Abstract
Arable land ecosystems are among the most important terrestrial systems. The issues of carbon sequestration and emission reductions in arable land ecosystems have received extensive attention. Countries around the world have actively issued policies to manage arable land ecosystems. At present, more than 100 countries have made carbon neutralization target commitments. Various arable land management measures and arable land planting strategies have important impacts on the carbon storage of arable land ecosystems. Research on arable land carbon is of great significance to global climate change. This study attempts to investigate the problems and deficiencies in the current research by summarizing a number of studies, including the main methods for the quantitative research of carbon sources and sinks as well as the influencing factors in these ecosystems. In this study, it is found that due to the differences of climate patterns, soil properties and management practices in arable land ecosystems, the factors affecting carbon sources and sinks are of great heterogeneity and complexity. Generally, variations in natural factors affect the carbon balance in different regions, while human management measures, such as irrigation, fertilization and the degree of agricultural mechanization, are the leading factors causing changes to carbon sources and sinks in these ecosystems. In addition, there are still great uncertainties in the evaluation of carbon sources and sinks in these ecosystems caused by different estimation models and methods. Therefore, emphasis should be placed on model parameter acquisition and method optimization in the future. This review provides a scientific basis for understanding carbon sources and sinks in arable land ecosystems, enhancing their carbon sink capacity and guiding low-carbon agriculture on arable land.
1. Introduction
Since the industrial revolution, great changes have taken place in human lifestyles, and the concentrations of greenhouse gases, such as carbon dioxide (CO2), methane (CH4) and nitrous oxide (N2O), in the atmosphere have increased significantly [1,2,3]. As a result, the transfer rate between different carbon pools and the amount of carbon has changed significantly, causing the carbon cycle and the entire ecosystem to enter a dynamic disequilibrium [4]. Fossil-fuel burning and land-use changes affect the amount of carbon in the atmosphere, and it is estimated that 30% of the carbon released by fossil-fuel burning and land-use changes was absorbed by terrestrial ecosystems during 2009–2018 [5]. Although the land may retain carbon, the terrestrial ecosystem releases carbon into the atmosphere, especially when extreme weather or disturbance events occur [4,6]. A related study showed that CO2, N2O and CH4 contribute 60%, 5% and 15% to global warming, respectively, and about 5%–20% of CO2 comes from soil every year [7]. Therefore, global warming has become the main focus of global climate change research [8,9]. Forests contain 2–4 times more carbon per unit area than arable lands [8], and thus many scholars have paid more attention to the carbon sequestration capacity of forest ecosystems [10,11]. It has been generally believed that compared with forest and grassland ecosystems, arable land ecosystems were a weaker carbon source or carbon sink and less valuable to the global carbon cycle, and therefore there has been less research on arable land ecosystem carbon-source and sink activities. However, a study of terrestrial ecosystems in the Northern Hemisphere found that forest and arable land ecosystems had significantly higher gross primary production (GPP) and net ecosystem productivity (NEP) than grassland and wetland ecosystems [12], indicating an important role for arable land ecosystems in climate-change mitigation.
Arable land accounts for 37% of the earth’s land area and is a main source of agricultural greenhouse-gas emissions [13], accounting for 30% of global emissions (direct and indirect), and has become an important part of any climate change adaptation and mitigation agenda [14]. Arable land ecosystems play a dual role in climate change. Large-scale farming and excessive use of chemical fertilizers and pesticides lead to a large amount of carbon loss and accelerate climate warming [15,16]. It is estimated that overall soil organic carbon (SOC) has decreased by 3% since the beginning of large-scale planting in the 19th century [17]. In Brazil, land-use changes caused by agriculture accounts for more than 2/3 of the country’s total carbon emissions [18]. In Australia’s agroecosystems, tillage has led to carbon loss for more than 40 years, and the total carbon loss on 10 cm topsoil is about 51% [19]. The carbon loss of arable land ecosystems around the world has become an urgent problem. In addition, arable land ecosystems have great carbon-sequestration potential in the context of reasonable farming practices, such as conservation tillage [19,20]. Therefore, it is necessary to reconstruct the initial balance of surface and atmospheric CO2 for arable land ecosystems by reducing greenhouse-gas effects and increasing soil carbon fixation [2].
The arable land ecosystem is one of the three major terrestrial ecosystems (along with the wetland ecosystem and the forest ecosystem), and can have a great impact on atmospheric carbon content [13,21]. In order to clarify the status of carbon sources and sinks in ecosystems, analyzing the generation, emission or absorption mechanisms of greenhouse gases in different ecosystems and their influencing factors, and estimating and evaluating the intensity of carbon sources and sinks are important research topics [8,11,21,22]. Existing research on carbon sources and sinks mainly focus on the comprehensive analysis of carbon changes in different regions during different periods [23,24,25]. The research has taken place on global, national and provincial scales [24,26,27], and has gradually extended to the county scale with the deepening of research methods and the enrichment of data sources [28]. The research has gradually expanded from forestland, shrubland and grassland ecosystems to arable land ecosystems [23,25,29,30,31]. Most studies on the carbon cycle of arable land ecosystems focus on soil carbon, mechanisms and the calculation of agricultural carbon emissions and the influencing factors [16,32]. The main methods for evaluating the carbon sources and sinks of an ecosystem include the eddy covariance technology method, the chamber method and mathematical modeling through the estimation of carbon absorption and carbon emission separately.
In order to provide sustainable solutions to managing arable land ecosystems, it is necessary to establish a scientific basis for strengthening the understanding of carbon sources and sinks of global arable land ecosystems. Therefore, the objectives of this study are to: (1) summarize the methods and research progress of carbon sources and sinks in arable land ecosystems; (2) explore the main controlling factors in changes to carbon sources and sinks in arable land ecosystems; and (3) propose a research focus on carbon pooling for arable land ecosystems in the future.
2. Relevant Research on Carbon Sources and Sinks in Arable Land Ecosystems
2.1. Related Concepts of Carbon in Ecosystems
The United Nations Framework Convention on climate change defines a “carbon source” as the process, activity or mechanism that releases greenhouse gases, aerosols or their precursors into the atmosphere, while “carbon sink” refers to the activity, process or mechanism of removing greenhouse gases, aerosols or their precursors from the atmosphere [33]. The knowledge acquired through the definition of carbon source and sink is that they are relative concepts. “Carbon source” refers to the matrix that releases carbon to the atmosphere in nature, and “carbon sink” refers to the deposit of carbon in nature.
Many scholars use two indicators, net primary productivity (NPP) and net ecosystem exchange (NEE) of vegetation, to describe the carbon sources and sinks of ecosystems [22,27,34,35]. NPP refers to the amount of organic matter in photosynthetic products fixed by plants per unit time and unit area after deducting the part consumed by the plants respiration [36]. It is an important index to evaluate the production capacity of the plant community under natural environment conditions and to measure the carbon sequestration capacity of vegetation [34,35]. Wang et al. [37] evaluated the temporal and spatial variation characteristics of the NPP of arable land ecosystems in China from 2001 to 2010 by combining MOD17A3 NPP data and GIS techniques, and observed that only 22% of NPP was significantly correlated with precipitation, and only 7% with temperature, indicating that arable land ecosystems were greatly affected by human activities. NEE is an important indicator to measure the carbon balance of ecosystems. It is the result of the balance between the total photosynthesis and total respiration of an ecosystem [38]. Zhang et al. [39] used a novel geospatial agricultural modeling system to calculate the NPP of crops, so as to estimate the NEE of arable land ecosystems. A positive value indicated that an area was a carbon source, while a negative value denoted a carbon sink. Li et al. [40] found that net radiation directly affected the seasonal variation of evapotranspiration and NEE in a winter wheat- summer maize system. Xu et al. [41] observed that in a rice–wheat system, seasonal variation in daily NEE and daytime NEE was directly affected by crop vegetation growth, and nighttime NEE and soil temperature at 10 cm during the wheat season exhibited a significant exponential relationship when accounting for grain removal and the return of straw to the field, indicating that the system was a weak carbon sink.
In addition, when calculating the carbon sources and sinks of arable land ecosystems, carbon absorption and carbon emission are usually estimated separately [2,26]. Carbon absorption is estimated based on crop-yield data, economic coefficient and carbon absorption rate, while carbon emission is estimated based on different carbon-emission pathways and combined with the carbon conversion coefficient [24]. The following parameters are often used [42]: biological yield is the total amount of dry matter (mostly above ground) harvested from crops per unit area of land; economic yield refers to the dry matter weight of grains or other organs of crops harvested for food or other uses per unit area of land; and economic coefficient is the ratio between economic yield and biological yield, varying with plant species, varieties, natural environment and cultivation measures. In general, the data of economic yield can be obtained from national or regional statistical data [24,26], but it is difficult to obtain the small-scale data [28]. Most of the carbon-emission coefficients of production activities are directly quoted from the research results of West (Oak Ridge National Laboratory) [43], Lal [44] and IPCC.
