Integrated Application of Biofloc Technology in Aquaculture: A Review
Abstract
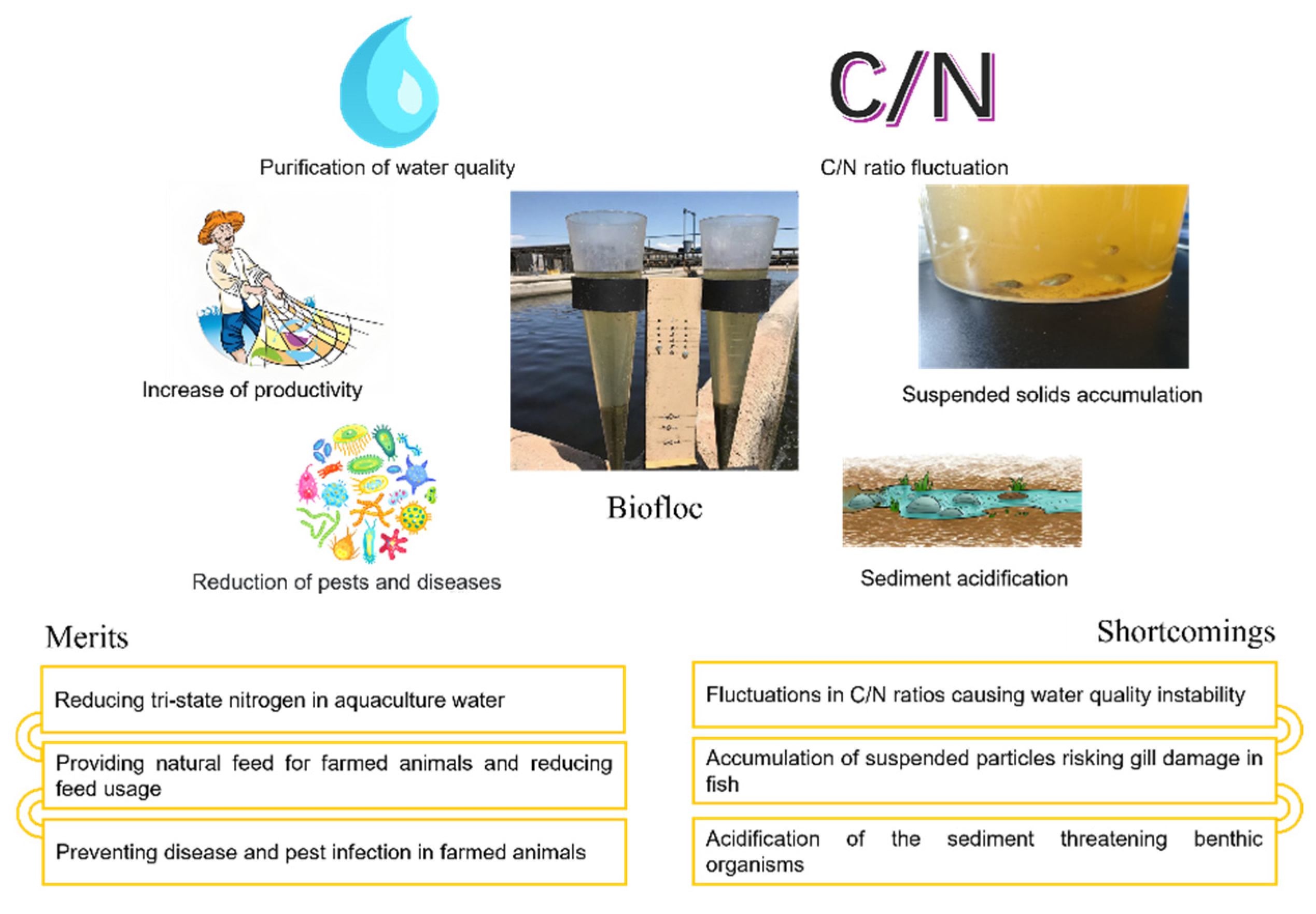
1. Introduction
2. Main Integrated Models of BFT in Aquaculture
2.1. BFT–Aquaponics Integration
2.2. BFT–Biofilm Technology Coupling
2.3. BFT–Integrated Multi-Trophic Aquaculture (IMTA) Coupling
2.4. BFT–Recirculating Aquaculture System (RAS) Hybrid System
3. Comparison Among Various Integrated Models
| Cultured Species | Combined Technology | Key Merits | Reference |
|---|---|---|---|
| Tilapia shrimp, Chinese carps, Crayfish, Crabs | Aquaponics |
| Martinez-Cordova, et al. [31] |
| Nile tilapia | Aquaponics |
| Martins, et al. [32] |
| Oreochromis niloticus | Aquaponics |
| Pinho, et al. [33] |
| Oreochromis niloticus | Aquaponics |
| Pinho, et al. [34] |
| Oreochromis spp., Litopenaeus vannamei | Aquaponics |
| Yu, et al. [35] |
| Oreochromis niloticus | Aquaponics |
| Pinho, et al. [39] |
| Oreochromis spp., Litopenaeus vannamei | Aquaponics |
| Pinho, et al. [41] |
| Litopenaeus vannamei, Mugil liza | Aquaponics |
| Legarda, et al. [44] |
| GIFT Tilapia, Oreochromis niloticus | Aquaponics |
| Saseendran, et al. [45] |
| Oreochromis niloticus | Aquaponics |
| Pinho, et al. [50] |
| Litopenaeus vannamei | Biofilm technology |
| de Lara, et al. [54] |
| Penaeus vannamei | Biofilm technology |
| Ramiro, et al. [55] |
| Oreochromis niloticus | Biofilm technology |
| Zheng, et al. [56] |
| Litopenaeus vannamei | Biofilm technology |
| Xu, et al. [57] |
| Litopenaeus vannamei | Biofilm technology |
| Lara, et al. [58] |
| Litopenaeus vannamei | Biofilm technology |
| Lara, et al. [59] |
| Anguilla marmorata | Biofilm technology |
| Jiang, et al. [61] |
| Salmo salar, Dicentrarchus labrax, Sparus aurata, Sciaenops ocellatus | IMTA |
| Khanjani, et al. [14] |
| Litopenaeus vannamei | IMTA |
| Sarkar, et al. [67] |
| Litopenaeus vannamei, Oreochromis niloticus | IMTA |
| Holanda, et al. [69] |
| Litopenaeus vannamei, Crassostrea gasar | IMTA |
| Costa, et al. [70] |
| Litopenaeus vannamei, Arthrospira platensis | IMTA |
| Holanda, et al. [71] |
| Oreochromis niloticus | RAS |
| Pai, et al. [76] |
| Oreochromis niloticus | RAS |
| Pai, et al. [78] |
4. Current Challenges and Further Research Dimensions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- National Bureau of Statistics of China. Statistical Communiqué of the People’s Republic of China on the 2024 National Economic and Social Development; National Bureau of Statistics of China: Beijing, China, 2025.
