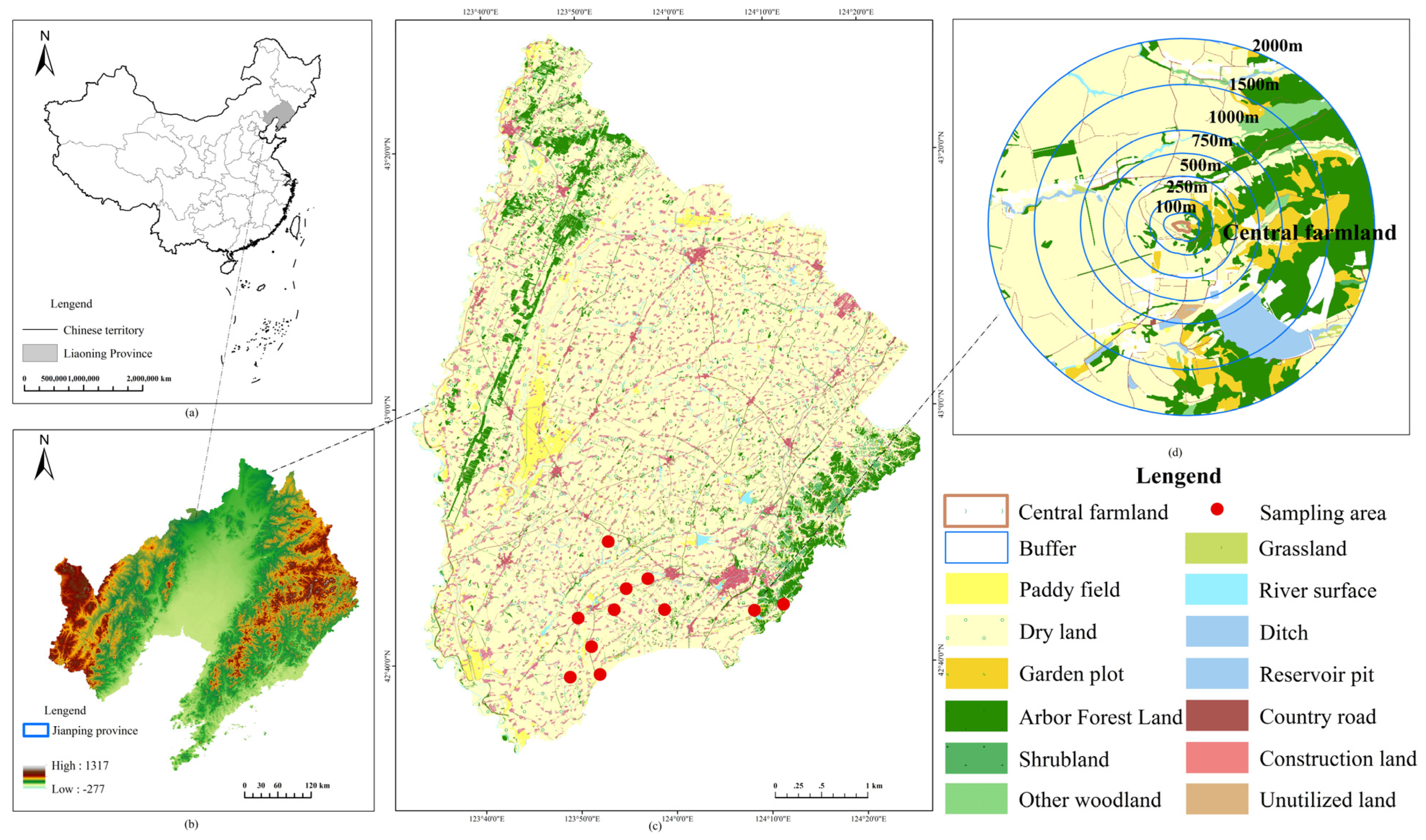
1. Introduction
The arable land system of sustainable development can greatly reduce the negative environmental impact caused by the continuous population growth. The arable land system is composed of intensive farmland and non-crop habitats (such as ditches, woodlands, grasslands, and among others.) [
1,
2,
3]. The arable land system coexists with the surrounding physical space (e.g., different land use types), and at the same time coexists with the natural ecology and other factors, and exchanges material energy and information to form micro-ecological space. In the past few decades, the increase in crop yields has been mainly achieved through agricultural intensification. A homogeneous arable land system has brought considerable productivity and also led to the loss of biodiversity [
4,
5]. The unsustainability of the arable land system is becoming more and more obvious in the context of the global popularity of COVID-19. Replanning a multi-functional landscape to form a reasonable landscape structure is an effective way to enhance the sustainability of the arable land system [
6,
7].