2.2. Research Status of Carbon Source and Sink Activities in Arable Land Ecosystems
Global terrestrial SOC stocks are about 1400–1500 PgC within 1 m depth soils, and are the largest carbon pool on the earth’s surface, 2–3 times greater than that of terrestrial vegetation and more than twice that of the atmospheric carbon pool [45]. Small changes in terrestrial stocks cause changes in CO2 concentrations in the atmosphere, thus affecting global climate [46]. SOC in arable land accounts for 10% of total organic carbon in soils [47]. Therefore, SOC has a certain function in the regulation of atmospheric CO2 concentrations. Whether an arable land ecosystem is a carbon source or sink largely depends on the balance between the fixation of arable land SOC and the release of greenhouse gases [31]. Therefore, research on the arable land SOC pool has gradually become the focus of the international community.
Many countries had completed the estimation of their SOC stocks on national or regional scales [48]. SOC stocks were mainly estimated by soil types, vegetation types or model methods, and the determination of relevant estimation factors was mainly obtained by collecting historical data and satellite images and through hyperspectral remote sensing technology. Song et al. [49] estimated that topsoil SOC stocks were about 5.1 PgC, based on the second soil survey data from 1979 to 1982 in China. Considering the entire arable land category, Tommaso et al. [50] estimated that the average SOC stock in the topsoil (30 cm) in Italy was 52.1 ± 17.4 Mg C ha−1, which was similar to that reported by other European countries. Sleutel et al. [51], combining SOC data with arable land area data, estimated that the SOC stocks of arable land was about 49,000 tons in Belgium. In France, Arrouays et al. [52] estimated SOC stocks at 0–30 cm soil depth according to land use and soil type using data from geo-referenced databases. The results showed that SOC stocks were 15–40 Mg C ha−1 in central France, and SOC stocks were 40–50 Mg C ha−1 in northern and southwestern regions.
Due to the strong carbon sequestration capacity of soil, a large number of studies on estimating soil carbon sequestration potential and on methods to achieve higher carbon sequestration have emerged. Some countries with large areas of crops and forestland have the potential to offset large greenhouse gas emissions by sequestering carbon in aboveground biomass and soil [53]. Lal [54] estimated that the total carbon sequestration potential of global arable land was 0.75–1.0 Pg C yr−1. In the United States, it was estimated the arable land has the potential to store 75–208 million metric tons of carbon equivalence per year, up to 8% of emissions [55]. In China, Lal [56] estimated the soil carbon-sequestration potential of agricultural and forest soils and concluded that the soil carbon-sequestration potential of China can offset about 25% of the annual emissions of fossil fuels. Wang et al. [57] predicted the carbon-sequestration potential of arable land soil under three management measures (nitrogen fertilizer application, straw returning and no tillage measures) in four agricultural regions of China using the existing field test survey results, which were about 12.1, 34.4 and 4.6 Tg C yr−1, respectively. Among the three measures, straw returning had the greatest carbon sequestration potential, with the capacity to offset 5.3% of China’s CO2 emissions from fossil fuel combustion in 1990. If more incentive policies could be formulated and implemented, China’s arable land soil carbon sequestration would be increased approximately twofold [57]. The 21st United Nations climate change conference proposed to increase the global soil organic matter (SOM) by 0.4 percentage points, pointing out that under the best management practices, this goal was expected to be achieved or even exceeded. Therefore, adopting optimal management practices will provide more opportunities to improve soil carbon sequestration.
3. Main Research Methods of Carbon Sources and Sinks in Arable Land Ecosystems
As a subsystem of terrestrial ecosystems, the arable land ecosystem is most closely related to human beings because it not only provides food, fiber, fuel and other products, but also supports and maintains the natural environment on which human beings depend for survival [26]. An arable land ecosystem can act as either a carbon source or a sink [58]. Quantitative analysis of carbon sources and sinks in arable land ecosystems can provide a basis for studying the temporal and spatial pattern changes and influencing factors. The quantifications are mainly conducted through direct observation and modeling methods. The advantages and disadvantages of research methods with regard to carbon sources and sinks in arable land ecosystems were showed in Figure 1.
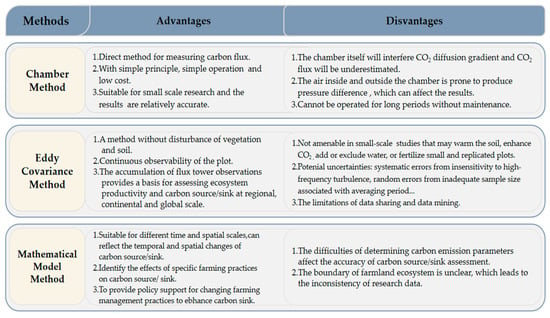
Figure 1.
Advantages and disadvantages of research methods of carbon source and sink in arable land ecosystems.
3.1. Direct Observation of Carbon Sources and Sinks among Arable Land Ecosystems
3.1.1. Chamber Method
The chamber method calculates the exchange rate of CO2 between ground and air by using a chamber of a certain volume to cover the surface to be measured. This isolates the air inside the chamber, and the exchange rate of CO2 is measured in the chamber. It is a direct method for measuring carbon flux [59]. Trace gas flux could be a useful indicator of ecosystem health, because it measures the material balance of vegetation and soils and the manner in which ecosystems respond to environmental pressure [60]. This method is currently the most popular technology in small-scale research [59]. Maljanen et al. [61] measured the annual CO2, N2O and CH4 dynamics of two organic agricultural soils with different soil characteristics by static-chamber method. In Malaysia, Melling et al. [62] used the closed-chamber method to measure the CO2 flux of forest, sago and oil palm ecosystems, which showed that the relative humidity of the forest, 5 cm soil temperature of sago and water-filled pores of oil palm were correlated with CO2 flux, indicating that land use affected the CO2 exchange between tropical peatlands and the atmosphere. In the Sanjiang Plain of Northeast China, Hao et al. [22] used the chamber method to select paddy fields and dry fields to study the seasonal variation of NEE, and quantitatively expressed the temporal variation law of arable land carbon sources and sinks. The results showed that the two types of arable land showed a weak carbon source in the non-growing season and a carbon sink in the growing season. In natural ecosystems, carbon sequestration during plant growth can promote an increase in carbon storage in soil [63]. However, for arable land ecosystems under anthropogenic management, there was evidence that carbon sequestration during crop growth cannot really increase the content of organic carbon in soil or improve the quality of organic carbon [22].
Since the chamber method can only measure the gas flux on the soil surface, it cannot obtain the gas flux from soil or from soil to atmosphere, which hinders understanding of the relation between soil depth and gas generation and movement in the soil profile [64,65]. Therefore, Granli [66] proposed a gradient method, in which Fick’s law was used to measure the gas flux in the soil profile based on the gas concentration gradient in the soil profile. Kusa et al. [64] compared the chamber method and the gradient method to measure N2O and CO2 fluxes in onion fields and corn fields. The results showed that the gradient method could be used to measure N2O flux (excluding high flux) and understand the seasonal variation law of CO2 flux. Wang et al. [67] combined these two methods to measure the greenhouse gas emissions and underground flux of 0–115 cm soil in the corn dry-farming system for two years in northern China, which showed that the 0–40 cm soil layer was the main site of CO2 production and CH4 absorption in arable soil. This discovery improved understanding of the processes of gas production and consumption in the soil–atmosphere system. However, the estimation of flux was very sensitive to changes in the soil gas diffusion coefficient. Accurate determination of the diffusion coefficient was also a challenge [68]. Since it was difficult to accurately estimate the diffusion coefficient through either modeling or experiment, this became the main source of error in the gradient method [25].
3.1.2. Eddy Covariance Method
The eddy covariance (EC) method refers to the vertical flux of a substance, which is the covariance between the concentration of the substance and its velocity. On the landscape scale, EC is the most widely used technology, and can comprehensively measure the trace gas flux in a large area [69]. This method provides a good opportunity to measure the NEE of the arable land system. If the NEE of the arable land ecosystem is greater than the amount of carbon released from the system in the form of agricultural practices and food consumption, there is potential for carbon sequestration [70]. EC is a direct measurement of CO2 exchange characterized by advantages such as the absence of interference in the environment and continuous observation of sample sites [71]. In the Midwest of the United States, Bernacchi et al. [70] assessed the net biological community productivity (NBP) of the corn/soybean no-tillage ecosystem by measuring the 6-year carbon flux and the carbon release related to agricultural practices. The results showed that the region was a large carbon sink. For the corn/soybean ecosystem, large-scale no-tillage systems could offset about 2% of the carbon emissions of the United States every year. Wang et al. [72] studied the net carbon budget of winter-wheat–summer-maize continuous cropping systems using EC, crop growth and soil respiration data on the North China Plain. The results showed that the winter wheat system was a carbon sink of 90 g C m−2, while the summer maize system was a carbon source of 167 g C m−2; therefore, the double-cropping system in this area was a carbon source of 77 g C m−2 on an annual basis, which was equivalent to the annual average loss rate of topsoil SOC stocks from 2003–2008 on comparison of the measured SOC data from 1998–2008. Although there are potential uncertainties in EC technology, including systematic errors from sensitivity to high-frequency turbulence, random errors from inadequate sample size associated with averaging period, and vertical and horizontal advection issues [73], the continuous operation of the flux network enables scientists to quantify the factors leading to interannual changes in annual and seasonal fluxes and to detect flux trends related to current environmental changes at the regional and global scales [60], providing an opportunity to understand the relationship between ecosystems and the atmosphere.