- Li, P.L.; Han, H.B.; Zhang, S.M.; Fang, H.; Fan, W.; Zhao, F.; Xu, C.F. Reviews on the development of digital intelligent fisheries technology in aquaculture. Aquac. Int. 2025, 33, 191. [Google Scholar] [CrossRef]
- Marimuthu, S.; Puvaneswari, S.; Lakshmanan, R. Effect of Biofloc Technology Enriches the Growth of Litopenaeus vannamei (Boone, 1931). Appl. Biochem. Biotechnol. 2024, 196, 3860–3890. [Google Scholar] [CrossRef] [PubMed]
- Azim, M.E.; Little, D.C. The biofloc technology (BFT) in indoor tanks: Water quality, biofloc composition, and growth and welfare of Nile tilapia (Oreochromis niloticus). Aquaculture 2008, 283, 29–35. [Google Scholar] [CrossRef]
- Ahmad, T.; Sethupathi, S.; Bashir, M.J.K.; Tan, S.Y. The role of oxidation on oil palm fiber biochar for ammoniacal nitrogen recovery from aquaculture wastewater. J. Water Process Eng. 2024, 60, 105091. [Google Scholar] [CrossRef]
- Yu, J.C.; Wang, Y.F.; Xiao, Y.S.; Li, X.; Xu, X.J.; Zhao, H.X.; Wu, L.L.; Li, J. Effects of chronic nitrate exposure on the intestinal morphology, immune status, barrier function, and microbiota of juvenile turbot (Scophthalmus maximus). Ecotoxicol. Environ. Saf. 2021, 207, 111287. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.B.; Choi, J.H.; Lee, J.H.; Jo, A.H.; Lee, J.W.; Choi, H.J.; Kang, Y.J.; Choi, C.Y.; Kang, J.C.; Lee, K.M.; et al. The use, application and efficacy of biofloc technology (BFT) in shrimp aquaculture industry: A review. Environ. Technol. Innov. 2024, 33, 103345. [Google Scholar] [CrossRef]
- Anandkumar, A.; Li, J.; Prabakaran, K.; Jia, Z.X.; Leng, Z.R.; Nagarajan, R.; Du, D.L. Accumulation of toxic elements in an invasive crayfish species (Procambarus clarkii) and its health risk assessment to humans. J. Food Compos. Anal. 2020, 88, 103449. [Google Scholar] [CrossRef]
- Yuan, H.; Cai, Y.; Liang, S.F.; Ku, J.S.; Qin, Y. Numerical Simulation and Analysis of Feeding Uniformity of Viscous Miscellaneous Fish Bait Based on EDEM Software. Agriculture 2023, 13, 356. [Google Scholar] [CrossRef]
- Yang, H.; Tan, T.; Du, X.P.; Feng, Q.; Liu, Y.L.; Tang, Y.D.; Bai, G.L.; Liu, Z.S.; Xia, S.B.; Song, S.X.; et al. Advancements in freshwater aquaculture wastewater management: A comprehensive review. Aquaculture 2025, 594, 741346. [Google Scholar] [CrossRef]
- Jin, L.L.; Sun, X.Z.; Ren, H.Q.; Huang, H. Biological filtration for wastewater treatment in the 21st century: A data-driven analysis of hotspots, challenges and prospects. Sci. Total Environ. 2023, 855, 158951. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.Y.; Liu, X.Q.; Chen, H.L.; Tian, X. Automatic cruise system for water quality monitoring. Int. J. Agric. Biol. Eng. 2018, 11, 244–250. [Google Scholar] [CrossRef]
