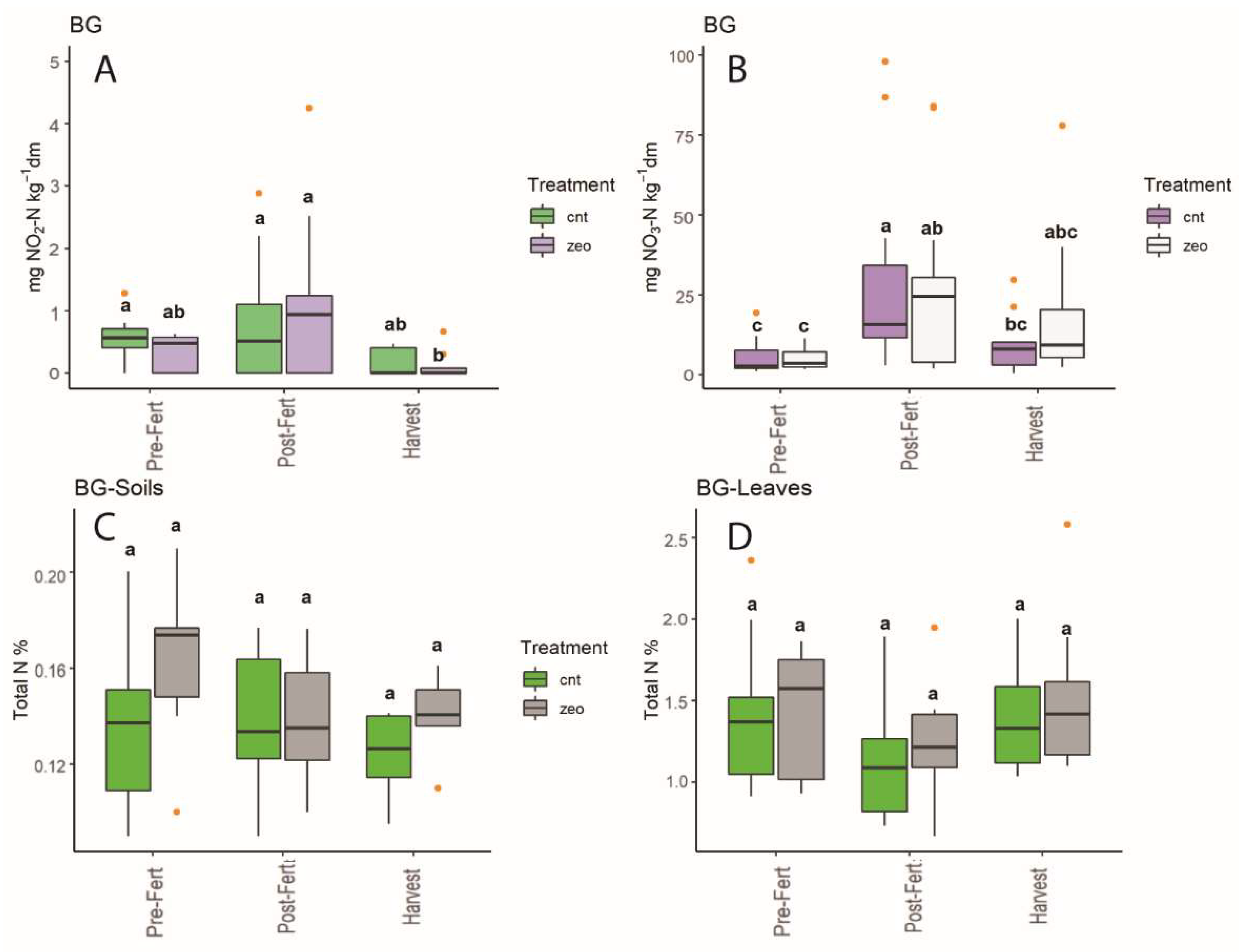
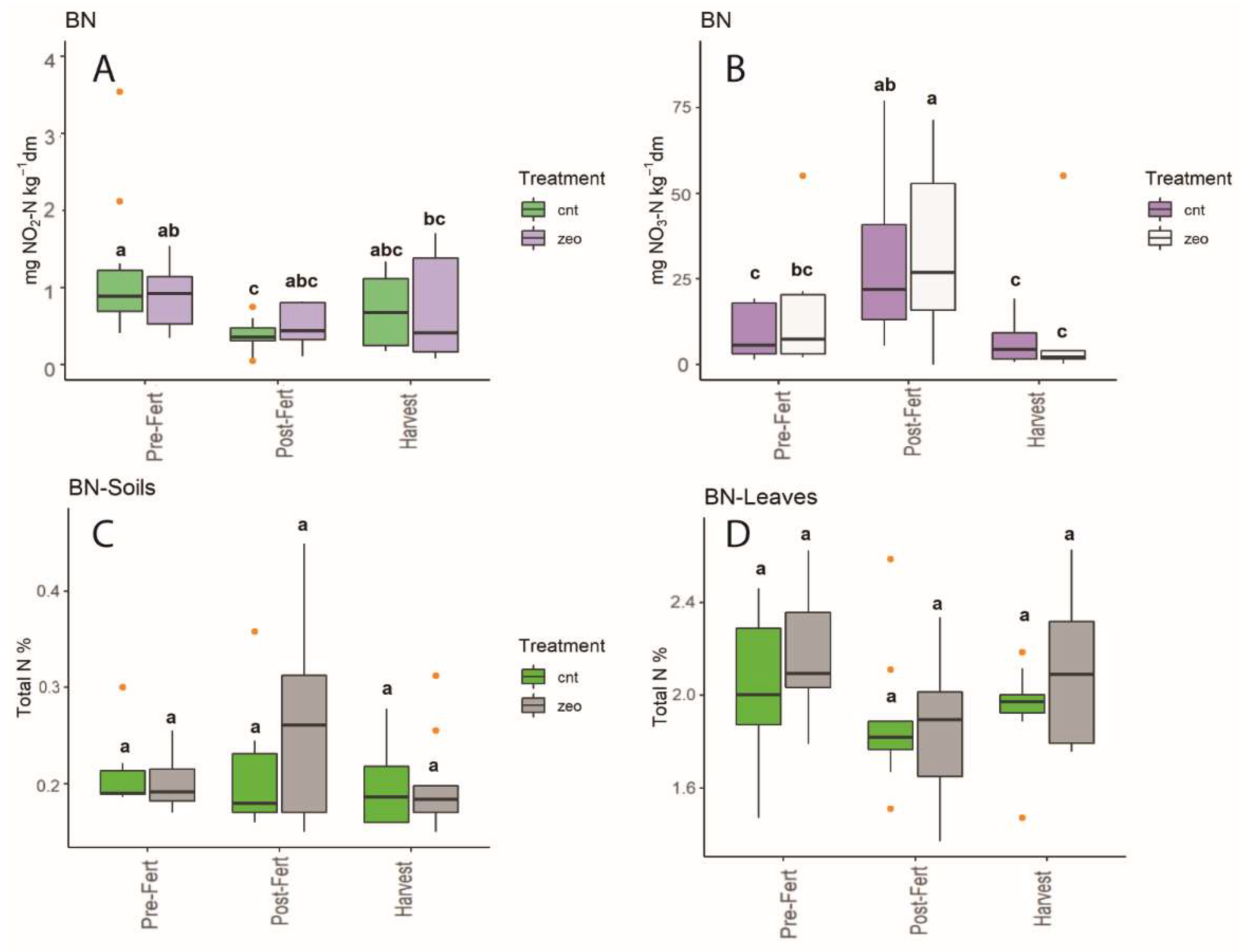
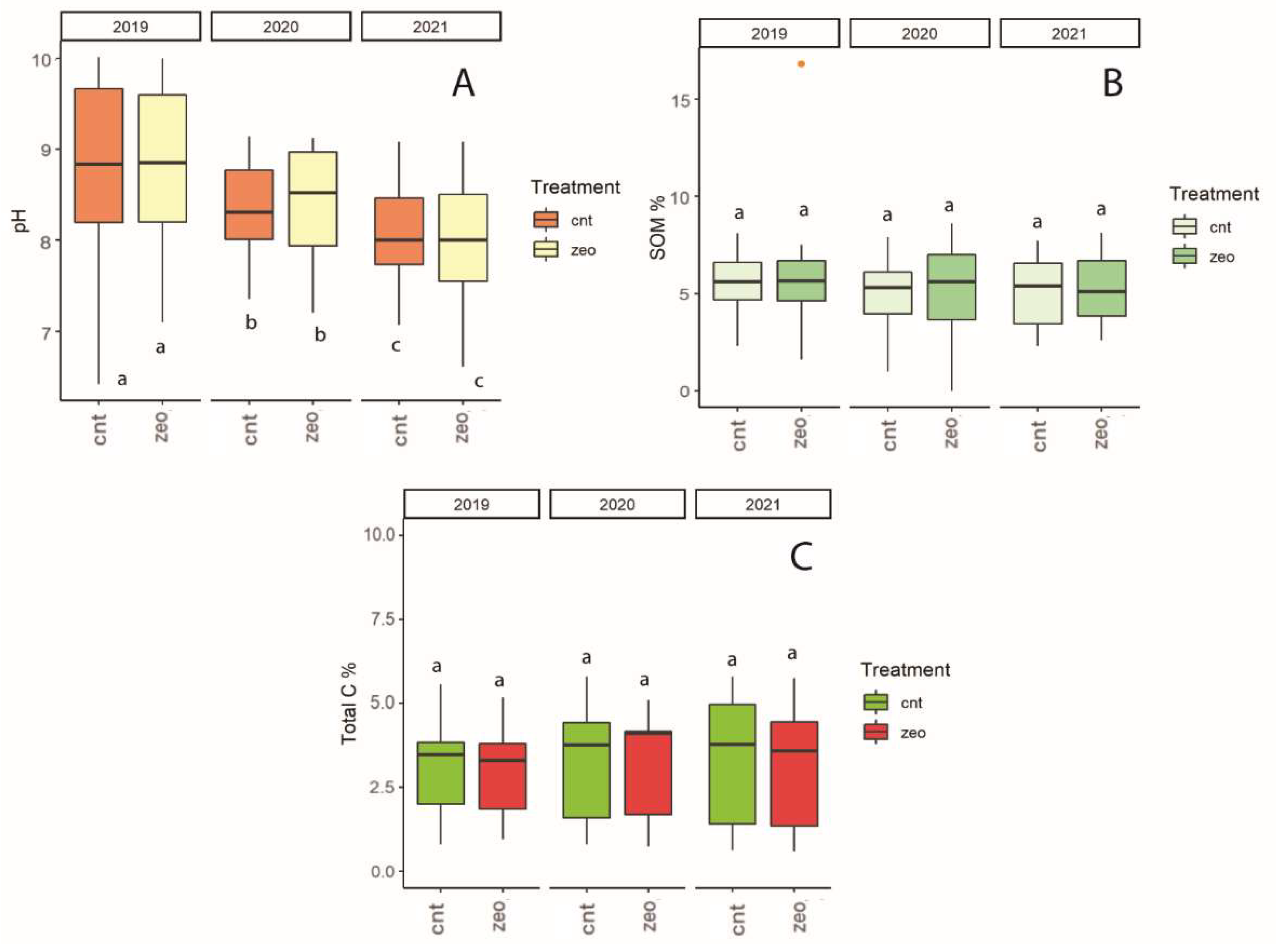
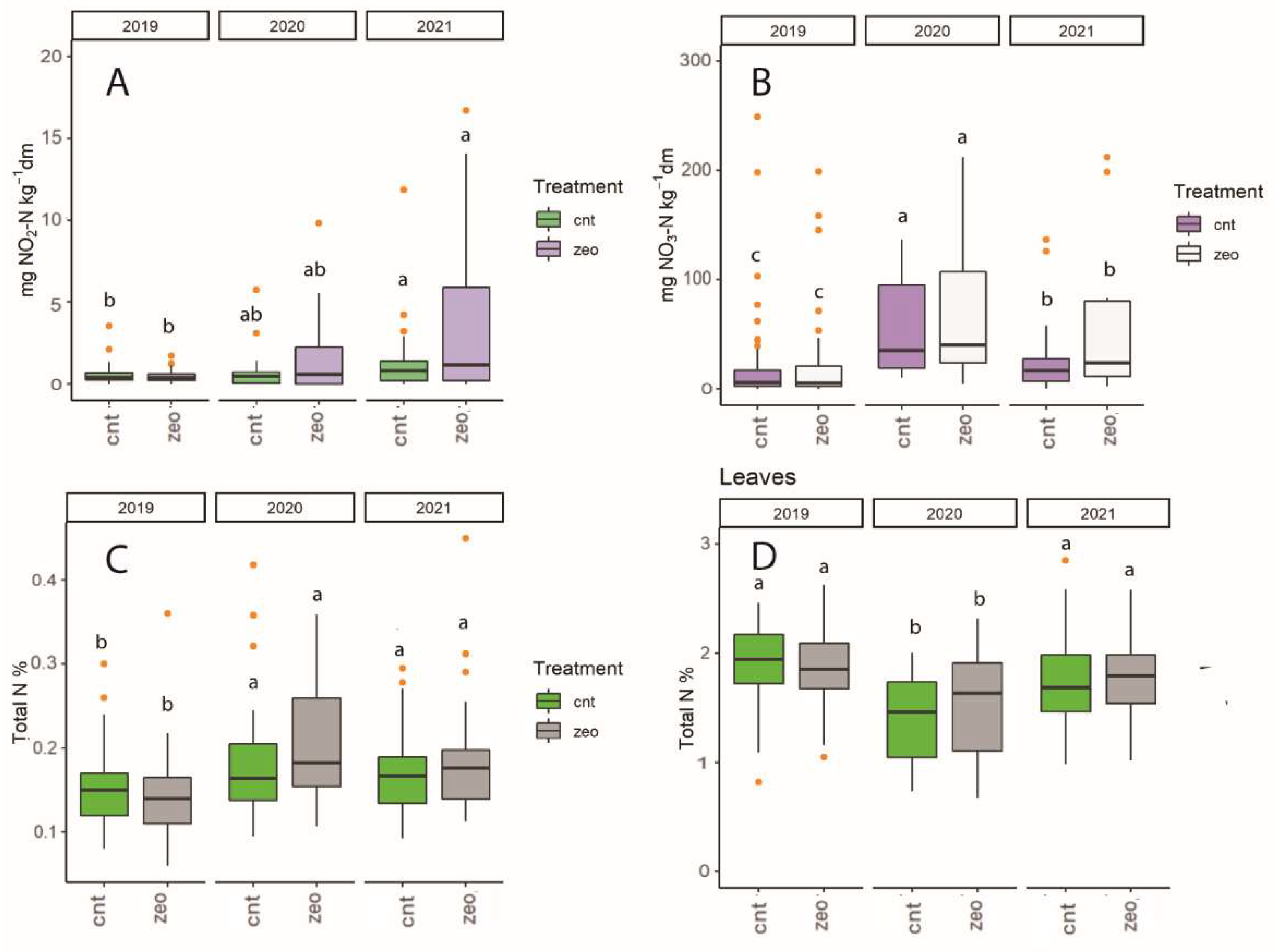
3.1. Dynamics at Each Experimental Site
3.2. Vegetative Measurements
3.3. Global Considerations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mustafa, A.; Hu, X.; Shah, S.A.A.; Abrar, M.M.; Maitlo, A.A.; Kubar, K.A.; Saeed, Q.; Kamran, M.; Naveed, M.; Boren, W.; et al. Long-term fertilization alters chemical composition and stability of aggregate-associated organic carbon in a Chinese red soil: Evidence from aggregate fractionation, C mineralization, and 13C NMR analyses. J. Soils Sediments 2021, 21, 2483–2496. [Google Scholar] [CrossRef]
- Drechsel, P.; Heffer, P.; Magen, H.; Mikkelsen, R.; Wichelns, D. Managing Water and Fertilizer for Sustainable Agricultural Intensification; IFA, IWMI, IPNI and IPI: Paris, France, 2015; ISBN 979-10-92366-02-0. [Google Scholar]
- Basso, B.; Dumont, B.; Cammarano, D.; Pezzuolo, A.; Marinello, F.; Sartori, L. Environmental and economic benefits of variable rate nitrogen fertilization in a nitrate vulnerable zone. Sci. Total Environ. 2016, 545, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Buresh, R.J.; Huang, J.; Yang, J.; Zou, Y.; Zhong, X.; Wang, G.; Zhang, F. Strategies for overcoming low agronomic nitrogen use efficiency in irrigated rice systems in China. Field Crop. Res. 2006, 96, 37–47. [Google Scholar] [CrossRef]
- Dawson, J.C.; Huggins, D.R.; Jones, S.S. Characterizing nitrogen use efficiency in natural and agricultural ecosystems to improve the performance of cereal crops in low-input and organic agricultural systems. Field Crop. Res. 2008, 107, 89–101. [Google Scholar] [CrossRef]
- Chien, S.H.; Teixeira, L.A.; Cantarella, H.; Rehm, G.W.; Grant, C.A.; Gearhart, M.M. Agronomic Effectiveness of Granular Nitrogen/Phosphorus Fertilizers Containing Elemental Sulfur with and without Ammonium Sulfate: A Review. Agron. J. 2016, 108, 1203–1213. [Google Scholar] [CrossRef]
