Earthworm Diversity, Forest Conversion and Agroforestry in Quang Nam Province, Vietnam
Abstract
1. Introduction
2. Material and Methods
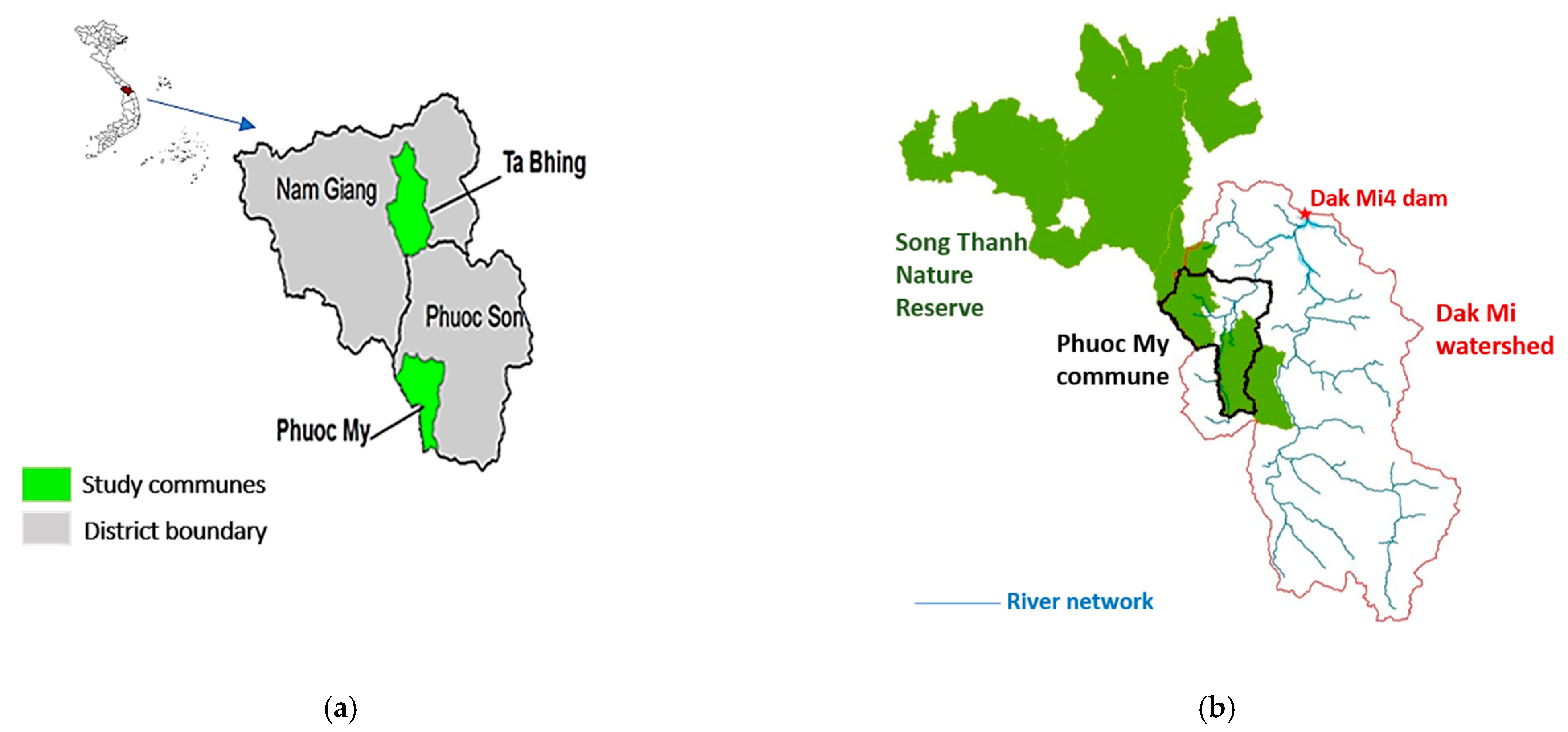
2.1. Study Sites
2.2. Land-Use History
2.3. Sample Plots by Habitat
2.4. Identification of Earthworm Species
2.5. Indicators for Earthworm Dominance and Diversity
2.6. Aboveground Litter Biomass as A Covariate
2.7. Statistical Analysis
3. Results
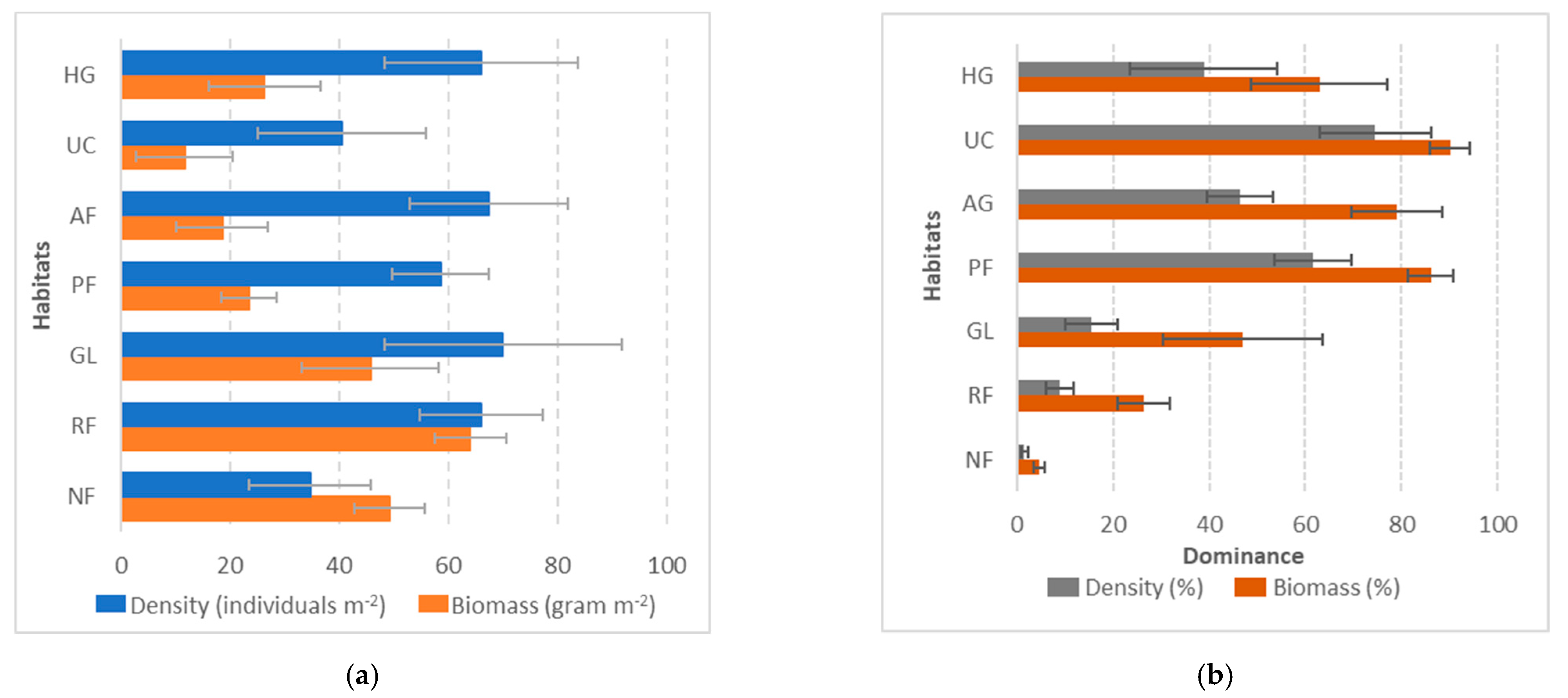
3.1. Species Occupancy by Habitat
3.2. Earthworm Density and Biomass by Habitat
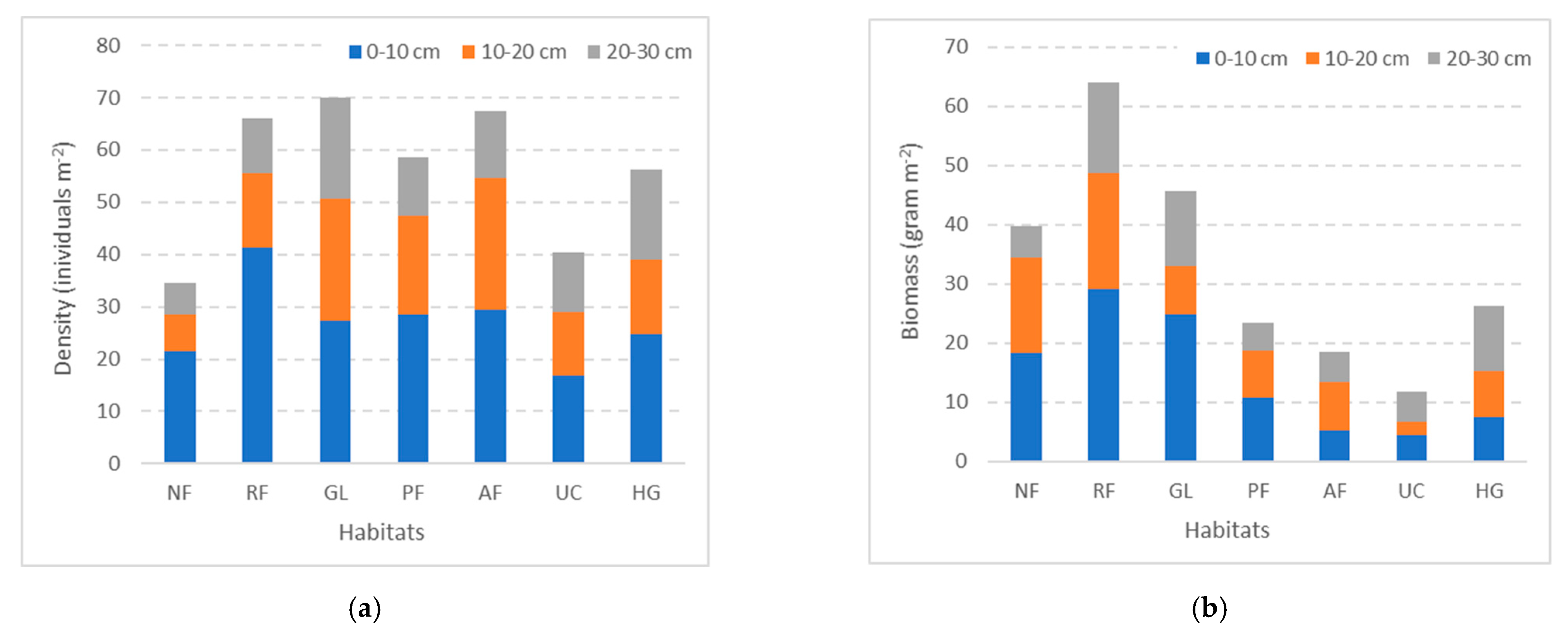
3.3. Earthworm Density and Biomass by Soil Depth
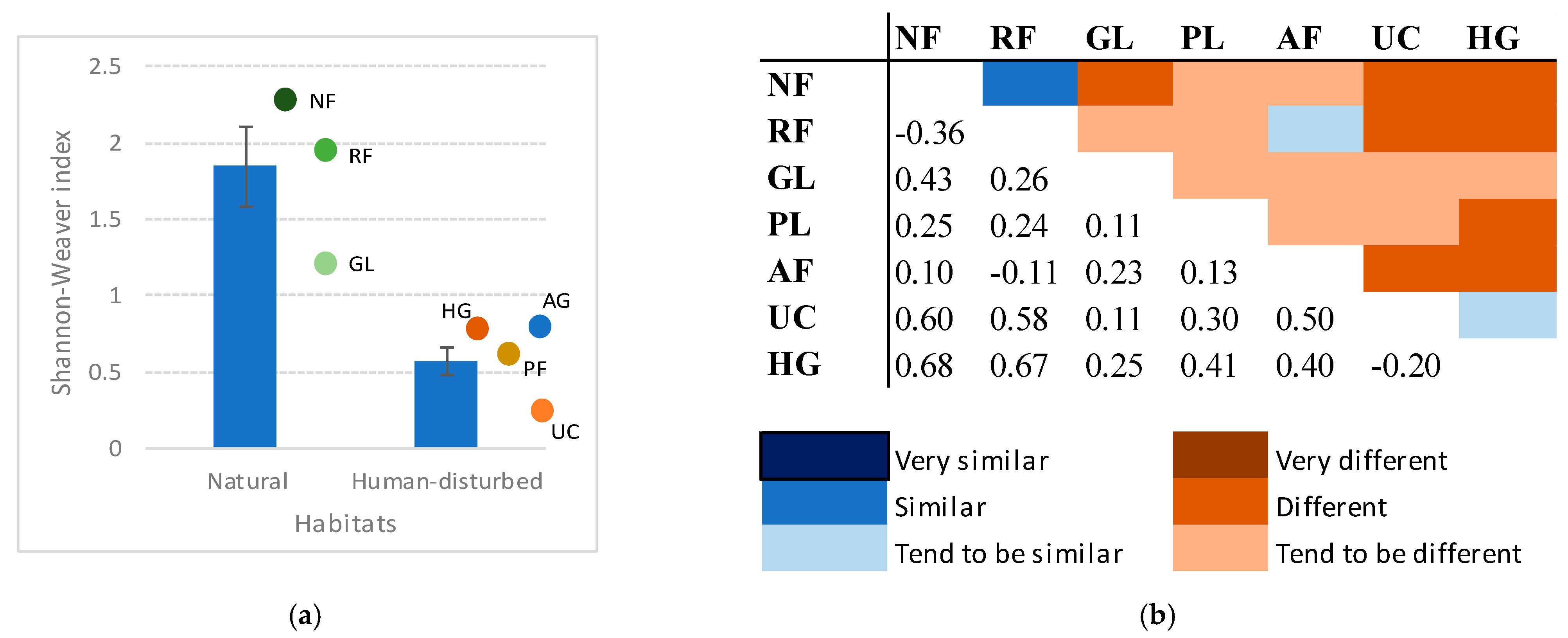
3.4. Species Diversity and Similarity Among Habitats
3.5. Variation among Types of Planted Forests
4. Discussion
4.1. Earthworm Diversity in Agroforestry
4.2. Potential Impact of P. corethrurus Dominance
4.3. Caveats in Assessing Earthworm-Habitat Linkage
4.4. Further Studies for the Unidentified Species
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Land Cover Types | Phuoc My | Ta Bhing | ||
|---|---|---|---|---|
| Ha | % | Ha | % | |
| Natural forest—rich | 7788 | 61.4 | 5766 | 36.5 |
| Logged over natural forest—medium | 1447 | 11.4 | 5307 | 33.6 |
| Logged over natural forest—poor | 492 | 3.9 | 917 | 5.8 |
| Regenerated natural forest | 704 | 5.6 | 328 | 2.1 |
| Planted forest | 798 | 6.3 | 1687 | 10.7 |
| Agroforestry | 267 | 2.2 | 149 | 1.0 |
| Home garden | 28 | 0.2 | 56 | 0.4 |
| Grasslands | 641 | 5.1 | 774 | 4.9 |
| Mixed annual crops | 390 | 3.1 | 693 | 4.4 |
| Paddy rice | 63 | 0.5 | 52 | 0.3 |
| Settlement, built up areas | 59 | 0.5 | 89 | 0.6 |
| Total (ha) | 12,677 | 15,818 | ||
| Habitat | Tree Cover (%) | No. of Sample Plots | Vegetation in Both Communes | |
|---|---|---|---|---|
| PM * | TB * | |||
| Natural forest (NF) | >60 | 5 | 10 | Fagaceae, Lauraceae, Meliaceae, Moraceae, Euphorbiaceae, Dipterocarpaceae, Sapindaceae |
| Regenerated forest (RF) | 10–30 | 5 | 10 | Bambosoideae such as Bambusa natans, Dendrocalamus patellaris, Neohouzeauna dullooa, and shrubs |
| Grassland (GL) | <10 | 1 | 3 | Shrub and grass |
| Planted forest (PF) | Young: <30, mature: >30 | 9 | 15 | In Phuoc My: Acacia (5 sample plots), Machilus odoratissima Nees (4); in Ta Bhing: Acacia (10), Melia azedarach (5). In both communes, Acacia variety is mostly the hybrid Acacia mangium x auriculiformis |
| Agroforestry (AF) | Young: <30, mature: >30 | 4 | 5 | Acacia-based with cassava, banana, or herbal plants (4), Melia-based with banana or cassava (2), agroforestry with mixed tree species such as Vernicia montana, Ficus racemose, Dimocarpus longan and annual crops such as cassava, banana, vegetables (3) |
| Upland annual crops (UC) | <10 | 3 | 5 | Key crops such as paddy rice, maize, and cassava |
| Home garden (HG) | >30 | 1 | 5 | Mostly diverse vegetable, annual crop and fruit trees such as mango, jackfruit, and longan trees |
| No | Indicators | Formula | Unit | Remark |
|---|---|---|---|---|
| 1 | Density per soil layer | Individual m−2 | nijk = number of individual of species i in soil block k layer j Nkj = total number of individuals from all species in soil block k layer j | |
| 2 | Biomass per soil layer | g m−2 | bijk = total biomass of species i in soil block k layer j | |
| 3 | Quantity dominance | % | ni = number of individuals of species i, N = total number of individuals of all species in the habitat | |
| 4 | Biomass dominance | % | bi = total biomass of species i, B = total biomass of all species in the habitat | |
| 5 | Occurrence frequency | % | si = number of sample plots having species i, S = total number of sample plots for the habitat. Range of values: >75% = very common species, >50–75% = common species, 25–50% = uncommon species, <25% = rare species | |
