Abstract
Microbially induced calcium carbonate precipitation (MICP) is recognized as a promising, environmentally sustainable technology with diverse applications in environmental engineering. A bibliometric analysis of 5373 publications indexed in Web of Science from 2005 to 2024 was conducted using CiteSpace and VOSviewer to identify research trends and hotspots in biomineralization and calcium carbonate (CaCO3) studies. The results showed exponential growth in publications, increasing from 96 in 2004 to 397 in 2024 and spanning 91 interdisciplinary research areas. China, United States of America, and Germany were identified as the leading contributors. Research evolution was categorized into five distinct phases, progressing from initial crystal formation investigations to the current emphasis on underlying microbial mechanisms. Trend analysis revealed four emerging research hotspots: interfaces (0.22), crystal morphology (0.18), amorphous calcium carbonate (0.05), and bacteria (0.02). Mechanisms of MICP across bacteria, fungi, and algae were examined, revealing diverse metabolic pathways, including urea hydrolysis, denitrification, and photosynthesis. These findings suggest a paradigm shift in research toward microbial diversity and the role of extracellular polymeric substances. This shift provides valuable insights for developing sustainable biotechnological applications in environmental remediation.
1. Introduction
As economic development continues, ecological degradation intensifies, creating an increased demand for environmentally sustainable technologies to control and remediate pollution. One such technology is microbially induced calcium carbonate precipitation (MICP), a naturally occurring form of biomineralization [1]. MICP facilitates carbonate precipitation via various metabolic pathways, such as photosynthesis, urea decomposition, ammonification, denitrification, sulfate reduction, anaerobic sulfide oxidation, and methane oxidation [2,3]. Due to its low environmental requirements, rapid reaction kinetics, operational simplicity, and potential for greenhouse gas mitigation, MICP has been widely adopted in civil and hydraulic engineering, especially for soil stabilization and concrete crack remediation. Recent MICP advances have enabled the effective removal of aquatic pollutants, such as fluoride, ammonium, nitrate, and heavy metals, through mechanisms like co-precipitation, lattice substitution, and bacterial metabolism. This underscores MICP’s significant potential in environmental and water treatment applications [4]. Consequently, focused research on microbial species, their mechanistic roles, and key environmental factors is essential to establish a robust technical foundation for practical water treatment implementations.
This review systematically examines two decades of biomineralization and calcium-related research through keyword co-occurrence network and trend factor analyses. The widespread adoption of MICP can be attributed to its ability to operate under mild environmental conditions, its rapid reaction kinetics, its minimal infrastructure requirements, its CO2 sequestration during mineralization, and its applicability across diverse engineering and remediation contexts, including soil reinforcement, concrete self-healing, and heavy metal immobilization in wastewater. However, several significant hurdles remain: microbial activity is inhibited by fluctuating temperature, pH, or salinity; successful results obtained in laboratory settings are rarely replicated under field conditions; the role of extracellular polymeric substances (EPS) in templating nucleation is unclear; economic viability is limited by nutrient and inoculum costs; and the long-term stability of minerals in dynamic environments is uncertain. These limitations underscore the urgent need for deeper mechanistic insight and more precise environmental control, the precise focus of this review. Unlike earlier reviews that focused on bacterial ureolysis, this review combines large-scale bibliometric analysis of 5373 publications from 2005 to 2024 with detailed mechanistic comparisons across bacterial, fungal, and algal systems. Special attention is given to previously overlooked fungal pathways. By linking bibliometric trends directly to practical insights, this review connects emerging research directions to field applications, particularly emphasizing EPS-mediated nucleation and multi-parameter optimization. This review systematically organizes research on biological calcium removal and explores the underlying mechanisms, aiming to provide clear research directions and insights for investigators in related fields.
2. Materials and Methods
2.1. Data Retrieval
The original data for this study were retrieved from the Web of Science Core Collection. The search was performed using the following parameters: TS (topic, including title, abstract, and keywords) = “biomineralization calcium” (abbreviated as BC). The search period was defined as 1 January 2005 to 31 December 2024. To ensure data quality and consistency, only publications of the types “article” and “review” were included, as these undergo rigorous peer review and contain standardized metadata (keywords, abstracts, and citations), which are essential for bibliometric analysis. Conference proceedings and patents were excluded due to incomplete metadata and inconsistent quality control, which could introduce bias in keyword co-occurrence and trend factor analyses. Only the publication types “article” and “review” were included. Complete records were exported in plain text format containing the following information for each article: title, author, year, keywords, source, and abstract. Duplicate screening was conducted using CiteSpace 6.3.R1’s automated duplicate detection algorithm, which identifies duplicates based on exact title matching, author name verification, publication year, source information, and DOI, when available. The initial retrieval yielded 5428 records. After removing duplicates, 5373 unique records remained for analysis (55 duplicates were removed, representing 1.0% of the initial dataset).
2.2. Publication Volume and Keyword Co-Occurrence Analysis
The growth pattern from 2005 to 2024 was determined by calculating the annual number of publications related to biomineralization and calcium [5]. Similar methods were applied to analyze the contributions of different countries and journals to this field of study. Keyword and term co-occurrence analyses were conducted to identify research hotspots and their relevance to subfields over time. Keywords from the collected papers were preprocessed using established methods [6]. All keywords were extracted using VOSviewer (version 1.6.20) [7]. During extraction, “keywords plus” was selected to improve matching accuracy; non-informative terms, such as “biomineralization” and “calcification,” were removed to increase analytical precision. Keywords that occurred more than 20 times, with a minimum co-occurrence frequency of 100, were selected for subsequent analysis. An author–keyword co-occurrence analysis of the 5373 papers was conducted using VOSviewer to generate network visualizations and elucidate the temporal relationships of research hotspots [8].
2.3. Trend Analysis
2.3.1. Annual Average Growth Rate
The average growth rate (AGR) of publications was calculated using Equation (1) to elucidate trends in biomineralization- and calcium-related research across topics, journals, and authors.
where B is the number of publications in the current year, and A is the number of publications n years ago.
For any given subject, attributes with upward trends necessarily have higher publication growth rates, as reflected by AGR.
2.3.2. Normalized Cumulative Frequency and Trend Factor
The normalized cumulative keyword frequency (NCF) is defined as the average number of times a keyword appears in 1000 publications over a period of time. It can be used to quantitatively characterize the popularity of a research topic. To visualize evolutionary keyword trends, publications from the past six years were divided into two groups of three years each (2019–2021 and 2022–2024). This division was chosen to: (1) provide a large enough sample size for a robust statistical analysis, (2) focus on the most recent trends relevant to future research directions, and (3) balance temporal resolution with statistical reliability, as is performed in established bibliometric methodologies. The trend factor was calculated as the logarithm of the ratio of the NCFs between the two periods [6]. If a keyword’s NCF is lower from 2019 to 2021 but higher from 2022 to 2024, its trend factor is positive, indicating an upward trend. The reverse indicates a downward trend. NCF and trend factors were calculated using Formulas (2)–(4):
where Fn(Ye-Ys) is the NCF from year Ys to year Ye; Fnyr represents the NCF of a year; fi represents the occurrence frequency of keywords.
3. Results
3.1. Evolution and Distribution of BC-Related Scientific Publications
The number of publications related to BC in the Web of Science Core Collection increased exponentially, rising from 96 in 2004 to 397 in 2024 (Figure 1). However, the annual growth rate slowed from 102% in 2005 to 22% in 2024, resulting in a cumulative total of 5373 publications. While the annual publication count continues to rise, the declining growth rate indicates that the field has entered a more stable developmental stage following a period of rapid expansion in the early 21st century. This transition is likely influenced by shifting research priorities and policy directions yet still maintains steady growth.
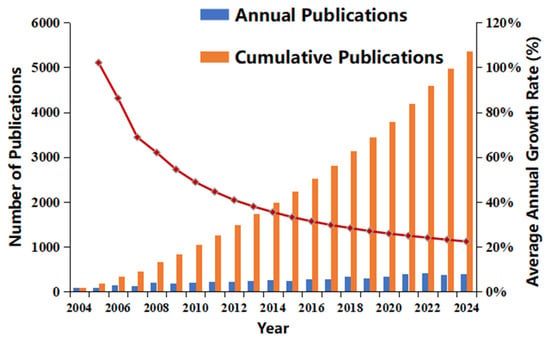
Figure 1.