As the dominant species in terrestrial ecosystems, arthropods provide a wide range of ecosystem services such as pest control, crop pollination, and decomposition to maintain soil fertility [
8,
9]. The non-crop habitats within an agro-ecological mosaic landscape have the potential to provide biodiversity and ES, which directly affect the stability of arable land system landscape structure and ecosystem [
10,
11]. The farmland landscape is the main component of the arable land system landscape, providing key ecosystem services, especially in the form of food. Compared with farmland, non-crop habitats have more diverse species, so they are considered the key areas for biodiversity conservation. Non-crop habitats can form corridor networks, connecting fragmented, isolated, or residual patches into a whole ecological region, which is convenient for biological migration in a landscape, thereby increasing gene exchange and the selection and utilization of different resource patches to maintain population stability [
12]. For example, shrublands, flower strips, and grasslands around farmland provide food sources for predators such as spiders and pelagic beetles, and overwintering sites, thereby regulating pests promptly by maintaining a stable population of natural enemies, and providing alternative food sources such as pollen for pollinators such as bees [
13,
14]. Bosch-Serra et al. summarized through a large number of cases that more than 63% of the species living in farmland depended on non-crop habitats [
15]. Studies have also shown that 20% of non-crop habitats in agricultural landscapes are the threshold for ecosystem processes associated with biodiversity conservation [
16,
17].
ES provided by agrobiodiversity and functional biodiversity can be enhanced by diversifying landscape elements such as non-crop habitats surrounding the fields [
18,
19]. Abundance, species richness, and evenness of epigaeic arthropods are all influenced by both habitat scale and landscape scale characteristics, although not necessarily to the same extent [
20,
21]. In regions with single landscape composition and configuration, biodiversity is more sensitive to landscape heterogeneity. The optimal scale of landscape heterogeneity affecting pollinator diversity in plain landform-dominated areas is 500 m [
22,
23], while in hilly landform-dominated areas, the optimal scale is 2000 m [
24]. In addition, compared with the habitat scale, the landscape (geomorphic type) scale has a more significant impact on pollinator diversity. The spatial scale has gradually become a key factor in the study of arthropod ecosystem services. In recent years, scholars have further proved that the increase in land-use intensity is beneficial to pests at the landscape scale by exploring the effects of agricultural intensification on crop insects and biological control at different scales [
25,
26]. However, low agricultural intensity management measures at the field scale, such as pesticide use, rotation intercropping, and organic farming, can alleviate these effects. The degree of biological control potentially achieved in afield is thus dependent on how epigaeic arthropods respond to these multi-scale factors, so determining the ideal arable land system landscape that would favor biodiversity conservation and ecosystem production becomes a complex task [
27,
28,
29].
On the landscape scale, the landscape index is considered to be an effective means to describe the landscape structure. There is an optimal scale for the response of epigaeic arthropod diversity to the landscape structure, but the optimal scale is not immutable [
30,
31]. It is mainly affected by the natural background characteristics such as topography, the characteristics of biological groups, and the differences in seasons and agricultural production behaviors [
32,
33]. At the same time, there is a minimum scale of landscape scale research, for example, scholars have found that there is no significant correlation between landscape heterogeneity and the interaction between predators and pests of epigaeic arthropods at the scale of 1000 m in the plain area [
34]. In this case, the study at the habitat scale can more appropriately reflect the impact of non-crop habitats on arthropods. The diversity of epigaeic arthropods at the habitat scale is mainly affected by habitat types and farmland margin characteristics. The results of the study on the effects of agroforestry systems on predatory arthropods showed that the abundance and diversity of arthropods in forest habitats were significantly higher than those in cultivated land [
35,
36]. Different types of habitat margins formed a variety of farmland margins, which have complex vegetation structures as a channel connecting adjacent two habitats. It not only has the common biological populations of adjacent two habitats, but also has its unique species, so it can support more abundant biological communities than the habitat center [
37]. The effects of landscape and habitat factors should be considered at the scale of significant correlation influence, because the outcome in terms of biological control ultimately depends on trophic and non-trophic links within interaction networks, which is a rarely addressed but important element to consider [
38,
39,
40].