| 6 | Shanon- Wiener index | - | ni = total of individuals of species i, N = total of individuals of all species in the habitat | |
| 7 | Similarity index | Where: , and | - | Rs = similarity level of species Rss = similarity of subspecies x (x’); y (y’) = number of species (number of subspecies) found only in one habitat z(z’) = number of species (number of subspecies) found in both habitats. Range of values: −1 to −0.7 = very similar, <−0.7 to −0.35 = similar, <−0.35 to 0 = tend to be similar, 0 to <0.35 = tend to be different, 0.35 to <0.7 = different, 0.7 to 1.0 = very different |
| Species | Occurrence Frequency by Habitat (%) | No. of Habitat * | ||||||
|---|---|---|---|---|---|---|---|---|
| NF | RF | GL | PF | AF | UC | HG | ||
| Family Rhinodrilidae | ||||||||
| Pontoscolex corethrurus | 16 | 48 | 74 | 92 | 89 | 76 | 94 | 7 |
| Family Moniligastridae | ||||||||
| Drawida beddardi | 34 | 30 | 3.5 | 4.5 | 4 | |||
| Family Megascolecidae | ||||||||
| Amynthas alluxus | 7 | 3 | 3 | 2.5 | 4 | |||
| Amynthas aspergillum | 25 | 32 | 20 | 18 | 6 | 2 | 10 | 7 |
| Amynthas cortices | 9 | 2 | 2 | 3 | ||||
| Amynthas divitopapillatus | 12 | 12 | 12 | 12 | 12 | 12 | 12 | 7 |
| Amynthas exiguus austrinus | 10 | 2 | 2 | |||||
| Amynthas exiguus chomontis | 2 | 10 | 2 | 5 | 4 | |||
| Amynthas falcipapillatus | 4 | 1 | ||||||
| Amynthas infantiloides | 4 | 3 | 2 | |||||
| Amynthas modiglianii | 13 | 34 | 30 | 6 | 8 | 7 | 10 | 7 |
| Amynthas zoysiae | 10 | 2 | 2 | |||||
| Amynthas wui | 6 | 1 | ||||||
| Amynthas sp.1 | 9 | 32 | 50 | 7 | 23 | 4 | 40 | 7 |
| Amynthas sp.2 | 24 | 18 | 2 | 2.5 | 4 | |||
| Amynthas sp.3 | 9 | 3 | 3 | 2 | 4 | |||
| Amynthas sp.4 | 9 | 1 | 2 | |||||
| Amynthas sp.5 | 20 | 11 | 2 | 3 | ||||
| Amynthas sp.6 | 6 | 6 | 2 | |||||
| Amynthas sp.7 | 4 | 1 | 2 | |||||
| Metapheretima tiencanhensis | 13 | 30 | 20 | 12 | 12 | 5 | ||
| Metaphire houlleti | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 7 |
| Metaphire sp.1 | 1 | 1 | ||||||
| Polypheretima sp.1 | 4 | 14 | 30 | 4 | 4 | |||
| Polypheretima taprobanae | 20 | 32 | 17 | 8 | 9 | 2 | 14 | 7 |
| Total number of species | 21 | 20 | 12 | 14 | 15 | 7 | 7 | |
| Species | NF | RF | GL | PF | AF | UC | HG | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n’ | b’ | n’ | b’ | n’ | b’ | n’ | b’ | n’ | b’ | n’ | b’ | n’ | b’ | |
| Pontoscolex corethrurus | ||||||||||||||
| 0–10 | 1.7 | 1.2 | 35.7 | 13.6 | 48.6 | 13.8 | 91.1 | 51.8 | 90.1 | 61.8 | 85.4 | 70.6 | 88.4 | 65.8 |
| 10–20 | 6.7 | 2.2 | 30.7 | 7.3 | 47.4 | 19.0 | 85.6 | 76.6 | 86.8 | 43.4 | 95.6 | 92.6 | 44.3 | 10.5 |
| 20–30 | 3.2 | 0.5 | 12.9 | 6.0 | 45.4 | 13.7 | 82.0 | 56.2 | 60.7 | 33.9 | 89.6 | 60.8 | 56.5 | 40.5 |
| Drawida beddardi | ||||||||||||||
| 0–10 | 2.3 | 0.9 | 11.7 | 3.7 | 0.4 | 1.1 | ||||||||
| 10–20 | 10.5 | 18.9 | 9.1 | 2.7 | 0.4 | 0.0 | ||||||||
| 20–30 | 4.7 | 1.4 | 9.1 | 1.1 | 2.2 | 0.4 | ||||||||
| Amynthas alluxus | ||||||||||||||
| 0–10 | 0.4 | 0.0 | 1.2 | 0.3 | ||||||||||
| 10–20 | 7.6 | 3.4 | 0.7 | 0.7 | ||||||||||
| 20–30 | 6.3 | 2.3 | ||||||||||||
| Amynthas aspergillum | ||||||||||||||
| 0–10 | 2.3 | 16.1 | 1.4 | 19.2 | 2.4 | 29.7 | 2.1 | 34.0 | 2.0 | 26.6 | ||||
| 10–20 | 6.7 | 30.9 | 6.7 | 59.8 | 1.3 | 25.7 | 0.2 | 8.5 | 0.7 | 48.1 | 2.2 | 34.0 | ||
| 20–30 | 8.4 | 72.1 | 7.8 | 70.9 | 1.7 | 35.4 | 0.8 | 20.4 | 0.7 | 29.3 | 0.6 | 13.4 | 2.0 | 17.8 |
| Amynthas corticis | ||||||||||||||
| 0–10 | 1.5 | 2.6 | ||||||||||||
| 10–20 | 1.4 | 1.6 | 0.3 | 1.1 | ||||||||||
| 20–30 | 0.5 | 0.4 | ||||||||||||
| Amynthas divitopapillatus | ||||||||||||||
| 0–10 | 0.8 | 1.3 | ||||||||||||
| 10–20 | 0.5 | 0.2 | ||||||||||||
| 20–30 | 0.5 | 0.2 | ||||||||||||