Annual publications and growth trends. (The red line indicates “Average Annual Growth Rate of %”.)
The corresponding authors of these publications were affiliated with 102 countries and regions. China had the most publications (1650), followed by the United States (1104) and Germany (633) (Figure 2). Notably, a high proportion of publications originated in the past five years (2020–2024), suggesting that this period may represent a significant phase in BC research. Among the top 20 publishing countries, publications from 2020 to 2024 accounted for an average of more than 36% of the total output, exceeding one-third; three countries surpassed 50%. Furthermore, India (57%), Iran (54%), and Russia (51%) surpassed China (46%), indicating these countries have placed a greater emphasis on this field during the past five years.
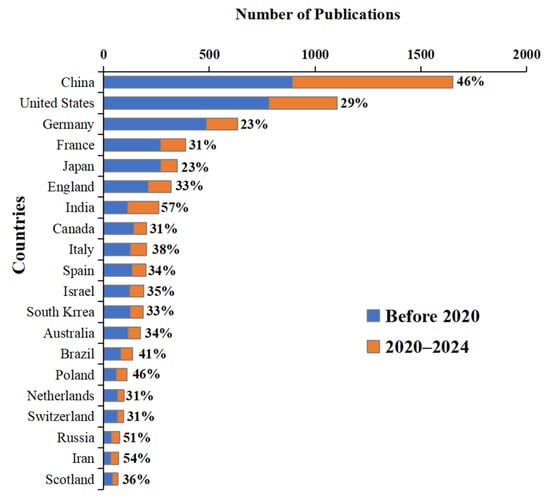
Figure 2.
Top 20 countries in terms of number of BC research publications.
Analysis of publications indicated that BC has been represented in 91 research fields, confirming its status as a highly interdisciplinary and cross-cutting research topic. Figure S1 shows that materials science was the most represented discipline with 1861 papers, followed by chemistry with 1614 papers and science and technology with 762 papers. Although publications from the past five years accounted for over one-third of the total, only ten new fields were introduced. This indicates that, although BC research has expanded in a multidisciplinary direction, the main research areas have remained concentrated in earlier core disciplines. Additionally, engineering-related publications exceeded those in science and technology, ranking third with 362 publications compared to 338. This indicates that increasing attention has been directed toward engineering applications of BC research.
3.2. Journal and Author Contributions
3.2.1. Journal Publications
The contribution of BC-related journals was evaluated based on the number of publications. Figure 3 shows BC-related publications in the top 10 journals over the past 20 years. Crystal Growth and Design published the most articles, with 167 papers. This was followed by Journal of Structural Biology and CrystEngComm, with 103 and 99 papers, respectively. Of these journals, SCIENTIFIC REPORTS had the latest start in terms of publication volume (2014), yet it showed the highest growth rate.
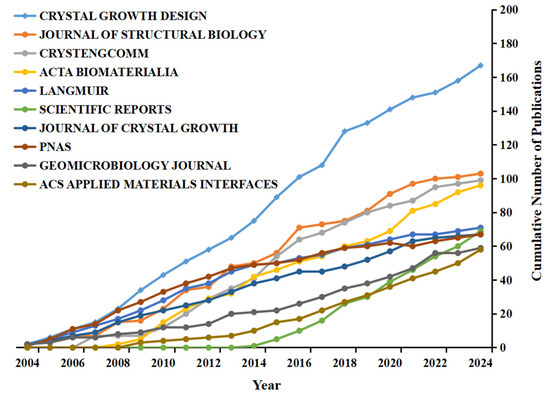
Figure 3.
Publication status of BC-related journal articles (top 10).
3.2.2. Author Contributions
Further analyses were conducted on the most productive researchers and their collaborative networks. Figure S2 shows that Tang R.K. was the most productive researcher with 93 publications, followed by H. Cölfen with 77. Consistent with China’s high research output noted in Section 3.1, five of the top 10 authors were from China. Y. Liu and Y. Zhang demonstrated substantial potential; their BC-related publications from the past five years constituted 50% and 59% of their total output, respectively. Between 2020 and 2024, their AGRs were 0.15 and 0.20, ranking them first and second among the 15 most productive authors. The disciplinary backgrounds of the most productive authors span biology, chemistry, and environmental science, reflecting the increasingly multidisciplinary nature of BC research.
We examined collaborative relationships among authors with more than 20 publications through co-author analysis using VOSviewer. The results indicated that H. Cölfen had the highest citation count (7088) and that China exhibited the highest degree of collaboration and the largest number of connections. While 33 authors published over 20 articles in the past 20 years, only 11 showed clear collaborative partnerships. BC is regarded as a multifaceted and comprehensive research topic for which extensive collaboration will be required for future progress.
3.3. BC Research Progress
A total of 226 highly relevant terms were extracted from publication titles, keywords, and abstracts to construct a co-occurrence network. Figure 4 shows that node size corresponds to term frequency, and the color scheme represents thematic clusters and temporal information. Warmer hues indicate more recent emergence. Through cluster analysis, the extracted terms were classified into five distinct thematic groups and are visually represented by different color zones: purple (left), green (upper), cyan (upper left), light blue (lower), and yellow (right). These clusters correspond to different developmental stages and research directions in the BC field. Therefore, the evolution of research over the past two decades can be interpreted through five primary aspects.
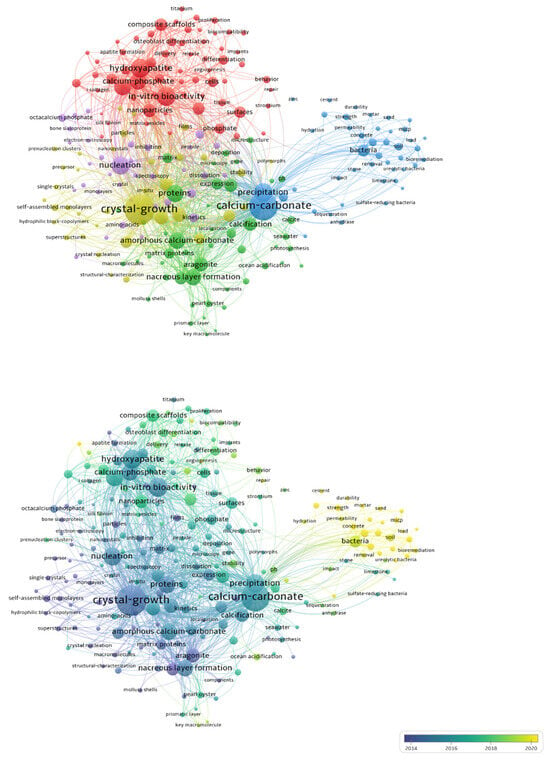
Figure 4.
Co-occurrence network diagram of terms frequently appearing in titles, keywords, and abstracts, and average time of appearance.
In the early 21st century, research focused primarily on crystal morphology and growth processes during mineral formation, emphasizing their chemical and physical aspects. Transitional studies addressed mechanisms such as nucleation hypotheses and the role of extracellular polymeric proteins in promoting crystal formation. Meanwhile, biomineralization research expanded into the biomedical field, as evidenced by the green cluster, which focuses primarily on bone regeneration and related topics. Over time, research increasingly emphasized calcium and phosphorus compounds in biomineralization processes, including calcium carbonate, calcification, and hydroxyapatite. Environmental factors, including pH variation during mineral formation, were systematically examined. Applied research on biocement also emerged. The most recent stage, represented by the yellow cluster, has primarily focused on MICP driven by urease-producing bacteria. This suggests that future research will focus more on microbial-induced calcium carbonate precipitation, including major microbial groups, influencing factors, and underlying mechanisms.