- Ferretti, G.; Keiblinger, K.M.; Zimmermann, M.; Di Giuseppe, D.; Faccini, B.; Colombani, N.; Mentler, A.; Zechmeister-Boltenstern, S.; Coltorti, M.; Mastrocicco, M. High resolution short-term investigation of soil CO2, N2O, NOx and NH3 emissions after different chabazite zeolite amendments. Appl. Soil Ecol. 2017, 119, 138–144. [Google Scholar] [CrossRef]
- Soares, J.R.; Cantarella, H.; de Campos Menegale, M.L. Ammonia volatilization losses from surface-applied urea with urease and nitrification inhibitors. Soil Biol. Biochem. 2012, 52, 82–89. [Google Scholar] [CrossRef]
- Sharma, L.K.; Bali, S.K. A review of methods to improve nitrogen use efficiency in agriculture. Sustainability 2017, 10, 58. [Google Scholar] [CrossRef]
- Cataldo, E.; Salvi, L.; Paoli, F.; Fucile, M.; Masciandaro, G.; Manzi, D.; Masini, C.M.; Mattii, G.B. Application of Zeolites in Agriculture and Other Potential Uses: A Review. Agronomy 2021, 11, 1547. [Google Scholar] [CrossRef]
- Shi, L.; Liu, L.; Yang, B.; Sheng, G.; Xu, T. Evaluation of Industrial Urea Energy Consumption (EC) Based on Life Cycle Assessment (LCA). Sustainability 2020, 12, 3793. [Google Scholar] [CrossRef]
- Bremmer, J.; Gonzalez-Martinez, A.; Jongeneel, R.; Huiting, H.; Stokkers, R.; Ruijs, M. Impact Assessment of EC 2030 Green Deal Targets for Sustainable Crop Production; 2021; ISBN 9789464470413. [Google Scholar]
- Reháková, M.; Čuvanová, S.; Dzivák, M.; Rimár, J.; Gaval’Ová, Z. Agricultural and agrochemical uses of natural zeolite of the clinoptilolite type. Curr. Opin. Solid State Mater. Sci. 2004, 8, 397–404. [Google Scholar] [CrossRef]
- Ferretti, G.; Galamini, G.; Medoro, V.; Coltorti, M.; Giuseppe, D.D.; Faccini, B. Impact of sequential treatments with natural and na-exchanged chabazite zeolite-rich tuff on pig-slurry chemical composition. Water 2020, 12, 310. [Google Scholar] [CrossRef]
- Rotondi, A.; Morrone, L.; Facini, O.; Faccini, B.; Ferretti, G.; Coltorti, M. Distinct particle films impacts on olive leaf optical properties and plant physiology. Foods 2021, 10, 1291. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, N.; Emekci, M.; Athanassiou, C.G. Applications of natural zeolites on agriculture and food production. J. Sci. Food Agric. 2017, 97, 3487–3499. [Google Scholar] [CrossRef] [PubMed]