| Amynthas exiguus austrinus | ||||||||||||||
| 0–10 | 10.5 | 3.5 | 0.5 | 0.1 | ||||||||||
| 10–20 | 5.8 | 2.7 | 2.4 | 0.1 | 0.7 | 0.2 | 3.3 | 0.3 | ||||||
| 20–30 | 18.8 | 1.5 | 18.2 | 2.8 | 0.6 | 0.1 | ||||||||
| Amynthas exiguus chomontis | ||||||||||||||
| 0–10 | ||||||||||||||
| 10–20 | 2.4 | 0.1 | 3.3 | 0.3 | ||||||||||
| 20–30 | 1.2 | 0.0 | 18.2 | 2.8 | ||||||||||
| Amynthas falcipapillatus | ||||||||||||||
| 0–10 | 1.4 | 1.0 | ||||||||||||
| 10–20 | ||||||||||||||
| 20–30 | ||||||||||||||
| Amynthas infantiloides | ||||||||||||||
| 0–10 | 0.8 | 5.8 | ||||||||||||
| 10–20 | 3.8 | 0.6 | 0.9 | 0.1 | 0.7 | 2.1 | ||||||||
| 20–30 | 9.4 | 0.7 | 1.4 | 0.0 | 1.1 | 4.1 | ||||||||
| Amynthas modiglianii | ||||||||||||||
| 0–10 | 5.1 | 13.9 | 16.8 | 27.7 | 13.2 | 26.1 | 1.7 | 7.8 | 2.0 | 17.5 | 1.0 | 10.5 | 0.3 | 0.4 |
| 10–20 | 2.9 | 10.7 | 2.3 | 2.7 | ||||||||||
| 20–30 | 5.1 | 10.8 | ||||||||||||
| Amynthas zoysiae | ||||||||||||||
| 0–10 | 0.9 | 0.1 | ||||||||||||
| 10–20 | 4.8 | 0.8 | 1.2 | 1.0 | ||||||||||
| 20–30 | 0.7 | 0.1 | ||||||||||||
| Amynthas wui | ||||||||||||||
| 0–10 | 0.1 | 0.0 | ||||||||||||
| 10–20 | ||||||||||||||
| 20–30 | ||||||||||||||
| Amynthas sp.1 | ||||||||||||||
| 0–10 | 1.5 | 0.1 | 1.9 | 0.1 | 5.3 | 0.1 | 0.7 | 0.1 | 1.4 | 0.2 | 3.1 | 0.5 | ||
| 10–20 | 2.9 | 0.5 | 5.8 | 0.2 | 24.7 | 1.6 | 7.1 | 1.6 | 2.3 | 0.3 | 0.6 | 0.7 | 50.0 | 50.0 |
| 20–30 | 2.7 | 0.0 | 10.5 | 0.4 | 17.9 | 0.6 | 8.4 | 0.9 | 9.8 | 3.1 | 22.2 | 15.5 | ||
| Amynthas sp.2 | ||||||||||||||
| 0–10 | 10.5 | 0.8 | 2.7 | 0.2 | 0.4 | 0.1 | ||||||||
| 10–20 | 11.5 | 1.6 | 4.2 | 0.2 | ||||||||||
| 20–30 | 3.1 | 0.1 | 1.7 | 0.1 | ||||||||||
| Amynthas sp.3 | ||||||||||||||
| 0–10 | 1.3 | 0.1 | ||||||||||||
| 10–20 | 2.9 | 0.3 | 0.8 | 0.1 | ||||||||||
| 20–30 | 3.7 | 0.1 | 1.0 | 4.2 | 4.5 | 4.5 | 2.1 | 0.8 | ||||||
| Amynthas sp.4 | ||||||||||||||
| 0–10 | 2.4 | 2.3 | ||||||||||||
| 10–20 | 1.0 | 0.9 | ||||||||||||
| 20–30 | ||||||||||||||
| Amynthas sp.5 | ||||||||||||||
| 0–10 | 19.7 | 0.9 | 1.0 | 0.1 | 9.1 | 1.2 | ||||||||
| 10–20 | 9.5 | 0.1 | 2.8 | 0.1 | 0.2 | 0.5 | 0.9 | 0.2 | ||||||
| 20–30 | 13.3 | 0.4 | 0.6 | 13.4 | ||||||||||
| Amynthas sp.6 | ||||||||||||||
| 0–10 | 1.4 | 0.2 | 0.5 | 0.0 | ||||||||||
| 10–20 | 1.9 | 0.2 | ||||||||||||
| 20–30 | 0.6 | 0.0 | ||||||||||||
| Amynthas sp.7 | ||||||||||||||
| 0–10 | 0.6 | 0.6 | ||||||||||||
| 10–20 | 1.9 | 1.5 | 0.4 | 0.1 | ||||||||||
| 20–30 | ||||||||||||||
| Metapheretima tiencanhensis | ||||||||||||||
| 0–10 | 0.3 | 1.5 | 4.2 | 6.8 | 6.1 | 2.2 | 0.9 | 1.9 | ||||||
| 10–20 | 3.9 | 4.1 | 1.6 | 2.5 | 1.0 | 1.8 | ||||||||
| 20–30 | 1.2 | 3.0 | 16.7 | 21.5 | ||||||||||
| Metaphire houlleti | ||||||||||||||
| 0–10 | 7.4 | 10.4 | 0.3 | 0.4 | 3.2 | 4.5 | 0.5 | 1.2 | ||||||
| 10–20 | 0.5 | 0.0 | 1.3 | 3.2 | ||||||||||
| 20–30 | 1.1 | 0.2 | ||||||||||||
| Metaphire sp1 | ||||||||||||||
| 0–10 | 0.1 | 0.9 | ||||||||||||
| 10–20 | ||||||||||||||
| 20–30 | ||||||||||||||
| Polypheretima sp.1 | ||||||||||||||
| 0–10 | 0.5 | 1.0 | 10.5 | 14.7 | 0.3 | 1.4 | ||||||||
| 10–20 | 1.9 | 0.9 | 0.4 | 0.6 | 14.3 | 45.4 | ||||||||
| 20–30 | 3.1 | 0.5 | 1.4 | 0.3 | 16.7 | 28.0 | 1.0 | 3.8 | ||||||
| Polypheretima taprobanae | ||||||||||||||
| 0–10 | 3.9 | 8.3 | 4.8 | 6.8 | 9.1 | 8.7 | 0.5 | 0.3 | 0.6 | 1.8 | 3.1 | 3.3 | ||
| 10–20 | 4.3 | 1.2 | 4.5 | 4.0 | 4.4 | 5.4 | 2.3 | 3.4 | 0.7 | 1.7 | 1.8 | 2.9 | ||
| 20–30 | 1.1 | 0.6 | 3.0 | 3.6 | 1.7 | 0.8 | 0.2 | 0.7 | 1.1 | 12.5 | 0.6 | 0.8 | 1.2 | 1.4 |
References
- Meyfroidt, P.; Lambin, E.F. Forest transition in Vietnam and displacement of deforestation abroad. Proc. Natl. Acad. Sci. USA 2009, 106, 16139–16144. [Google Scholar] [CrossRef] [PubMed]