3.2. Mathematical Model of Carbon Sources and Sinks among Arable Land Ecosystems
For the estimation of carbon budgets of arable land ecosystems in a region, the mathematical model estimation method is mainly used. It estimates carbon absorption and carbon emission with relevant statistical data such as crop sowing area, crop yield and agricultural-production input [26,31]. The estimation of carbon absorption is mainly obtained by establishing mathematical models of the biomass and carbon absorption rate of various crops, while carbon emission is estimated by multiplying the use of various agricultural materials by their corresponding carbon emission coefficients [24,28]. It is necessary to include carbon emissions from agricultural operations and inputs in the calculation, because changes in carbon emissions from agricultural operations can affect the net flux of carbon to the atmosphere by enhancing or reducing carbon sequestration [43]. In terms of index selection, chemical fertilizer, pesticide, plastic film, diesel, agricultural machinery and agricultural irrigation are usually selected [2]. The emission caused by crop growth is determined by the sowing area and its corresponding carbon emission coefficient, the carbon stocks of crop vegetation are mainly affected by planting area and carbon density, the planting area is affected by land use change and the carbon density is affected by crop biomass [74]. Zhao and Qin [24] estimated the carbon-source and sink status of arable land ecosystems using the statistical data of crop yield and agricultural input from 1981 to 2001 in China’s coastal areas, finding that reductions in agricultural planting area and increases in agricultural input weakened the carbon-sink function of arable land ecosystems. Wang et al. [75] used a quantitative analysis method to estimate the NEP and NPP of arable land ecosystems in Virginia in the United States, concluding that these areas changed slowly from carbon-source to carbon-sink status.
Since the carbon-sink function of crops played an important role in mitigating climate change [26], and there were differences in carbon-emission and carbon-absorption rates of different crops, quantifying the typical emissions of some food crops provides a starting point for exploring the potential for reducing the carbon emissions of food crops [76]. She et al. [26] divided China into six typical regions, namely northeast, north, northwest, middle-lower reaches of Yangtze River, southwest and south, then collected and analyzed the carbon cost data of main crops, estimated the carbon-sink and source effects of arable land and quantitatively evaluated the carbon inputs and outputs of crop-production systems. The results showed that the major crop production was a net carbon sink of 236.32 Tg C yr−1. The total annual net carbon sink of rice, wheat and corn was about 165.76 Tg C, to which rice was the highest contributor, accounting for 48.71%. In the study of Liu et al. [77], optimization of crop-production structure by increasing the production area of soybean and reducing the production area of corn showed that 26% of nitrogen fertilizer use, 28% of active nitrogen loss and 19% of greenhouse gas emissions could be reduced. Some studies have found that the residue carbon input of rice straw and root system was higher than that of dry crops (rain-fed crops that mainly rely on natural precipitation), such as wheat, rape, cotton, corn and soybean [78,79]. Wu et al. [32] also found that the SOC of rice crop fields was higher than that of soybean and significantly higher than that of sesame and cotton, indicating that rice was more conducive to carbon fixation than dry crops, and soybean was the most suitable dry crop for carbon fixation. The SOC content in paddy soil was higher than that in dry land, mainly because paddy soil is an anaerobic and low-temperature environment that inhibits the activity of microorganisms and reduces the mineralization rate of SOC, and is thus conducive to carbon sequestration [80]. The SOC of soybean is higher than that of other dry crops, which may be because legumes have a large number of rhizobium bacteria attached to their roots, and during growth, nitrogen in the air is fixed to the roots, which can increase soil nitrogen and soil organic matter, further improving soil structure and reducing soil erosion [81]. Although we know that planting soybeans would potentially lead to more carbon sequestration, taking China as an example, converting corn planting areas to soybean planting areas is an unattractive option (net benefits averaging US $1485 ha−1 for maize versus US $1086 ha−1 for soy) [77]. Providing financial incentives can perhaps play a key role in encouraging small farmers to expand soybean planting areas [77]. This highlights the necessity of further optimizing crop-planting structures and achieving better environmental benefits through macro policy control. Figure 2 shows the process of carbon input, carbon fixation and carbon output in arable land ecosystems. Farming practices directly affect the plantation carbon pool and soil carbon pool, which then affect the atmospheric carbon pool. Different farming practices will lead to a change from source to sink in an arable system.
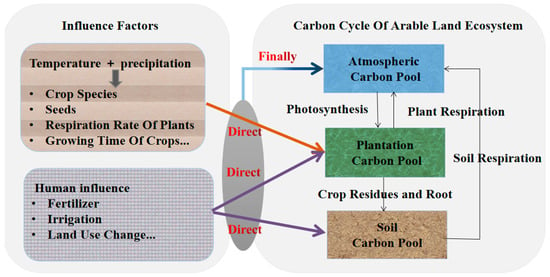
Figure 2.
The diagram depicts the flow of carbon between the soil carbon pool, the atmospheric carbon pool and the plantation carbon pool in arable land ecosystems.
4. Main Factors Affecting the Dynamics of Carbon Sources and Sinks in Arable Land Ecosystems
The factors affecting carbon sources and sinks in arable land ecosystems have long been the focus of research. Only clarification of the impact of different factors on carbon sources and sinks can provide a direction for guidance and regulation for reducing carbon emissions. The main factors affecting changes in carbon sources and sinks in arable land ecosystems include natural factors and human management measures. These factors will directly or indirectly affect temporal and spatial changes in carbon-source and sink activities in arable ecosystems.
4.1. Effects of Natural Environmental Factors on Arable Land Carbon
The rate of soil carbon loss is related to the soil environment, which is strongly controlled by climatic conditions [19]. Climate controls NPP above and below the ground, and thus the input of organic matter, while climate also contributes to carbon loss by driving the output of organic matter through microbial activity in the soil [82,83]. A large number of studies have confirmed a negative correlation between SOC and temperature in arable land ecosystems. Low temperature may reduce the mineralization of SOC through thermodynamic mechanism [3,20,82,84]. This conclusion was verified in arable-land ecosystems in different regions [85,86,87], and was also confirmed in forest ecosystems [82] and shrub ecosystems [30]. However, the situation was different in some low-temperature areas. Increasing temperature could stimulate the input of plant productivity [88], which was more conducive to the accumulation of SOC. On the Qinghai Tibet Plateau, Nie et al. [89] concluded that the increase of annual average temperature had a positive impact on SOC density, which might ascribe to the increase of soil carbon input exceeded the carbon loss caused by elevated temperature. Therefore, the increase of temperature might make the soil accumulate carbon under warming conditions. In addition, the types suitable for crop growth will vary under different temperatures, leading to differences in photosynthetic rate and carbon absorption rate. For example, temperature led to differences in crop structure between South and North China, further resulting in differences in carbon sources and sinks in arable land ecosystems [24]. In the research of Wang et al. [72], although the season length of maize (113 days) was 52% shorter than that of wheat (235 days), more than 55% of CO2 emissions come from maize season, and the interaction of soil temperature and moisture better explained the variations of the ecosystem respiration and soil respiration from the relatively colder and drier wheat growing season to the warmer and wetter maize growing season.
The impact of precipitation on SOC cannot be ignored, because water is the basic driver of almost all chemical and biological processes, including plant growth and survival, photosynthesis, microbial activities and soil respiration [90]. Precipitation changes might directly (by promoting microbial growth and activity) and indirectly (by improving plant productivity and soil carbon input) affect the soil carbon cycle [21]. When the increase of organic matter input from biomass exceeds the increase of biological activity, SOC would be accumulated [85]. Precipitation is generally positively correlated with SOC, which can be explained that when water resource is limited, plants grow slowly and contribute less organic matter to the soil [86]. Drought inhibits ecosystem productivity and respiration. Since ecosystem productivity is usually more sensitive to drought than respiration, drought may significantly reduce the intensity of terrestrial ecosystem carbon sink and even convert it into a carbon source [6]. However, in low-temperature ecosystems, dewdrops could significantly supplement the water required for vegetation growth [91], so precipitation might not be the main factor affecting SOC accumulation. At present, one of the most severe challenges is the increase in the frequency, intensity and duration of extreme climate events, which is also one of the most significant features of global climate change [6]. The strong dependence on precipitation and temperature highlights the carbon sensitivity of arable land ecosystems under future climate change. It is necessary to further discuss how to better describe the response of arable land ecosystem to extreme climate.
4.2. Impact of Human Management Measures on Arable Land Carbon
4.2.1. Tillage Measures
In arable land ecosystems, it is generally believed that agricultural farming strategies have more far-reaching impacts on SOC than natural factors [85,92]. The decrease of SOC content in arable land systems was mainly caused by cultivation. Tillage changes the quality and quantity of carbon input in soil and the physical properties of soil that affect carbon decomposition [19]. In the process of cultivation, the topsoil environment is often changing. It was generally believed that the loss of SOC mainly occurs in the 0–30 cm soil layer [93,94]. Tillage methods could also significantly affect SOC content [95]. Traditional farming methods, such as fallow in bare land, burning or removal of crop residues and inverted farming, have promoted the loss of SOM [20,96]. By reducing soil tillage and adopting conservation tillage measures, such as retaining crop residues in arable land, SOC can be fixed [2,13,19].