- Kesraoui-Ouki, S.; Cheeseman, C.R.; Perry, R. Natural zeolite utilisation in pollution control: A review of applications to metals’ effluents. J. Chem. Technol. Biotechnol. 1994, 59, 121–126. [Google Scholar] [CrossRef]
- Colombani, N.; Di Giuseppe, D.; Faccini, B.; Ferretti, G.; Mastrocicco, M.; Coltorti, M. Estimated Water Savings in an Agricultural Field Amended With Natural Zeolites. Environ. Process. 2016, 3, 617–628. [Google Scholar] [CrossRef]
- Perez-Caballero, R.; Gil, J.; Benitez, C.; Gonzalez, J.L. The effect of adding zeolite to soils in order to improve the N-K nutrition of olive trees. Preliminary results. Am. J. Agric. Biol. Sci. 2008, 3, 321–324. [Google Scholar] [CrossRef][Green Version]
- Passaglia, E. Zeoliti Naturali, Zeolititi e Loro Applicazioni; Arvan: Gardena, CA, USA, 2008; ISBN 9788887801194. [Google Scholar]
- Colombani, N.; Mastrocicco, M.; Giambastiani, B.M.S. Predicting Salinization Trends in a Lowland Coastal Aquifer: Comacchio (Italy). Water Resour. Manag. 2015, 29, 603–618. [Google Scholar] [CrossRef]
- Gholamhoseini, M.; Ghalavand, A.; Khodaei-Joghan, A.; Dolatabadian, A.; Zakikhani, H.; Farmanbar, E. Zeolite-amended cattle manure effects on sunflower yield, seed quality, water use efficiency and nutrient leaching. Soil Tillage Res. 2013, 126, 193–202. [Google Scholar] [CrossRef]
- Nakhli, S.A.A.; Delkash, M.; Bakhshayesh, B.E.; Kazemian, H. Application of Zeolites for Sustainable Agriculture: A Review on Water and Nutrient Retention. Water Air Soil Pollut. 2017, 228, 464. [Google Scholar] [CrossRef]
- Mirzaei Aminiyan, M.; Safari Sinegani, A.A.; Sheklabadi, M. Aggregation stability and organic carbon fraction in a soil amended with some plant residues, nanozeolite, and natural zeolite. Int. J. Recycl. Org. Waste Agric. 2015, 4, 11–22. [Google Scholar] [CrossRef]