- Barbier, E.B.; Burgess, J.C.; Grainger, A. The forest transition: Towards a more comprehensive theoretical framework. Land Use Policy 2010, 27, 98–107. [Google Scholar] [CrossRef]
- Meyfroidt, P.; Lambin, E.F. Global forest transition: Prospects for an end to deforestation. Annu. Rev. Environ. Resour. 2011, 36, 343–371. [Google Scholar] [CrossRef]
- Tomich, T.P.; Thomas, D.E.; Van Noordwijk, M. Environmental services and land use change in Southeast Asia: From recognition to regulation or reward? In Proceedings of the Agriculture, Ecosystems and Environment; Elsevier: Amsterdam, The Netherlands, 2004; Volume 104, pp. 229–244. [Google Scholar]
- Leimona, B.; van Noordwijk, M.; de Groot, R.; Leemans, R. Fairly efficient, efficiently fair: Lessons from designing and testing payment schemes for ecosystem services in Asia. Ecosyst. Serv. 2015, 12, 16–28. [Google Scholar] [CrossRef]
- Bruijnzeel, L.A. Hydrological functions of tropical forests: Not seeing the soil for the trees? In Proceedings of the Agriculture, Ecosystems and Environment; Elsevier: Amsterdam, The Netherlands, 2004; Volume 104, pp. 185–228. [Google Scholar]
- Wall, D.H.; Nielsen, U.N. Biodiversity and ecosystem services: Is it the same below ground? Nat. Educ. Knowl. 2012, 3, 8. [Google Scholar]
- Delgado-Baquerizo, M.; Bardgett, R.D.; Vitousek, P.M.; Maestre, F.T.; Williams, M.A.; Eldridge, D.J.; Lambers, H.; Neuhauser, S.; Gallardo, A.; García-Velázquez, L.; et al. Changes in belowground biodiversity during ecosystem development. Proc. Natl. Acad. Sci. USA 2019, 116, 6891–6896. [Google Scholar] [CrossRef] [PubMed]
- Wagg, C.; Bender, S.F.; Widmer, F.; Van Der Heijden, M.G.A. Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc. Natl. Acad. Sci. USA 2014, 111, 5266–5270. [Google Scholar] [CrossRef] [PubMed]
- Greacen, E.; Sands, R. Compaction of forest soils. A review. Aust. J. Soil Res. 1980, 18, 163–189. [Google Scholar] [CrossRef]
- Ruiz, S.; Schymanski, S.J.; Or, D. Mechanics and energetics of soil penetration by earthworms and plant roots: Higher rates cost more. Vadose Zone J. 2017, 16, vzj2017.01.0021. [Google Scholar] [CrossRef]
- World Bank. Country Forest Note Vietnam; World Bank: Washington DC, USA, 2019. [Google Scholar]
- To, X.P.; Tran, H.N. Forest Land Allocation in the Context of Forestry Sector Restructuring: Opportunities for Forestry Development and Upland Livelihood Improvement; Tropenbos International Vietnam: Hue City, Vietnam, 2014. [Google Scholar]
- de Jong, W.; Do, D.S.; Trieu, V.H. Forest Rehabilitation in Vietnam: Histories, Realities and Future; Center for International Forestry Research (CIFOR): Jakarta, Indonesia, 2006. [Google Scholar]
- Le, D.M.; Nguyen, M.H.; Tran, T. Deforestation in the Central Highlands: Implications for minority ethnic communities and a number of recommendations. In Proceedings of the 14th International Conference on Humanities and Social Sciences 2018 (IC-HUSO 2018), Khon Kaen, Thailand, 22–23 November 2018; pp. 491–507. [Google Scholar]
- Catacutan, D.C.; Do, T.H.; Simelton, E.; Hanh, V.T.; Hoang, T.L.; Patton, I.; Hairiah, K.; Le, T.T.; van Noordwijk, M.; Nguyen, M.P.; et al. Assessment of the Livelihoods and Ecological Conditions of Bufferzone Communes in Song Thanh National Reserve, Quang Nam Province, and Phong Dien Natural Reserve, Thua Thien Hue Province; World Agroforestry (ICRAF): Hanoi, Vietnam, 2017. [Google Scholar]
- Nguyen, H.T.; Yen, H.M. Song Thanh Nature Reserve. In Evidence-Based Conservation. Lessons from the Lower Mekong; Sunderland, T.C.H., Sayer, J., Hoang, M.H., Eds.; Center for International Forestry Research: Bogor, Indonesia, 2013; pp. 29–38. [Google Scholar]
- FAO. Vietnam Forestry Outlook Study; FAO: Bangkok, Thailand, 2009. [Google Scholar]