No tillage and less tillage have significant effects on the increase of SOC stocks and the change of microbial biomass carbon [19,31]. West and Post [43] estimated that with the conversion from traditional tillage to no tillage, the global SOC retention rate was 57 ± 14 g cm−2 yr−1. Dachraoui and Sombrero [2] compared the carbon footprint of corn under traditional tillage and no tillage management, showed that no tillage system reduced greenhouse gas emissions and contributed to carbon sequestration in the soil at the depth of 0-30 cm. Zhang et al. [97] also noted that SOC of the topsoil (0–30 cm) increased significantly under no tillage system compared with conventional tillage. In addition, increasing the complexity of crop rotation and straw return could also increase SOC and reduce greenhouse gas emissions [98]. In monoculture system, crops uptake less than half the amount of nitrogen fertilizers normally, through crop rotation, other types of crops could absorb nitrogen during the fallow period of bare land, also straw might lead to the richness and diversity of plant litter and increase the acquisition of carbon [58]. Because different natural factors shape the background of different arable land ecosystems, the spatial variability affecting site characteristics must be considered in the land use planning and implementation of strengthening carbon sequestration, so as to scientifically and effectively increase the carbon sink capacity of arable land ecosystems. Table 1 lists the potential amounts of carbon sequestration under different farming practices.

Table 1.
Estimation of potentials for agricultural carbon sequestration by different management practices.
4.2.2. Fertilization Measures
The impacts of fertilization on carbon source and sink of arable land ecosystems are mainly reflected in two aspects [19,80]. First, it improves the vegetative environment for plant growth and increases biomass, so as to increase the input of soil organic residues and promote the accumulation of organic carbon. Second, by affecting the population, quantity and activity of soil microorganisms, it has an impact on soil respiration. Many researchers have reported positive effects on SOC sequestration due to increased fertilizer and organic inputs. Morell et al. [103] found that after 15 years of application of mineral nitrogen, the amount of carbon retained in the soil increased due to the increase of crop residue production. Trost et al. [104] concluded that the combination of irrigation and fertilization may lead to a significant increase in SOC content, especially in light soil with low initial organic carbon content. Yue et al. [15] showed in a meta-analysis that nitrogen application significantly increased the total carbon storage of soil by 5.82%, and increased the carbon content of aboveground and underground parts of plants by 25.65% and 15.93% respectively. Globally, nitrogen addition significantly increased aboveground net primary productivity by 52.38%, indicating that with the increase of nitrogen deposition, terrestrial ecosystems may be enhanced as carbon sinks. Moharana et al. [105] observed that the SOC accumulation effects of farmyard manure (FYM) and FYM + NPK (N: nitrogen; P: phosphorus; K: potassium) treatments were better than that of NPK alone.
In intensive agriculture, production depended on the extensive use of synthetic fertilizers, especially nitrogen fertilizer [106]. Some studies found that nitrogen addition was considered to be the largest contributor to the impact of different management practices on carbon emissions, the emissions caused by nitrogen fertilizer exceed 50% of the total emissions [107,108]. Due to the decline of nutrient use efficiency, the use of chemical fertilizer to maintain crop yield has been increasing, which leads to higher direct emissions of greenhouse gases from soil [109]. The mechanism of nitrogen fertilizer affecting CO2 emission is that nitrogen application promotes microbial growth and soil respiration that depends on the SOM content. When the soil carbon source is sufficient, applying N fertilizer will promote soil respiration and increase CO2 emission, while when the carbon source is insufficient, soil respiration is inhibited [110]. Jiang et al. [107] found that increasing the amount of nitrogen application could improve rice yield, but when the amount of nitrogen application exceeded 225 kg N ha−1, it had little impacts on rice yield and even caused some adverse effects. This highlights the importance of improving nitrogen management practices, preventing economic losses to crop producers, thus achieving a balance between reducing carbon emissions and expanding net carbon sinks in arable land ecosystems.
4.2.3. Irrigation Measures
Irrigation and its scheduling affect soil and crop water status, thereby affecting microbial function and greenhouse gas emissions [111]. Proper soil moisture would enhance root respiration and microbial activities, accelerate the decomposition of SOM and increase CO2 emission [104]. However, high humidity reduces soil aeration and inhibits soil respiration and CO2 emissions [112]. The effect of irrigation on carbon sequestration appear to be highly dependent on location/conditions [72]. In arid areas or soil with low initial carbon content, irrigation can increase the content of SOC [16,113]. Wetting the soil with irrigation after drought could release the accumulated SOM during drought periods and produce a large amount of nutrients and organic carbon [114]. In desert areas, due to large soil pores, the infiltration of moisture can transport SOM and fine particles to deep layers [20]. However, in areas with humid climate and high initial SOC content, irrigation might lead to a decrease in SOC [115].
The commonly used irrigation methods in arable land include border irrigation, sprinkler irrigation and drip irrigation, which may cause different effects on arable land carbon. In southwestern Nebraska, Gillabel et al. [16] compared the carbon stocks between irrigation and dryland management treatment, it was found that the carbon stocks of irrigation were 25% higher than that of dryland cultivation, and the carbon input of crop residue under drip irrigation was estimated to be 2.5 times higher than that under drought. However, it was found that increasing carbon input under irrigation could not improve the level of large aggregates and the greater carbon stock was mainly due to the higher carbon sequestration in micro aggregates. The impact of tillage damage on the overall level was greater than the increased residue input under irrigation [16]. Li et al. [111] concluded the CO2 flux and cumulative emission of drip irrigation plot were significantly higher than that of border irrigation plot. The increase of CO2 emission of drip irrigation might due to the better water and soil environment created by irrigation, resulting in higher plant root respiration and stronger microbial activity. However, there were no unified conclusions on the impacts of irrigation method on CO2 emission. Li et al. [116] reported that under the condition of film covering, the CO2 emission of drip irrigation in clay loam was lower than that of flood irrigation. Therefore, information on different management practices and other irrigation systems in different regions is needed to more accurately understand the overall impact of irrigation on soil carbon storage.
4.2.4. Land Use Change
Land use change is considered to be the second largest cause of carbon emissions after fuel consumption [117]. Houghton et al. [118] concluded that 156 Pg C was released into the atmosphere globally due to land use change and management during 1850–2000. The growing population’s demand for food, fiber and fuel had accelerated the transformation of natural land into managed land, such as from forest or natural grassland to pasture or arable land [119]. The transformation from natural ecosystems to agricultural ecosystems would consume organic carbon pool, mainly due to: (I) low return of biomass carbon, (II) large loss of organic carbon caused by erosion, mineralization and leaching, and (III) large changes in soil temperature and water status [14]. Some studies had also focused on changes in carbon stocks between specific ecosystems. Don et al. [120] found that the conversion from virgin forest to arable land resulted in 25% to 30% SOC loss by a meta-analysis using 385 existing studies in tropical land. Clair et al. [121] found that the replacement of existing forest land by rape field would lead to net emissions, while the replacement of existing arable land by perennial miscanthus and short rotation shrub would produce significant net greenhouse gas benefits. In a study on the black soil area in Northeast China, Song et al. [84] found that the conversion of grassland to arable land would lead to the loss of C and N in 0–30 cm soil layer to a certain extent. DeFries et al. [122] concluded that 25–30% of the carbon in the topsoil would be released into the atmosphere when the forest was transformed into permanent arable land. It could be seen that the mutual transformation of forest and arable land will strengthen or reduce the carbon sequestration capacity of soil to a certain extent. Houghton and Nassikas [123] used land use change rate and carbon density data to compare the interaction between different ecosystems, considering five land use types: Arable land, pasture, plantation, industrial wood and fuelwood. The results showed that the net carbon flux of land use change from 1850 to 2015 was 145 ± 16 Pg C. Most of the emissions came from tropical regions (102 ± 5.8 Pg C). The average global net emissions in the last decade (2006-2015) were 1.11 (±0.35) Pg C yr−1, including the net carbon source in tropical regions (1.41 ± 0.17 Pg C yr−1), the net carbon sink in northern mid-latitudes regions (−0.28 ± 0.21 Pg C yr−1), and the neutrality in southern mid-latitudes regions. Recently, some studies combined RS and GIS to obtain land use change information and then estimate carbon emissions. Zhu et al. [124] combined remote sensing, GIS and IPCC method to quantify changes in vegetation carbon storage and SOC storage resulting from land use change during 1970–2010 in Zhejiang province of China. The result showed that land use change has resulted in huge amounts of carbon emissions, mainly caused by decrease of farmland with high SOC content, attributing to urban expansion. Li et al. [125] used the land use change data from 2000–2020 of Anhui province in China, evaluated the net carbon emissions and clarified the carbon emission effect from three aspects of carbon footprint, ecological carrying capacity and ecological deficit. They found that forestland is the main carbon sink, while construction land is the main carbon source, also the carbon footprint of increases rapidly, the ecological carrying capacity changes slowly, and the ecological deficit becomes larger and larger, indicated that with economic development, carbon emissions from construction land will become more and more significant, and the low-carbon development will face great pressure. The improvement of the availability and quality of multi-spatial and multi-temporal remote sensing data and the emergence of new analysis technologies have deepened the understanding of impact of land use change on carbon emissions [124].
5. Problems and Prospects of Carbon Source and Sink Research in Arable Land Ecosystems
5.1. Research Problems of Carbon Source and Sink in Arable Land Ecosystems
Although many scholars have made significant progress and achieved important results in the research of carbon source and sink in arable land ecosystems, due to the differences of natural and social environment in different regions, there still remains insufficient understanding on mechanisms of carbon cycle in arable land system and the influencing factors of carbon source and sink changes. In particular, due to the difficulty of obtaining statistical data, the change of carbon source and sink at small scales is not well understood, so it cannot provide guidance for carbon sequestration and emission reduction of arable land system. There is still a lot of room for improvement in relevant research. Also, balancing the need for agricultural products and other land use with reducing carbon emissions is a big challenge in developing sustainable management in arable land system.
