Influence of Vegetation Cover and Soil Properties on Water Infiltration: A Study in High-Andean Ecosystems of Peru
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Field Methods
2.3. Hydraulic Models
2.3.1. Modified Kostiakov Model
2.3.2. Philip Model
2.3.3. Horton Model
2.4. Statistical Analysis and Model Fitting
3. Results
3.1. Associated Physicochemical Characteristics
3.2. Hydraulic Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fausak, L.K.; Bridson, N.; Diaz-Osorio, F.; Jassal, R.S.; Lavkulich, L.M. Soil health—A perspective. Front. Soil Sci. 2024, 4, 1462428. [Google Scholar] [CrossRef]
- Dong, X.; Martin, J.B.; Cohen, M.J.; Tu, T. Bedrock mediates responses of ecosystem productivity to climate variability. Commun. Earth Environ. 2023, 4, 114. [Google Scholar] [CrossRef]
- Hou, D. Soil health and ecosystem services. Soil Use Manag. 2023, 39, 1259–1266. [Google Scholar] [CrossRef]
- Telo da Gama, J. The Role of Soils in Sustainability, Climate Change, and Ecosystem Services: Challenges and Opportunities. Ecologies 2023, 4, 552–567. [Google Scholar] [CrossRef]
- Burbano-Orjuela, H. El suelo y su relación con los servicios ecosistémicos y la seguridad alimentaria. RCIA 2016, 33, 117–124. [Google Scholar] [CrossRef]
- Huamán-Carrión, M.L.; Espinoza-Montes, F.; Barrial-Lujan, A.I.; Ponce-Atencio, Y. Influence of altitude and soil characteristics on organic carbon storage capacity of high Andean natural pastures. Sci. Agropecu. 2021, 12, 83–90. [Google Scholar] [CrossRef]
- Lal, R. Managing soils for resolving the conflict between agriculture and nature: The hard talk. Eur. J. Soil Sci. 2020, 71, 1–9. [Google Scholar] [CrossRef]
- Cervantes, R.; Sánchez, J.M.; Alegre, J.; Rendón, E.; Baiker, J.R.; Locatelli, B.; Bonnesoeur, V. Contribución de los Ecosistemas Altoandinos en la Provisión del Servicio Ecosistémico de Regulación Hídrica. Ecol. Apl. 2022, 20, 137–146. [Google Scholar] [CrossRef]
- Jia, Z.; Weng, B.; Yan, D.; Peng, H.; Dong, Z. The effects of different factors on soil water infiltration properties in High Mountain Asia: A meta-analysis. CATENA 2024, 234, 107583. [Google Scholar] [CrossRef]
- Páez-Bimos, S.; Villacís, M.; Morales, O.; Calispa, M.; Molina, A.; Salgado, S.; De Bievre, B.; Delmelle, P.; Muñoz, T.; Vanacker, V. Vegetation effects on soil pore structure and hydraulic properties in volcanic ash soils of the high Andes. Hydrol. Process. 2022, 36, e14678. [Google Scholar] [CrossRef]
- Bate, D.B.; Barrett, J.E.; Poage, M.A.; Virginia, R.A. Soil phosphorus cycling in an Antarctic polar desert. Geoderma 2008, 144, 21–31. [Google Scholar] [CrossRef]
- Muñoz-Gómez, F.A.; Figueroa-Casas, A.; Pérez, E.H.; Rengifo-Cañizalez, E. Susceptibilidad a la erosión en dos agroecosistemas altoandinos del Cauca. Rev. Investig. Univ. Quindío 2010, 20, 9–17. [Google Scholar] [CrossRef]
- Oñate-Valdivieso, F.; Oñate-Paladines, A.; Díaz, R. Soil degradation in andean watersheds: A case study using remote sensing. Front. Earth Sci. 2024, 12, 1325189. [Google Scholar] [CrossRef]
- Rust, N.; Lunder, O.E.; Iversen, S.; Vella, S.; Oughton, E.A.; Breland, T.A.; Glass, J.H.; Maynard, C.M.; McMorran, R.; Reed, M.S. Perceived Causes and Solutions to Soil Degradation in the UK and Norway. Land 2022, 11, 131. [Google Scholar] [CrossRef]
- Sahagún-Sánchez, F.J.; Reyes-Hernández, H. Impactos por cambio de uso de suelo en las áreas naturales protegidas de la región central de la Sierra Madre Oriental, México. CienciaUAT 2018, 12, 6–21. Available online: https://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S2007-78582018000100006 (accessed on 29 May 2025). [CrossRef]
- Wen, L.; Peng, Y.; Zhou, Y.; Cai, G.; Lin, Y.; Li, B. Study on soil erosion and its driving factors from the perspective of landscape in Xiushui watershed, China. Sci. Rep. 2023, 13, 8182. [Google Scholar] [CrossRef] [PubMed]
- Medina Quispe, P.R.; Arizapana-Almonacid, M.A.; Nosetto, M.D. Changes in the Soil Organic Carbon of Grasslands in the High Andes of Peru after Their Conversion to Croplands and Their Environmental Controls. Grasses 2024, 3, 35–44. [Google Scholar] [CrossRef]
- Torres, J.; Gutierrez, J.A.; Beltran, H.A. Compactación, Una de las causas más comunes de la degradación del suelo. Cienc. Agropecu. 2017, 3, 18–22. Available online: https://revistas.ucundinamarca.edu.co/index.php/Ciencias_agropecuarias/article/view/225 (accessed on 29 May 2025).