- Shi, B.; Sreeram, V.; Zhao, D.A.; Duan, S.L.; Jiang, J.M. A wireless sensor network-based monitoring system for freshwater fishpond aquaculture. Biosyst. Eng. 2018, 172, 57–66. [Google Scholar] [CrossRef]
- Khanjani, M.H.; Zahedi, S.; Mohammadi, A. Integrated multitrophic aquaculture (IMTA) as an environmentally friendly system for sustainable aquaculture: Functionality, species, and application of biofloc technology (BFT). Environ. Sci. Pollut. Res. 2022, 29, 67513–67531. [Google Scholar] [CrossRef] [PubMed]
- Khanjani, M.H.; Sharifinia, M. Biofloc technology as a promising tool to improve aquaculture production. Rev. Aquac. 2020, 12, 1836–1850. [Google Scholar] [CrossRef]
- Kasan, N.A.; Dagang, A.N.; Abdullah, M.I. Application of biofloc technology (BFT) in shrimp aquaculture industry. IOP Conf. Ser. Earth Environ. Sci. 2018, 196, 012043. [Google Scholar] [CrossRef]
- Khanjani, M.H.; Sharifinia, M.; Hajirezaee, S. Recent progress towards the application of biofloc technology for tilapia farming. Aquaculture 2022, 552, 738021. [Google Scholar] [CrossRef]
- Iber, B.T.; Ikyo, B.C.; Nor, M.N.M.; Abdullah, S.R.S.; Shafie, M.S.B.; Manan, H.; Abdullah, M.H.D.I.; Kasan, N.A. Application of Biofloc technology in shrimp aquaculture: A review on current practices, challenges, and future perspectives. J. Agric. Food Res. 2025, 19, 101675. [Google Scholar] [CrossRef]
- Korbee, N.; Bautista, B.; García-Sánchez, M.; Cobos, P.; Ferres-García, J.L.; Figueroa, F.L.; Medrano, E. Evaluating hydroponics and aquaponics: Comparative insights into sustainability and strawberry quality. Agric. Water Manag. 2025, 312, 109412. [Google Scholar] [CrossRef]
- Tang, Y.; Ju, C.; Mei, R.; Zhao, L.; Liu, J.; Yang, Y.; Guo, X.; Su, C.; Cheng, Y.; Liu, Q. Exploring the optimal integrated multi-trophic aquaculture (IMTA) patterns benefiting culture animals and natural water environment. Aquaculture 2024, 589, 741011. [Google Scholar] [CrossRef]
- Roy, S.M.; Choi, H.; Kim, T. Review of state-of-the-art improvements in recirculating aquaculture systems: Insights into design, operation, and statistical modeling approaches. Aquaculture 2025, 605, 742545. [Google Scholar] [CrossRef]
- Tiwari, H.; Pophali, G.R. Review of improved hydrodynamics in moving bed biofilm reactors (MBBRs) for wastewater treatment: A way forward. J. Environ. Chem. Eng. 2025, 13, 116751. [Google Scholar] [CrossRef]
