Abstract
Volcanic rock is a natural mineral material which has garnered interest for its potential application in water treatment due to its unique physicochemical properties. In this study, we prepared a polysilicate aluminum chloride (PSAC) coagulant using volcanic rock which exhibited good coagulation–flocculation performance. Further investigation into the influence of synthetic parameters, such as calcination temperature, reaction time, and alkali types, on the structure and performance of a volcanic rock-derived coagulant was conducted. Techniques including Scanning Electron Microscopy, Energy-Dispersive Spectroscopy, Fourier-Transform Infrared Spectroscopy, and X-Ray Diffraction were utilized to characterize it. Also, a ferron-complexation timed spectrophotometric method was used to study the distribution of aluminum species in the coagulant. Results indicated that the volcanic rock that was treated with acidic and alkaline solutions had the potential to form PSAC with Al-OH, Al-O-Si, Fe-OH, and Fe-O-Si bonds, which influenced the coagulation–flocculation efficiency. An acid leaching temperature of 90 °C, 8 mL of 2 mol/L NaOH, a reaction time of 0.5 h, and a reaction temperature of 60 °C were conducive to the preparation. A higher temperature could result in a higher proportion of Alb species, and, at 100 °C, the Ala, Alc, and Alb were 29%, 24%, and 47%, respectively, achieving a residual turbidity lower than 1 NTU at an appropriate dosage, as well as a reduction of over 0.1 to 0.018 in the level of UV254. The findings of this study provide a feasible method to prepare a flocculant using volcanic rock. Further application is expected to yield good results in wastewater/water treatment.
1. Introduction
As the essence of life, water underpins the sustenance of human life and fosters urban development [1]. As environmental consciousness escalates globally, the deterioration of water resources has prompted governments to enact stringent regulations and policies. These regulations and policies are aimed at mitigating water pollution and enhancing water treatment. Contemporary water treatment methods cover three main areas: physical, chemical, and biological processes [2,3]. Physical treatments involve separating contaminants from water using techniques like filtration, membrane separation, and activated carbon adsorption [4,5]. Chemical methods utilize chemical principles to eliminate pollutants, such as flocculation and ion exchange [2,6,7]. Biological approaches, on the other hand, leverage microorganisms to decompose organic matter [8]. Examples include biofilm reactors and biological contact oxidation systems [9]. Among these methods, flocculation is notable for its simplicity and effectiveness [10,11,12]. Nevertheless, the plethora of flocculants available on the market exhibits varying degrees of performance [13]. As water pollution intensifies, the efficacy of many flocculants no longer meets the stringent treatment standards required. Consequently, there is an imperative to develop a novel coagulant that surpasses the limitations of its traditional counterparts. This will help cater to the expanding scale of water treatment efforts and ensure the attainment of superior water quality standards.
Polysilicate is an outdated flocculant, and its development has significantly lagged behind that of conventional aluminum- and iron-based flocculants. One of the main reasons for this is that polysilicate flocculants undergo further polymerization during storage, resulting in inactivation [14]. Due to their poor stability, the application of polysilicate flocculants in the field of water treatment is limited [15]. Experimental research has revealed that introducing metal cations into polysilicate flocculants can greatly reduce their tendency to polymerize and become inactive [16]. Polysilicate-based flocculants are formed by polymerizing metal salt solutions with polysilicate solutions [17,18]. Different metal salts incorporated into polysilicate can yield flocculants with varying water treatment effects [19,20]. The unique network structure and high molecular weight of polysilicate [21], when combined with metal salt ions, endow the final product with similar structural advantages and effectively overcome the drawback of polysilicate’s easy inactivation, thereby exhibiting excellent performance in the water treatment process. Further discussion on the advantages of polymeric silicate coagulants has been presented in our previously published results, including a comparison with aluminum- or iron-based coagulants in terms of performance and cost [22].
Volcanic rock, a widely distributed mineral resource in mines, is rich in key elements such as silicon, aluminum, and oxygen [23]. Its high content of beneficial components indicates significant application potential. Using volcanic rock as a water treatment reagent not only has a solid scientific basis but also embodies the concept of environmental protection through resource recycling. Volcanic rock contains a substantial amount of silicon. The potential of employing physicochemical methods to extract silicon from volcanic rock and combining it with aluminum and iron to form more efficient coagulants is significant. Importantly, silicate-based synthetic coagulants have faced challenges with destabilization [15], which could be overcome because the diverse silicon species in rocks are more likely to interact with other metals compared to those from pure chemicals. Precisely controlling the extraction process would facilitate achieving this goal, and a good water purification performance could be attained. However, for a long time, the preparation of coagulants has been closely tied to the strict control of preparation conditions. For example, the influence of reaction temperature, reaction time, and alkaline conditions on coagulation is not well understood. While various preparation conditions have been established for many minerals, it is more challenging to control the new silicon interactions when using a new mineral. It is for this reason that we propose this study, with the hope of developing a new coagulant for water treatment.
Iron and aluminum flocculants are the most widely applied category of flocculants on the current market, and carrying out improvement work on them in terms of performance enhancement or cost reduction holds significant positive implications for advancing water treatment technology. However, through market research, it has been found that there is a severe shortage of truly effective modification measures specifically targeting iron and aluminum flocculants. This is precisely the purpose of this study: to explore and implement improvement measure schemes.
In this study, we prepared a polysilicate aluminum chloride (PSAC) flocculant using a hydrochloric acid and alkaline solution. We investigated the physicochemical properties of various types of PSAC which was prepared under different conditions using volcanic rock material. We utilized characterization technologies, including Scanning Electron Microscopy (SEM), Energy-Dispersive Spectroscopy (EDS), Fourier-Transform Infrared Spectroscopy (FT-IR), and X-Ray Diffraction (XRD). We evaluated the flocculation performance and obtained the optimal preparation conditions.
2. Materials and Methods
2.1. Materials
The main reagents used in this study are hydrochloric acid (Tianjin Hengxing Chemical Reagent Manufacturing Co., Ltd., Tianjin, China), sodium hydroxide (Xilong Scientific Co., Ltd., Shantou, China), o-phenanthroline (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China), hydroxylamine hydrochloride (Guangdong Chemical Reagent Engineering Technology Research and Development Center, Shantou, China), 8-hydroxy-7-iodo-5-quinoline sulfonic acid (Sinopharm Chemical Reagent Co., Ltd.), anhydrous sodium acetate (Sinopharm Chemical Reagent Co., Ltd.), hydrochloric acid (Shanghai Huanfa Chemical Industry Co., Ltd., Shanghai, China), and potassium bromide (Tianjin Kemio Chemical Reagent Co., Ltd., Tianjin, China). The commercial product, volcanic rock, was purchased from TianchiYuanyi Co., Ltd., Urumqi, China.
The main instruments used are as follows: a pH meter (ST2100, OHAUS International Trade Co., Ltd., Shanghai, China), a dual-beam UV spectrophotometer (TU-1901, Puxi General Instrument Co., Ltd., Jinan, China), a six-port coagulation experiment stirrer (MY3000-6, Wuhan Meiyu Instrument Co., Ltd., Wuhan, China), a turbidity meter (HACH2100Q, Hach Company, Loveland, CO, USA), scanning electron microscopes (SEM, S-4800, Hitachi, Japan and EM-30Plus, Korea Limited company COXEM Co., Ltd., Daejeon, Republic of Korea), a Fourier-transform infrared spectrometer (FTIR, TENSOR II, Bruker Optics, Ettlingen, Germany), an X-ray diffractometer (XRD, D8 advance, Bruker Optics, Ettlingen, Germany), and a freeze dryer (FD, LGJ-18A-50, Shanghai Jipu Electronic Technology Co., Ltd., Shanghai, China).