3.4. Evolution of BC Research Popularity
Increasing Popularity of BC
A bibliometric analysis was conducted to calculate trend factors and normalized cumulative frequencies (NCF) for keywords in BC research. The 20 BCs with the highest occurrence frequencies among the 5373 publications were selected for the calculations. Over time, research associated with several specific keywords has gained prominence (Figure 5). Of the 20 selected BCs, only four exhibited positive trend factors; the remaining 16 showed negative values.
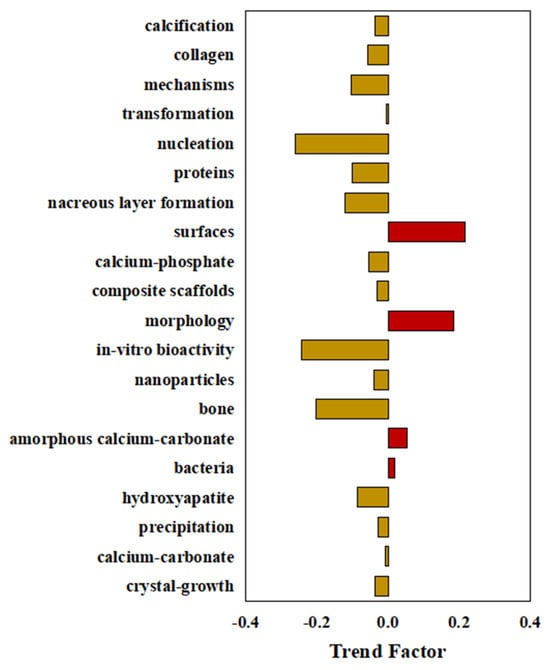
Figure 5.
Evolutionary trends of the top 20 BCs based on trend factors.
Specifically, research focusing on microscopic mechanisms, particularly interfaces, crystal morphology, amorphous calcium carbonate, and bacteria, has demonstrated an increasingly prominent trend. Of these topics, the keyword “interface” exhibited the highest positive trend factor, at 0.22. Its normalized cumulative frequency increased markedly from 28.41 in 2019 to 46.65 in 2024, indicating a growing emphasis on interfacial processes in research. The next highest trend factors were observed for “morphology” at 0.18, followed by “amorphous calcium carbonate” at 0.05 and “bacteria” at 0.02. The latter two keywords maintained relatively high normalized cumulative frequency values of 57.68 and 60.22, respectively. These values reflect the established prevalence of and rising interest in microbial mechanisms and amorphous calcium carbonate phases. Together, these trends suggest that future studies on microbially induced calcium carbonate precipitation will prioritize investigations into microbiological mechanisms and crystal morphology. This research direction may be further supported by ongoing technological and methodological advances that enable more detailed mechanistic investigations.
In contrast, several traditionally prominent research areas, including nucleation, bioactivity, and osteogenesis, have shown significant declines in trend factors ranging from −0.20 to −0.26 in recent years, despite maintaining high cumulative publication frequencies (all exceeding 50).
3.5. Mechanisms of Calcium Carbonate Precipitation Induced by Different Types of Microorganisms
The microbial species examined in this section were selected through a multi-criteria process that emphasized scientific relevance and representativeness. We prioritized microorganisms with substantial scholarly influence, specifically those with over fifty citations in our bibliometric dataset, reflecting their importance in the research community. The selection also encompasses organisms representative of key metabolic pathways that support MICP, such as ureolysis, denitrification, photosynthesis, and amino acid degradation. To demonstrate the phylogenetic breadth of MICP-capable organisms, we included species that are taxonomically diverse, spanning bacteria, fungi, and algae. Additionally, we gave preference to species with clearly documented applications in environmental or engineering contexts. Together, these selected microorganisms exemplify fundamental MICP mechanisms across taxonomic groups and provide cases for the mechanistic discussions in subsequent subsections.
3.5.1. Calcium Carbonate Precipitation Mechanisms Induced by Bacteria and Fungi Based on Nitrogen and Sulfur Cycles
- (1)
- Urea Hydrolysis
Urea hydrolysis is a series of complex enzymatic reactions catalyzed by urease and carbonic anhydrase (Figure 6). Urease hydrolyzes one mole of urea into one mole of ammonia and carbamate. The carbamate then spontaneously hydrolyzes to yield one mole of ammonia and carbonic acid. Carbonic anhydrase converts carbonic acid to bicarbonate, while ammonia hydrolysis generates two moles of ammonium and hydroxide. This increases the local pH, inducing calcium carbonate precipitation in the presence of soluble Ca2+ (Equations (5)–(11)). Under unfavorable conditions, cells permit calcium ion entry and accumulation to maintain survival, which leads to excessive proton efflux. Subsequently, calcium is actively exported to compensate for proton loss. Carbonate ion secretion requires a low proton concentration and high calcium ion levels in the microenvironment. There, carbonate supersaturation induces calcium carbonate precipitation on cell surfaces [9]. Exopolymers, biofilms, and even inactive spores can provide nucleation sites for these reactions [10,11].
CO(NH2)2 + H2O → NH2COOH + NH3
NH2COOH + H2O → NH3 + H2CO3
H2CO3 → HCO3− + H+
2NH3 + 2H2O → 2NH4+ + 2OH−-
HCO3− + H+ + 2NH4+ + 2OH− → CO32− + 2NH4+ + 2H2O
Ca2+ + Cell → Cell − Ca2+
Cell − Ca2+ + CO32− → Cell − CaCO3
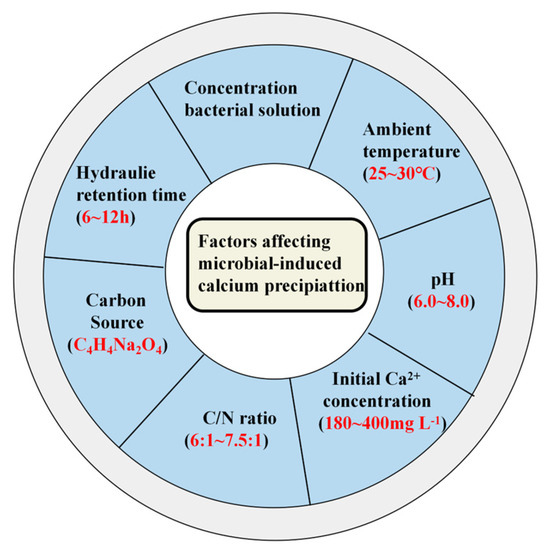
Figure 6.
Factors affecting different bacteria in the MICP process (red indicates optimal conditions).
- (2)
- Ammonification
Another microbial pathway is ammonification, in which amino acids are broken down to produce CO2 and ammonia through microbial metabolism. The hydrolysis of ammonia yields ammonium and hydroxide ions around cells, creating supersaturation that favors the precipitation of calcium carbonate (Equations (12)–(15)) [12].
Amino acids + O2 → NH3 + CO2 + H2O
NH3 + H2O → NH4+ + OH−
CO2 + OH− → HCO3−
Ca2+ +HCO3− → CaCO3 + H+
Myxococcus xanthus has been reported to utilize this mechanism during growth by precipitating uranium as uranyl minerals [13]. This process can protect concrete structures exposed to radioactive waste and maintain stability.
- (3)
- Denitrification
In the denitrification pathway, MICP is facilitated by using NO3− as a terminal electron acceptor during the oxidation of organic matter. This process produces NO2, CO2, and OH− (Equation (16)). Bacteria create an alkaline microenvironment by consuming H+ in the presence of soluble calcium ions [13]. Ersan [14] found that after adding denitrifying and expanded clay particle-immobilized Pseudomonas aeruginosa and nitrogen-reducing Diaphorobacter nitroreducens sealed microcracks in the 200–250 μm range, reducing permeability reduced by 42% and 47%, respectively. Separately, Singh et al. [15] reported that P. stutzeri mediates calcium carbonate precipitation via a two-stage denitrification mechanism, which effectively mitigates the inhibition associated with nitrite accumulation.