In addition, there are some problems in the studies of carbon source and sink of arable land system, such as fuzzy system boundary, incomplete accounting index and parameter inconsistent with reality, making the differences between results, and even making the results are not comparable. The measurement accuracy of carbon absorption and emission is relatively at a low level, mainly because most scholars used the production activities input coefficient or carbon respiration coefficient of different crops published by the IPCC for calculation, and did not take into account the differences caused by climate or soil conditions, inducing larger errors in carbon emission and carbon absorption estimates. Besides, while most studies considered the impacts of agricultural machinery and chemical fertilizer factors on carbon emissions, but did not consider the carbon emissions caused by the power consumption of people engaged in agriculture production; however some studies included the latter factors, providing different estimates.
5.2. Research Prospects of Carbon Source and Sink in Arable Land Ecosystems
In view of the problems existing in quantification of the carbon source and sink of arable ecosystems, the research on the following aspects should be strengthened.
First, the accuracy of data calculation shall be improved. When determining the carbon conversion rate of different agricultural inputs, the conversion coefficient should be adjusted according to different soil texture, climate and farming conditions. At the same time, we should comprehensively analyze the role of various influencing factors. Models and methods should be compared to select the best method according to different research scales.
Second, the use of high-tech can not only improve the accuracy of research, but also provide a more scientific basis for the rational development of agriculture and the protection of the global ecological environment. Since there are considerable uncertainties in the global long-term and large-scale study of arable land system, combining models with remote sensing and GIS technology provides a good opportunity to evaluate the spatial and temporal distribution pattern of carbon sink and carbon source activities in arable land system.
Finally, the impact of management measures on the carbon source and sink of arable land ecosystems shall be considered. Due to the complexity and diversity of influencing factors of arable land carbon source and sink, the research results cannot be blindly generalized. To determine the amount of irrigation, fertilizer application and mechanized use according to local conditions, it is necessary to carry out studies at different scales.
Author Contributions
Conceptualization, S.W. and Q.Z.; methodology, X.J. and Y.Z.; validation, Z.B.; formal analysis, M.Z. and Z.M.; investigation, C.H.; writing—original draft preparation, X.L. and S.W.; writing—review and editing, S.W. and X.L.; visualization, X.G. and W.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the China Postdoctoral Science Foundation (Grant No. 2019M660782), National Science and Technology Basic Resources Survey Program of China (Grant No. 2019FY101300), Doctoral research start-up fund project of Liaoning Provincial Department of Science and Technology(Grant No. 2021-BS-136), and Young Scientific and Technological Talents Project of Liaoning Province (Grant No. LSNQN201910 and LSNQN201914).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Acknowledgments
Thank you to the reviewers for their valuable feedback on the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; p. 1535.
- Dachraoui, M.; Sombrero, A. Effect of tillage systems and different rates of nitrogen fertilisation on the carbon footprint of irrigated maize in a semiarid area of Castile and Leon, Spain. Soil Till. Res. 2020, 196, 104472. [Google Scholar] [CrossRef]
- Lal, R. Soil carbon dynamics in cropland and rangeland. Environ. Pollut. 2002, 116, 353–362. [Google Scholar] [CrossRef]
- Luo, Y.Q.; Xia, J.Y. A dynamic disequilibrium hypothesis for terrestrial carbon cycle. Biodivers. Sci. 2020, 28, 1405–1416. [Google Scholar] [CrossRef]
- Friedlingstein, P.; O’Sullivan, M.; Jones, M.W.; Andrew, R.M.; Hauck, J.; Olsen, A.; Peters, G.P.; Peters, W.; Pongratz, J.; Sitch, S.; et al. Global Carbon Budget 2019. Earth Syst. Sci. Data 2019, 11, 1783–1838. [Google Scholar] [CrossRef] [Green Version]
- Piao, S.L.; Zhang, X.P.; Chen, A.P.; Liu, Q.; Lian, X.; Wang, X.H.; Peng, S.S.; Wu, X.C. The impacts of climate extremes on the terrestrial carbon cycle: A review. China Earth Sci. 2019, 62, 1551–1563. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Hu, C.S.; Zhang, J.B.; Dong, W.B.; Wang, Y.Y.; Song, L.N. Research advances on source/sink intensities and greenhouse effects of CO2, CH4 and N2O in agricultural soils. Chin. J. Eco-Agric. 2011, 19, 966–975. [Google Scholar] [CrossRef]
- Houghton, R.A.; Hackler, J.L. Sources and sinks of carbon from land-use change in China. Glob. Biogeochem. Cycles 2003, 17, 1034. [Google Scholar] [CrossRef]
- Bradford, M.A.; Wieder, W.R.; Bonan, G.B.; Fierer, N.; Raymond, P.A.; Crowther, T.W. Managing uncertainty in soil carbon feedbacks to climate change. Nat. Clim. Change 2016, 6, 751–758. [Google Scholar] [CrossRef]
- Piao, S.L.; Ito, A.; Li, S.G.; Huang, Y.; Ciais, P.; Wang, X.H.; Peng, S.S.; Nan, H.J.; Zhao, C.; Ahlström, A.; et al. The carbon budget of terrestrial ecosystems in East Asia over the last two decades. Biogeosciences 2012, 9, 3571–3586. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.G.; Loveland, T.R.; Kurtz, R.M. Contemporary carbon dynamics in terrestrial ecosystems in the Southeastern Plains of the United States. Environ. Manag. 2004, 33, S442–S456. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, G.R.; Zhu, X.J.; Wang, Q.F. Spatial patterns and regional characteristics of carbon exchange fluxes in terrestrial ecosystems in the Northern Hemisphere. Quat. Res. 2014, 34, 710–722. [Google Scholar]
- Smith, P.; Martino, D.; Cai, Z.; Gwary, D.; Janzen, H.; Kumar, P.; McCarl, B.; Ogle, S.; O’Mara, F.; Rice, C.; et al. Greenhouse gas mitigation in agriculture. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 789–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lal, R.; Negassa, W.; Lorenz, K. Carbon and agriculture Carbon sequestration in soils. Curr. Opin. Environ. Sustain. 2015, 15, 79–86. [Google Scholar] [CrossRef]
- Yue, K.; Peng, Y.; Peng, C.; Yang, W.; Peng, X.; Wu, F. Stimulation of terrestrial ecosystem carbon storage by nitrogen addition: A meta-analysis. Sci. Rep. 2016, 6, 19895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillabel, J.; Denef, K.; Brenner, J.; Merckx, R.; Paustian, K. Carbon Sequestration and Soil Aggregation in Center-Pivot Irrigated and Dryland Cultivated Farming Systems. Soil Sci. Soc. Am. J. 2007, 71, 1020–1028. [Google Scholar] [CrossRef] [Green Version]
- Mullen, R.W.; Thomason, W.E.; Raun, W.R. Estimated increase in atmospheric carbon dioxide due to worldwide decrease in soil organic matter. Commun. Soil Sci. Plant Anal. 1999, 30, 1713–1719. [Google Scholar] [CrossRef]
- Cerri, C.E.P.; Sparovek, G.; Bernoux, M.; Easterling, W.E.; Melillo, J.M.; Cerri, C.C. Tropical agriculture and global warming: Impacts and mitigation options. Sci. Agricola 2007, 64, 83–99. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, E.; Sun, O. Soil carbon change and its responses to agricultural practices in Australian agro-ecosystems: A review and synthesis. Geoderma 2010, 155, 211–223. [Google Scholar] [CrossRef]
- Li, D.; Shao, M. Soil organic carbon and influencing factors in different landscapes in an arid region of northwestern China. CATENA 2014, 116, 95–104. [Google Scholar] [CrossRef]
- Smith, K.R.; Waring, B.G. Broad-Scale Patterns of Soil Carbon (C) Pools and Fluxes Across Semiarid Ecosystems are Linked to Climate and Soil Texture. Ecosystems 2018, 22, 742–753. [Google Scholar] [CrossRef]
- Hao, Q.J.; Wang, Y.S.; Song, C.C.; Jiang, C.S. CO2 budget in agro-ecosystems in the Sanjiang Plain. J. Agro-Environ. Sci. 2007, 26, 1556–1560. [Google Scholar]
- Mosier, A.R.; Halvorson, A.D.; Reule, C.A.; Liu, X.J. Net Global Warming Potential and Greenhouse Gas Intensity in Irrigated Cropping Systems in Northeastern Colorado. J. Environ. Qual. 2006, 35, 1584–1598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, R.Q.; Qin, M.Z. Temporospatial variation of partial carbon source /sink of farm land ecosystem in coastal China. J. Ecol. Rural Environ. 2007, 23, 7. [Google Scholar]