2.2. Preparation of Coagulant
The preparation method for the PSAC flocculant involves several steps. First, 20 g of volcanic rock is placed in a porcelain crucible and calcined in a muffle furnace. Once the calcination process is complete, the crucible is removed and allowed to cool to room temperature. The calcined volcanic rock is then ground into a fine powder for further use. Next, in a beaker, 50 milliliters of hydrochloric acid is mixed with 150 milliliters of pure water to dilute the acid. This mixture is heated to 50 °C with the help of an electromagnetic heating stirrer. The diluted hydrochloric acid mixture is then poured over the volcanic rock powder, and stirring is initiated while the mixture is heated to 90 °C. The temperature is maintained at 90 °C with continuous stirring for 2 h. After stirring, the beaker is removed and allowed to stand overnight. After filtration, the yellow liquid obtained is the required volcanic rock acid extract. To proceed, 10 milliliters of the acid extract is taken and placed in a separate beaker on an electromagnetic heating stirrer. To this, 12 milliliters of a 2 mol/L sodium hydroxide solution is slowly added. The polymerization temperature is controlled at 60 °C, and stirring is continued for 10 min. Following stirring, the beaker is removed and allowed to stand at room temperature for 24 h to mature. An oily yellow liquid will be observed, indicating the formation of the PSAC flocculant.
To understand the structural characteristics of our product, we used SEM, FTIR, and XRD to investigate the surface, chemical groups, and crystalline structure of the coagulant, respectively. Furthermore, Al species distribution in the polyaluminum silicate chloride was measured using a ferron-complexation timed spectrophotometric method, following a detailed procedure performed by Zhou et al. [24] using a Ferron method. Al species were divided into three categories: Ala, Alb, and Alc. Ala represents the monomeric hydroxy complex of aluminum in the form of free ions. Alb denotes the polynuclear hydroxy complex of aluminum with a low polymerization degree. Alc signifies the aluminum polymer with a high polymerization degree.
2.3. Coagulation Test
The water sample was collected from Yuehu Lake at Hunan University of Science and Technology, located in Xiangtan City, Hunan Province, China. The water was clear and odorless, indicating good initial water quality. The basic characteristics of the water quality include a pH value around 7, a turbidity value of around 10 NTU, total phosphate of around 0.5 mg/L, and an algal optical density of around 0.05 cm−1.
Coagulation operations were carried out utilizing a six-port stirrer, with the rapid-mixing stage set at 1 min at an agitation speed of 300 per minute (rpm), followed by a slow-mixing stage lasting 15 min at a speed of 70 rpm. Upon completion of the coagulation stirring, the mixture was allowed to stand for 30 min. Subsequently, a clarified water sample was taken approximately 3–4 cm below the surface of the supernatant to measure its residual turbidity. The supernatant was then filtered through a 0.45 µm syringe filter, and the UV254 absorbance was measured using a dual-beam UV spectrophotometer at a wavelength of 254 nm.
3. Results and Discussion
3.1. SEM Results
This study employed SEM to examine the morphological features of volcanic rock, silicon-containing PSAC, and silicon-free PSAC. The results are presented in Figure 1, Figure 2 and Figure 3. Figure 1a,b depict SEM micrographs of volcanic rock at magnifications of 500× and 1000×, respectively, revealing an uneven surface topography characterized by particles of varying sizes and surface impurities. This uneven structure enhances the contact area, facilitating complete acid soaking during the leaching process, which improves the efficiency of extracting valuable components from the volcanic rock and mitigates resource wastage due to incomplete leaching. Moreover, Figure 1c,d present the energy spectrum and elemental distribution maps of the volcanic rock, demonstrating the presence of carbon, oxygen, silicon, aluminum, and magnesium, with carbon, oxygen, and silicon being the most abundant. Notably, the aluminum content surpasses that of other metals, indicating the feasibility of utilizing volcanic rock for flocculant production.
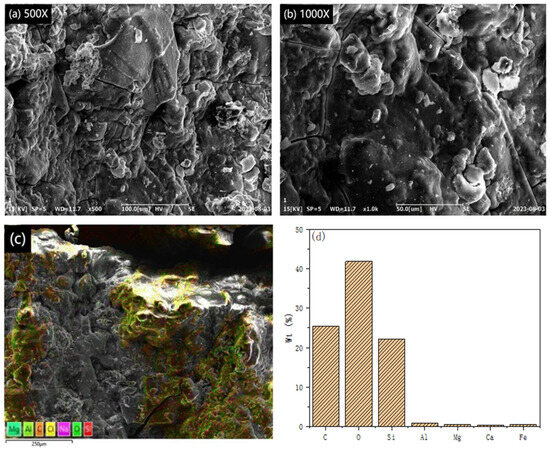
Figure 1.
SEM images, EDS energy spectrum, and elemental distribution of igneous rock at different magnifications: (a) 500×, (b) 1000×, (c) energy spectrum, and (d) proportion of elements.
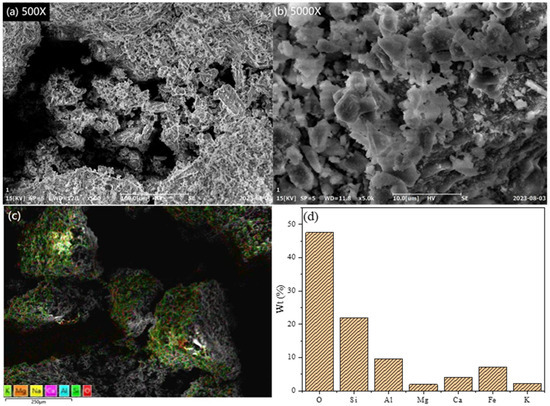
Figure 2.
SEM images, EDS energy spectrum, and elemental distribution of PSAC at different magnifications: (a) 500×, (b) 1000×, (c) energy spectrum, and (d) proportion of elements.
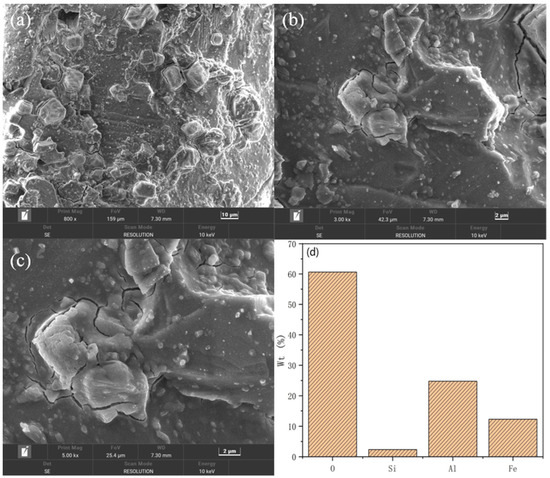
Figure 3.
SEM images, EDS energy spectrum, and elemental distribution of silicon-free PSAC at different magnifications: (a) 800×, (b) 1000×, (c) 5000×, and (d) proportion of elements.