(CH3COOH)2Ca + NO3− → CaCO3 + N2 + CO2 + H2O + OH−
- (4)
- Sulfur Reduction Process Based on Sulfur Cycle
In organic-rich, anaerobic environments, dissimilatory sulfate reduction allows sulfate-reducing bacteria (SRB) to indirectly produce calcium carbonate minerals when calcium is present (Equation (17)). The sulfate-reducing bacterium Desulfovibrio sp. mediates calcium carbonate precipitation through dissimilatory sulfate reduction in gypsum. After sulfate ions are removed, the calcium ions that are liberated during gypsum dissolution react with CO2 within the resulting alkaline microenvironment. This induces calcium carbonate precipitation [16].
CaSO4 + H2O + CO2 → CaCO3 + H2S + S + O2
Certain Desulfovibrio species, including magnetotactic ones, produce extracellular iron sulfide particles via biologically induced mineralization while synthesizing intracellular magnetite crystals through biologically controlled mineralization [17]. Furthermore, Alshalif et al. [18] reported that incorporating SRB into concrete increased its compressive strength by 13% and reduced its permeability by 8.5%. Tambunan et al. [19] achieved significant increases in compressive (60.87%) and flexural (52.30%) strength by incorporating SRB isolated from domestic acidic water [20].
- (5)
- Methane Oxidation Process
In marine and freshwater sediments, the concentration of CO2 is primarily regulated by methane-oxidizing bacteria under both aerobic and anaerobic conditions. Under aerobic conditions, methane monooxygenase converts methane to methanol. In the periplasm, methanol is converted to formate (as a carbon source) via sequential enzymatic reactions. Subsequently, formate dehydrogenase oxidizes formate (which is in equilibrium with formic acid) to CO2. The resulting CO2 is incorporated into carbonate, which precipitates calcium carbonate in the presence of Ca2+ near cells (Equations (18)–(23)) [21].
CH4 + O2 → CH3OH + H2O
CH3OH → CHOH
CHOH + H2O → HCOO− + H+
HCOO− + H2O → HCOOH + OH
HCOOH → CO2
Ca2+ + CO2 + 2OH− → CaCO3 + H2O
3.5.2. Algae-Based Photosynthesis
In addition to heterotrophic bacterial metabolism, the role of MICP in autotrophic pathways, such as photosynthesis and methane oxidation, is well documented. In aquatic environments, cyanobacteria and microalgae primarily drive MICP through photosynthesis. These photosynthetic microorganisms primarily precipitate CaCO3 through the bicarbonate-carbonate equilibrium pathway. In this process, HCO3− diffuses across the cell membrane and dissociates into CO2 and OH- in the cytoplasm, a reaction catalyzed by carbonic anhydrase (CA). This subsequently induces calcium carbonate precipitation due to the resulting increase in local pH [22,23] (Equations (24)–(26)).
Ca2+ + HCO3− → CaCO3 + CO2 + H2O
Ca2+ + HCO3− + OH− → CaCO3 + H2O
2HCO3− → CO2 + CO32− + H2O
Srinivas et al. [24] used Synechococcus elongatus and Arthrospira platensis in a solution of 200 mM CaCl2 and pH 10.12 to repair concrete cracks, achieving 75–80% efficacy [25].
3.6. Factors and Mechanisms Influencing Microbial Calcium Carbonate Precipitation
Biomineralization occurs in various natural environments, and involves microorganisms with different metabolic pathways. Microbial CaCO3 precipitation is typically classified into three mechanisms: biologically controlled, biologically induced, and biologically mediated [3,23,26]. Biologically controlled mineralization can occur intracellularly, extracellularly, or at intercellular boundaries. It is commonly found in calcareous algae, where extracellular polymers or vesicles facilitate nucleation [27]. Biologically mediated mineralization arises from interactions between organic matrices and organic or inorganic compounds, independent of direct intracellular or extracellular biological control [28,29]. Biologically induced mineralization, on the other hand, involves carbonate precipitation driven by microbial metabolites that interact with environmental ions or compounds [30]. This mechanism predominates in microbial CaCO3 precipitation and is widespread in soils and oceans [31]. MICP is influenced by temperature, pH, ion concentrations (particularly Ca2+), dissolved inorganic carbon, carbon sources, availability of nucleation sites, and urease activity [4].
The earliest microorganism capable of MICP that was isolated from a natural environment was a calcium bacterium that was later named Pseudomonas calcis [32,33]. After this discovery, extensive research on bacteria-dominated MICP expanded. Liu et al. [4] conducted a systematic review of the distribution and influencing factors of MICP-capable bacteria from 2009 to 2022. Their findings revealed that bacterial metabolism and urease activity peaked near 30 °C. Extreme temperatures were found to impair both processes, thereby inhibiting CaCO3 formation [34]. Additionally, alkaline conditions (pH 7.0–8.0) favor CaCO3 precipitation by promoting HCO3− conversion to CO32−. Higher initial Ca2+ concentrations enhance CaCO3 formation, and removal rates scale with the initial concentration. However, low Ca2+ levels suppress precipitation [35]. High C/N ratios promote denitrification and CaCO3 formation, whereas low ratios limit the supply of electrons, thereby reducing denitrification efficiency and CaCO3 precipitation [36]. The type of organic matter differentially affects MICP. For example, low-molecular-weight compounds, such as disodium succinate (C4H4Na2O4) enhance CaCO3 precipitation efficiency. Adequate hydraulic retention time (HRT) enables MICP bacteria to complete denitrification and CaCO3 precipitation. Generally, longer HRT improves Ca2+ and pollutant removal (Figure 6).
MICP has proven effective in addressing a variety of engineering and environmental challenges. In concrete repair, denitrifying bacteria have sealed microcracks ranging from 200 to 250 μm in width, reducing permeability by 42 to 47 percent [14], and photosynthetic microalgae have achieved 75 to 80 percent crack closure [24]. Fungal systems excel in heavy metal removal. Neurospora crassa removed 93.2% of calcium with Cd2+ co-precipitation, and Penicillium chrysogenum CS1 eliminated 98.8% of lead. Soil stabilization benefits from integrated approaches such as MICP combined with straw checkerboard barriers to restore degraded lands. In water treatment, the simultaneous removal of fluoride, nitrate, and calcium exceeded 90 percent efficiency [2,3]. These outcomes underscore the need for systematic parameter optimization to translate laboratory success to field conditions.
Effective pollutant removal in flow-through systems requires the systematic optimization of multiple operating variables. Response surface methodology (RSM) and orthogonal designs are powerful tools for this purpose. They enable researchers to map interactions among temperature, pH, calcium concentration, and urea dosage—interactions that single-factor studies often overlook. These multi-variable approaches maximize carbonate yield and reduce reagent costs through targeted synergy. However, laboratory-derived optima frequently underperform in the field. Natural environments introduce uncontrolled fluctuations in temperature, pH, and ionic strength. Competing ions or dissolved organics in wastewater can suppress or inadvertently enhance precipitation. Microbial consortia in open systems can shift metabolic dominance, and large-scale reactors can exacerbate mass-transfer constraints and substrate gradients. Several field-adapted strategies merit attention to overcome these discrepancies: immobilizing MICP strains on porous substrates, such as expanded clay or biochar, to improve survival; adjusting parameters in real time using sensor feedback; assembling multi-species consortia with complementary metabolic roles; and encapsulating cells to preserve activity under stress. These refinements align with the growing emphasis in research on interfacial dynamics and microbial ecology, as identified in our bibliometric survey. They pave the way for robust, commercially viable MICP deployment.