- Eslami, M.; Khorassani, R.; Coltorti, M.; Malferrari, D.; Faccini, B.; Ferretti, G.; Di Giuseppe, D.; Fotovat, A.; Halajnia, A. Leaching behaviour of a sandy soil amended with natural and NH4+ and K+ saturated clinoptilolite and chabazite. Arch. Agron. Soil Sci. 2017, 64, 1142–1151. [Google Scholar] [CrossRef]
- Ferretti, G.; Di Giuseppe, D.; Natali, C.; Faccini, B.; Bianchini, G.; Coltorti, M. C-N elemental and isotopic investigation in agricultural soils: Insights on the effects of zeolitite amendments. Chem. Der Erde 2017, 77, 45–52. [Google Scholar] [CrossRef]
- Tsintskaladze, G.; Eprikashvili, L.; Mumladze, N.; Gabunia, V.; Sharashenidze, T.; Zautashvili, M.; Kordzakhia, T.; Shatakishvili, T. Nitrogenous zeolite nanomaterial and the possibility of its application in agriculture. Ann. Agrar. Sci. 2017, 15, 365–369. [Google Scholar] [CrossRef]
- Manjaiah, K.M.; Mukhopadhyay, R.; Paul, R.; Datta, S.C.; Kumararaja, P.; Sarkar, B. Clay minerals and zeolites for environmentally sustainable agriculture. Modif. Clay Zeolite Nanocompos. Mater. Environ. Pharm. Appl. 2019, 309–329. [Google Scholar] [CrossRef]
- Milosevic, T.; Milosevic, N. The effect of zeolite, organic and inorganic fertilizers on soil chemical properties, growth and biomass yield of apple trees. Plant Soil Environ. 2009, 55, 528–535. [Google Scholar] [CrossRef]
- Ferretti, G.; Faccini, B.; Antisari, L.V.; Di Giuseppe, D.; Coltorti, M. 15N natural abundance, nitrogen and carbon pools in soil-sorghum system amended with natural and NH4+-enriched zeolitites. Appl. Sci. 2019, 9, 4524. [Google Scholar] [CrossRef]
- Faccini, B.; Di Giuseppe, D.; Ferretti, G.; Coltorti, M.; Colombani, N.; Mastrocicco, M. Natural and NH4+-enriched zeolitite amendment effects on nitrate leaching from a reclaimed agricultural soil (Ferrara Province, Italy). Nutr. Cycl. Agroecosyst. 2018, 110, 327–341. [Google Scholar] [CrossRef]
- Hazrati, S.; Tahmasebi-Sarvestani, Z.; Mokhtassi-Bidgoli, A.; Modarres-Sanavy, S.A.M.; Mohammadi, H.; Nicola, S. Effects of zeolite and water stress on growth, yield and chemical compositions of Aloe vera L. Agric. Water Manag. 2017, 181, 66–72. [Google Scholar] [CrossRef]