- Betanzos-Simon, J.E.; Ney Ríos, J.; Benegas Negri, L.A.; Jiménez-Trujillo, J.A.; Pérez-Sánchez, E.; Martínez-Salinas, A.; Sepúlveda López, C.J. Comportamiento de la infiltración del recurso hídrico en diferentes usos de suelo en paisajes ganaderos. Rev. AIA 2022, 25, 130–131. [Google Scholar] [CrossRef]
- Litt, G.F.; Ogden, F.L.; Mojica, A.; Hendrickx, J.M.H.; Kempema, E.W.; Gardner, C.B.; Bretfeld, M.; Regina, J.A.; Harrison, J.B.J.; Cheng, Y.; et al. Land cover effects on soil infiltration capacity measured using plot scale rainfall simulation in steep tropical lowlands of Central Panama. Hydrol. Process. 2020, 34, 878–897. [Google Scholar] [CrossRef]
- Naharuddin, N.; Massiri, S.D.; Pribadi, H.; Maiwa, A.; Ihsan, M. Evaluation of Rainwater Harvesting and Bio-pore Infiltration Holes for Flood Mitigation and Soil Conservation. JCHE 2024, 2, 142–151. [Google Scholar] [CrossRef]
- Nawaz, M.F.; Bourrié, G.; Trolard, F. Soil compaction impact and modelling. A review. Agron. Sustain. Dev. 2013, 33, 291–309. [Google Scholar] [CrossRef]
- Carranza-Patiño, M.; Aragundi-Sabando, L.; Macias-Barrera, K.; Paredes-Sarabia, E.; Villegas-Ramírez, A. Conservación y Manejo Sostenible del Suelo en la Agricultura: Una Revisión Sistemática de Prácticas Tradicionales y Modernas. COCIRI 2024, 5, 1–28. [Google Scholar] [CrossRef]
- Basche, A.D.; DeLonge, M.S. Comparing infiltration rates in soils managed with conventional and alternative farming methods: A meta-analysis. PLoS ONE 2019, 14, e0215702. [Google Scholar] [CrossRef] [PubMed]
- Sabogal-Dunin-Borkowski, A. Desarrollo sostenible del páramo peruano: Estudio de caso de los páramos de Pacaipampa, Frías y Huancabamba, departamento de Piura, Perú. KAWSAYPACHA 2023, 12, A-004. [Google Scholar] [CrossRef]
- Guzman, C.D.; Hoyos-Villada, F.; Da Silva, M.; Zimale, F.A.; Chirinda, N.; Botero, C.; Morales Vargas, A.; Rivera, B.; Moreno, P.; Steenhuis, T.S. Variability of soil surface characteristics in a mountainous watershed in Valle del Cauca, Colombia: Implications for runoff, erosion, and conservation. J. Hydrol. 2019, 576, 273–286. [Google Scholar] [CrossRef]
- Bonnesoeur, V.; Locatelli, B.; Guariguata, M.R.; Ochoa-Tocachi, B.F.; Vanacker, V.; Mao, Z.; Stokes, A.; Mathez-Stiefel, S.-L. Impacts of forests and forestation on hydrological services in the Andes: A systematic review. For. Ecol. Manag. 2019, 433, 569–584. [Google Scholar] [CrossRef]
- SENAMHI. Descarga de Datos. Available online: https://www.senamhi.gob.pe/site/descarga-datos/ (accessed on 16 May 2025).
- Boñon, G.A. Geomorfología: Memoria Descriptiva del Mapa Geomorfológico del Departamento de Cajamarca; Zonificación Ecológica Económica; Gobierno Regional de Cajamarca: Cajamarca, Peru, 2011. [Google Scholar]
- Rojas, W.P.; Boñon, G.A. Estudio de Suelos y Capacidad de Uso Mayor del Departamento de Cajamarca; Zonificación Ecológica Económica; Gobierno Regional de Cajamarca: Cajamarca, Peru, 2011. [Google Scholar]
- Boñon, A. Cobertura Vegetal y Uso Actual Departamento de Cajamarca; Gobierno Regional de Cajamarca: Cajamarca, Peru, 2011. [Google Scholar]
- MIDAGRI Perfil Productivo Regional. Available online: https://siea.midagri.gob.pe/siea_bi/ (accessed on 24 July 2024).
- Burga-Cieza, A.M.; Burga Cieza, J.; Iglesias-Osores, S.; Alcalde-Alfaro, V.W.; Martínez-Sovero, G.; Dávila-Estela, L.; Villena-Velásquez, J.J. Estructura, diversidad y endemismo de la flora del relicto Los Lanches del bosque montano Las Palmas, Cajamarca, Perú. Cienc. Amaz. 2021, 9, 43–58. [Google Scholar] [CrossRef]
- Rufasto-Peralta, Y.L.; Baselly-Villanueva, J.R.; Alva-Mendoza, D.M.; Seminario-Cunya, A.; Elera-Gonzales, D.G.; Villena-Velásquez, J.J. Estimación de la calidad de sitio de Cinchona pubescens (Rubiaceae), en el bosque montano La Palma, Chota, Perú. Lilloa 2023, 60, 259–279. [Google Scholar] [CrossRef]
- Method 9045D; Soil and Waste pH 2004. USEPA: Washington, DC, USA, 2004.