As previously noted, bacterial MICP primarily proceeds via urea hydrolysis, sulfate reduction, denitrification, and ammonification. In addition to metabolic pathways, EPS-mediated nucleation plays a crucial role in determining the location, size, and polymorphism of biogenic CaCO3 [37] (Figure S3). Bacteria secrete EPS, which is composed primarily of polysaccharides, proteins, lipids, and organic acids and is enriched in carboxyl, hydroxyl, and amino functional groups. These groups serve as heterogeneous nucleation templates that reduce the biomineralization energy barrier, accelerate the mineralization cycle, and increase precipitation rates [38]. For example, the negatively charged amino acid-derived groups in EPS can precipitate Ca2+ in saturated or unsaturated solutions as CaCO3 through chelation and electrostatic interactions. These groups serve as effective nucleation sites. Additionally, humic acids in EPS contain redox-active quinone groups that accelerate electron transfer and promote MICP [2,39,40]. The formation of specific crystal polymorphs, including vaterite, aragonite and calcite, is governed by the presence of Mg2+ [41]. Hao et al. [42] further confirmed through studies of calcium carbonate precipitation studies, induced separately by cells and extracellular polymers, that electrostatic adsorption primarily causes nucleation in both cases. However, EPS significantly influences the crystal forms of calcium carbonate precipitation. Different biochemical components within EPS play distinct roles in calcium carbonate precipitation. Proteins, particularly those rich in acidic amino acids such as aspartate and glutamate, provide high-affinity Ca2+ binding sites through their carboxyl groups. This initiates heterogeneous nucleation despite proteins comprising only 20–40% of EPS mass. Conversely, polysaccharides, which comprise 40–60% of EPS, have a lower affinity for Ca2+ binding per unit mass, but they provide extensive hydroxyl and carboxyl groups that facilitate crystal growth following nucleation. Studies have shown that protein–polysaccharide interactions create synergistic effects: proteins initiate nucleation, and polysaccharides support subsequent crystal development. These interactions collectively optimize the MICP process. W. Yu et al. [11] further demonstrated that EPS polysaccharides (e.g., trehalose and sodium alginate) enhance mineralization rates and promote CaCO3 nucleation without altering mineral phases or crystal morphology. Li et al. [43] proposed a bacteria-induced biomineralization model based on carbonic anhydrase-inhibited Pseudomonas (Gram-positive) and E. coli (Gram-negative). This model indicates that cell walls are the primary determinants of CaCO3 polymorphs, and that extracellular secretions/metabolites are key promoters (Figure S4).
Research on CaCO3 precipitation has been dominated by urease-producing bacteria. In contrast, urease-producing fungi remain understudied in this context. Fungi generally tolerate temperature, salinity, and drought better than bacteria do [44,45]. Furthermore, the mineralization of fungal mycelium in specific environments is crucial because it provides an abundance of nucleation sites [46,47,48]. Urease-producing fungi inhabit diverse environments, including soil, cement, and rock. CaCO3-precipitating fungal isolates include Neurospora crassa, Penicillium chrysogenum, Fusarium cerealis, Phoma herbarum, Mucor hiemalis, and Cladosporium sp. [44,49,50,51,52]. Early studies characterized the urease activity of Aspergillus nidulans, which has an optimum at pH 8.0–8.5, similar to bacterial urease [53]. Aspergillus niger exhibits broader thermal tolerance, maintaining over 70% of its activity after 30 min at 65 °C, 90 days at 4 °C, or 28 days at −20/−80 °C [54]. Although it is optimal at ~pH 8, fungal MICP exhibits broad pH tolerance, with precipitation tightly coupled to pH variation. For example, Li et al. [49] reported that Neurospora crassa achieved a calcium removal rate of 93.2% after 12 days of cultivation under sufficient urea, with removal amount reaching 46.62 ± 2.57 μmol/L, pH ranging from 5.5 to 9.22, and simultaneous co-precipitation of Cd2+ ions. Other fungi exhibit comparable heavy metal removal. Pestalotiopsis sp. and Myrothecium gramineum co-precipitate heavy metals at pH 5.5. Penicillium chrysogenum CS1 achieved 98.8% lead removal at pH 9.22 [44]. Trichoderma reesei grows on concrete plates and tolerates extreme pH (up to 13) and 30 °C for calcium carbonate precipitation [45] (Figure 7). Fusarium cerealis, Phoma herbarum, and Mucor hiemalis tolerate alkalinity at pH 8.3–10.6 (except one M strain at pH 10.6), with the P strain showing the highest calcium removal (62.5%) and accumulation (2.9 mmol/g dry weight), while the F strain exhibited the greatest strontium removal (60.72%) [51]. Ye et al. [52] found that Cladosporium sp. mainly induced precipitates composed of calcite and vaterite. Mechanistic studies of fungal-induced CaCO3 precipitation and polymorph formation lag behind bacterial research. Nonetheless, fungi offer substantial potential due to greater environmental tolerance and broader applications. Bacterial insights can guide fungal MICP research, with tailored fungal applications highly promising.
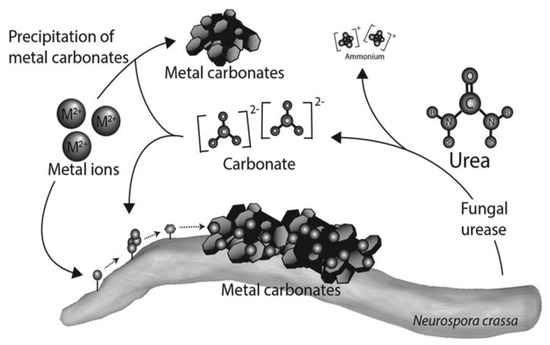
Figure 7.
Mechanism of heavy metal coprecipitation by fungal MICP based on urease.
Although bacteria and fungi demonstrate significant potential in MICP, other microorganisms, including photosynthetic microalgae with autotrophic metabolism, also play a role [55,56]. Microalgae produce various forms of calcium carbonate biomineralization. This process involves bicarbonate-carbonate exchange, membrane diffusion, and calcium-catalyzed dissociation. These steps generate alkaline microenvironments that induce calcium carbonate precipitation [57]. Microalgae also enable CO2 sequestration as a green strategy [58]. Calcifying algae include microalgae and macroalgae. Research on microalgae predominantly focuses on Synechococcus, while studies on macroalgae primarily investigate coccolithophores. Both groups form protective calcium carbonate structures known as coccoliths or calcite through secretion and precipitation processes [59]. These structures play crucial roles in carbon cycling and contribute to marine sediments [60], making calcifying algae the most promising species for inducing calcium carbonate precipitation to form biocement. Beyond inherently calcifying algae, non-calcifying species increasingly demonstrate induced CaCO3 precipitation. Furthermore, environmental factors such as temperature, pH, Ca2+, and CO32−, modulate microalgal calcification [61].
Among them, Santomauro et al. [62] used Scenedesmus obliquus and found that aragonite forms in the presence of algae. The presence of zinc ions promotes the formation of this mineral type. Irfan et al. [56] investigated the optimal conditions for inducing calcium carbonate in Chlorella kessleri, reporting that calcium carbonate production peaked at 25.18 g with a calcium removal rate of 96% on day 9 at 23 °C and pH 10.62. Xu et al. [63] cultivated Chlorella sp. and Sporosarcina pasteurii symbiotically at a 3:2 inoculation ratio under pH 9.0 with 1 g/L glucose. This increased Chlorella biomass and enhanced calcium ion removal, with a calcification rate of 0.3514, producing calcite as the predominant crystal phase. Arumugam et al. [64] examined the precipitation ability of eight microalgae (three Synechococcus, two Spirulina, two Scenedesmus, and Chlorella) and found optimal induction at pH 8.5–10.2. The two best-performing strains, one Synechococcus and one Chlorella, demonstrated daily calcium ion removal rates of 28 and 16 mg, respectively. Srinivas et al. [24] used Synechococcus elongatus and Arthrospira platensis to repair concrete and found that, with 200 mM calcium chloride at pH 10.12, they achieved 75–80% crack repair efficiency, demonstrating considerable potential for application.
Biologically controlled mineralization is prevalent in calcareous algae, where calcite nucleates and grows perpendicular to the cell surface [65,66]. Intracellular biologically controlled mineralization involves compartmentalized crystallization in specialized vesicles that direct biomineral nucleation. This process occurs in many eukaryotes, which are typically tissue-forming multicellular organisms [67,68]. In these organisms, cellular activity largely controls mineral nucleation, growth, morphology, and final positioning [69]. The formed mineral particles are synthesized intracellularly or deposited on or within organic matrices or vesicles at specific positions relative to the cell. However, Benzerara et al. [70], in a survey of intracellular CaCO3 inclusions across 68 phylogenetically diverse cyanobacterial strains, identified amorphous intracellular CaCO3 exhibiting two distinct distribution patterns: cytoplasmic dispersion, as observed in Candidatus Gloeomargarita lithophora, and polar localization, exemplified by Thermosynechococcus elongatus BP-1. This morphological distinction suggests the existence of divergent biomineralization mechanisms. These results reveal a greater complexity of cyanobacterial biomineralization than was previously thought and warrant further study.