- MONRE. Vietnam’s Fifth National Report to the United Nations Convention on Biological Diversity (Reporting Period: 2009–2013); MONRE: Hanoi, Vietnam, 2014. [Google Scholar]
- Bartz, M.L.C.; Brown, G.G.; da Rosa, M.G.; Filho, O.K.; James, S.W.; Decaëns, T.; Baretta, D. Earthworm richness in land-use systems in Santa Catarina, Brazil. Appl. Soil Ecol. 2014, 83, 59–70. [Google Scholar] [CrossRef]
- Shakir, S.H.; Dindal, D.L. Density and biomass of earthworms in forest and herbaceous microecosystems in central New York, North America. Soil Biol. Biochem. 1997, 29, 275–285. [Google Scholar] [CrossRef]
- Zhang, H.; Schrader, S. Earthworm effects on selected physical and chemical properties of soil aggregates. Biol. Fertil. Soils 1993, 15, 229–234. [Google Scholar] [CrossRef]
- Blouin, M.; Hodson, M.E.; Delgado, E.A.; Baker, G.; Brussaard, L.; Butt, K.R.; Dai, J.; Dendooven, L.; Peres, G.; Tondoh, J.E.; et al. A review of earthworm impact on soil function and ecosystem services. Eur. J. Soil Sci. 2013, 64, 161–182. [Google Scholar] [CrossRef]
- Lavelle, P.; Barois, I.; Cruz, I.; Fragoso, C.; Hernandez, A.; Pineda, A.; Rangel, P. Adaptive strategies of Pontoscolex corethrurus (Glossoscolecidae, Oligochaeta), a peregrine geophagous earthworm of the humid tropics. Biol. Fertil. Soils 1987, 5, 188–194. [Google Scholar] [CrossRef]
- González, G.; Huang, C.Y.; Zou, X.; Rodríguez, C. Earthworm invasions in the tropics. In Biological Invasions Belowground: Earthworms as Invasive Species; Springer: Berlin/Heidelberg, Germany, 2006; pp. 47–56. ISBN 1402052820. [Google Scholar]
- Bhadauria, T.; Ramakrishnan, P.S. Earthworm population dynamics and contribution to nutrient cycling during cropping and fallow phases of shifting agriculture (Jhum) in North-East India. J. Appl. Ecol. 1989, 26, 505. [Google Scholar] [CrossRef]
- Nunes, D.H.; Pasini, A.; Benito, N.P.; Brown, G.G. Earthworm diversity in four land use systems in the region of Jaguapitã, Paraná State, Brazil. Caribb. J. Sci. 2006, 42, 331–338. [Google Scholar]
- Singh, S.; Singh, J.; Vig, A.P. Effect of abiotic factors on the distribution of earthworms in different land use patterns. J. Basic Appl. Zool. 2016, 74, 41–50. [Google Scholar] [CrossRef]
- Tao, Y.; Gu, W.; Chen, J.; Tao, J.; Xu, Y.J.; Zhang, H. The influence of land use practices on earthworm communities in saline agriculture soils of the west coast region of China’s Bohai Bay. Plant Soil Environ. 2013, 59, 8–13. [Google Scholar] [CrossRef]
- Rajkhowa, D.J.; Bhattacharyya, P.N.; Sarma, A.K.; Mahanta, K. Diversity and distribution of earthworms in different soil habitats of Assam, North-East India, an Indo-Burma biodiversity hotspot. Proc. Natl. Acad. Sci. India Sect. B-Biol. Sci. 2015, 85, 389–396. [Google Scholar] [CrossRef]
- Dewi, W.S.; Senge, M. Earthworm diversity and ecosystem services under threat. Rev. Agric. Sci. 2015, 3, 25–35. [Google Scholar] [CrossRef]
- ICEM. Strategic Environmental Assessment of the Quang Nam Province Hydropower Plan for the Vu Gia-Thu Bon River Basin; ICEM: Hanoi, Vietnam, 2008. [Google Scholar]
- Mulia, R.; Khasanah, N.; Catacutan, D. Alternative forest plantation systems for Southcentral Coast of Vietnam: Projections of growth and production using the WaNuLCAS model. In Towards Low-Emission Landscapes in Vietnam; Mulia, R., Simelton, E., Eds.; World Agroforestry (ICRAF) Vietnam, World Agroforestry (ICRAF) Southeast Asia Regional Program: Hanoi, Vietnam, 2018; pp. 45–60. [Google Scholar]
- Górny, M.; Grüm, L. Methods in Soil Zoology; Elsevier Science: Amsterdam, The Netherlands, 1993. [Google Scholar]
- Gates, G.E. Burmese earthworms: An introduction to the systematics and biology of Megadrile Oligochaetes with special reference to Southeast Asia. Trans. Am. Philos. Soc. 1972, 62, 1. [Google Scholar] [CrossRef]