Figure 2a,b show SEM micrographs of PSAC at 500× and 1000× magnifications, respectively. The images reveal a rough PSAC surface characterized by unevenly distributed granules and a complex morphology adorned with numerous small pores. This intricate surface structure arises from the presence of polysilicic acid, which imparts a high molecular weight and complexity to PSAC molecules. These features create abundant attachment sites for metal cations such as aluminum and iron, which contribute to the rough texture. Figure 2c,d present the EDS spectrum and partial elemental distribution of the PSAC flocculant, confirming the even distribution of oxygen, silicon, aluminum, and iron on its surface. This uniformity suggests that these elements are effectively aggregated through heating and stirring, forming the flocculant’s active components and enhancing its flocculation efficacy. Comparing Figure 1d and Figure 2d, we can see that a notable increase in aluminum and iron content in the PSAC indicates the successful extraction of effective components from volcanic rock via acid leaching and the subsequent PSAC flocculant preparation.
Figure 3 shows SEM images and elemental distribution graphs of desilicified PSAC. In contrast to the PSAC flocculant, Figure 3a,c show that desilicified PSAC has reduced surface roughness, with discernible cubic particles. These particles are likely remnants of aluminum silicate, which indicates incomplete silicon removal. As Figure 3d illustrates, desilication significantly reduces silicon content, with oxygen, aluminum, and iron becoming the primary constituents. This composition highlights the flocculant’s fundamental action mechanisms during flocculation processes.
3.2. FTIR Analysis
This study employed FTIR spectroscopy to investigate the phase structures of volcanic rock, silicon-containing PSAC, and silicon-free PSAC materials, as depicted in Figure 4. The FTIR spectrum of volcanic rock reveals characteristic peaks that provide insights into its chemical composition. The peak at 3610 cm−1 corresponds to the bending vibration of H-O-H [25], suggesting a lower concentration of crystalline and structural water. A prominent peak at 1029 cm−1 and another at 467 cm−1 further confirm the presence of Si-O-Al/Mg and Si-O plane vibrations [26,27,28]. In contrast, the FTIR spectra of the PSAC flocculant and silicon-free PSAC flocculant exhibit distinct features. The peaks at 3413 cm−1 and 3415 cm−1, respectively, correspond to the stretching vibration of -OH groups, indicating the formation of hydroxyl complexes during the hydrolysis of aluminum and iron ions in the flocculants [29]. The presence of a bending vibration peak at 1633 cm−1 for H-O-H [30] suggests the incorporation of water molecules as adsorbed water within the flocculants. This confirms the existence of hydroxyl complexes in PSAC, which contribute to its flocculation properties. Notably, the peak representing Si-O-Fe and Si-O-Al bonds shifts from 1029 cm−1 in volcanic rock to 1106 cm−1 in PSAC and 1103 cm−1 in silicon-free PSAC materials [31,32]. This shift indicates that the bonding between these elements undergoes fracture and recombination during the preparation process, leading to the formation of new tetrahedral structures. The structure included the Si in a high valence state, which has the possibility to enhance the flocculation by coupling Si, Al, and Fe. The emergence of this new peak validates the successful polymerization of the PSAC flocculant. Furthermore, absorption peaks at 604 cm−1 in the silicon-free PSAC materials suggest the superposition of Fe-O vibration peaks, Fe-OH characteristic absorption peaks, and Al-OH bending vibration peaks [33]. This indicates strong interactions between the hydroxyl complexes of aluminum and iron ions, which enhance the flocculation capacity of PSAC. Overall, the FTIR analysis provides valuable insights into the chemical transformations and structural changes that occur during the preparation of PSAC flocculants from volcanic rock.
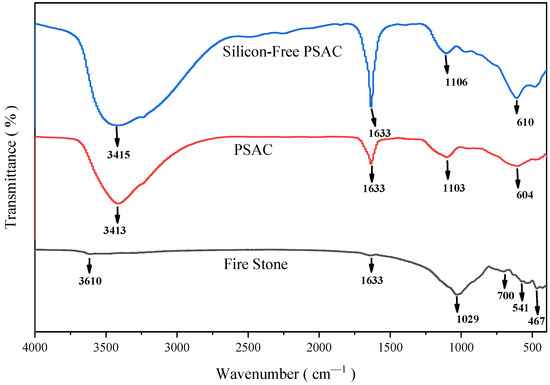
Figure 4.
FTIR spectra of volcanic rock, PSAC, and desilicified PSAC flocculant.
3.3. XRD Analysis
In this study, XRD was employed to characterize and analyze volcanic rock, silicon-containing PSAC, and silicon-free PSAC materials. The XRD patterns obtained were analyzed using Jade 6 software. By comparing the patterns with standard references, the overall chemical composition and molecular structure of the samples were determined, with the results shown in Figure 5, Figure 6 and Figure 7.
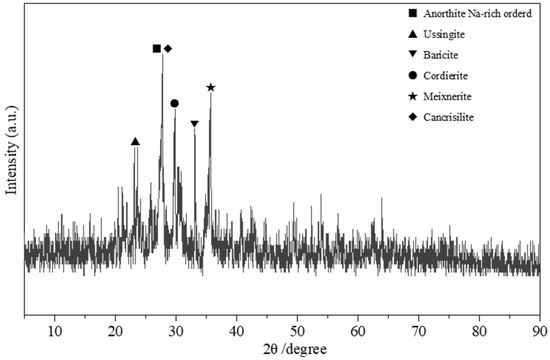
Figure 5.
XRD patterns of the volcanic rocks.
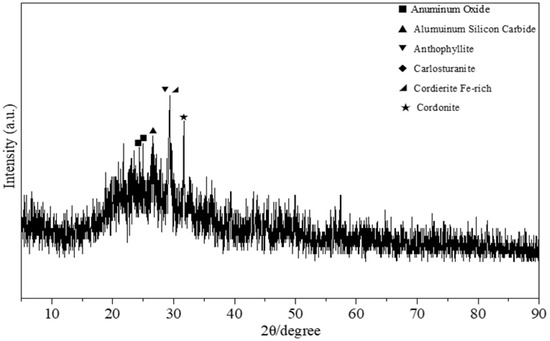
Figure 6.
XRD pattern of the PSAC.
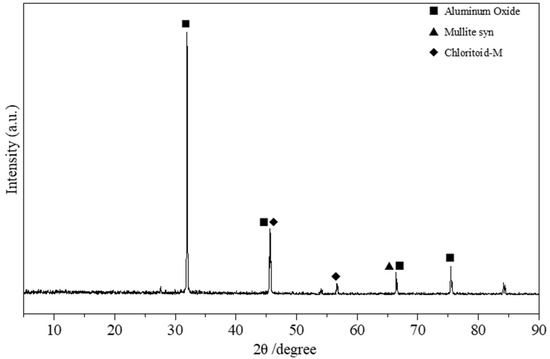
Figure 7.
XRD results of the silicon-free PSAC flocculant.
Figure 5 presents the XRD pattern of the volcanic rock mineral material. From the pattern, it can be seen that the characteristic diffraction peak at 2θ = 27.884° may represent sodium-rich analcime containing elements such as calcium, sodium, aluminum, and silicon (PDF 20-0528). The characteristic peak at a diffraction angle of 2θ = 27.681° may correspond to kanklitasite containing elements like aluminum and sodium (PDF 46-1381). The peak at 2θ = 33.165° may represent barite containing elements such as iron and phosphorus (PDF 29-0705). The characteristic diffraction peak at 2θ = 29.614° may belong to the mineral pyrope containing magnesium–aluminum silicate, with the chemical formula Mg2Al4Si5O18 (PDF 12-0303). The peak at 2θ = 35.611° may be attributed to mesolite containing elements such as silicon, aluminum, and oxygen (PDF 50-1684). The characteristic diffraction peak at 2θ = 22.922° may belong to ulexite containing elements like sodium, aluminum, and silicon (PDF 28-1037). The mineral analysis is largely consistent with the EDS elemental analysis of the volcanic rock.