In this study, to make landscape design on the spatial scale related to arthropod species, we focused on the influence mechanism of landscape structure at different scales (landscape and habitat) on the diversity of epigaeic arthropods. More specifically, we propose the following research objectives: (1) On the landscape scale, we analyze the scale effect of the response of epigaeic arthropod diversity to landscape heterogeneity, and determine the optimal scale and minimum scale of the impact. (2) On the habitat scale, we predicted that: (i) the differences in epigaeic arthropod community structures in different habitat types were analyzed. (ii) The margin effect affects the distribution of epigaeic arthropods. (3) On the field scale, the vegetation structure of field margin strips was the main factor affecting the differences in epigaeic arthropods. (4) Clarify the landscape and environmental factors affecting epigaeic arthropods at two scales and construct the optimal model.
4. Discussion
The arable land system can be interpreted as a heterogeneous region composed of habitat patches with certain biodiversity and intensive agricultural land, and the regional scope is the key. The interaction between species and environment at different scales is different, and the appropriate scale analysis is particularly important [
35,
44]. Studies have shown that landscape structure affects the dynamic evolution and species richness of complex populations on a large scale [
21,
45,
46]. The analysis of plant and bird diversity in the agricultural landscape in Europe shows that landscape heterogeneity has a scale effect on biodiversity [
3]. Species diversity has a strong scale dependence on landscape heterogeneity, due to the limited range of activities, the research on epigaeic arthropods is mostly on a small scale [
47,
48]. The studies on epigaeic arthropods at the habitat scale show that the implementation of compound planting pattern and other methods is conducive to the formation of microhabitats suitable for survival such as natural enemies (such as spiders, armor), and plays an important role in increasing biodiversity, especially natural enemy diversity. In addition to increasing crop diversity, on the field scale, the construction of adjacent non-crop habitats in farmland is the key to affecting natural enemy diversity and pest biological control in farmland [
49,
50]. Therefore, the diversity and community composition of epigaeic arthropods in arable land system landscapes are affected by different scale factors, and landscape reconstruction should be carried out from multiple scales.
In general, most of the current studies on agricultural biodiversity at home and abroad focused on large-scale sampling [
51,
52,
53], while in small and medium-scale, different habitat types were used to distinguish and discuss [
54,
55]. How to combine margin characteristics, habitat types, and landscape structure to comprehensively discuss the landscape at different levels, which requires profound exploration and solution.
For studies aiming to describe the arable land system landscape structure, we propose metrics that reveal more information about connectivity, fragmentation, and diversity, such as contagion index, patch richness density, and Shannon’s diversity index, as they jointly reflect how a given matrix facilitates or hinders migration and re-colonization of habitat patches or remnants [
56]. In this study, the relationship between the diversity of epigaeic arthropods and landscape structure in the northern plains was found at different landscape scales, which supported the previous study [
57]. The smaller the degree of human disturbance, the higher the connectivity, the richer the patch types, the greater the number of epigaeic arthropod species, and the greater the Shannon diversity index. The higher the landscape heterogeneity, the richer the diversity of epigaeic arthropods.