- Sims, R.W.; Easton, E.G. A numerical revision of the earthworm genus Pheretima auct. (Megascolecidae: Oligochaeta) with the recognition of new genera and an appendix on the earthworms collected by the Royal Society North Borneo Expedition. Biol. J. Linn. Soc. 1972, 4, 169–268. [Google Scholar] [CrossRef]
- Pham, T.H. The earthworm fauna of Quang Nam—Da Nang, Hanoi National University of Education. Ph.D. Thesis, Hanoi National University, Hanoi, Vietnam, 1995. [Google Scholar]
- Nguyen, T.T.; Tran, B.T.T.; Nguyen, A.D. Earthworms of the “acaecate” Pheretima group in Vietnam (Oligochaeta: Megascolecidae), with description of a new species from the Mekong delta. Zootaxa 2014, 3866, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Thai, T. Earthworms of Vietnam (Systematic, Fauna, Distribution and Zoogeographic). Ph.D.Thesis, Lomonosov Moscow State University, Moscow, Russia, 1983. [Google Scholar]
- Thai, T.B. New species of the genus Pheretima in Vietnam. Zool. J. 1984, 63, 1317–1327. [Google Scholar]
- Thai, T.B. New species and subspecies of the genus Pheretima (Oligochaeta, Megascolecidae) from Vietnam. Zool. J. 1984, 63, 613–617. [Google Scholar]
- Nguyen, T.T.; Nguyen, A.D.; Tran, B.T.T.; Blakemore, R.J. A comprehensive checklist of earthworm species and subspecies from Vietnam (Annelida: Clitellata: Oligochaeta: Almidae, Eudrilidae, Glossoscolecidae, Lumbricidae, Megascolecidae, Moniligastridae, Ocnerodrilidae, Octochaetidae). Zootaxa 2016, 4140, 1–92. [Google Scholar] [CrossRef]
- Thai, T.B. Description of five new species of the acaecate earthworms of the genus Pheretima Kinberg in Vietnam and key to the species of acaecate Pheretima recorded from Indochinese area. J. Biol. 1996, 18, 1–6. [Google Scholar]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949. [Google Scholar]
- Stugren, B.; Radulescu, M. Metode matematice in zoogeografia regionala. Studies Univ. Babes-Bolyai Biol. 1961, 18, 8–24. [Google Scholar]
- Gonzalez, G.; Zou, X. Plant and litter influences on earthworm abundance and community structure in a tropical wet forest. Biotropica 1999, 31, 486–493. [Google Scholar] [CrossRef]
- Dechaine, J.; Ruan, H.; Sanchez-De Leon, Y.; Zou, X. Correlation between earthworms and plant litter decomposition in a tropical wet forest of Puerto Rico. Pedobiologia (Jena) 2005, 49, 601–607. [Google Scholar] [CrossRef]
- Sangha, K.K.; Jalota, R.K.; Midmore, D.J. Litter production, decomposition and nutrient release in cleared and uncleared pasture systems of central Queensland, Australia. J. Trop. Ecol. 2006, 22, 177–189. [Google Scholar] [CrossRef][Green Version]
- Van Noordwijk, M.; Hairiah, K. Rapid carbon stock appraisal. In Negotiation-Support Toolkit for Learning Landscapes; van Noordwijk, M., Lusiana, B., Leimona, B., Dewi, S., Wulandari, D., Eds.; World Agroforestry (ICRAF), Southeast Asia Regional Program: Bogor, Indonesia, 2013. [Google Scholar]
- Chakravarty, S.; Prakash, R.; Vineeta, N.A.; Pala, G.S. Litter production and decomposition in tropical forest. In Handbook of Research on the Conservation and Restoration of Tropical Dry Forests; Bhadouria, R., Ed.; IGI Global: Hershey, PA, USA, 2020; pp. 193–212. [Google Scholar]
- Cardinael, R.; Umulisa, V.; Toudert, A.; Olivier, A.; Bockel, L.; Bernoux, M. Revisiting IPCC Tier 1 coefficients for soil organic and biomass carbon storage in agroforestry systems. Environ. Res. Lett. 2018, 13, 124020. [Google Scholar] [CrossRef]
- Barros, E.; Curmi, P.; Hallaire, V.; Chauvel, A.; Lavelle, P. The role of macrofauna in the transformation and reversibility of soil structure of an oxisol in the process of forest to pasture conversion. Geoderma 2001, 100, 193–213. [Google Scholar] [CrossRef]