Figure 6 shows the XRD pattern of the PSAC flocculant material. The various elements present in the volcanic rock are mixed into the PSAC material, existing in different polymeric states within the PSAC, leading to a somewhat cluttered XRD pattern. To provide a more comprehensive understanding, it is noted that, as shown in Figure 6 (assuming it is a relevant reference for comparison, if not, this part can be adjusted or removed based on the actual context), the PSAC material also contains a variety of substances. Starting with the analysis of the XRD pattern in Figure 6, the characteristic peaks at diffraction angles of 2θ = 24.921° and 2θ = 25.463° are similar to the diffraction peaks in the standard pattern for alumina (PDF 31-0026). The characteristic diffraction peak at 2θ = 26.570° may represent silicon aluminum carbide (PDF 35-1073), with the chemical formula Al4Si2C5. The peak at 2θ = 26.356° may correspond to compounds contained in kyanite (PDF 45-1343), which includes elements such as magnesium, iron, silicon, and oxygen. The characteristic diffraction peak at 2θ = 29.355° is similar to the diffraction peaks in the standard pattern for ferroan amphibole (PDF 09-0472), a mineral rich in iron, aluminum, silicon, and magnesium. The peak at 2θ = 39.491° is similar to the diffraction peaks of Carlosturanite (PDF 38-0404), a mineral rich in magnesium, iron, and silicon. Regarding the characteristic diffraction peak at 2θ = 31.589°, it is similar to the diffraction peaks in the standard pattern for MgAl2(PO4)2(OH)2 (PDF 14-0313). The elements mentioned in these results are largely consistent with the findings of the EDS elemental analysis, indicating that the PSAC flocculant is primarily a composite product synthesized from elements such as aluminum, iron, magnesium, silicon, and oxygen. In addition, in comparison with the XRD pattern of the silicon-free PSAC flocculant in terms of peak intensity and sharpness (see Figure 7), the XRD pattern of PSAC displayed a fiber-bundle-like shape with broad peaks. This shape was regarded as a notable indicator of the presence of a greater number of polymeric compounds in the coagulants [34]. Furthermore, in the subsequent test, the proportion of polymeric species is significantly higher in silicon-containing PSAC than in silicon-free PSAC, a finding that is supported by the XRD results.
Figure 7 shows the XRD pattern of the porous PSAC without silicon. Notably, the XRD pattern appears less complex compared to that of the PSAC material containing silicon. The diffraction peaks observed at 2θ angles of 31.819°, 45.618°, 66.493°, and 75.372° align well with the standard pattern for alumina, as indicated by Powder Diffraction File (PDF) card number 47–1292. Furthermore, characteristic diffraction peaks are observed at 2θ angles of 66.488° and 45.691°, which correspond to the diffraction peaks in the standard pattern for chlorite (PDF 14-0062). Chlorite is a mineral abundant in iron-rich silicate–aluminate salts, with the primary component having the chemical formula FeAl2SiO5(OH)2. Additionally, the diffraction peak at 2θ = 66.514° might be associated with the diffraction peak in the standard pattern for mullite (Al6Si2O13, PDF 15-0776). Although most of the silicon was removed, a small quantity of residual silicon is still present in the material, as indicated by the XRD and EDS results.
3.4. Species Analysis
3.4.1. The Influence of Reaction Temperature
The study employed the Al–Ferron method to investigate the impact of polymerization temperature on the distribution and transformation of aluminum species in the flocculant. As shown in Figure 8, it was found that PSAC contained a higher proportion of Ala. The change in polymerization temperature had a significant influence on the distribution of aluminum species in PSAC. At ambient temperature, the proportions of Alc and Ala were 21% and 52%, respectively. As the polymerization temperature increased, the proportion of Alc remained relatively stable, whereas the proportion of Ala decreased and the proportion of Alb increased. When the polymerization temperature reached 100 °C, the proportions of Ala and Alc were relatively low (29% and 24%, respectively), while the proportion of Alb was relatively high, reaching 47%. This suggests that temperature changes significantly affected the proportions of Ala and Alb in PSAC. At lower polymerization temperatures, the majority of aluminum species in PSAC existed in the form of low-polymerization-state aluminum. However, as the polymerization temperature increased to 60 °C or higher, the distribution of aluminum species in the PSAC flocculant underwent notable changes. The proportion of Alb increased, while the proportion of Ala decreased. This is because the rise in temperature facilitated the transformation of Ala to Alb to some extent, thereby enhancing the flocculating performance of the PSAC.
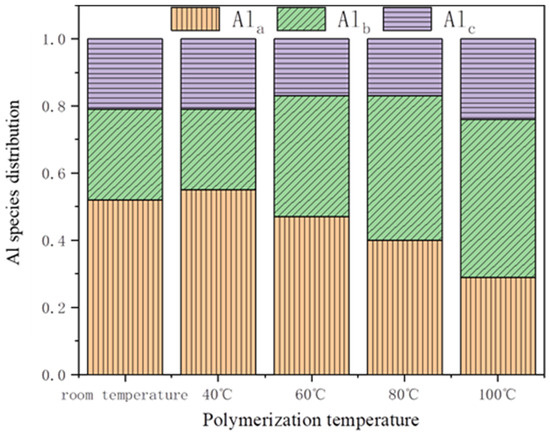
Figure 8.
Distributions of Al species in different types of flocculants that are prepared by varyingtemperature.
3.4.2. The Influence of Alkali Dosage and Silicon
The study employed the Al–Ferron method to investigate the effects of alkali dosage and the presence of silicon on the distribution and transformation of aluminum species within the flocculant. The results, presented in Figure 9, clearly demonstrate that the amount of alkali significantly influences the form of aluminum in the PSAC flocculant. In PSAC, the majority of aluminum exists in the form of Ala, with only a minor portion of Al3+ combining with OH− in water to form complexes during hydrolysis. Specifically, PSAC treated with 4 mL of NaOH exhibits the highest proportion of Ala, reaching 82%, whereas PSAC treated with 8 mL of NaOH shows a notable increase in Alb, reaching 36%, and a slight increase in Alc, reaching 17%. This phenomenon can be attributed to the gradual increase in OH− concentration in the solution as the alkali dosage increases, leading to the continuous polymerization of free Al3+ and Ala with OH− to form Alb and Alc.
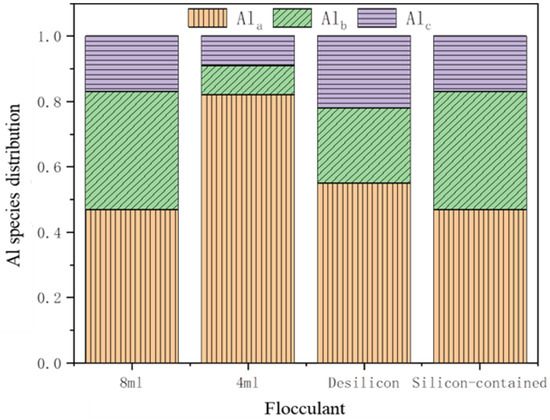
Figure 9.
Distributions of Al species in different types of flocculants that are prepared by varying alkali dosage.