- Ozbahce, A.; Tari, A.F.; Gönülal, E.; Simsekli, N.; Padem, H. The effect of zeolite applications on yield components and nutrient uptake of common bean under water stress. Arch. Agron. Soil Sci. 2014, 61, 615–626. [Google Scholar] [CrossRef]
- El-Sherpiny, M.; Baddour, A.G.; El-Kafrawy, M.M. Effect of Zeolite Soil addition under Different Irrigation Intervals on Maize Yield (zea mays L.) and some Soil Properties. J. Soil Sci. Agric. Eng. 2020, 11, 793–799. [Google Scholar] [CrossRef]
- Rodrigues, M.; Torres, L.D.N.D.; Damo, L.; Raimundo, S.; Sartor, L.; Cassol, L.C.; Arrobas, M. Nitrogen Use Efficiency and Crop Yield in Four Successive Crops Following Application of Biochar and Zeolites. J. Soil Sci. Plant Nutr. 2021, 21, 1053–1065. [Google Scholar] [CrossRef]
- Minardi, S.; Haniati, I.L.; Nastiti, A.H.L. Adding manure and zeolite to improve soil chemical properties and increase soybean yield. SainsTanah-J. Soil Sci. Agroclimatol. 2020, 17, 1. [Google Scholar] [CrossRef]
- Sun, Y.; Xia, G.; He, Z.; Wu, Q.; Zheng, J.; Li, Y.; Wang, Y.; Chen, T.; Chi, D. Zeolite amendment coupled with alternate wetting and drying to reduce nitrogen loss and enhance rice production. Field Crop. Res. 2019, 235, 95–103. [Google Scholar] [CrossRef]
- Ferreira, I.Q.; Arrobas, M.; Moutinho-Pereira, J.M.; Correia, C.M.; Rodrigues, M.Â. The effect of nitrogen applications on the growth of young olive trees and nitrogen use efficiency. Turk. J. Agric. For. 2020, 44, 278–289. [Google Scholar] [CrossRef]
- Fernández-Escobar, R.; Benlloch, M.; Herrera, E.; García-Novelo, J.M. Effect of traditional and slow-release N fertilizers on growth of olive nursery plants and N losses by leaching. Sci. Hortic. 2004, 101, 39–49. [Google Scholar] [CrossRef]
- Fernández-Escobar, R. Use and abuse of nitrogen in olive fertilization. Acta Hortic. 2011, 888, 249–257. [Google Scholar] [CrossRef]
- Othman, Y.A.; Leskovar, D. Nitrogen management influenced root length intensity of young olive trees. Sci. Hortic. 2019, 246, 726–733. [Google Scholar] [CrossRef]