- Hauser, S. Distribution and activity of earthworms and contribution to nutrient recycling in alley cropping. Biol. Fertil. Soils 1993, 15, 16–20. [Google Scholar] [CrossRef]
- Hauser, S.; Asawalam, D.O.; Vanlauwe, B. Spatial and temporal gradients of earthworm casting activity in alley cropping systems. Agrofor. Syst. 1998, 41, 127–137. [Google Scholar] [CrossRef]
- Marsden, C.; Martin-Chave, A.; Cortet, J.; Hedde, M.; Capowiez, Y. How agroforestry systems influence soil fauna and their functions-a review. Plant Soil 2020, 453, 29–44. [Google Scholar] [CrossRef]
- Norgrove, L.; Csuzdi, C.; Forzi, F.; Canet, M.; Gounes, J. Shifts in soil faunal community structure in shaded cacao agroforests and consequences for ecosystem function in Central Africa. Trop. Ecol. 2009, 50, 71–78. [Google Scholar]
- Sánchez-De León, Y.; De Melo, E.; Soto, G.; Johnson-Maynard, J.; Lugo-Pérez, J. Earthworm populations, microbial biomass and coffee production in different experimental agroforestry management systems in Costa Rica. Caribb. J. Sci. 2006, 42, 397–409. [Google Scholar]
- Cardinael, R.; Hoeffner, K.; Chenu, C.; Chevallier, T.; Béral, C.; Dewisme, A.; Cluzeau, D. Spatial variation of earthworm communities and soil organic carbon in temperate agroforestry. Biol. Fertil. Soils 2019, 55, 171–183. [Google Scholar] [CrossRef]
- Tian, G.; Olimah, J.A.; Adeoye, G.O.; Kang, B.T. Regeneration of earthworm populations in a degraded soil by natural and planted fallows under humid tropical conditions. Soil Sci. Soc. Am. J. 2000, 64, 222–228. [Google Scholar] [CrossRef]
- Price, G.W.; Gordon, A.M. Spatial and temporal distribution of earthworms in a temperate intercropping system in southern Ontario, Canada. Agrofor. Syst. 1998, 44, 141–149. [Google Scholar] [CrossRef]
- Taheri, S.; Pelosi, C.; Dupont, L. Harmful or useful? A case study of the exotic peregrine earthworm morphospecies Pontoscolex corethrurus. Soil Biol. Biochem. 2018, 116, 277–289. [Google Scholar] [CrossRef]
- Nguyen, V.T. The Earthworm Fauna of Binh Tri Thien Region; Hanoi National University of Education: Hanoi, Vietnam, 1994. [Google Scholar]
- Thai, T.B. Species diversity of earthworms in Vietnam. In Proceedings of the National Workshop on the Basic Issues in Life Science; Hanoi Science and Technics Publishing House: Hanoi, Vietnam, 2000; pp. 307–311. [Google Scholar]
- Thai, T.B.; Huynh, T.K.H.; Nguyen, D.A. Remarks of earthworms on the islands in southern of Vietnam. In Proceedings of the National Workshop on the Basic Issues in Life Science; Hanoi Science and Technics Publishing House: Hanoi, Vietnam, 2004; pp. 757–760. [Google Scholar]
- Fragoso, C.; Kanyonyo, J.; Moreno, A.; Senapati, B.K.; Blanchart, E.; Rodriguez, C. A survey of tropical earthworms: Taxonomy, biogeography and environmental plasticity. In Earthworm Management in Tropical Agroecosystems; Lavelle, P., Brussaard, L., Hendrix, P., Eds.; CAB International: Wallingford, UK, 1999; pp. 27–55. [Google Scholar]
- Williamson, M.; Fitter, A. The varying success of invaders. Ecology 1996, 77, 1661–1666. [Google Scholar] [CrossRef]
- Alegre, J.C.; Pashanasi, B.; Lavelle, P. Dynamics of soil physical properties in Amazonian agroecosystems inoculated with earthworms. Soil Sci. Soc. Am. J. 1996, 60, 1522–1529. [Google Scholar] [CrossRef]
- Blanchart, E.; Lavelle, P.; Braudeau, E.; Le Bissonnais, Y.; Valentin, C. Regulation of soil structure by geophagous earthworm activities in humid savannas of Cote d’Ivoire. Soil Biol. Biochem. 1997, 29, 431–439. [Google Scholar] [CrossRef]
- Chauvel, A.; Grimaldi, M.; Barros, E.; Blanchart, E.; Desjardins, T.; Sarrazin, M.; Lavelle, P. Pasture damage by an Amazonian earthworm. Nature 1999, 398, 32–33. [Google Scholar] [CrossRef]