- Liang, N.; Nakadai, T.; Hirano, T.; Qu, L.; Koike, T.; Fujinuma, Y.; Inoue, G. In situ comparison of four approachesto estimating soil CO2 efflux in a northern larch (Larix kaemferi Sarg.) forest. Agric. For. Meteorol. 2004, 123, 97–117. [Google Scholar] [CrossRef]
- She, W.; Wu, Y.; Huang, H.; Chen, Z.D.; Cui, G.X.; Zheng, H.B.; Guan, C.Y.; Chen, F. Integrative analysis of carbon structure and carbon sink function for major crop production in China’s typical agriculture regions. J. Clean. Prod. 2017, 162, 702–708. [Google Scholar] [CrossRef]
- Bandaru, V.; West, T.O.; Ricciuto, D.M.; Izaurralde, R.C. Estimating crop net primary production using national inventory data and MODIS-derived parameters. ISPRS J. Photogramm. 2013, 80, 61–71. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Li, B.; Zhang, R.Q. Spatiotemporal evolution and influencing factors of agricultural carbon emissions in Hebei Province at county scale. Chin. J. Eco-Agric. 2022, 30, 570–581. [Google Scholar]
- Zhu, J.B.; He, H.D.; Zhang, F.W.; Li, H.Q.; Li, Y.N.; Yang, Y.S.; Wang, C.Y.; Zhang, G.R.; Luo, F.L. Effect of temperature difference between day and night on NEE and its variation characteristics in alpine shrubland in Qinghai-Tibetan plateau. Res. Soil Water Conversat. 2020, 27, 232–238. [Google Scholar]
- Ge, J.L.; Xu, W.T.; Liu, Q.; Tang, Z.Y.; Xie, Z.Q. Patterns and environmental controls of soil organic carbon density in Chinese shrublands. Geoderma 2020, 363, 114161. [Google Scholar] [CrossRef]
- Zhang, H.H.; Yan, C.R.; Zhang, Y.Q.; Wang, J.B.; He, W.Q.; Chen, B.Q.; Liu, E.K. Effect of no tillage on carbon sequestration and carbon balance in farming ecosystem in dryland area of northern China. Trans. Chin. Soc. Agric. Eng. 2015, 31, 240–247. [Google Scholar]
- Wu, Z.; Liu, Y.; Han, Y.; Zhou, J.; Liu, J.; Wu, J. Mapping farmland soil organic carbon density in plains with combined cropping system extracted from NDVI time-series data. Sci. Total Environ. 2020, 754, 142120. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. Carbon sequestration options under the clean development mechanism to address land degradation. In World Soil Resources Reports 92; FAO and IFAD: Rome, Italy, 2000. [Google Scholar]
- Zhang, M.; Huang, X.J.; Chuai, X.W.; Xie, X.L.; Zhu, Z.Y.; Wang, Y. Spatial distribution and changing trends of net ecosystem productivity in China. Geogr. Geo-Inf. Sci. 2020, 36, 69–74. [Google Scholar]
- Xu, Y.Q.; Xiao, F.J.; Yu, L. Review of spatio-temporal distribution of net primary productity in forest ecosystem and its responses to climate change in China. Acta Ecol. Sin. 2020, 40, 4710–4723. [Google Scholar]
- Lieth, H.; Whittaker, R.H. Primary Productivity of the Biosphere; Springer: Berlin, Germany, 1975. [Google Scholar]
- Wang, Y.H.; Shi, X.Z.; Wang, M.Y.; Zhao, Y.C. Spatio-temporal variation of NPP in cropland ecosystem of China during the years from 2001 to 2010. Acta Pedol. Sin. 2017, 54, 319–330. [Google Scholar]
- Zhu, J.B.; Li, H.; Zhang, F.; He, H.; Li, Y.; Yang, Y.; Zhang, G.; Wang, C.; Luo, F. Little direct effect of diurnal temperature amplitude on growing seasonal CO2 fluxes in alpine humid shrubland, Qinghai-Tibetan Plateau. Ecol. Res. 2020, 35, 603–612. [Google Scholar] [CrossRef]
- Zhang, X.S.; Izaurralde, R.C.; Manowitza, D.H.; Sahajpal, R.; West, T.O.; Thomson, A.M.; Xu, M.; Zhao, K.G.; LeDuc, S.D.; Williams, J.R. Regional scale cropland carbon budgets: Evaluating a geospatial agricultural modeling system using inventory data. Environ. Model. Softw. 2015, 63, 199–216. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Wang, R.H.; Li, Z.Z.; Xu, Y. Characteristics and Influencing Factors of Evapotranspiration and Net CO2 Exchange in Winter Wheat Summer Maize Field. Trans. Chin. Soc. Agric. Mach. 2022, 53, 331–339. [Google Scholar]
- Xu, X.B.; Yang, G.S.; Sun, X.X. Analysis of net ecosystem CO2 exchange (NEE) in the rice-wheat rotation agroecosystem of the Lake Taihu Basin, China. Acta Ecol. Sin. 2015, 35, 6655–6665. [Google Scholar]
- Zhang, B.D. Biological yield, economic yield, economic coefficient. Hebei Agric. 1999, 3, 26. [Google Scholar]
- West, T.O.; Post, W.M. Soil Organic Carbon Sequestration Rates by Tillage and Crop Rotation: A Global Data Analysis. Soil Sci. Soc. Am. J. 2002, 66, 1930–1946. [Google Scholar] [CrossRef] [Green Version]
- Lal, R. Carbon emission from farm operations. Environ. Int. 2004, 30, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Lal, R. Soil Carbon Sequestration impacts on global climate change and food security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Were, K.; Bui, D.T.; Oystein, B.; Dick, B.; Singh, R. A comparative assessment of support vector regression, artificial neural networks, and random forests for predicting and mapping soil organic carbon stocks across an Afromontane landscape. Ecol. Indic. 2015, 52, 394–403. [Google Scholar] [CrossRef]
- Tang, H.Y.; Liu, Y.; Li, X.M.; Muhammad, A.; Huang, G.Q. Carbon sequestration of cropland and paddy soils in China: Potential, driving factors, and mechanisms. Greenh. Gases Sci. Technol. 2019, 9, 872–885. [Google Scholar] [CrossRef]
- Vandenbygaart, A.J.; Gregorich, E.G.; Angers, D.A.; Stoklas, U.F. Uncertainty analysis of soil organic carbon stock change in Canadian cropland from 1991 to 2001. Glob. Change Biol. 2004, 10, 983–994. [Google Scholar] [CrossRef]
- Song, G.H.; Li, L.Q.; Pan, G.X.; Zhang, Q. Topsoil Organic Carbon Storage of China and Its Loss by Cultivation. Biogeocheistry 2005, 74, 47–62. [Google Scholar] [CrossRef]
- Tommaso, C.; Gardin, L.; Perugini, L.; Quaratino, R.; Vaccari, F.P.; Miglietta, F.; Riccardo, V. Soil organic carbon stock assessment for the different cropland land uses in Italy. Biol. Fert. Soils 2011, 48, 9–17. [Google Scholar]
- Sleutel, S.; Neve, S.D.; Hofman, G. Estimates of carbon stock changes in Belgian cropland. Soil Use Manag. 2010, 19, 166–171. [Google Scholar] [CrossRef]
- Arrouays, D.; Deslais, W.; Badeau, V. The carbon content of topsoil and its geographical distribution in France. Soil Use Manag. 2010, 17, 7–11. [Google Scholar] [CrossRef]
- Antle, J.; Capalbo, S.; Mooney, S.; Elliott, E.; Paustian, K. Spatial heterogeneity, contract design, and the efficiency of carbon sequestration policies for agriculture. J. Environ. Econ. Manag. 2003, 46, 231–250. [Google Scholar] [CrossRef]
- Lal, R. Carbon Management in Agricultural Soils. Mitig. Adapt. Strat. Glob. Change 2007, 12, 303–322. [Google Scholar] [CrossRef]
- Lal, R.; Kimble, J.M.; Follett, R.F. The potential of U.S. cropland to sequester carbon and mitigate the greenhouse effect. Ann Arbor Press 2001, 16, 47–54. [Google Scholar]
- Lal, R. Offsetting China’s CO2 Emissions by soil carbon sequestration. Clim. Change 2004, 65, 263–275. [Google Scholar] [CrossRef]
- Wang, X.; Lu, F.; Han, B.; Ouyang, Z. Carbon sequestration by cropland soil in China: Potential and feasibilty. In IOP Conference Series. Earth and Environmental Science; IOP Publishing: Bristol, UK, 2009; Volume 6. [Google Scholar]
- Singh, J.; Kumar, S. Responses of soil microbial community structure and greenhouse gas fluxes to crop rotations that include winter cover crops. Geoderma 2020, 385, 114843. [Google Scholar] [CrossRef]
- Luther-Mosebach, J.; Kalinski, K.; Gröngröft, A.; Eschenbach, A. CO2 fluxes in subtropical dryland soils—A comparison of the gradient and the closed-chamber method. J. Plant Nutr. Soil Sci. 2018, 181, 21–30. [Google Scholar] [CrossRef]
- Baldocchi, D. Measuring fluxes of trace gases and energy between ecosystems and the atmosphere—The state and future of the eddy covariance method. Glob. Change Biol. 2015, 20, 3600–3609. [Google Scholar] [CrossRef]
- Maljanen, M.; Komulainenb, V.M.; Hytonen, J.; Martikainena, P.J.; Laine, J. Carbon dioxide, nitrous oxide and methane dynamics in borealorganic agricultural soils with different soil characteristics. Soil Biol. Biochem. 2004, 36, 1801–1808. [Google Scholar] [CrossRef]
- Melling, L.; Hatano, R.; Goh, K.J. Soil CO2 flux from three ecosystems in tropical peatland of Sarawak, Malaysia. Tellus B 2005, 57, 1–11. [Google Scholar] [CrossRef]
- Ma, X.H.; Lv, X.G.; Yang, Q.; Yan, M.H. Carbon cycle of a marsh in Sanjiang Plian. Sci. Geogr. Sin. 1996, 16, 323–330. [Google Scholar]
- Kusa, K.; Sawamoto, T.; Hu, R.G.; Hatano, R. Comparison of the closed-chamber and gas concentration gradient methods for measurement of CO2 and N2O fluxes in two upland field soils. Soil Sci. Plant Nutr. 2008, 54, 777–785. [Google Scholar] [CrossRef]