3.7. Mechanisms and Challenges of MICP in Sediment Remediation
Sediments are major reservoirs and secondary sources of contaminants in aquatic ecosystems. Their fine particles, high organic matter content, and redox gradients foster unique microenvironments that support microbial metabolism and mineralization. In sediment MICP, the microporous structure restricts the diffusion of oxygen and ions. This creates spatially variable carbonate nucleation and localized pH shifts. Sediments rich in fine-grained clay or organic material often promote CaCO3 precipitation around microbial colonies. In contrast, coarse-grained, sandy sediments allow for quicker diffusion but often result in less stable crystal development.
The CaCO3 precipitates formed via MICP can effectively trap heavy metals, such as lead, cadmium, and zinc, as well as phosphorus and organochlorine compounds, through coprecipitation and adsorption. This sequestration reduces the movement of pollutants across the sediment-water interface and limits their release into the water column. For instance, ref. [43] demonstrated that MICP significantly reduced the mobility of lead and zinc in estuarine sediments by encapsulating these metals within carbonate minerals and promoting ion exchange within crystal structures. Similarly, Wilcox et al. [71] observed that sulfate reducing bacteria improved MICP efficiency under anaerobic conditions, producing dense calcite formations that effectively stabilized metal(loid)s in mining waste.
The complex composition of EPS in sediments, which contains an abundance of humic acids, polysaccharides, and amino acids, provides numerous nucleation sites and functional groups for adsorption. These components influence the morphology of CaCO3 crystals (vaterite, calcite, and aragonite) and the coprecipitation of contaminants. As electron shuttles, humic substances facilitate metal integration into carbonates, thereby enhancing the stability of biogenic minerals.
However, sediment MICP faces several challenges. These include uneven distribution of Ca2+ and dissolved inorganic carbon due to diffusion barriers, competition between aerobic and anaerobic microbes for the same substrate, and potential ecological impacts from elevated pH and Ca2+. Future research should prioritize the development of controlled-release microbial additives and AI-based models that can predict carbonate distribution in complex sediment matrices. Integrating MICP with phytoremediation or biochar amendments could improve long-term stability and ecological compatibility.
Recent studies highlight the potential of MICP for microplastic immobilization. CaCO3 coatings on microplastics reduce resuspension, promote sediment settling, and minimize transport to overlying waters [61]. These findings illustrate MICP’s growing importance in managing traditional and emerging contaminants in sedimentary environments.
4. Conclusions
A bibliometric analysis suggests that future research into biomineralization and calcium will focus on microbial mechanisms and amorphous calcium carbonate. Microorganisms such as bacteria, fungi and algae have tremendous potential to induce calcium carbonate precipitation. This could provide a practical solution for facilitating mineralization removal in various pollution scenarios, provided the appropriate species are selected. In addition to environmental factors, extracellular polymers play a pivotal role in promoting calcium carbonate precipitation processes. Future investigations should prioritize this aspect to advance sustainable biotechnological applications in environmental remediation.
Despite these ascending trends, significant methodological hurdles continue to impede progress. Keywords related to metagenomics and microbial community analysis appear fewer than 20 times, reflecting the difficulty of culturing non-model MICP consortia and resolving their cooperative interactions. Terms describing EPS composition and protein–polysaccharide interactions also appear rarely, reflecting the challenges of extracting, purifying and structurally resolving these complex polymers. Most telling of all is the near absence of terms relating to in situ observation and real-time monitoring (fewer than 15 occurrences), which signals the underdevelopment of imaging techniques capable of resolving transient nucleation at mineral–microbe boundaries. Examples of such techniques include in situ AFM and cryo-TEM. Although ‘interface’ (0.22) and ‘morphology’ (0.18) show strong upward momentum, their low absolute frequencies confirm that these critical domains are still in the early stages. Therefore, sustained investment in analytical innovation is imperative to convert bibliometric signals into mechanistic clarity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w17233332/s1, Figure S1: Proportion of publications in different research fields.; Figure S2: Contributions of the author in this field of research (top 15).; Figure S3: The role of bacteria in crystal nucleation.; Figure S4: Model of calcium carbonate biomineralization induced by Bacillus subtilis (left) and Escherichia coli (right).
Author Contributions
R.X.: Conceptualization, Writing—original draft, Project administration, Funding acquisition. G.J.: Formal analysis, Methodology, Visualization, Writing—original draft. Z.L.: Supervision, Visualization, Project administration. W.C.: Project administration, Supervision. Z.J.: Writing—review and editing. R.D.: Conceptualization, Writing—review and editing. M.K.: Project administration, Supervision. L.X.: Conceptualization, Project administration, Funding acquisition, Writing—review and editing. H.Y.: Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This project was jointly supported by National Key Research and Development Program of China (2023YFC2907800), CNUC-ECUT State Key Laboratory of Nuclear Resources and Environment Joint Innovation Fund (2023NRE-LH-14), National Science Foundation of China (42407333).
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
The authors declare that no Gen AI was used in the creation of this manuscript.
Conflicts of Interest
Authors Rui Xiao, Guoping Jiang, Mumtaz Khan and Lechang Xu were employed by the company Beijing Research Institute of Chemical Engineering and Metallurgy. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflict of interest.
References
- Li, Z.; Li, T. New insights into microbial induced calcium carbonate precipitation using Saccharomyces cerevisiae. Front. Microbiol. 2022, 13, 904095. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Su, J.; Ali, A.; Zhang, R.; Yang, W.; Xu, L.; Zhao, T. Microbially induced calcium precipitation based simultaneous removal of fluoride, nitrate, and calcium by Pseudomonas sp. WZ39: Mechanisms and nucleation pathways. J. Hazard. Mater. 2021, 416, 125914. [Google Scholar] [CrossRef] [PubMed]
- Castro-Alonso, M.J.; Montañez-Hernandez, L.E.; Sanchez-Muñoz, M.A.; Macias Franco, M.R.; Narayanasamy, R.; Balagurusamy, N. Microbially Induced Calcium Carbonate Precipitation (MICP) and Its Potential in Bioconcrete: Microbiological and Molecular Concepts. Front. Mater. 2019, 6, 126. [Google Scholar] [CrossRef]