- ISO 11265:1994; Soil Quality—Determination of the Specific Electrical Conductivity. International Organization for Standardization: Geneva, Switzerland, 1994.
- NOM-021-RECNAT-2000; Norma Oficial Mexicana Que Establece Las Especificaciones de Fertilidad, Salinidad y Clasificación de Suelos. Estudios, Muestreo y Análisis. SEMARNAT: Mexico City, Mexico, 2002.
- Saxton, K.E.; Rawls, W.J. Soil Water Characteristic Estimates by Texture and Organic Matter for Hydrologic Solutions. Soil Sci. Soc. Amer J. 2006, 70, 1569–1578. [Google Scholar] [CrossRef]
- ASTM D3385-18; Standard Test Method for Infiltration Rate of Soils in Field Using Double-Ring Infiltrometer. ASTM International: West Conshohocken, PA, USA, 2018. [CrossRef]
- King, P. Comparison of methods for measuring severity of water repellence of sandy soils and assessment of some factors that affect its measurement. Soil Res. 1981, 19, 275. [Google Scholar] [CrossRef]
- Bisdom, E.B.A.; Dekker, L.W.; Schoute, J.F.T. Water repellency of sieve fractions from sandy soils and relationships with organic material and soil structure. Geoderma 1993, 56, 105–118. [Google Scholar] [CrossRef]
- Wendroth, O.; Nielsen, D.R. Time and Space Series. In Methods of Soil Analysis: Part 4: Physical Methods; Dane, J.H., Topp, G.C., Eds.; Soil Science Society of America: Madison, WI, USA, 2018; pp. 119–137. ISBN 978-0-89118-893-3. [Google Scholar]
- Sihag, P.; Tiwari, N.K.; Ranjan, S. Estimation and inter-comparison of infiltration models. Water Sci. 2017, 31, 34–43. [Google Scholar] [CrossRef]
- Philip, J.R. Sorptivity and Algebraic Infiltration Equations. Soil Sci. 1957, 84, 257–264. [Google Scholar] [CrossRef]
- Bach, L.B.; Wierenga, P.J.; Ward, T.J. Estimation of the Philip Infiltration Parameters from Rainfall Simulation Data. Soil Sci. Soc. Am. J. 1986, 50, 1319–1323. [Google Scholar] [CrossRef]
- Horton, R.E. An Approach Toward a Physical Interpretation of Infiltration-Capacity. Soil Sci. Soc. Am. J. 1941, 5, 399–417. [Google Scholar] [CrossRef]
- Martin, M.; Chanzy, A.; Lassabatere, L.; Legout, A.; Pousse, N.; Ruy, S. Characterization and prediction of hydraulic properties of traffic-compacted forest soils based on soil information and traffic treatments. Ann. For. Sci. 2024, 81, 47. [Google Scholar] [CrossRef]
- Bolar, K. STAT: Interactive Document for Working with Basic Statistical Analysis, version 0.1.0; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar] [CrossRef]
- de Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research, version 1.3-7; R Foundation for Statistical Computing: Vienna, Austria, 2006. [Google Scholar] [CrossRef]
- Dinno, A. Dunn.Test: Dunn’s Test of Multiple Comparisons Using Rank Sums, version 1.3.6; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar] [CrossRef]
- Taiyun. Taiyun/Corrplot. Available online: https://github.com/taiyun/corrplot (accessed on 28 May 2025).
- Vand, A.S.; Sihag, P.; Singh, B.; Zand, M. Comparative Evaluation of Infiltration Models. KSCE J. Civ. Eng. 2018, 22, 4173–4184. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Ma, Z.; Lv, M. Response mechanism of soil structural heterogeneity in permafrost active layer to freeze–thaw action and vegetation degradation. CATENA 2023, 230, 107250. [Google Scholar] [CrossRef]
- Dias, P.M.S.; Portela, J.C.; Gondim, J.E.F.; Batista, R.O.; Rossi, L.S.; Medeiros, J.L.F.; Farias, P.K.P.; Mota, P.J.; Bandeira, D.J.D.C.; Filho, L.C.D.A.L.; et al. Soil Attributes and Their Interrelationships with Resistance to Root Penetration and Water Infiltration in Areas with Different Land Uses in the Apodi Plateau, Semiarid Region of Brazil. Agriculture 2023, 13, 1921. [Google Scholar] [CrossRef]
- Lucas, M.; Nguyen, L.T.T.; Guber, A.; Kravchenko, A.N. Cover crop influence on pore size distribution and biopore dynamics: Enumerating root and soil faunal effects. Front. Plant Sci. 2022, 13, 928569. [Google Scholar] [CrossRef]
- Lopes, E.; Carvalho Marenzi, R.; Marques de Almeida, T.C. Comparison of soil use in the infiltration of rainwater: Pasture and forest. Rev. Fac. Nac. Agron. Medellín 2018, 71, 8593–8600. [Google Scholar] [CrossRef]
- Jarvis, N.J.; Moeys, J.; Hollis, J.M.; Reichenberger, S.; Lindahl, A.M.L.; Dubus, I.G. A Conceptual Model of Soil Susceptibility to Macropore Flow. Vadose Zone J. 2009, 8, 902–910. [Google Scholar] [CrossRef]