- Fernández-Escobar, R.; García-Novelo, J.M.; Molina-Soria, C.; Parra, M.A. An approach to nitrogen balance in olive orchards. Sci. Hortic. 2012, 135, 219–226. [Google Scholar] [CrossRef]
- Lopes, J.I.; Arrobas, M.; Brito, C.; Gonçalves, A.; Silva, E.; Martins, S.; Raimundo, S.; Rodrigues, M.Â.; Correia, C.M. Mycorrhizal Fungi were More Effective than Zeolites in Increasing the Growth of Non-Irrigated Young Olive Trees. Sustainability 2020, 12, 10630. [Google Scholar] [CrossRef]
- Ferretti, G.; Keiblinger, K.M.; Faccini, B.; Di Giuseppe, D.; Mentler, A.; Zechmeister-Boltenstern, S.; Coltorti, M. Effects of Different Chabazite Zeolite Amendments to Sorption of Nitrification Inhibitor 3,4-Dimethylpyrazole Phosphate (DMPP) in Soil. J. Soil Sci. Plant Nutr. 2020, 20, 973–978. [Google Scholar] [CrossRef]
- Galamini, G.; Ferretti, G.; Medoro, V.; Tescaro, N.; Faccini, B.; Coltorti, M. Isotherms, Kinetics, and Thermodynamics of NH4+ Adsorption in Raw Liquid Manure by Using Natural Chabazite Zeolite-Rich Tuff. Water 2020, 12, 2944. [Google Scholar] [CrossRef]
- Stamatakis, M.G.; Stamataki, I.S.; Giannatou, S.; Vasilatos, C.; Drakou, F.; Mitsis, I.; Xinou, K. Characterization and evaluation of chabazite- and mordenite-rich tuffs, and their mixtures as soil amendments and slow release fertilizers. Arch. Agron. Soil Sci. 2016, 63, 735–747. [Google Scholar] [CrossRef]
- Malferrari, D.; Laurora, A.; Brigatti, M.F.; Coltorti, M.; Di Giuseppe, D.; Faccini, B.; Passaglia, E.; Vezzalini, M.G. Open-field experimentation of an innovative and integrated zeolitite cycle: Project definition and material characterization. Rend. Lincei 2013, 24, 141–150. [Google Scholar] [CrossRef]
- GeoViewer—Geoportale. Available online: https://geoportale.regione.emilia-romagna.it/mappe/geo-viewer?layer_id=0f536ddad2c442d293b6afa0119edde5 (accessed on 22 August 2022).
- World Reference Base.|FAO SOILS PORTAL|Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/soils-portal/data-hub/soil-classification/world-reference-base/en/ (accessed on 16 August 2022).