- Liu, Y.; Ali, A.; Su, J.-F.; Li, K.; Hu, R.-Z.; Wang, Z. Microbial-induced calcium carbonate precipitation: Influencing factors, nucleation pathways, and application in waste water remediation. Sci. Total Environ. 2023, 860, 160439. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, S.; Yu, P.; Wang, D.; Hu, B.; Zheng, P.; Zhang, M. A bibliometric analysis of emerging contaminants (ECs) (2001−2021): Evolution of hotspots and research trends. Sci. Total Environ. 2024, 907, 168116. [Google Scholar] [CrossRef]
- Zhu, J.-J.; Dressel, W.; Pacion, K.; Ren, Z.J. ES&T in the 21st Century: A Data-Driven Analysis of Research Topics, Interconnections, And Trends in the Past 20 Years. Environ. Sci. Technol. 2021, 55, 3453–3464. [Google Scholar] [CrossRef]
- van Eck, N.J.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef]
- Marcal, J.; Bishop, T.; Hofman, J.; Shen, J. From pollutant removal to resource recovery: A bibliometric analysis of municipal wastewater research in Europe. Chemosphere 2021, 284, 131267. [Google Scholar] [CrossRef]
- Hammes, F.; Verstraete, W. Key roles of pH and calcium metabolism in microbial carbonate precipitation. Rev. Environ. Sci. Biotechnol. 2002, 1, 3–7. [Google Scholar] [CrossRef]
- Anbu, P.; Kang, C.-H.; Shin, Y.-J.; So, J.-S. Formations of calcium carbonate minerals by bacteria and its multiple applications. SpringerPlus 2016, 5, 250. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Huang, X.; Zhou, L.; Zhang, L.; Zheng, X.; Luo, W. Effects of trehalose and sodium alginate on microbially induced carbonate precipitation. Environ. Res. 2024, 263, 120145. [Google Scholar] [CrossRef]
- Zhu, T.; Dittrich, M. Carbonate Precipitation through Microbial Activities in Natural Environment, and Their Potential in Biotechnology: A Review. Front. Bioeng. Biotechnol. 2016, 4, 4. [Google Scholar] [CrossRef]
- Turick, C.E.; Berry, C.J. Review of concrete biodeterioration in relation to nuclear waste. J. Environ. Radioact. 2016, 151, 12–21. [Google Scholar] [CrossRef]
- Erşan, Y.; Boon, N.; De Belie, N. Microbial self-healing concrete: Denitrification as an enhanced and environment-friendly apporach. In Proceedings of the 5th International conference on Self-Healing Materials (ICSHM 2015), Durham, NC, USA, 22–24 June 2015. [Google Scholar]
- Singh, R.; Yoon, H.; Sanford, R.A.; Katz, L.; Fouke, B.W.; Werth, C.J. Metabolism-Induced CaCO3 Biomineralization During Reactive Transport in a Micromodel: Implications for Porosity Alteration. Environ. Sci. Technol. 2015, 49, 12094–12104. [Google Scholar] [CrossRef] [PubMed]
- Perito, B.; Mastromei, G. Molecular Basis of Bacterial Calcium Carbonate Precipitation. In Molecular Biomineralization: Aquatic Organisms Forming Extraordinary Materials; Müller, W.E.G., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 113–139. [Google Scholar]
- Sakaguchi, T.; Arakaki, A.; Matsunaga, T. Desulfovibrio magneticus sp. nov., a novel sulfate-reducing bacterium that produces intracellular single-domain-sized magnetite particles. Int. J. Syst. Evol. Microbiol. 2002, 52, 215–221. [Google Scholar] [CrossRef]
- Alshalif, A.; Irwan, J.M.; Othman, N.; Anneza, L. Isolation of Sulphate Reduction Bacteria (SRB) to Improve Compress Strength and Water Penetration of Bio-Concrete. MATEC Web Conf. 2016, 47, 01016. [Google Scholar] [CrossRef]
- Tambunan, T.; Irwan, J.M.; Othman, N. Mechanical properties of sulphate reduction bacteria on the durability of concrete in chloride condition. MATEC Web Conf. 2019, 258, 01024. [Google Scholar] [CrossRef]
- O’Connell, M.; McNally, C.; Richardson, M.G. Biochemical attack on concrete in wastewater applications: A state of the art review. Cem. Concr. Compos. 2010, 32, 479–485. [Google Scholar] [CrossRef]
- Ersan, Y.C. Overlooked Strategies in Exploitation of Microorganisms in the Field of Building Materials. In Ecological Wisdom Inspired Restoration Engineering; Achal, V., Mukherjee, A., Eds.; Springer: Singapore, 2019; pp. 19–45. [Google Scholar]
- Dhami, N.K.; Reddy, M.S.; Mukherjee, A. Application of calcifying bacteria for remediation of stones and cultural heritages. Front. Microbiol. 2014, 5, 304. [Google Scholar] [CrossRef]
- Achal, V.; Mukherjee, A.; Kumari, D.; Zhang, Q. Biomineralization for sustainable construction—A review of processes and applications. Earth-Sci. Rev. 2015, 148, 1–17. [Google Scholar] [CrossRef]
- Srinivas, K.; Alengaram, U.J.; Ibrahim, S.; Vello, V.; Phang, S.-M. Feasibility study on the use of microalgae as an external crack healing agent for cement mortar rehabilitation. J. Sustain. Cem.-Based Mater. 2023, 13, 17–32. [Google Scholar] [CrossRef]
- Seifan, M.; Samani, A.K.; Berenjian, A. Bioconcrete: Next generation of self-healing concrete. Appl. Microbiol. Biotechnol. 2016, 100, 2591–2602. [Google Scholar] [CrossRef]
- Gao, X.; Pan, Z.; Gong, P.; Jiang, Y.; Li, C. Microbially induced carbonate precipitation process and mechanism. Carsologica Sin. 2022, 41, 441–452. [Google Scholar]
- Weiner, S.; Dove, P.M. An Overview of Biomineralization Processes and the Problem of the Vital Effect. Biomineralization 2003, 54, 1–29. [Google Scholar] [CrossRef]
- Dupraz, C.; Reid, R.P.; Braissant, O.; Decho, A.W.; Norman, R.S.; Visscher, P.T. Processes of carbonate precipitation in modern microbial mats. Earth-Sci. Rev. 2009, 96, 141–162. [Google Scholar] [CrossRef]
- Sun, L.; Qin, S.; Liu, Z.; Zhao, H. Research progress on algal biomineralization. Trans. Oceanol. Limnol. 2023, 45, 164–171. [Google Scholar] [CrossRef]
- Frankel, R.B.; Bazylinski, D.A. Biologically Induced Mineralization by Bacteria. Rev. Mineral. Geochem. 2003, 54, 95–114. [Google Scholar] [CrossRef]
- Arias, D.; Cisternas, L.A.; Rivas, M. Biomineralization Mediated by Ureolytic Bacteria Applied to Water Treatment: A Review. Crystals 2017, 7, 345. [Google Scholar] [CrossRef]
- Drew, G.H. The Action of some Denitrifying Bacteria in Tropical and Temperate Seas, and the Bacterial Precipitation of Calcium Carbonate in the Sea. J. Mar. Biol. Assoc. UK 1911, 9, 142–155. [Google Scholar] [CrossRef][Green Version]
- Kellerman, K.F.; Smith, N.R. Bacterial precipitation of calcium carbonate. J. Wash. Acad. Sci. 1914, 4, 400–402. [Google Scholar]
- Zhang, J.; Shi, X.; Chen, X.; Huo, X.; Yu, Z. Microbial-Induced Carbonate Precipitation: A Review on Influencing Factors and Applications. Adv. Civ. Eng. 2021, 2021, 9974027. [Google Scholar] [CrossRef]
- Su, J.; Zhang, R.; Hu, X.; Ali, A.; Wang, Z. Calcium precipitation to remove fluorine in groundwater: Induced by Acinetobacter sp. H12 as a template. Korean J. Chem. Eng. 2022, 39, 655–663. [Google Scholar] [CrossRef]
- Dang, Y.; Zhang, R.; Wu, S.; Liu, Z.; Qiu, B.; Fang, Y.; Sun, D. Calcium effect on anaerobic biological treatment of fresh leachate with extreme high calcium concentration. Int. Biodeterior. Biodegrad. 2014, 95, 76–83. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Ali, A.; Su, J.; Huang, T.; Hou, C.; Li, X. Microbial-induced calcium precipitation: Bibliometric analysis, reaction mechanisms, mineralization types, and perspectives. Chemosphere 2024, 362, 142762. [Google Scholar] [CrossRef]
- Zhang, C.; Li, X.; Lyu, J.; Li, F. Comparison of carbonate precipitation induced by Curvibacter sp. HJ-1 and Arthrobacter sp. MF-2: Further insight into the biomineralization process. J. Struct. Biol. 2020, 212, 107609. [Google Scholar] [CrossRef]