Furthermore, Figure 9 reveals significant differences in the aluminum species between the two PSAC samples: the one containing silicon and the other without. Under the same alkali dosage, the PSAC with silicon has the highest proportion of Ala, at 47%, and the lowest proportion of Alc, at 17%. In contrast, the PSAC without silicon also has the highest proportion of Ala, at 55%, but a slightly higher proportion of Alc, at 22%. Notably, the proportion of Alb in the PSAC with silicon is significantly higher than that in the PSAC without silicon, with a difference of 13%. Based on these findings, it can be inferred that the PSAC with silicon contains more silicates, which increase the molecular weight of PSAC. During the preparation process, these silicates facilitate the transformation of Ala to Alb and hinder the transformation of Alb to Alc. Most aluminum-based flocculants are effective in removing turbidity, UV254, or dissolved organic carbon from water, and the Alb species, which is the medium-polymeric form, still plays a major role [34]. This study clearly demonstrated that silicon could increase the content of this form. This presents a new approach to enhancing the preparation and performance of aluminum-based flocculants. It can be seen that using volcanic rock to prepare aluminum-based flocculants is of great significance.
3.5. The Optimal Conditions for Synthesis of PSAC
To achieve a stable and high-performing composite silica–alumina–iron flocculant, a series of experimental studies was conducted to explore the intricate effects of various factors on its properties and coagulation performance. These factors included the calcination temperature of the rock, the temperature of acid leaching, and the type and dosage of alkali used, as well as the polymerization time and temperature. The coagulation performance of the flocculant was rigorously evaluated based on two key indicators: the efficiency of removing turbidity and the efficiency of removing UV254 from the treated water. These metrics provided a quantitative assessment of the flocculant’s ability to clarify and purify water. Through meticulous experimentation and analysis, the optimal preparation conditions for the PSAC flocculant were identified. These conditions, which resulted in the flocculant exhibiting the most favorable coagulation performance, were carefully determined to ensure the production of an effective and reliable flocculant.
From the perspective of the development of existing research [22], these preparation parameters play a crucial role in altering the structural distribution and charge properties of flocculants. Flocculation theories mainly encompass adsorption bridging, net-capture sweeping, and charge neutralization, and these pollutant-removal mechanisms are closely related to the structure of flocculants. Therefore, it is imperative to explore and optimize preparation conditions to achieve the optimal configuration of the structural and physicochemical properties of flocculants.
3.5.1. Effect of Calcination Temperature
Under experimental conditions where the acid leaching temperature and time were set at 90 °C and 2 h, respectively, and the polymerization time and temperature were set at 10 min and 60 °C, respectively, 12 mL of 2 mol/L sodium hydroxide solution was added as the alkali condition. The effects of calcination temperatures (uncalcined, 200 °C, 400 °C, and 600 °C) on the flocculant performance at a dosing range of 0.5 mL/L to 5 mL/L were studied. The results are shown in Figure 10.
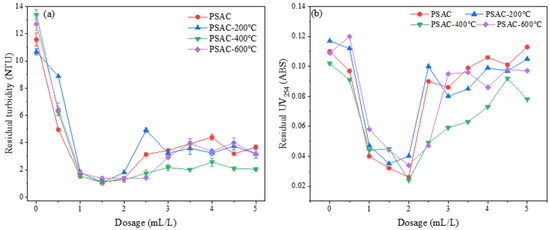
Figure 10.
Effect of calcination temperature on the removal of (a) turbidity and (b) UV254.
Figure 10a demonstrates the influence of varying calcination temperatures on turbidity removal from water. It was found that, under identical dosing conditions, changes in calcination temperature had minimal impact on the turbidity removal efficiency of the PSAC flocculant. Within a specific dosing range, the turbidity in the water decreased significantly as the flocculant dosage increased. The main reason for the decrease should be attributed to the over-dosing effect. Such a flocculation phenomenon was often observed in subsequent experiments. Optimal turbidity removal was achieved at a dosage of 1.5 mL/L, beyond which the residual turbidity in the water began to rise, indicating a pronounced instability phenomenon.
Figure 10b reveals the effect of different calcination temperatures on the removal of UV254 from water. The results show that the PSAC flocculants prepared at various calcination temperatures achieved the best UV254 removal at a dosage of 2 mL/L, maintaining the residual UV254 concentration in the water within the range of 0.02 to 0.04. Notably, the flocculants prepared without calcination and at 400 °C calcination exhibited the best UV254 removal performance, with no significant difference between them. When the flocculant dosage exceeded 2 mL/L, the efficiency of UV254 removal from the water began to decline, again demonstrating a clear instability.
Based on these findings, it can be inferred that calcining the volcanic rock did not effectively purify the material by removing impurities. Considering both the pollutant removal efficiency in water and the principles of energy conservation and environmental protection, it was decided not to use calcination of volcanic rock as a preparation condition for the PSAC flocculant in subsequent experiments.
3.5.2. Effect of Acid Leaching Temperature
Under the experimental conditions where the acid leaching time was 2 h, the polymerization time and temperature were 10 min and 60 °C, respectively, and 12 mL of 2 mol/L sodium hydroxide solution was added to provide the alkali condition, the impacts of acid leaching temperatures 50 °C, 70 °C, and 90 °C on the performance of the flocculant within a dosing range of 0.5 mL/L to 5 mL/L were investigated. The results are presented in Figure 11.
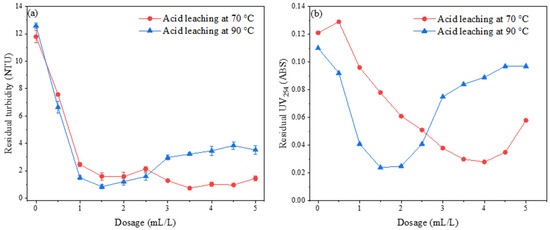
Figure 11.
Effect of acid leaching temperature on the removal of (a) turbidity and (b) UV254.
Figure 11a displays the influence of varying acid leaching temperatures on turbidity removal from water. The findings indicate that, within the dosing range of 0.5 to 2.5 mL/L, the PSAC flocculant prepared under the 90 °C acid leaching condition exhibited a slightly superior turbidity removal effect. However, as the dosing amount increased further, an evident instability phenomenon emerged. When the dosing amount surpassed 2.5 mL/L, the PSAC flocculant prepared under the 70 °C acid leaching condition began to outperform in terms of turbidity removal, demonstrating a stable flocculation effect with the residual turbidity in the water fluctuating within a certain range.
Figure 11b illustrates the influence of different acid leaching temperatures on the removal of UV254 from water. The results showed that the ultimate efficiency of the removal of UV254 by both flocculants was not significantly different. However, there was a notable variation in the optimal dosing amount required. The PSAC prepared under the 90 °C acid leaching condition achieved optimal UV254 removal at a dosing amount of 1.5 mL/L, beyond which instability was observed. In contrast, the optimal dosing amount for the PSAC prepared under the 70 °C acid leaching condition was 4 mL/L, and the efficiency of the removal of UV254 by these two PSACs varied significantly at different dosing amounts. Overall, the PSAC prepared under the 90 °C acid leaching condition demonstrated a superior overall performance.
From these observations, it can be inferred that varying acid leaching temperatures alter the concentration of effective components in the acid leaching solution, subsequently impacting the flocculation performance of the resulting PSAC flocculant. Considering both the dosage requirements and the removal of pollutants in water, the optimal acid leaching temperature of 90 °C is chosen as the preparation condition for subsequent experiments in the production of the PSAC flocculant.
3.5.3. Effect of Alkali Types
Under the experimental conditions with an acid leaching temperature and time set at 90 °C and 2 h, respectively, and a polymerization time and temperature set at 10 min and 60 °C, respectively, the effects of 2 mol/L sodium hydroxide solution and 2 mol/L calcium oxide suspension, with volumes of 8 mL and 12 mL, respectively, on the flocculation performance of the PSAC flocculant were studied. The dosing range was 0.5 mL/L to 5 mL/L. The results are shown in Figure 12.