- Hosen, Y.; Tsuruta, H.; Minami, K. Effects of the depth of NO and N2O productions in soil on their emission rates to the atmosphere: Analysis by a simulation model. Nutr. Cycl. Agroecosystems 2000, 57, 83–98. [Google Scholar] [CrossRef]
- Granli, T. The experimental basis, nitrous oxide from agriculture. Nor. J. Agric. 1994, 12, 22–29. [Google Scholar]
- Wang, Y.Y.; Li, X.X.; Dong, W.X.; Wu, D.M.; Hu, C.S.; Zhang, Y.M.; Luo, Y.Q. Depth dependent greenhouse gas production and consumption in an upland cropping system in northern China. Geoderma 2018, 319, 100–112. [Google Scholar] [CrossRef]
- Pingintha, N.; Leclerc, M.Y.; Beasley, J.P., Jr.; Zhang, G.S.; Senthong, C. Assessment of the soil CO2 gradient method for soil CO2 efflux measurements: Comparison of six models in the calculation of the relative gas diffusion coefficient. Tellus B 2010, 62, 47–58. [Google Scholar] [CrossRef]
- Myklebust, M.C.; Hipps, L.E.; Ryel, R.J. Comparison of eddy covariance, chamber, and gradient methods of measuring soil CO2 efflux in an annual semi-arid grass, Bromus tectorum. Agric. For. Meteorol. 2008, 148, 1894–1907. [Google Scholar] [CrossRef]
- Bernacchi, C.J.; Hollinger, S.E.; Meyers, T. The conversion of the corn/soybean ecosystem to no-till agriculture may result in a carbon sink. Glob. Change Biol. 2010, 11, 1867–1872. [Google Scholar]
- Baldocchi, D. Assessing the eddy covariance technique for evaluating carbon dioxide exchange rates of ecosystems: Past, present and future. Glob. Change Biol. 2003, 9, 479–492. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.Y.; Hu, C.S.; Dong, W.S.; Li, X.X.; Zhang, Y.M.; Qin, S.P.; Oenema, O. Carbon budget of a winter-wheat and summer-maize rotation cropland in the North China Plain. Agric. Ecosyst. Environ. 2015, 206, 33–45. [Google Scholar] [CrossRef]
- Loescher, H.W.; Law, B.E.; Mahrt, L.; Hollinger, D.Y.; Campbell, J.; Wofsy, S.C. Uncertainties in, and interpretation of, carbon flux estimates using the eddy covariance technique. J. Geophys. Res.-Atmos. 2006, 111, D21S90. [Google Scholar] [CrossRef] [Green Version]
- Luo, H.L. Advances on carbon storage in crops of China. Ecol. Environ. Sci. 2014, 23, 692–697. [Google Scholar]
- Wang, J.; Howard, E.E. Estimating carbon source-sink transition during secondary succession in a Virginia valley. Plant Soil 2013, 362, 135–147. [Google Scholar] [CrossRef]
- Hillier, J.; Hawes, C.; Squire, G.; Hiton, A.; Wale, S.; Smith, P. The carbon footprints of food crop production. Int. J. Agric. Sustain. 2009, 7, 107–118. [Google Scholar] [CrossRef]
- Liu, Z.T.; Ying, H.; Chen, M.Y.; Bai, J.; Xue, Y.F.; Yin, Y.L.; Batchelor, W.D.; Yang, Y.; Bai, Z.H.; Du, M.X.; et al. Optimization of China’s maize and soy production can ensure feed sufficiency at lower nitrogen and carbon footprints. Nat. Food 2021, 2, 426–433. [Google Scholar] [CrossRef]
- Jarecki, M.K.; Lal, R. Crop management for soil carbon sequestration. Crit. Rev. Plant Sci. 2003, 22, 471–502. [Google Scholar] [CrossRef]
- Chen, S.; Xu, C.M.; Yan, J.X.; Zhang, X.G.; Zhang, X.F.; Wang, D.Y. The influence of the type of crop residue on soil organic carbon fractions: An 11-year field study of rice-based cropping systems in southeast China. Agric. Ecosyst. Environ. 2016, 223, 261–269. [Google Scholar] [CrossRef]
- Yan, X.; Zhou, H.; Zhu, Q.H.; Wang, X.F.; Zhang, Y.Z.; Yu, X.C.; Peng, X. Carbon sequestration efficiency in paddy soil and upland soil under long-term fertilization in southern China. Soil Till. Res. 2013, 130, 42–51. [Google Scholar] [CrossRef]
- Bezdicek, D.F.; Power, J.F. Opportunities for meeting crop nitrogen needs from symbiotic nitrogen fixation [by Rhizobium-legume]. ASA Spec. Publ. Am. Soc. Agron. 1984, 46, 49–59. [Google Scholar]
- Jackson, R.B.; Lajtha, K.; Susan, E.; Hugelius, C.G.; Kramer, M.G.; Piñeiro, G. The ecology of soil carbon: Pools, vulnerabilities, and biotic and abiotic controls. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 419–445. [Google Scholar] [CrossRef] [Green Version]
- Vaughan, E.; Matos, M.; Ríos, S.; Santiago, C.; Marín-Spiotta, E. Clay and climate are poor predictors of regional-scale soil carbon storage in the US Caribbean. Geoderma 2019, 354, 113841. [Google Scholar] [CrossRef]
- Song, X.-D.; Yang, F.; Ju, B.; Li, D.-C.; Zhao, Y.-G.; Yang, J.-L.; Zhang, G.-L. The influence of the conversion of grassland to cropland on changes in soil organic carbon and total nitrogen stocks in the Songnen Plain of Northeast China. CATENA 2018, 171, 588–601. [Google Scholar] [CrossRef]
- Guo, N.; Shi, X.; Zhao, Y.; Xu, S.; Wang, M.; Zhang, G.; Wu, J.; Huang, B.; Kong, C. Environmental and Anthropogenic Factors Driving Changes in Paddy Soil Organic Matter: A Case Study in the Middle and Lower Yangtze River Plain of China. Pedosphere 2017, 27, 926–937. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Gong, X.; Niu, Y.; Chen, Y.; Shi, X.; Li, W. Storage, pattern and driving factors of soil organic carbon in an ecologically fragile zone of northern China. Geoderma 2019, 343, 155–165. [Google Scholar] [CrossRef]
- Wang, M.; Su, Y.; Yang, X. Spatial Distribution of Soil Organic Carbon and Its Influencing Factors in Desert Grasslands of the Hexi Corridor, Northwest China. PLoS ONE 2014, 9, e94652. [Google Scholar] [CrossRef] [PubMed]
- Piao, S.; Fang, J.; Ciais, P.; Peylin, P.; Huang, Y.; Sitch, S.; Wang, T. The carbon balance of terrestrial ecosystems in China. Nature 2009, 458, 1009–1013. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Yang, L.; Li, F.; Xiong, F.; Li, C.; Zhou, G. Storage, patterns and controls of soil organic carbon in the alpine shrubland in the Three Rivers Source Region on the Qinghai-Tibetan Plateau. CATENA 2019, 178, 154–162. [Google Scholar] [CrossRef]
- Gerten, D.; Luo, Y.; Le Maire, G.; Parton, W.J.; Keough, C.; Weng, E.; Beier, C.; Ciais, P.; Cramer, W.; Dukes, J.S.; et al. Modelled effects of precipitation on ecosystem carbon and water dynamics in different climatic zones. Glob. Change Biol. 2008, 14, 2365–2379. [Google Scholar] [CrossRef]
- Li, Y.S. Experimental Study on the Influence of Alpine Meadow Cover Change on Water Cycle in Typical Permafrost Area of Qinghai Tibet Plateau; Institute of environment and Engineering in cold and arid areas, Chinese Academy of Sciences: Beijing, China, 2007. [Google Scholar]
- Torn, M.S.; Swanston, C.W.; Castanha, C.; Trumbore, S.E. Storage and Turnover of Organic Matter in Soil. Biophysico-Chemical Processes Involving Natural Nonliving Organic Matter in Environmental Systems; Wiley Publishing: Hoboken, NJ, USA, 2009; pp. 219–272. [Google Scholar]
- Doran, J.W.; Elliott, E.T.; Paustian, K. Soil microbial activity, nitrogen cycling, and long-term changes in organic carbon pools as related to fallow tillage management. Soil Till. Res. 1998, 49, 3–18. [Google Scholar] [CrossRef]
- Mikhailova, E.A.; Bryant, R.B.; Vassenev, I.I.; Schwager, S.J.; Post, C.J. Cultivation effects on soil carbon and nitrogen contents at depth in the Russian Chernozem. Soil Sci. Soc. Am. J. 2000, 64, 738–745. [Google Scholar] [CrossRef]
- Bai, Y.X.; Zhou, Y.C. The main factors controlling spatial variability of soil organic carbon in a small karst watershed, Guizhou Province, China. Geoderma 2020, 357, 113938. [Google Scholar] [CrossRef]
- Boubehziz, S.; Khanchoul, K.; Benslama, M.; Benslama, A.; Marchetti, A.; Francaviglia, R.; Piccini, C. Predictive mapping of soil organic carbon in Northeast Algeria. CATENA 2020, 190, 104539. [Google Scholar] [CrossRef]
- Zhang, L.M.; Zhuang, Q.L.; He, Y.J.; Yu, D.S.; Zhao, Q.Y.; Shi, X.Z.; Xing, S.H.; Wang, G.X. Toward optimal soil organic carbon sequestration with effects of agricultural management practices and climate change in Tai-Lake paddy soils of China. Geoderma 2016, 275, 28–39. [Google Scholar] [CrossRef] [Green Version]
- Baggs, E.M.; Watson, C.A.; Rees, R.M. The fate of nitrogen from incorporated cover crop and green manure residues. Nutr. Cycl. Agroecosystems 2000, 56, 153–163. [Google Scholar] [CrossRef]
- Yan, H.M.; Cao, M.K.; Liu, J.Y.; Tao, B. Potential and sustainability for carbon sequestration with improved soil management in agricultural soils of China. Agric. Ecosyst. Environ. 2007, 121, 325–335. [Google Scholar] [CrossRef]
- Batjes, N.H. Mitigation of atmospheric CO2 concentrations by increased carbon sequestration in the soil. Biol. Fert. Soils 1998, 27, 230–235. [Google Scholar] [CrossRef]
- Watson, R.T.; Noble, I.R.; Bolin, B.; Ravindranath, N.H.; Dokken, D.J. Land Use, Land-Use Change, and Forestry: A Special Report of the IPCC; Cambridge University Press: Cambridge, UK, 2000; Available online: https://digitallibrary.un.org/record/466920?ln=en (accessed on 20 March 2022).