Landscape attributes are significantly different with the different landscape scales concerned by the research. The larger the selected landscape scale is, the closer the landscape attributes are to the global common level. The smaller the selected landscape scale is, the more obvious the uniqueness of landscape attributes is [
58]. Therefore, there is an optimal scale for the response of biodiversity to landscape structure at the landscape scale, but this scale is not unique. In the existing studies, Clough et al. considered that the radius range of 500 m was the appropriate scale to study the response of spiders to landscape structure [
22], and there were also studies on the impact of landscape elements on the distribution of beetle diversity at the scale of about 1000 m [
59]. In this study, when the radius is 100 m, the landscape elements have no significant impact on the diversity of epigaeic arthropods. Between 250 m and 1500 m, with the increase of radius, the correlation between each landscape index and biodiversity index increased as a whole, and the correlation was the most obvious at 1500 m, and the correlation decreased at 2000 m. Therefore, we believe that 1500 m is the optimal scale of landscape elements affecting the diversity of epigaeic arthropods. Multi-scale studies are conducive to eliminating misjudgments under general rules, so as to comprehensively evaluate species diversity and find appropriate scales for biodiversity conservation [
60,
61].
In terms of which scale should be used in future works that address how landscape features affect epigaeic arthropods (e.g., [
62,
63,
64]), we found an optimal scale for the landscape scale. For the habitat scale, domestic and foreign scholars have done a lot of research on the impact of different habitats on epigaeic arthropods, mainly concentrating on the natural, semi-natural, and artificial three habitat types, and discussed the impact of different habitat types on the diversity of epigaeic arthropods [
65,
66,
67]. A large number of studies have found that different land use types have different impacts on soil fauna communities, especially the distribution characteristics of dominant groups and common groups in various land-use types [
68,
69].
This is further complemented in our study, for example, according to the distribution characteristics of habitat classification,
Harpalus sinicus, Pheropsophus occiptalis, Holotrichia oblita Faldermann, Chrysacris liaoningensis, etc., as the same category, mainly distributed in the orchard. Non-crop habitats have more epigaeic arthropod diversity [
70] due to their own vegetation conditions and less human disturbance, namely margin effects. At the same time, the spillover effect also affects the diversity of epigaeic arthropods in the surrounding farmland habitats. We concluded that the community structure of epigaeic arthropods in grassland, shrubland, arbor land, and their adjacent farmland habitats in the arable land system landscape of the plain area was similar.
As a way of spatial connection of different types of habitats, the margin is of great significance in the ecological process, and its community groups and the overall number are higher than those of adjacent habitats, namely the margin effect [
37,
71]. Margin not only contains the characteristics and components of adjacent habitats, but also forms more environmental characteristics that are not included in adjacent habitats according to their characteristics, resulting in more functions and characteristics [
72]. In this study, the number of individuals and species (S) of epigaeic arthropods at the margin of four types of non-crop-farmland habitats (orchard, shrub land, arbor forest land, and grassland) were significantly higher than those of adjacent farmland and other non-tillage habitats, and the margin effect was significant. The study area benefited from the three-north shelter forest system in northern China. Previous studies focused on the impact of farmland shelter forests on biodiversity or arable land yield. Constrained by the inherent thinking of local farmers, herbaceous plant clumps separated from farmland management (fertilization, pesticides, etc.) are considered negative factors of the agricultural ecosystem. This study provides a new idea for farmers to implement farmland management measures on the habitat scale.
Vegetation structure in non-crop habitats, as a major habitat control factor, is considered to be an environmental variable affecting biodiversity and ecosystem services in farmland [
73,
74,
75]. Garratt et al. argued that complex and continuous understory vegetation can significantly improve the diversity of pollinators and natural enemy groups of epigaeic arthropods in farmland [
71]. Tougeron et al. believed that at the habitat scale, the margin type or the distance to the margin affected the activity density and parasitic rate of beetles and spiders [
3]. On the habitat scale, we found that there was a significant relationship between the distribution of epigaeic arthropod community and the vegetation structure through the redundancy analysis (RDA). The Shannon diversity and Pielou evenness of herb vegetation were important factors affecting the composition of the epigaeic arthropod community. The composition of heterogeneous herb vegetation structure can often provide diversified microhabitats for a variety of taxonomic groups, thus providing a broader niche for the habitat survival of more organisms [
61,
76,
77]. In addition, due to the weak migration ability of most epigaeic arthropods, it is necessary to strengthen the protection of habitat scale, especially the restoration and protection of plant diversity in habitat, so as to play a positive role in improving the biodiversity of epigaeic arthropod communities in arable land system landscape to a certain extent.