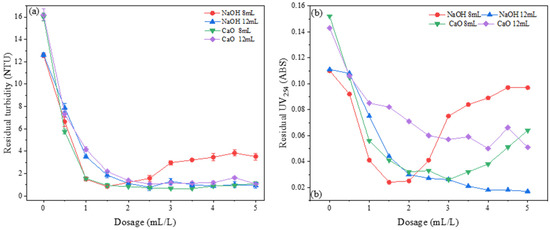
Figure 12.
Effect of alkaline type on removal of (a) turbidity and (b) UV254.
Figure 12a shows the influence of CaO- and NaOH-alkalized PSAC flocculants on the removal of turbidity from water. The results indicate that both 8 mL CaO-alkalized and 8 mL NaOH-alkalized PSAC flocculants achieved the best turbidity removal effect at a dosage of 1.5 mL/L. As the dosage increased, the turbidity removal effect of the 8 mL NaOH-alkalized PSAC flocculant initially increased and then decreased. The turbidity removal effects of the other three flocculants fluctuated within a certain range as the dosage increased. The residual turbidity remained within the range of 0.7–2 NTU.
Figure 12b shows the influence of CaO- and NaOH-alkalized PSAC flocculants on the removal of UV254 from water samples. The results indicated that there was a significant difference in the removal effects of the four PSAC flocculants for UV254 in water samples. The 8 mL NaOH-alkalized and 8 mL CaO-alkalized flocculants showed significant instability in their removal effect as the dosage increased. Within the dosage range of 0–2.2 mL, the NaOH-alkalized flocculant had a better removal effect for UV254 than the CaO-alkalized flocculant. The PSAC flocculants treated with 12 mL of NaOH and 12 mL of CaO showed a gradual stabilization in their UV254 removal effect as the dosage increased. Among them, the PSAC flocculant treated with 12 mL NaOH achieved the best removal effect for UV254 at a dosage of 5 mL. The residual UV254 concentration in the water fell below 0.02.
From these results, it can be concluded that the alkali agent had a significant impact on the removal of UV254 by the PSAC flocculant, while its effect on turbidity removal was relatively minor. Considering the overall removal of turbidity and UV254, as well as the dosage, NaOH was selected as the alkali agent for the acid leaching solution in subsequent experiments to prepare the optimal PSAC flocculant.
3.5.4. Effect of Alkali Dosage
In the experimental conditions where the acid leaching temperature and time were set at 90 °C and 2 h, respectively, and the polymerization time and temperature were set at 10 min and 60 °C, respectively, the effects of 2 mol/L sodium hydroxide solution at 4 mL, 8 mL, 12 mL, and 16 mL on the flocculation performance of the PSAC flocculant at a dosing range of 0.5 mL/L to 5 mL/L were studied. The results are shown in Figure 13.
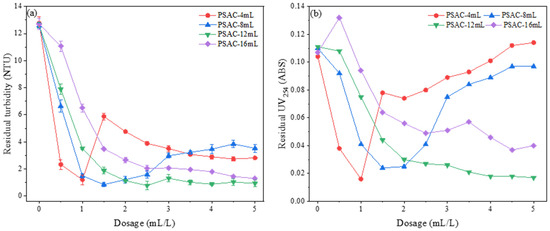
Figure 13.
Effect of the amount of alkali content on the removal of (a) turbidity and (b) UV254.
Figure 13a shows the influence of PSAC flocculants prepared with different alkali dosages on the removal of turbidity from water. The results indicate that PSAC-4 initially achieved the best turbidity removal effect as the dosage increased. However, it quickly exhibited an instability phenomenon, with the turbidity removal effect starting to decrease and gradually stabilizing. This may be attributed to the incomplete alkali treatment of PSAC-4. Similarly, the turbidity removal effect of PSAC-8 also showed an instability phenomenon with increasing dosage, but the overall turbidity removal effect was relatively stable. PSAC-8 achieved the best turbidity removal effect at a dosage of 1.5 mL/L, with the residual turbidity controlled below 1 NTU. The turbidity removal effects of PSAC-12 and PSAC-16 showed a trend of initially increasing and then stabilizing, with the best turbidity removal effect achieved at a dosage of 5 mL/L.
Figure 13b demonstrates the influence of PSAC flocculants with different alkali dosages on the removal of UV254 from water. The results indicate that PSAC-16 had the worst flocculation effect, while PSAC-4 and PSAC-8 flocculants showed good removal effects for UV254 in water at lower dosages, both controlling the UV254 content in water below 0.02. PSAC-12 also achieved a similar removal effect, but the required dosage reached 5 mL/L, which was much higher than the dosages required for PSAC-4 and PSAC-8. From these results, it can be concluded that the amount of alkali significantly impacts the flocculation performance of the PSAC flocculant. PSAC with incomplete alkali treatment exhibits unstable purification effects, with significant fluctuations in performance as the dosage increases. Within a certain range, the higher the degree of alkali treatment, the better the flocculation performance of PSAC, and the more effective it is at removing pollutants from water. Considering both the removal effect of pollutants in water and the dosage factor, the alkali condition with 8 mL of NaOH was selected as the optimal preparation condition for subsequent synthesis of PSAC.
3.5.5. Effect of Polymerization Time
The experimental conditions were set at an acid leaching temperature of 90 °C for a duration of 2 h. Additionally, 8 mL of 2 mol/L sodium hydroxide solution was added, and the polymerization temperature was kept at 60 °C. Under these conditions, the study investigated the impact of varying polymerization times (0 h, 10 min, 0.5 h, 1 h, 2 h, and 4 h) on the flocculation performance of the PSAC flocculant within a dosing range of 0.5 mL/L to 5 mL/L. The results of this investigation are presented in Figure 14.
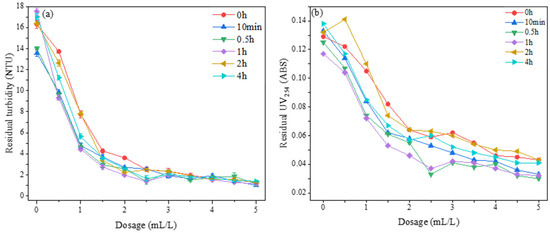
Figure 14.
Effect of polymerization time on the removal of (a) turbidity and (b) and UV254.
Figure 14a shows the effect of PSAC flocculants prepared with different polymerization times on the removal of turbidity from water. The results indicated that changes in polymerization time had little impact on the turbidity removal efficiency of the PSAC flocculant at a given dosage. As the dosage increased, the turbidity in the water decreased significantly. With the dosage over 3 mL/L, all PSAC flocculants prepared under the various polymerization times exhibited good turbidity removal effects, with the residual turbidity in the water primarily remaining within the range of 1–1.5 NTU.
Figure 14b illustrates the effect of PSAC flocculants with varying polymerization times on the removal of UV254 from water. The findings reveal that, at the same dosage, the removal of UV254 from water was notably better for flocculants with polymerization times of 1 h and 0.5 h compared to those with other polymerization times, although the difference was not substantial. When the dosage exceeded 2.5 mL/L, the flocculant with a polymerization time of 0.5 h demonstrated a slightly superior UV254 removal effect compared to that with a polymerization time of 1 h. Taking into account the pollutant removal efficiency and considerations related to energy conservation and environmental protection, a polymerization time of 0.5 h was chosen as the optimal preparation condition for subsequent experiments involving the preparation of the PSAC flocculant.