- Escurra, M.E. Situación de la ganadería lechera en Cajamarca. Rev. Investig. Vet. Perú 2001, 12, 21–26. [Google Scholar] [CrossRef]
- Ladeira, F.S.B.; Mescolotti, P.C.; Do Nascimento Pupim, F.; De Faria, L.M.D.M.; Assine, M.L. Paleosols record dry and humid paleoenvironments during the Upper Pleistocene in the Brazilian Pantanal. CATENA 2022, 212, 106113. [Google Scholar] [CrossRef]
- Thierfelder, C.; Wall, P.C. Effects of conservation agriculture techniques on infiltration and soil water content in Zambia and Zimbabwe. Soil Tillage Res. 2009, 105, 217–227. [Google Scholar] [CrossRef]
- Fukumasu, J.; Jarvis, N.; Koestel, J.; Kätterer, T.; Larsbo, M. Relations between soil organic carbon content and the pore size distribution for an arable topsoil with large variations in soil properties. Eur. J. Soil Sci. 2022, 73, e13212. [Google Scholar] [CrossRef]
- Mesele, H.; Grum, B.; Aregay, G.; Berhe, G.T. Evaluation and comparison of infiltration models for estimating infiltration capacity of different textures of irrigated soils. Environ. Syst. Res. 2024, 13, 26. [Google Scholar] [CrossRef]
- Romay, C.; Ezquerra-Canalejo, A.; Botta, G.F. Sensitivity Analysis of Performance Indices of Surge-Flow Irrigation with System Variables Using the SIRMOD Model. Agronomy 2024, 14, 1509. [Google Scholar] [CrossRef]
- Béjar Pulido, S.J.; Silva, I.C.; Yánez Díaz, M.I.; Robles, E.O.L. Evaluación y predicción de la infiltración en un Andosol bajo diferentes usos de suelo. Remexca 2021, 12, 1171–1183. [Google Scholar] [CrossRef]
- Sun, D.; Yang, H.; Guan, D.; Yang, M.; Wu, J.; Yuan, F.; Jin, C.; Wang, A.; Zhang, Y. The effects of land use change on soil infiltration capacity in China: A meta-analysis. Sci. Total Environ. 2018, 626, 1394–1401. [Google Scholar] [CrossRef] [PubMed]
- Garate-Quispe, J.S.; Bejar Chura, N.; Pillco Sardon, N.; Herrera-Machaca, M.; Dueñas Linares, H. Diferencias en la capacidad de infiltración del suelo en dos tipos de cobertura vegeal en la Amazonía peruana. Folia Amaz. 2022, 31, 227–241. [Google Scholar] [CrossRef]
- Allton, K.E.; Harris, J.A.; Rickson, R.J.; Ritz, K. The effect of microbial communities on soil hydrological processes: A microcosm study utilising simulated rainfall. Geoderma 2007, 142, 11–17. [Google Scholar] [CrossRef]
- Jeffery, S.; Harris, J.A.; Rickson, R.J.; Ritz, K. Effects of soil-surface microbial community phenotype upon physical and hydrological properties of an arable soil: A microcosm study. Eur. J. Soil Sci. 2010, 61, 493–503. [Google Scholar] [CrossRef]
- Young, I.M.; Crawford, J.W. Interactions and Self-Organization in the Soil-Microbe Complex. Science 2004, 304, 1634–1637. [Google Scholar] [CrossRef]
- Chenu, C. Clay- or sand-polysaccharide associations as models for the interface between micro-organisms and soil: Water related properties and microstructure. Geoderma 1993, 56, 143–156. [Google Scholar] [CrossRef]
- Hu, X.; Huang, L.; Chen, H.; Chen, L.; Fallgren, P.H. Effects of soil bulk density and corresponding soil infiltration rate on the migration and transformation of gibberellic acid. J. Contam. Hydrol. 2025, 269, 104488. [Google Scholar] [CrossRef]
- Ridley, A. Legume-based farming in Southern Australia: Developing sustainable systems to meet environmental challenges. Soil Biol. Biochem. 2004, 36, 1213–1221. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, Y.; Sui, X.; Frey, B.; Song, F. Effects of Land Conversion on Soil Microbial Community Structure and Diversity in Songnen Plain, Northeast China. Sustainability 2022, 14, 10767. [Google Scholar] [CrossRef]
- Louisson, Z.; Hermans, S.M.; Buckley, H.L.; Case, B.S.; Taylor, M.; Curran-Cournane, F.; Lear, G. Land use modification causes slow, but predictable, change in soil microbial community composition and functional potential. Environ. Microbiome 2023, 18, 30. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, C.; Zuo, S.; Ji, Y.; Liu, W.; Huang, D. Heavy grazing reduces soil bacterial diversity by increasing soil pH in a semi-arid steppe. PeerJ 2024, 12, e17031. [Google Scholar] [CrossRef]
- Ghosh, T.; Das, B.; Roy, D.; Chakraborty, D.; Sethi, D. Evaluation of infiltration models based on simple multicriteria decision making across various soil types and land uses in India. Environ. Earth Sci. 2024, 83, 567. [Google Scholar] [CrossRef]