- Hallaire, V.; Curmi, P.; Duboisset, A.; Lavelle, P.; Pashanasi, B. Soil structure changes induced by the tropical earthworm Pontoscolex corethrurus and organic inputs in a Peruvian ultisol. Eur. J. Soil Biol. 2000, 36, 35–44. [Google Scholar] [CrossRef]
- Sparovek, G.; Lambais, M.R.; Silva, Á.P.d.; Tormena, C.A. Earthworm (Pontoscolex corethrurus) and organic matter effects on the reclamation of an eroded oxisol. Pedobiologia (Jena) 1999, 43, 698–704. [Google Scholar]
- Brown, G.G.; James, S.W.; Pasini, A.; Nunes, D.H.; Benito, N.P.; Martins, P.T.; Sautter, K. Exotic, peregrine, and invasive earthworms in Brazil: Diversity, distribution, and effects on soils and plants. Caribb. J. Sci. 2006, 42, 339–358. [Google Scholar]
- Bamgbose, O.; Odukoya, O.; Arowolo, T. Earthworms as bio-indicators of metal pollution in dump sites of Abeokuta City, Nigeria. Rev. Biol. Trop 2000, 48, 229–234. [Google Scholar]
- Suthar, S.; Singh, S.; Dhawan, S. Earthworms as bioindicator of metals (Zn, Fe, Mn, Cu, Pb and Cd) in soils: Is metal bioaccumulation affected by their ecological category? Ecol. Eng. 2008, 32, 99–107. [Google Scholar] [CrossRef]
- Fründ, H.-C.; Graefe, U.; Tischer, S. Earthworms as bioindicators of soil quality. In Biology of Earthworms; Karaca, A., Ed.; Springer: Berli/Heidelberg, Germany, 2011; pp. 261–278. [Google Scholar]
- Hirano, T.; Tamae, K. Earthworms and soil pollutants. Sensors 2011, 11, 11157–11167. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Tran, T.T.B.; Lam, H.D.; Nguyen, A.D. Four new species of Amynthas earthworms in southeastern Vietnam (Annelida, Oligochaeta, Megascolecidae). Zootaxa 2020, 4790, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y. New earthworm species of Amynthas (Clitellata: Megascolecidae) from Nam Phouin National Protected Area, Laos. J. Asia-Pac. Biodivers. 2019, 12, 353–356. [Google Scholar] [CrossRef]



| Habitat | Number of Sample Plots | Litter Production (ton ha−1 year−1) | |
|---|---|---|---|
| Average | SE | ||
| Regenerated Forest (RF) | 4 | 5.8 | 0.26 |
| Grassland (GL) | 18 | 0.7 | 0.05 |
| Planted Forest (PF) | 37 | 4.4 | 0.35 |
| Upland Crops (UC) | 11 | 2.7 | 0.45 |
| Home Garden (HG) | 8 | 3.4 | 0.87 |
| Earthworm Indicators | Acacia | Melia | Machilus |
|---|---|---|---|
| No. of sample plots | 15 | 5 | 4 |
| No. of species | 13 | 7 | 9 |
| Frequency of occurrence of P. corethrurus (%) | 97 | 72 | 100 |
| No. of species with occurrence frequency ≥25% | 1 | 3 | 2 |
| Density (individuals m−2) | 80 | 17 | 59 |
| Biomass (gram m−2) | 23 | 27 | 27 |
| Shannon-Wiener index | 0.48 | 0.95 | 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulia, R.; Hoang, S.V.; Dinh, V.M.; Duong, N.B.T.; Nguyen, A.D.; Lam, D.H.; Thi Hoang, D.T.; van Noordwijk, M. Earthworm Diversity, Forest Conversion and Agroforestry in Quang Nam Province, Vietnam. Land 2021, 10, 36. https://doi.org/10.3390/land10010036
Mulia R, Hoang SV, Dinh VM, Duong NBT, Nguyen AD, Lam DH, Thi Hoang DT, van Noordwijk M. Earthworm Diversity, Forest Conversion and Agroforestry in Quang Nam Province, Vietnam. Land. 2021; 10(1):36. https://doi.org/10.3390/land10010036
Chicago/Turabian StyleMulia, Rachmat, Sam Van Hoang, Van Mai Dinh, Ngoc Bich Thi Duong, Anh Duc Nguyen, Dang Hai Lam, Duyen Thu Thi Hoang, and Meine van Noordwijk. 2021. "Earthworm Diversity, Forest Conversion and Agroforestry in Quang Nam Province, Vietnam" Land 10, no. 1: 36. https://doi.org/10.3390/land10010036
APA StyleMulia, R., Hoang, S. V., Dinh, V. M., Duong, N. B. T., Nguyen, A. D., Lam, D. H., Thi Hoang, D. T., & van Noordwijk, M. (2021). Earthworm Diversity, Forest Conversion and Agroforestry in Quang Nam Province, Vietnam. Land, 10(1), 36. https://doi.org/10.3390/land10010036