3.5.6. Effect of Polymerization Temperature
Under the experimental conditions where the acid leaching time and temperature were fixed at 2 h and 90 °C, respectively, 8 mL of a 2 mol/L sodium hydroxide solution was added, and the polymerization time was set to 0.5 h. The study investigated the impact of varying polymerization temperatures, specifically 20 °C, 40 °C, 60 °C, 80 °C, and 100 °C, on the flocculation performance of the PSAC flocculant within a dosing range of 0.5 mL/L to 5 mL/L. The results are shown in Figure 15.
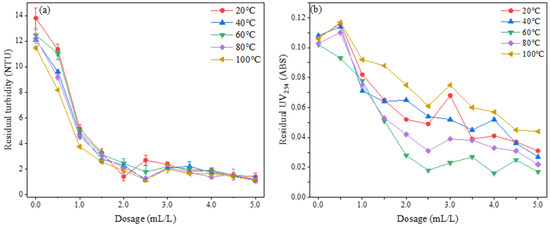
Figure 15.
Effect of polymerization temperature on the removal of (a) turbidity and (b) UV254.
Figure 15a illustrates the influence of the PSAC flocculant prepared at different polymerization temperatures on the removal of turbidity from water. The results demonstrated that the PSAC prepared at 20 °C achieved the optimal turbidity removal effect at a dosage of 2 mL/L, reducing the residual turbidity in water to below 1 NTU. However, at a dosage of 2.5 mL/L, the turbidity removal performance of PSAC prepared at 20 °C exhibited instability. In contrast, PSAC prepared at 40 °C, 60 °C, 80 °C, and 100 °C demonstrated satisfactory turbidity removal effects. Notably, upon reaching a dosage of 3 mL/L, a slight rebound in residual turbidity was observed, which subsequently stabilized as the dosage increased further.
Figure 1b illustrates the impact of PSAC flocculant prepared at varying polymerization temperatures on the removal of UV254 from water. The results indicated that, within the dosing range of 1 to 1.5 mL/L, there was no discernible difference in the UV254 removal efficiency among the flocculants. However, as the dosage surpassed this range, the PSAC flocculant prepared at 60 °C emerged as the most effective, achieving the optimal UV254 removal at a dosage of 2.5 mL/L. It is concluded that both excessively high and low polymerization temperatures can negatively affect the UV254 removal of the PSAC flocculant. Consequently, an appropriate increase in polymerization temperature enhances the flocculation performance of PSAC. Given the removal efficiency of UV254, a polymerization temperature of 60 °C is considered the optimal condition for preparing the PSAC flocculant in subsequent experimental efforts. An appropriate temperature strengthens the metallic species distribution and electrical properties of flocculants and improves their flocculation performance.
3.6. Others
At present, the coagulation technology faces enormous challenges when treating both slightly and severely polluted water (such as wastewater from industrial parks) in a cost—effective way. In this study, the prepared the volcanic rock-derived coagulant has potential for treatment of other pollutants, for example, the total phosphorus (TPs). The efficiency of the removal of TPs can reach over 97% for treatment of lightly or heavily polluted water. It shows the great potential in treating the key pollutants from industrial wastewater and domestic sewage.
4. Conclusions
This study has centered on the preparation of flocculants from volcanic rock, exploring how preparation parameters like acid leaching temperature, alkalizing agent, dosage, polymerization time, and temperature affect Polysilicate Aluminum Chloride (PSAC) flocculants’ performance. We found that volcanic rock without prior calcination works well; 90 °C acid leaching boosts effective component leaching and flocculation; 2 mol/L NaOH at 8 mL dosage enhances pollutant removal; 0.5 h is optimal for UV254 removal in polymerization; and 60 °C gives high pollutant removal. The optimal conditions are non-calcined volcanic rock, 90 °C acid leaching, 2 mol/L NaOH at 8 mL, 0.5 h polymerization, and 60 °C polymerization, producing effective flocculants for turbidity and UV254 removal as well as phosphorus, providing a reference for treatment of industrial wastewater and domestic sewage. For further development, in the short term, we aim to set up a pilot-scale production to validate large-scale feasibility and estimate costs. In the medium term, we may conduct industrial trials, compare costs and benefits with traditional flocculants, and set targets for cost reduction and performance improvement. In the long term, we may expand the market share and continuously improve based on feedback. Overall, this study provides a reference for the industrial application of volcanic rock-based flocculants.
Author Contributions
Z.Y. and L.Z.: data curation; validation. L.Z. and Z.Y., as co-first authors, contributed to the paper equally. X.Y., X.W., X.L. and Y.Z.: funding acquisition; investigation. G.Z.: conceptualization; methodology; investigation; supervision; funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Hunan Provincial Educational Commission Foundation (No. 21A0324) and the China Railway Urban Construction Group No. 1 Engineering Co., Ltd., Foundation (No. D123ED).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
Authors Lei Zhou, Xiaoyong Liu, Xiaoben Yang and Xuewen Wu were employed by the company China Railways Urban Construction Group No. 1 Engineering Co., Ltd. Authors Zhangrui Yang and Yong Zhou were employed by the company Shenzhen Changlong Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declare that this study received funding from China Railway Urban Construction Group No. 1 Engineering Co., Ltd. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Abbreviations
The following abbreviations are used in this manuscript:
| PSAC | Polysilicate Aluminum Chloride |
| SEM | Scanning Electron Microscopy |
| EDS | Energy-Dispersive Spectroscopy |
| FTIR | Fourier-Transform Infrared Spectroscopy |
| XRD | X-Ray Diffraction |
| UV254 | UV Absorbance at 254 nm |
| TP | Total Phosphorus |
References
- Falkenmark, M. Water resilience and human life support-global outlook for the next half century. Int. J. Water Resour. Dev. 2020, 36, 377–396. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Jeevanantham, S.; Karishma, S.; Tajsabreen, B.; Yaashikaa, P.; Reshma, B. Effective water/wastewater treatment methodologies for toxic pollutants removal: Processes and applications towards sustainable development. Chemosphere 2021, 280, 130595. [Google Scholar] [CrossRef]
- Ahmed, S.; Mofijur, M.; Nuzhat, S.; Chowdhury, A.T.; Rafa, N.; Uddin, M.A.; Inayat, A.; Mahlia, T.; Ong, H.C.; Chia, W.Y.; et al. Recent developments in physical, biological, chemical, and hybrid treatment techniques for removing emerging contaminants from wastewater. J. Hazard. Mater. 2021, 416, 125912. [Google Scholar] [CrossRef]
- Cevallos-Mendoza, J.; Amorim, C.G.; Rodríguez-Díaz, J.M.; Montenegro, M.d.C.B.S.M. Removal of contaminants from water by membrane filtration: A review. Membranes 2022, 12, 570. [Google Scholar] [CrossRef] [PubMed]