In the main grain-producing areas of northern China, the plain area represented by Changtu County in the study area undertakes the important task of ensuring regional and even national food security. Agricultural production is characterized by a small number of planting plants and livestock species to replace the natural state of biodiversity, resulting in a wide range of environmental structure that tends to simplify and single [
78,
79]. In addition, due to China’s relevant policies, the farmland management measures implemented by farmers are limited to the arable land they own, which makes the management of arable land a single scale and lacks systematicness and integrity. Our research results clarify the key points of cropland system protection at multiple scales, and provide a detailed reference for the implementation of policies related to the sustainable development of cropland systems in northern China in the future. Optimizing arable land system landscape structure and enhancing landscape heterogeneity are effective ways to protect biodiversity and ecosystem services [
80]. Of course, there is no “one-size-fits-all” answer to the preservation of agricultural biodiversity. The appropriate scale is the optimal lever for the protection of agricultural biodiversity, which promotes the accurate implementation of agricultural management measures at different scales. Combined with the macro layout at the landscape scale and the dynamic adjustment at the habitat scale, the overall landscape layout, and microenvironment control can be used to maintain regional agricultural biodiversity and enhance the sustainability of the agricultural system, so as to improve the agricultural ecological environment and ensure the safety of the food system.
In the future, this study can classify and discuss epigaeic arthropods, and further clarify the interaction between different functional groups at different scales. In addition, climate, soil, and other environmental variables can also be introduced to make a more comprehensive discussion of other regions. In addition, this study chose to obtain biodiversity data during the crop maturity period (September). In future research, data from other seasons can be combined to enhance the broad applicability of the research conclusions.
5. Conclusions
Discussing the response of epigaeic arthropod diversity to environmental changes at multiple spatial scales in detail is essential for the sustainable development of the arable land system in the context of global change and biodiversity crisis. Based on landscape data and biodiversity data, from the perspective of biodiversity conservation, we used scale leverage (landscape, habitat, and field) to clarify the direction of efficient implemen-tation of agricultural system management measures in the northern plains of China.
We conclude that at the landscape scale, the landscape structure has a significant scale effect on the diversity of epigaeic arthropods, and 1500 m is the optimal radius for improving the biodiversity level by optimizing the landscape structure. On this scale, the largest patch index, patch richness density, contagion index, interspersion and juxtaposi-tion index, and Shannon diversity index significantly affected the biodiversity of epigaeic arthropods. The greater the landscape heterogeneity, the higher the level of epigaeic ar-thropod diversity. This result can point out the direction for the large-scale changes in landscape patterns in the study area. 100 m radius is the habitat scale to explore the factors affecting the diversity of epigaeic arthropods. On this scale, habitat types affect the diver-sity of epigaeic arthropods, and have a significant impact on the abundance and commu-nity structure richness of epigaeic arthropods, as well as the relative density between spe-cies. The number of epigaeic arthropods in the shrubland is the most abundant, the farmland adjacent to the orchard has the largest number of species, and the number of epigaeic arthropods of different species in the orchard is more evenly distributed. The rational layout of non-crop habitat patches and farmland is an effective way to protect the diversity of epigaeic arthropods at the field scale. To some extent, our study showed that field margin strips should be the unique cornerstones of agro-ecological landscape design strategies on this scale. The species and quantity distribution of epigaeic arthropods have obvious margin effects, and the herb vegetation structure of farmland margin is the main influencing factor for this effect. On the field scale, Shannon diversity, Pielou even-ness, average height, average cover, and Margalef richness of herb vegetation are important environmental factors affecting the diversity and community structure of epigaeic arthro-pods.
Biodiversity is the basis for a sustainable arable land system. Our findings underscore that, in agricultural landscapes dominated by arable land, combining precise implementation at various scales with comprehensive coordination at multiple scales has greater potential to support regional biodiversity than focusing on a single scale. We believe that using spatial scales to protect biodiversity is consistent with the reality of agricultural management, which is of great significance to the rational distribution of the arable land system.