- Vishwakarma, D.K.; Yadav, D.; Kumar, R.; Kumar, R.; Bhat, S.A.; Mirzania, E.; Kuriqi, A. Assessing the performance of various infiltration models to improve water management practices. Paddy Water Environ. 2025, 23, 77–93. [Google Scholar] [CrossRef]
- Alaoui, A.; Caduff, U.; Gerke, H.H.; Weingartner, R. Preferential Flow Effects on Infiltration and Runoff in Grassland and Forest Soils. Vadose Zone J. 2011, 10, 367–377. [Google Scholar] [CrossRef]
- Corradini, C.; Flammini, A.; Morbidelli, R.; Govindaraju, R.S. A conceptual model for infiltration in two-layered soils with a more permeable upper layer: From local to field scale. J. Hydrol. 2011, 410, 62–72. [Google Scholar] [CrossRef]
- Orfánus, T.; Zvala, A.; Čierniková, M.; Stojkovová, D.; Nagy, V.; Dlapa, P. Peculiarities of Infiltration Measurements in Water-Repellent Forest Soil. Forests 2021, 12, 472. [Google Scholar] [CrossRef]
- Al-Janabi, A.M.S.; Halim Ghazali, A.; Yusuf, B. Modified models for better prediction of infiltration rates in trapezoidal permeable stormwater channels. Hydrol. Sci. J. 2019, 64, 1918–1931. [Google Scholar] [CrossRef]
- Alves, M.A.B.; Borella, D.R.; Paulista, R.S.D.; Almeida, F.T.D.; Souza, A.P.D.; Carvalho, D.F.D. Water Infiltration in Different Soil Covers and Management in the Cerrado–Amazon Ecotone, Brazil. Soil Syst. 2024, 8, 31. [Google Scholar] [CrossRef]
- Mirbabaei, S.M.; Shahrestani, M.S.; Zolfaghari, A.; Abkenar, K.T. Relationship between soil water repellency and some of soil properties in northern Iran. CATENA 2013, 108, 26–34. [Google Scholar] [CrossRef]
- Kořenková, L.; Urík, M. Basic soil properties as a factor controlling the occurrence and intensity of water repellency in rankers of the White Carpathians. Folia For. Pol. 2015, 57, 129–137. [Google Scholar] [CrossRef][Green Version]
- Roper, M.M.; Davies, S.L.; Blackwell, P.S.; Hall, D.J.M.; Bakker, D.M.; Jongepier, R.; Ward, P.R. Management options for water-repellent soils in Australian dryland agriculture. Soil Res. 2015, 53, 786. [Google Scholar] [CrossRef]
- Flores-Mangual, M.L.; Lowery, B.; Bockheim, J.G.; Pagliari, P.H.; Scharenbroch, B. Hydrophobicity of Sparta Sand under Different Vegetation Types in the Lower Wisconsin River Valley. Soil Sci. Soc. Am. J. 2013, 77, 1506–1516. [Google Scholar] [CrossRef]
- Fonseca, F.; Castro, M.; Alves, L.; Castro, J.; De Figueiredo, T. Impacts of Extensive Sheep Grazing on Soil Physical and Chemical Quality in Open Mountain Forests, NE Portugal. Span. J. Soil Sci. 2023, 13, 11632. [Google Scholar] [CrossRef]
- González-Sosa, M.; González-Barrios, P.; Bentancur, O.J.; Pérez-Bidegain, M. Differential effects on soil water repellency of Eucalyptus and Pinus plantations replacing natural pastures. Rev. Bras. Ciência Solo 2024, 48, e0230070. [Google Scholar] [CrossRef]
- Li, S.; Liu, X.; Wu, X.; Lu, J.; Abdelrhman, A.A.; Liang, G. Factors governing soil hydrological function under long-term tillage practices: Insight into soil water repellency. Soil Sci. Soc. Amer J. 2023, 87, 781–796. [Google Scholar] [CrossRef]
- Danielsen, A.S.; Hermansen, C.; Weber, P.L.; Mikstas, D.; Pesch, C.; De Carvalho Gomes, L.; Gutierrez, S.; Nielsen, P.H.; Greve, M.H.; Møldrup, P.; et al. Soil Water Repellency in Natural and Semi-Natural Habitats: A Nexus Between Abiotic Factors and Prokaryotic Communities. Eur. J. Soil Sci. 2025, 76, e70063. [Google Scholar] [CrossRef]
- Razipoor, E.; Mukherjee, S.; Schütt, B. Spatiotemporal Variability of Soil Water Repellency in Urban Parks of Berlin. Soil Syst. 2025, 9, 31. [Google Scholar] [CrossRef]
- Sone, J.S.; Sanches de Oliveira, P.T.; Pereira Zamboni, P.A.; Motta Vieira, N.O.; Altrão Carvalho, G.; Motta Macedo, M.C.; Romeiro de Araujo, A.; Baptaglin Montagner, D.; Alves Sobrinho, T. Effects of Long-Term Crop-Livestock-Forestry Systems on Soil Erosion and Water Infiltration in a Brazilian Cerrado Site. Sustainability 2019, 11, 5339. [Google Scholar] [CrossRef]
- Sánchez-García, C.; Doerr, S.H.; Urbanek, E. The effect of water repellency on the short-term release of CO2 upon soil wetting. Geoderma 2020, 375, 114481. [Google Scholar] [CrossRef]
- MapBiomas Peru Project Collection 3.0. Available online: https://peru.mapbiomas.org (accessed on 18 July 2025).