- Stoquart, C.; Servais, P.; Bérubé, P.R.; Barbeau, B. Hybrid membrane processes using activated carbon treatment for drinking water: A review. J. Membr. Sci. 2012, 411, 1–12. [Google Scholar] [CrossRef]
- Qian, Y.; Wan, P.; Hursthouse, A.S.; Zhu, G. A novel zero-valent iron/titanium tannate composite for deep removal of nitrogen from water. Chem. Eng. J. 2025, 508, 160937. [Google Scholar] [CrossRef]
- Lv, L.; Luo, H.; Zhu, G. Characterization and application of titanium tannate (Ti-TA) metallic phenolic network for removal of ammonia nitrogen. J. Water Process Eng. 2025, 73, 107703. [Google Scholar] [CrossRef]
- Osborne, M.R.; Champlain, D.; Garrett, C.; Hall, R.; Hengst, G.; McCarty, P.; Miller, J.; Moreau, C.; Pedri, J.; Poole, C. Biological Wastewater Treatment and Reactive Nitrogen. Master’s Thesis, University of Maryland, College Park, MD, USA, 2009. [Google Scholar]
- Dong, H.; Tian, Y.; Lu, J.; Zhao, J.; Tong, Y.; Niu, J. Bioaugmented biological contact oxidation reactor for treating simulated textile dyeing wastewater. Bioresour. Technol. 2024, 404, 130916. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Hong, C.; Ling, W.; Hu, J.; Feng, W.; Xing, Y.; Wang, Y.; Zhao, C.; Feng, L. Research progress in improving sludge dewaterability: Sludge characteristics, chemical conditioning and influencing factors. J. Environ. Manag. 2024, 351, 119863. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, G.; Ren, B.; Zhang, P.; Hursthouse, A. A cationic polymer enhanced PAC for the removal of dissolved aquatic organic carbon and organic nitrogen from surface waters. Can. J. Chem. Eng. 2019, 97, 955–966. [Google Scholar] [CrossRef]
- Iwuozor, K.O. Prospects and challenges of using coagulation-flocculation method in the treatment of effluents. Adv. J. Chem. -Sect. A 2019, 2, 105–127. [Google Scholar] [CrossRef]
- Lee, C.S.; Robinson, J.; Chong, M.F. A review on application of flocculants in wastewater treatment. Process Saf. Environ. Prot. 2014, 92, 489–508. [Google Scholar] [CrossRef]
- Li, H.; Tian, Y.; Xie, J.; Wu, Y.; Gao, Q. Overview of Commonly Used Flocculants in Sewage Treatment. Chem. Eng. Des. Commun. 2023, 49, 38–40. [Google Scholar]
- Zhu, G.; Liu, J.; Zhou, T.; Zhang, P.; Ren, B.; Zheng, H.; Liu, Y.; Li, X. The preparation and characterization of a new solid coagulant: Polymeric ferric silicate sulfate. Spectrosc. Spectr. Anal. 2016, 36, 2455–2461. [Google Scholar]
- Yi, X.; Ma, Z.; Zhang, S. Research progress on inorganic silica flocculants. Fine Spec. Chem. 2009, 10, 29–32+2. [Google Scholar]
- Gao, B.-Y.; Yue, Q.-Y.; Wang, B.; Chu, Y. Poly-aluminum-silicate-chloride (PASiC)—A new type of composite inorganic polymer coagulant. Colloids Surf. A Physicochem. Eng. Asp. 2003, 229, 121–127. [Google Scholar] [CrossRef]
- Zouboulis, A.; Tzoupanos, N. Polyaluminium silicate chloride—A systematic study for the preparation and application of an efficient coagulant for water or wastewater treatment. J. Hazard. Mater. 2009, 162, 1379–1389. [Google Scholar] [CrossRef]
- Gao, B.-Y.; Yue, Q.-Y.; Wang, Y. Coagulation performance of polyaluminum silicate chloride (PASiC) for water and wastewater treatment. Sep. Purif. Technol. 2007, 56, 225–230. [Google Scholar] [CrossRef]
- Tolkou, A.; Zouboulis, A. Synthesis and coagulation performance of composite poly-aluminum-ferric-silicate-chloride coagulants in water and wastewater. Desalination Water Treat. 2015, 53, 3309–3318. [Google Scholar] [CrossRef]
- Wu, L.; Gao, Y.; Xu, X.; Deng, J.; Liu, H. Excellent coagulation performance of polysilicate aluminum ferric for treating oily wastewater from Daqing gasfield: Responses to polymer properties and coagulation mechanism. J. Environ. Manag. 2024, 356, 120642. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.R.; Long, Y.Z.; Yang, X.B.; Liu, J.F.; Zhu, G.C. Preparation and application of polymeric silicate coagulant: A short review. Environ. Eng. Res. 2024, 29, 230672. [Google Scholar] [CrossRef]
- Dalmora, A.C.; Ramos, C.G.; Plata, L.G.; da Costa, M.L.; Kautzmann, R.M.; Oliveira, L.F.S. Understanding the mobility of potential nutrients in rock mining by-products: An opportunity for more sustainable agriculture and mining. Sci. Total Environ. 2020, 710, 136240. [Google Scholar] [CrossRef]
- Zhou, W.; Gao, B.; Yue, Q.; Liu, L.; Wang, Y.J.C.; Physicochemical, S.A.; Aspects, E. Al-Ferron kinetics and quantitative calculation of Al (III) species in polyaluminum chloride coagulants. Colloids Surf. A Physicochem. Eng. Asp. 2006, 278, 235–240. [Google Scholar] [CrossRef]
- He, N. Research on the Properties of Polymer Permeable Concrete in Red Mud Slag Base. Master’s Thesis, South China University of Technology, Guangzhou, China, 2022. [Google Scholar]
- Zhang, H.; Lin, H.; Li, Q.; Cheng, C.; Shen, H.; Zhang, Z.; Zhang, Z.; Wang, H. Removal of refractory organics in wastewater by coagulation/flocculation with green chlorine-free coagulants. Sci. Total Environ. 2021, 787, 147654. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, H.; Sun, C.; Tong, Y.; Deng, J.; Wu, L.; Sun, L.; Guo, S.; Liu, H. Evaluation of new hydrophobic association inorganic composite material as coagulant for oilfield wastewater treatment. Sep. Purif. Technol. 2021, 275, 119126. [Google Scholar] [CrossRef]
- Liang, D.; Wang, Y.; Chen, M.; Xie, X.; Li, C.; Wang, J.; Yuan, L. Dry reforming of methane for syngas production over attapulgite-derived MFI zeolite encapsulated bimetallic Ni-Co catalysts. Appl. Catal. B Environ. 2023, 322, 122088. [Google Scholar] [CrossRef]
- Li, Y.; Miao, Y.; Yang, L.; Zhao, Y.; Wu, K.; Lu, Z.; Hu, Z.; Guo, J. Recent advances in the development and antimicrobial applications of metal–phenolic networks. Adv. Sci. 2022, 9, 2202684. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Zhang, W.; Zhang, Y.; Wang, L. Synthesis and characterization of a new inorganic-organosilicon-aluminum composite flocculant. Appl. Chem. Ind. 2022, 51, 759–763. [Google Scholar] [CrossRef]
- Feng, Q.; Guo, K.; Gao, Y.; Liu, B.; Yue, Q.; Shi, W.; Feng, C.; Zhou, J.; Wang, G.; Gao, B.J.S. Effect of coagulation treatment on sludge dewatering performance: Application of polysilicate and their mechanism. Sep. Purif. Technol. 2022, 301, 121954. [Google Scholar] [CrossRef]
- Tian, X.; Han, C.Y.; Zhang, L.D.; Du, W.Z.; Li, Z.H. A study on the preparation and flocculation properties of boracic poly-aluminum-ferric-silicate. Adv. Mater. Res. 2013, 726, 3025–3029. [Google Scholar] [CrossRef]
- Xu, L. Preparation of Flocculant Polysilicate Aluminium Ferrric from Fly Ash and Its Application in Wastewater Treatment. Master’s Thesis, North University of China, Taiyuan, China, 2013. [Google Scholar]
- Zhu, G.C.; Wang, Q.; Yin, J.; Li, Z.W.; Zhang, P.; Ren, B.Z.; Fan, G.D.; Wan, P. Toward a better understanding of coagulation for dissolved organic nitrogen using polymeric zinc-iron-phosphate coagulant. Water Res. 2016, 100, 201–210. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).