- Tarocco, A.; Aprea, A. Carta dei Suoli Della Regione Emilia-Romagna; Regione Emilia-Romagna: Bologna, Italy, 2021. [Google Scholar]
- Pavan, V.; Marletto, V. Rapporto IdroMeteoClima Emilia-Romagna. Dati 2019; Osservatorio Clima di ARPAE: Rimini, Italy, 2020. [Google Scholar]
- Pavan, V. Rapporto IdroMeteoClima Emilia-Romagna. Dati 2020; Osservatorio Clima di ARPAE: Rimini, Italy, 2021. [Google Scholar]
- Pavan, V. Rapporto IdroMeteoClima Emilia-Romagna. Dati 2021; Osservatorio Clima di ARPAE: Rimini, Italy, 2022. [Google Scholar]
- Di Giuseppe, D.; Faccini, B.; Mastrocicco, M.; Colombani, N.; Coltorti, M. Reclamation influence and background geochemistry of neutral saline soils in the Po River Delta Plain (Northern Italy). Environ. Earth Sci. 2014, 72, 2457–2473. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer International Publishing: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- De Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research. R Package Version 1.2-0. 2016. Available online: http://CRAN.R-project.org/package=agricolae (accessed on 11 April 2022).
- Schloerke, B.; Cook, D.; Larmarange, J.; Briatte, F.; Marbach, M.; Thoen, E.; Elberg, A.; Toomet, O.; Crowley, J.; Hofmann, H.; et al. GGally: Extension to Ggplot2 2021. Available online: https://CRAN.R-project.org/package=Ggally (accessed on 11 April 2022).
- Vincent, Q. Vu Ggbiplot: A Ggplot2 Based Biplot 2011. Available online: http://github.com/vqv/ggbiplot (accessed on 11 April 2022).
- Horikoshi, M.; Ggfortify, Y.T. Data Visualization Tools for Statistical Analysis Results; 2016; Available online: https://CRAN.R-project.org/package=ggfortify (accessed on 11 April 2022).
- Eldor, A.P. Soil Microbiology, Ecology, and Biochemistry; Academic Press: Burlington, NJ, USA, 2007; ISBN 9780125468077. [Google Scholar]
- Bateman, A.S.; Kelly, S.D. Fertilizer nitrogen isotope signatures. Isot. Environ. Health Stud. 2007, 43, 237–247. [Google Scholar] [CrossRef]
- Shan, Y.; Huang, M.; Suo, L.; Zhao, X.; Wu, L. Composition and variation of soil δ15N stable isotope in natural ecosystems. CATENA 2019, 183, 104236. [Google Scholar] [CrossRef]
- Amundson, R.; Austin, A.T.; Schuur, E.A.G.; Yoo, K.; Matzek, V.; Kendall, C.; Uebersax, A.; Brenner, D.; Baisden, W.T. Global patterns of the isotopic composition of soil and plant nitrogen. Glob. Biogeochem. Cycles 2003, 17, 1031. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon Isotope Discrimination and Photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Riehl, S.; Pustovoytov, K.E.; Weippert, H.; Klett, S.; Hole, F. Drought stress variability in ancient Near Eastern agricultural systems evidenced by δ13C in barley grain. Proc. Natl. Acad. Sci. USA 2014, 111, 12348–12353. [Google Scholar] [CrossRef]
- Busch, F.A.; Holloway-Phillips, M.; Stuart-Williams, H.; Farquhar, G.D. Revisiting carbon isotope discrimination in C3 plants shows respiration rules when photosynthesis is low. Nat. Plants 2020, 6, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Singh, B. Effect of water stress on carbon isotope discrimination and Rubisco activity in bread and durum wheat genotypes. Physiol. Mol. Biol. Plants 2009, 15, 281–286. [Google Scholar] [CrossRef]
- Jakkula, V.; Zeolites, W.S.P. Potential Soil Amendments for Improving Nutrient and Water Use Efficiency and Agriculture Productivity. Available online: https://www.researchgate.net/publication/325544295_Zeolites_Potential_soil_amendments_for_improving_nutrient_and_water_use_efficiency_and_agriculture_productivity (accessed on 11 April 2022).
- Hatfield, J.L.; Follet, R.F. Nitrogen in the Environment; The Scientific World Journal: London, UK, 2018; ISBN 9780333227794. [Google Scholar]
- Prisa, D. Optimised fertilisation with zeolitites containing Plant Growth Promoting Rhizobacteria (PGPR) in Ranunculus asiaticus. GSC Biol. Pharm. Sci. 2020, 10, 096–102. [Google Scholar] [CrossRef]
- Choo, L.N.L.K.; Ahmed, O.H.; Talib, S.A.A.; Ghani, M.Z.A.; Sekot, S. Clinoptilolite zeolite on tropical peat soils nutrient, growth, fruit quality, and yield of Carica papaya L. CV. sekaki. Agronomy 2020, 10, 1320. [Google Scholar] [CrossRef]