- Tovar, C.; Seijmonsbergen, A.C.; Duivenvoorden, J.F. Monitoring land use and land cover change in mountain regions: An example in the Jalca grasslands of the Peruvian Andes. Landsc. Urban Plan. 2013, 112, 40–49. [Google Scholar] [CrossRef]
- Arizapana-Almonacid, M.A.; Pariona-Antonio, V.H.; Castañeda-Tinco, I.; Ascención Mendoza, J.C.; Gutiérrez Gómez, E.; Ramoni-Perazzi, P. Land cover changes and comparison of current landscape metrics in a region of the Central Andes affected by population migration. Ann. GIS 2024, 30, 105–120. [Google Scholar] [CrossRef]
- SENAMHI. Modulo para la Estimación de Curvas de Intensidad-Duración-Frecuencia (IDF). Available online: https://idesep.senamhi.gob.pe/dhi-idf/ (accessed on 21 July 2025).
- Dionizio, E.A.; Costa, M.H. Influence of Land Use and Land Cover on Hydraulic and Physical Soil Properties at the Cerrado Agricultural Frontier. Agriculture 2019, 9, 24. [Google Scholar] [CrossRef]
- SENAMHI Datos Hidrometeorológicos a Nivel Nacional. Available online: https://www.senamhi.gob.pe/?p=estaciones (accessed on 16 May 2025).
- Registry-Migration.Gbif.Org GBIF Secretariat. GBIF Backbone Taxonomy. 2023. Available online: https://doi.org/10.15468/39OMEI (accessed on 21 July 2025). [CrossRef]
- Herrera, C.; Morocho, V.; Vidari, G.; Bicchi, C.; Gilardoni, G. Phytochemical Investigation of Male and Female Hedyosmum Scabrum (Ruiz & Pav.) Solms Leaves from Ecuador. Chem. Biodivers. 2018, 15, e1700423. [Google Scholar] [CrossRef] [PubMed]
- Pinto, E.; Pérez, Á.J.; Ulloa Ulloa, C.; Cuesta, F. Árboles Representativos de los Bosques Montanos del Noroccidente de Pichincha-Ecuador; CONDESAN: Quito, Ecuador, 2018. [Google Scholar]
- Universidad del Azuay Herbario Azuay. Available online: https://herbario.uazuay.edu.ec/muestras/244-dicotiledonae-rubiaceae-palicourea-amethystina-ruiz-pav-dc (accessed on 21 July 2025).
- Aguirre Mendoza, Z.; Quirola Kirby, G.; Armando Jaramillo, N.; Ventimilla Ramos, D.; Aguirre Mendoza, Z.; Quirola Kirby, G.; Armando Jaramillo, N.; Ventimilla Ramos, D. Regeneración Natural de Especies Forestales En Un Bosque Andino En El Sur de Ecuador. Arnaldoa 2024, 31, 105–119. [Google Scholar] [CrossRef]
- IUCN. IUCN Red List of Threatened Species: Hedyosmum Scabrum. Available online: https://www.iucnredlist.org/species/193975218/194006227 (accessed on 21 July 2025).
- León, B. Lauraceae endémicas del Perú. Rev Peru Biol 2006, 13, 380s–388s. [Google Scholar] [CrossRef]