- Nurmi, J.T.; Tratnyek, P.G. Electrochemical Properties of Natural Organic Matter (NOM), Fractions of NOM, and Model Biogeochemical Electron Shuttles. Environ. Sci. Technol. 2002, 36, 617–624. [Google Scholar] [CrossRef]
- Yan, H.; Cao, J.; Teng, M.; Meng, L.; Zhao, L.; Chi, X.; Han, Z.; Tucker, M.E.; Zhao, H. Calcium ion removal at different sodium chloride concentrations by free and immobilized halophilic bacteria. Water Res. 2023, 229, 119438. [Google Scholar] [CrossRef]
- Bai, H.; Liu, D.; Zheng, W.; Ma, L.; Yang, S.; Cao, J.; Lu, X.; Wang, H.; Mehta, N. Microbially-induced calcium carbonate precipitation by a halophilic ureolytic bacterium and its potential for remediation of heavy metal-contaminated saline environments. Int. Biodeterior. Biodegrad. 2021, 165, 105311. [Google Scholar] [CrossRef]
- Hao, Z.; Su, Y.; Liu, S.; Zhang, X. Roles of bacteria and extracellular polymeric substance in calcium carbonate formation: Insights from the effects of calcium source and deposition rate on nucleation. Biochem. Eng. J. 2024, 202, 109160. [Google Scholar] [CrossRef]
- Li, X.; He, X.; Ren, K.; Dong, H.; Lian, B. Mechanisms of carbonate precipitation induced by two model bacteria. Chem. Geol. 2023, 628, 121461. [Google Scholar] [CrossRef]
- Qian, X.; Fang, C.; Huang, M.; Achal, V. Characterization of fungal-mediated carbonate precipitation in the biomineralization of chromate and lead from an aqueous solution and soil. J. Clean. Prod. 2017, 164, 198–208. [Google Scholar] [CrossRef]
- Luo, J.; Chen, X.; Crump, J.; Zhou, H.; Davies, D.G.; Zhou, G.; Zhang, N.; Jin, C. Interactions of fungi with concrete: Significant importance for bio-based self-healing concrete. Constr. Build. Mater. 2018, 164, 275–285. [Google Scholar] [CrossRef]
- Menon, R.R.; Luo, J.; Chen, X.; Zhou, H.; Liu, Z.; Zhou, G.; Zhang, N.; Jin, C. Screening of Fungi for Potential Application of Self-Healing Concrete. Sci. Rep. 2019, 9, 2075. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Cao, J.; Yu, F.; Ma, J.; Zhang, D.; Tang, Y.; Zheng, J. Microbial biomanufacture of metal/metallic nanomaterials and metabolic engineering: Design strategies, fundamental mechanisms, and future opportunities. J. Mater. Chem. B 2021, 9, 6491–6506. [Google Scholar] [CrossRef] [PubMed]
- Sokol, N.W.; Slessarev, E.; Marschmann, G.L.; Nicolas, A.; Blazewicz, S.J.; Brodie, E.L.; Firestone, M.K.; Foley, M.M.; Hestrin, R.; Hungate, B.A.; et al. Life and death in the soil microbiome: How ecological processes influence biogeochemistry. Nat. Rev. Microbiol. 2022, 20, 415–430. [Google Scholar] [CrossRef]
- Li, Q.; Csetenyi, L.; Gadd, G.M. Biomineralization of Metal Carbonates by Neurospora crassa. Environ. Sci. Technol. 2014, 48, 14409–14416. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Csetenyi, L.; Paton, G.; Gadd, G. CaCO3 and SrCO3 bioprecipitation by fungi isolated from calcareous soil. Environ. Microbiol. 2015, 17, 3082–3097. [Google Scholar] [CrossRef]
- Zhao, J.; Csetenyi, L.; Gadd, G.M. Fungal-induced CaCO3 and SrCO3 precipitation: A potential strategy for bioprotection of concrete. Sci. Total Environ. 2022, 816, 151501. [Google Scholar] [CrossRef]
- Ye, P.; Xiao, F.; Wei, S. Biomineralization and Characterization of Calcite and Vaterite Induced by the Fungus Cladosporium sp. YPLJS-14. Minerals 2023, 13, 1344. [Google Scholar] [CrossRef]
- Creaser, E.H.; Porter, R.L. The purification of urease from Aspergillus nidulans. Int. J. Biochem. 1985, 17, 1339–1341. [Google Scholar] [CrossRef]
- Smith, P.T.; King Jr, A.D.; Goodman, N. Isolation and characterization of urease from Aspergillus niger. Microbiology 1993, 139, 957–962. [Google Scholar] [CrossRef]
- Ariyanti, D.; Handayani, N.; Hadiyanto, H. Feasibility of Using Microalgae for Biocement Production through Biocementation. Bioprocess. Biotech. 2012, 2, 2. [Google Scholar] [CrossRef]
- Irfan, M.F.; Hossain, S.M.Z.; Khalid, H.; Sadaf, F.; Al-Thawadi, S.; Alshater, A.; Hossain, M.M.; Razzak, S.A. Optimization of bio-cement production from cement kiln dust using microalgae. Biotechnol. Rep. 2019, 23, e00356. [Google Scholar] [CrossRef]
- De Muynck, W.; Verbeken, K.; De Belie, N.; Verstraete, W. Influence of urea and calcium dosage on the effectiveness of bacterially induced carbonate precipitation on limestone. Ecol. Eng. 2010, 36, 99–111. [Google Scholar] [CrossRef]
- Li, S.; Li, C.; Yao, D.; Wang, S. Feasibility of microbially induced carbonate precipitation and straw checkerboard barriers on desertification control and ecological restoration. Ecol. Eng. 2020, 152, 105883. [Google Scholar] [CrossRef]
- Paasche, E.; Brubak, S.J.P. Enhanced calcification in the coccolithophorid Emiliania huxleyi (Haptophyceae) under phosphorus limitation. Phycologia 1994, 33, 324–330. [Google Scholar] [CrossRef]
- Gal, A. Looking away from the streetlight—New insights into marine calcification. New Phytol. 2018, 220, 5–7. [Google Scholar] [CrossRef]
- Nur, M.M.A.; Dewi, R.N. Opportunities and challenges of microalgae in biocement production and self-repair mechanisms. Biocatal. Agric. Biotechnol. 2024, 56, 103048. [Google Scholar] [CrossRef]
- Santomauro, G.; Baier, J.; Huang, W.; Pezold, S.; Bill, J. Formation of Calcium Carbonate Polymorphs Induced by Living Microalgae. J. Biomater. Nanobiotechnol. 2012, 3, 413–420. [Google Scholar] [CrossRef]
- Xu, P.; Fan, H.; Leng, L.; Fan, L.; Liu, S.; Chen, P.; Zhou, W. Feasibility of microbially induced carbonate precipitation through a Chlorella-Sporosaricina co-culture system. Algal Res. 2020, 47, 101831. [Google Scholar] [CrossRef]
- Arumugam, K.; Mohamad, R.; Ashari, S.E.; Tan, J.S.; Mohamed, M.S. Bioprospecting microalgae with the capacity for inducing calcium carbonate biomineral precipitation. Asia-Pac. J. Chem. Eng. 2022, 17, e2767. [Google Scholar] [CrossRef]
- Borowitzka, M.A.; Larkum, A.W.D.; Nockolds, C.E. A scanning electron microscope study of the structure and organization of the calcium carbonate deposits of algae. Phycologia 1974, 13, 195–203. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Morphological and Cytological Aspects of Algal Calcification. In International Review of Cytology; Bourne, G.H., Danielli, J.F., Jeon, K.W., Eds.; Academic Press: Cambridge, MA, USA, 1982; Volume 74, pp. 127–162. [Google Scholar]
- Lowenstam, H.A.; Weiner, S. On Biomineralization; Oxford University Press: Oxford, UK, 1989. [Google Scholar]
- Mann, S. Biomineralization Principles and Concepts in Bioinorganic Materials Chemistry; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Decho, A.W. Overview of biopolymer-induced mineralization: What goes on in biofilms? Ecol. Eng. 2010, 36, 137–144. [Google Scholar] [CrossRef]
- Benzerara, K.; Skouri-Panet, F.; Li, J.; Férard, C.; Gugger, M.; Laurent, T.; Couradeau, E.; Ragon, M.; Cosmidis, J.; Menguy, N.; et al. Intracellular Ca-carbonate biomineralization is widespread in cyanobacteria. Proc. Natl. Acad. Sci. USA 2014, 111, 10933–10938. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, S.M.; Mulligan, C.N.; Neculita, C.M. Microbially Induced Calcium Carbonate Precipitation as a Bioremediation Technique for Mining Waste. Toxics 2024, 12, 107. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).