- Sepaskhah, A.R.; Barzegar, M. Yield, water and nitrogen-use response of rice to zeolite and nitrogen fertilization in a semi-arid environment. Agric. Water Manag. 2010, 98, 38–44. [Google Scholar] [CrossRef]
- Ferretti, G.; Galamini, G.; Deltedesco, E.; Gorfer, M.; Fritz, J.; Faccini, B.; Mentler, A.; Zechmeister-Boltenstern, S.; Coltorti, M.; Keiblinger, K.M. Gross Ammonification and Nitrification Rates in Soil Amended with Natural and NH4-Enriched Chabazite Zeolite and Nitrification Inhibitor DMPP. Appl. Sci. 2021, 11, 2605. [Google Scholar] [CrossRef]




| Phase | % | St.dev | ||
|---|---|---|---|---|
| DA (g cm−3) | 0.56 | chabazite | 68.5 | 0.9 |
| HR (%) | 34.2 | phillipsite | 1.8 | 0.4 |
| analcime | 0.6 | 0.3 | ||
| CEC (meq g−1) | TZC | 70.9 | ||
| Ca2+ | 1.46 | mica | 5.3 | 0.6 |
| Mg2+ | 0.04 | K-feldspar | 9.7 | 0.7 |
| Na+ | 0.07 | plagioclase | - | |
| K+ | 0.6 | pyroxene | 2.9 | 0.4 |
| Total | 2.17 | calcite | - | |
| volcanic glass | 11.2 | 1.0 |
| SL | BG | BN | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | SOM (%) | pH | SOM (%) | pH | SOM (%) | ||||||||
| Pre-Fert | CNT | 8.01° | ±0.09 | 3.59° | ±1.12 | 8.83° | ±0.29 | 4.76ab | ±1.32 | 8.84° | ±1.21 | 6.44b | ±0.03 |
| ZEO | 7.93° | ±0.37 | 4.04° | ±0.48 | 8.77° | ±0.22 | 5.51ab | ±2.04 | 8.86° | ±1.24 | 6.30b | ±0.33 | |
| Post-Fert | CNT | 7.71° | ±0.43 | 5.05b | ±0.80 | 8.66b | ±0.19 | 5.26b | ±0.30 | 8.65° | ±0.92 | 6.97° | ±0.38 |
| ZEO | 8.27° | ±0.55 | 3.18b | ±2.72 | 8.67b | ±0.08 | 5.22b | ±0.94 | 8.45° | ±0.96 | 7.64° | ±0.63 | |
| Harvest | CNT | 7.56° | ±0.24 | 3.21c | ±0.62 | 8.77ab | ±0.05 | 5.91° | ±0.51 | 8.47° | ±1.11 | 5.35b | ±2.37 |
| ZEO | 7.56° | ±0.48 | 3.02c | ±0.51 | 8.84ab | ±0.13 | 6.16° | ±0.25 | 8.43° | ±1.09 | 4.79b | ±3.32 | |
| Tree Height (cm) | Number of Branches | Average Branches Length (cm) | ∑ Branches Length (cm) | ||
|---|---|---|---|---|---|
| Cv. Nostrana di Brisighella | ZEO | 141.48 | 63.39 | 30.63 | 1918.05 |
| CNT | 134.09 | 51.78 | 30.09 | 1597.65 |
| Tree Height (cm) | Number of Branches | Average Branches Length (cm) | ∑ Branches Length (cm) | ||
|---|---|---|---|---|---|
| Cv. Montebudello | ZEO | 121.14 | 49.86 | 24.71 | 1193.14 |
| CNT | 92.00 | 20.00 | 22.39 | 441.86 | |
| Cv. Farneto | ZEO | 114.31 | 69.69 | 22.75 | 1592.50 |
| CNT | 84.54 | 44.38 | 20.37 | 972.15 | |
| Cv. Montecapra | ZEO | 104.50 | 62.63 | 22.71 | 1368.38 |
| CNT | 99.29 | 47.00 | 21.55 | 1060.50 |
| Tree Height (cm) | Number of Branches | Average Branches Length (cm) | ∑ Branches Length (cm) | ||
|---|---|---|---|---|---|
| Cv. Capolga | ZEO | 86 | 20.76 | 14.65 | 392.18 |
| CNT | 83.1 | 18.95 | 13.92 | 347.18 | |
| Cv. Colombina | ZEO | 74.88 | 10.53 | 15.63 | 233.82 |
| CNT | 63.77 | 6.46 | 11.33 | 141.58 | |
| Cv. Correggiolo | ZEO | 102.38 | 9.25 | 21.04 | 291.38 |
| CNT | 80.43 | 7.79 | 15.76 | 204.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medoro, V.; Ferretti, G.; Galamini, G.; Rotondi, A.; Morrone, L.; Faccini, B.; Coltorti, M. Reducing Nitrogen Fertilization in Olive Growing by the Use of Natural Chabazite-Zeolitite as Soil Improver. Land 2022, 11, 1471. https://doi.org/10.3390/land11091471
Medoro V, Ferretti G, Galamini G, Rotondi A, Morrone L, Faccini B, Coltorti M. Reducing Nitrogen Fertilization in Olive Growing by the Use of Natural Chabazite-Zeolitite as Soil Improver. Land. 2022; 11(9):1471. https://doi.org/10.3390/land11091471
Chicago/Turabian StyleMedoro, Valeria, Giacomo Ferretti, Giulio Galamini, Annalisa Rotondi, Lucia Morrone, Barbara Faccini, and Massimo Coltorti. 2022. "Reducing Nitrogen Fertilization in Olive Growing by the Use of Natural Chabazite-Zeolitite as Soil Improver" Land 11, no. 9: 1471. https://doi.org/10.3390/land11091471
APA StyleMedoro, V., Ferretti, G., Galamini, G., Rotondi, A., Morrone, L., Faccini, B., & Coltorti, M. (2022). Reducing Nitrogen Fertilization in Olive Growing by the Use of Natural Chabazite-Zeolitite as Soil Improver. Land, 11(9), 1471. https://doi.org/10.3390/land11091471