- MINAM. Guía de Evaluacion Del Estado de Los Ecosistemas de Bosques Relictos: Altoandino, Mesoandino, Montano de Vertiente Occidental; MINAM: Lima, Peru, 2022. [Google Scholar]
- Ruiz, J. Guía Para La Siembra y Aprovechamiento de Pastos Cultivados y Mejorados En Zonas Altoandinas; Dirección de Investigación en Ecosistemas de Montaña–Instituto Nacional de Investigación en Glaciares y Ecosistemas de Montaña (DIEM-INAIGEM): Huaraz, Peru, 2022. [Google Scholar]
- Undersander, D.; Cosgrove, D.; Cullen, E.; Grau, C.; Rice, M.E.; Renz, M.; Sheaffer, C.; Shewmaker, G.; Sulc, M. Alfalfa Management Guide; American Society of Agronomy, Inc., Crop Science Society of America, Inc., Soil Science Society of America, Inc.: Madison, WI, USA, 2011; ISBN 978-0-89118-179-8. [Google Scholar]
- Wedderburn, M.; Crush, J.; Pengelly, W.; Walcroft, J. Root Growth Patterns of Perennial Ryegrasses under Well-Watered and Drought Conditions. N. Z. J. Agric. Res. 2010, 53, 377–388. [Google Scholar] [CrossRef]
- Weaver, J.E. Root Development of Field Crops, 1st ed.; McGraw Hill: New York, NY, USA, 1926. [Google Scholar]
- Blanco-Canqui, H.; Wilke, K.; Holman, J.; Creech, C.F.; Obour, A.K.; Anderson, L. Grazing Cover Crops: How Does It Influence Soils and Crops? Agron. J. 2023, 115, 2801–2828. [Google Scholar] [CrossRef]
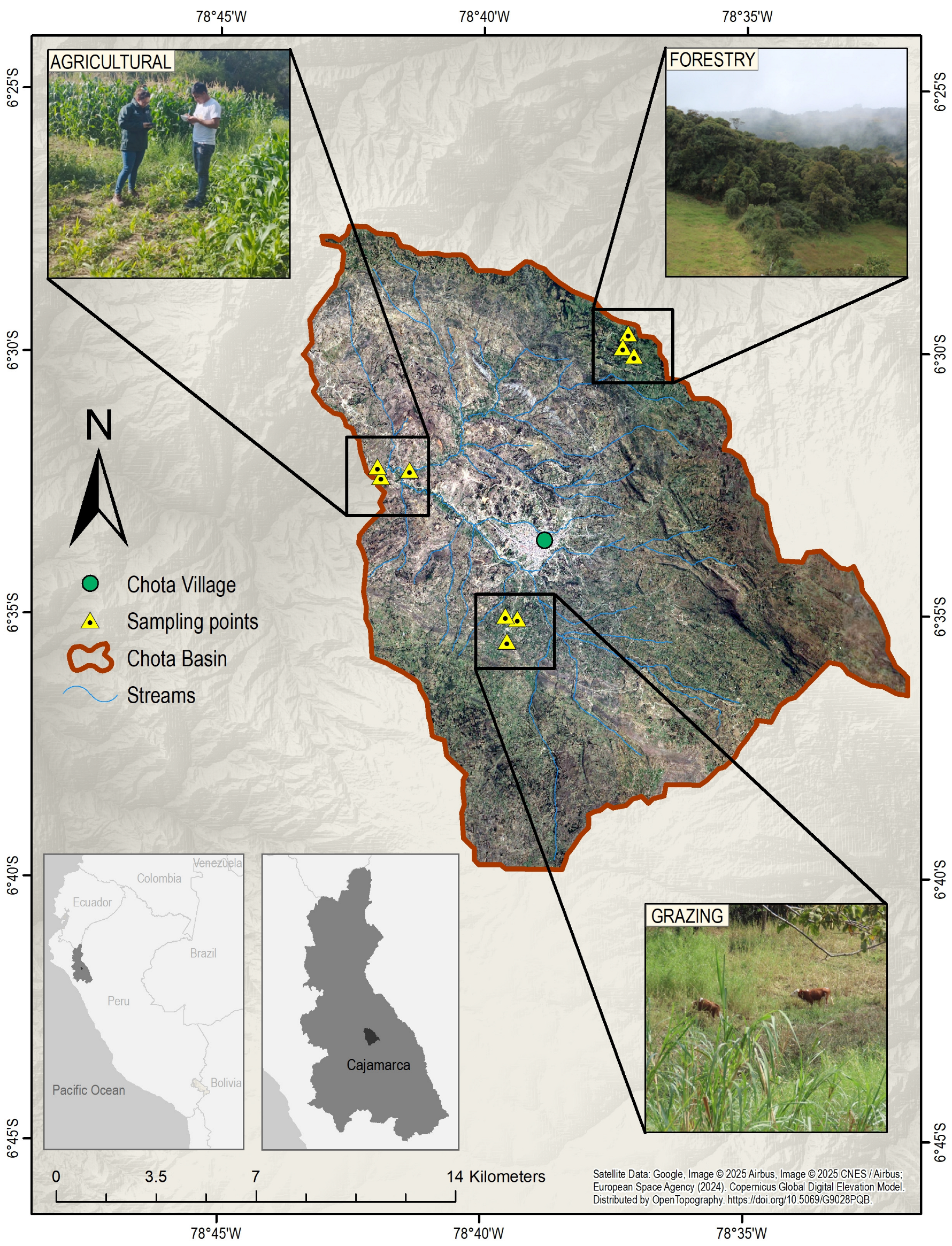




| Land Cover | Symbol | Length | Latitude | Altitude (masl) | Predominant Species |
|---|---|---|---|---|---|
| Agricultural | A | 6°29′37.91″ | 78°37′14.89″ | 3010 | Area dominated by the cultivation of potatoes (Solanum tuberosum), ollucos (Ullucus tuberosus), corn (Zea mays) and vegetables [31,32]. |
| 6°29′54.01″ | 78°37′20.90″ | 2980 | |||
| 6°30′3.62″ | 78°37′8.10″ | 3985 | |||
| Forestry | F | 6°32′15.35″ | 78°41′23.57″ | 2246 | Area dominated by endemic species (Hedyosmum scabrum, Palicourea amethystina, Weinmannia elliptica, Lauraceae, Myrtaceae, and Melastomataceae, Brachyotum coronatum, Cyathea caracasana, Axinaea nitida, and Ocotea jumbillensis) [33,34]. |
| 6°32′22.72″ | 78°41′55.81″ | 2240 | |||
| 6°32′11.35″ | 78°42′0.19″ | 2245 | |||
| Grazing | G | 6°35′4.34″ | 78°39′19.56″ | 2380 | Area dominated by established crops of alfalfa, ryegrass, and clover [31,32]. |
| 6°35′1.15″ | 78°39′33.47″ | 2395 | |||
| 6°35′29.97″ | 78°39′31.41″ | 2390 |