- Wang, X.B.; Wu, X.L.; Zhao, Q.S.; Deng, X.Z.; Cai, Y.D. Effects of cropland-use management on potentials of soil carbon sequestration and carbon emission mitigation in China. Sci. Agric. Sin. 2011, 44, 2284–2293. [Google Scholar]
- Morell, F.J.; Cantero-Martínez, C.; Lampurlanés, J.; Plaza-Bonilla, D.; Álvaro-Fuentes, J. Soil carbon dioxide flux and organic carbon content: Effects of tillage and nitrogen fertilization. Soil Sci. Soc. Am. J. 2011, 75, 2223–2229. [Google Scholar] [CrossRef]
- Trost, B.; Prochnow, A.; Drastig, K.; Meyer-Aurich, A.; Ellmer, F.; Baumecker, M. Irrigation, soil organic carbon and N2O emissions. A review. Agron. Sustain. Dev. 2013, 33, 733–749. [Google Scholar] [CrossRef] [Green Version]
- Moharana, P.C.; Sharma, B.M.; Biswas, D.R.; Dwivedi, B.S.; Singh, R.V. Long-term effect of nutrient management on soil fertility and soil organic carbon pools under a 6-year-old pearl millet–wheat cropping system in an Inceptisol of subtropical India. Field Crop. Res. 2012, 136, 32–41. [Google Scholar] [CrossRef]
- Chen, X.P.; Cui, Z.L.; Fan, M.S.; Vitousek, P.; Zhao, M.; Ma, W.Q.; Wang, Z.L.; Zhang, W.J.; Yan, X.Y.; Yang, J.C.; et al. Producing more grain with lower environmental costs. Nature 2014, 514, 486–489. [Google Scholar] [CrossRef]
- Jiang, Z.H.; Zhong, Y.M.; Yang, J.P.; Wu, Y.X.Y.; Li, H.; Zheng, L. Effect of nitrogen fertilizer rates on carbon footprint and ecosystem service of carbon sequestration in rice production. Sci. Total Environ. 2019, 670, 210–217. [Google Scholar] [CrossRef]
- Cheng, K.; Pan, G.; Smith, P.; Luo, T.; Li, L.; Zheng, J.; Yan, M. Carbon footprint of China’s crop production—An estimation using agro-statistics data over 1993–2007. Agric. Ecosyst. Environ. 2011, 142, 231–237. [Google Scholar] [CrossRef]
- Mandal, S.; Roy, S.; Das, A.; Ramkrushna, G.I.; Lal, R.; Verma, B.C.; Kumar, A.; Singh, R.K.; Layek, J. Energy efficiency and economics of rice cultivation systems under subtropical Eastern Himalaya. Energy Sustain. Dev. 2015, 28, 115–121. [Google Scholar] [CrossRef]
- Niu, S.; Wu, M.; Han, Y.I.; Xia, J.; Zhang, Z.H.E.; Yang, H.; Wan, S. Nitrogen effects on net ecosystem carbon exchange in a temperate steppe. Glob. Change Biol. 2010, 16, 144–155. [Google Scholar] [CrossRef]
- Li, C.J.; Xiong, Y.W.; Huang, Q.Z.; Xu, X.; Huang, G.H. Impact of irrigation and fertilization regimes on greenhouse gas emissions from soil of mulching cultivated maize (Zea mays L.) field in the upper reaches of Yellow River, China. J. Clean. Prod. 2020, 259, 120873. [Google Scholar] [CrossRef]
- Trost, B.; Prochnow, A.; Baumecker, M.; Meyer-Aurich, A.; Drastig, K.; Ellmer, F. Effects of nitrogen fertilization and irrigation on N2O emissions from a sandy soil in Germany. Arch. Agron. Soil Sci. 2015, 61, 569–580. [Google Scholar] [CrossRef]
- Denef, K.; Stewart, C.E.; Brenner, J.; Paustian, K. Does long-term center-pivot irrigation increase soil carbon stocks in semi-arid agro-ecosystems? Geoderma 2008, 145, 121–129. [Google Scholar] [CrossRef]
- Schimel, J.; Balser, T.C.; Wallenstein, M. Microbial stress-response physiology and its implications for ecosystem function. Ecology 2007, 88, 1386–1394. [Google Scholar] [CrossRef]
- Dersch, G.; Böhm, K. Effects of agronomic practices on the soil carbon storage potential in arable farming in Austria. Nutr. Cycl. Agroecosystems 2001, 60, 49–55. [Google Scholar] [CrossRef]
- Li., Z.G.; Zhang, R.H.; Wang, X.J.; Chen, F.; Tian, C.Y. Growing season carbon dioxide exchange in flooded non-mulching and non-flooded mulching cotton. PLoS ONE 2012, 7, e50760. [Google Scholar]
- Watson, R.T.; Noble, I.R.; Bolin, B.; Ravindramath, N.H.; Verardo, D.J.; Dokken, D.J. Land Use, Land-use Change, and Forestry (A Special Report of the IPCC); Cambridge University Press: Cambridge, UK, 2000; Available online: https://archive.ipcc.ch/ipccreports/sres/land_use/index.php?idp=0 (accessed on 20 March 2022).
- Houghton, R.A. Why are estimates of the terrestrial carbon balance so different? Glob. Change Biol. 2003, 9, 500–509. [Google Scholar] [CrossRef]
- Smith, P.; House, J.I.; Bustamante, M.; Sobocká, J.; Harper, R.; Pan, G.; Pugh, T.A. Global change pressures on soils from land use and management. Glob. Change Biol. 2016, 22, 1008–1028. [Google Scholar] [CrossRef] [PubMed]
- Don, A.; Schumacher, J.; Freibauer, A. Impact of tropical land-use change on soil organic carbon stocks—A meta-analysis. Glob. Change Biol. 2015, 17, 1658–1670. [Google Scholar] [CrossRef] [Green Version]
- Clair, S.S.; Hillier, J.; Smith, P. Estimating the pre-harvest greenhouse gas costs of energy crop production. Biomass Bioenergy 2008, 32, 442–452. [Google Scholar] [CrossRef]
- DeFries, R.S.; Asner, G.P.; Houghton, R.A. Ecosystems and land use change. Wash. DC Am. Geophys. Union Geophys. Monogr. Ser. 2004, 153, 337–344. [Google Scholar] [CrossRef]
- Houghton, R.A.; Nassikas, A.A. Global and regional fluxes of carbon from land use and land cover change 1850–2015. Glob. Biogeochem. Cycles 2017, 31, 456–472. [Google Scholar] [CrossRef]
- Zhu, E.Y.; Deng, J.S.; Zhou, M.M.; Gan, M.Y.; Jiang, R.W.; Wang, K.; Shahtahmassebi, A. Carbon emissions induced by land-use and land-cover change from 1970 to 2010 in Zhejiang, China. Sci. Total Environ. 2019, 646, 930–939. [Google Scholar] [CrossRef]
- Li, Y.M.; Shen, Y.S.; Wang, S.H. Spatio-temporal characteristics and effects of terrestrial carbon emissions based on land use change in Anhui province. J. Soil Water Conserv. 2022, 36, 182–188. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).