| Land Cover | Sand % | Clay % | Silt % | SOM % | TC g·kg−1 | pH | EC mS·m−1 | Ca2+ cmol(+) kg−1 | Mg2+ cmol(+) kg−1 | Na+ cmol(+) kg−1 | K+ cmol(+) kg−1 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Agricultural | 39.2 ± 8.4 ab | 34.7 ± 7.1 b | 26 ± 2.1 | 5.03 ± 2.86 b | 35.7 ± 17.4 c | 6.4 ± 0.6 b | 10.7 ± 9 | 13.9 ± 5.98 b | 0.87 ± 0.43 | 0.09 ± 0.05 | 0.43 ± 0.23 b |
| Forestry | 47.2 ± 6.3 a | 31.4 ± 4.8 b | 21.4 ± 2.3 | 17.96 ± 6.50 a | 131.7 ± 21.9 a | 7.4 ± 0.2 a | 32.5 ± 2.7 | 56.34 ± 12.12 a | 3.11 ± 1.46 | 0.06 ± 0.01 | 2.62 ± 0.32 a |
| Grazing | 28.5 ± 4.6 b | 46.8 ± 2.3 a | 24.6 ± 6.1 | 10.81 ± 0.79 ab | 71.9 ± 2.8 b | 7.6 ± 0.5 a | 25.4 ± 11.5 | 48.22 ± 10.33 a | 2.14 ± 0.33 | 0.21 ± 0.12 | 2.05 ± 0.69 a |
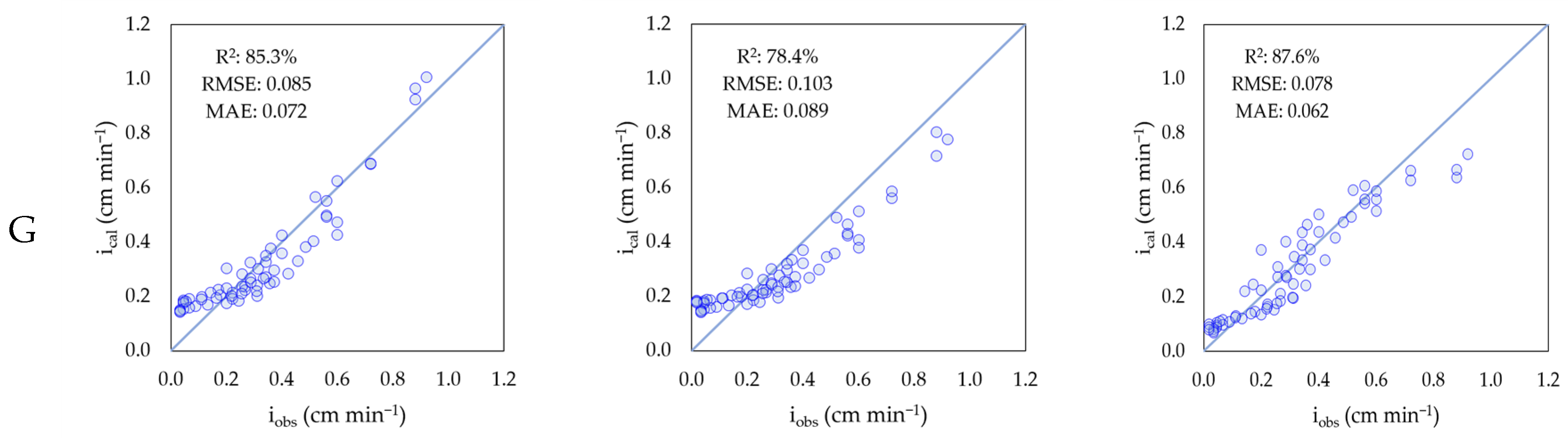
| Model | Parameter | Agricultural | Forestry | Grazing |
|---|---|---|---|---|
| Modified Kostiakov | k | 1.639 ± 0.117 | 2.93 ± 0.418 | 1.558 ± 0.076 |
| a | −0.568 ± 0.062 | −0.791 ± 0.129 | −0.559 ± 0.052 | |
| fc | 0.051 ± 0.016 | 0.096 ± 0.064 | 0.032 ± 0.013 | |
| Philip | S | 2.231 ± 0.266 | 2.813 ± 0.095 | 2.288 ± 0.09 |
| A | 0.08 ± 0.014 | 0.038 ± 0.025 | 0.042 ± 0.018 | |
| Horton | f0 | 0.736 ± 0.053 | 0.932 ± 0.037 | 0.73 ± 0.054 |
| fc | 0.051 ± 0.016 | 0.059 ± 0.064 | 0.032 ± 0.013 | |
| k | 0.028 ± 0.003 | 0.039 ± 0.008 | 0.032 ± 0.007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chávez-Collantes, A.; Vásquez Lozano, D.J.; Velarde-Apaza, L.D.; Cuevas, J.-P.; Solórzano, R.; Flores-Marquez, R. Influence of Vegetation Cover and Soil Properties on Water Infiltration: A Study in High-Andean Ecosystems of Peru. Water 2025, 17, 2280. https://doi.org/10.3390/w17152280
Chávez-Collantes A, Vásquez Lozano DJ, Velarde-Apaza LD, Cuevas J-P, Solórzano R, Flores-Marquez R. Influence of Vegetation Cover and Soil Properties on Water Infiltration: A Study in High-Andean Ecosystems of Peru. Water. 2025; 17(15):2280. https://doi.org/10.3390/w17152280
Chicago/Turabian StyleChávez-Collantes, Azucena, Danny Jarlis Vásquez Lozano, Leslie Diana Velarde-Apaza, Juan-Pablo Cuevas, Richard Solórzano, and Ricardo Flores-Marquez. 2025. "Influence of Vegetation Cover and Soil Properties on Water Infiltration: A Study in High-Andean Ecosystems of Peru" Water 17, no. 15: 2280. https://doi.org/10.3390/w17152280
APA StyleChávez-Collantes, A., Vásquez Lozano, D. J., Velarde-Apaza, L. D., Cuevas, J.-P., Solórzano, R., & Flores-Marquez, R. (2025). Influence of Vegetation Cover and Soil Properties on Water Infiltration: A Study in High-Andean Ecosystems of Peru. Water, 17(15), 2280. https://doi.org/10.3390/w17152280