- Ogello, E.O.; Outa, N.O.; Obiero, K.O.; Kyule, D.N.; Munguti, J.M. The prospects of biofloc technology (BFT) for sustainable aquaculture development. Sci. Afr. 2021, 14, e01053. [Google Scholar] [CrossRef]
- Shi, M.; Ruan, Y.; Wu, B.; Ye, Z.; Zhu, S. Performance Evaluation of Hydrodynamic Vortex Separator at Different Hydraulic Retention Times Applied in Recirculating Biofloc Technology System. Trans. Asabe 2017, 60, 1737–1747. [Google Scholar] [CrossRef]
- Yu, Y.B.; Choi, J.H.; Lee, J.H.; Jo, A.H.; Lee, K.M.; Kim, J.H. Biofloc Technology in Fish Aquaculture: A Review. Antioxidants 2023, 12, 398. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.J.; Wen, G.L.; Su, H.C.; Xu, Y.; Hu, X.J.; Cao, Y.C. Effect of Input C/N Ratio on Bacterial Community of Water Biofloc and Shrimp Gut in a Commercial Zero-Exchange System with Intensive Production of Penaeus vannamei. Microorganisms 2022, 10, 1060. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.K.; Wasielesky, W.; Tesser, M.B. Fish culture in biofloc technology (BFT): Insights on stocking density carbon sources, C/N ratio, fish nutrition and health. Aquac. Fish. 2024, 9, 522–533. [Google Scholar] [CrossRef]
- Shamsuddin, M.; Hossain, M.B.; Rahman, M.; Kawla, M.S.; Shufol, M.B.A.; Rashid, M.M.; Asadujjaman, M.; Rakib, M.R.J. Application of Biofloc Technology for the culture of Heteropneustes fossilis (Bloch) in Bangladesh: Stocking density, floc volume, growth performance, and profitability. Aquac. Int. 2022, 30, 1047–1070. [Google Scholar] [CrossRef]
- Nisar, U.; Peng, D.M.; Mu, Y.T.; Sun, Y. A Solution for Sustainable Utilization of Aquaculture Waste: A Comprehensive Review of Biofloc Technology and Aquamimicry. Front. Nutr. 2022, 8, 791738. [Google Scholar] [CrossRef] [PubMed]
- Anila, M.; Daramola, O. Applications, technologies, and evaluation methods in smart aquaponics: A systematic literature review. Artif. Intell. Rev. 2024, 58, 25. [Google Scholar] [CrossRef]
- Martinez-Cordova, L.R.; Emerenciano, M.G.C.; Miranda-Baeza, A.; Pinho, S.M.; Garibay-Valdez, E.; Martínez-Porchas, M. Advancing toward a more integrated aquaculture with polyculture > aquaponics > biofloc technology > FLOCponics. Aquac. Int. 2023, 31, 1057–1076. [Google Scholar] [CrossRef]
- Martins, V.B.; Dartora, A.; Lasala, M.D.; Morais, K.F.; de Andrade, J.I.A.; Jatobá, A. Cultivation of vegetables in an integrated biofloc system with nile tilapia. Aquaculture 2025, 596, 741818. [Google Scholar] [CrossRef]
- Pinho, S.M.; de Lima, J.P.; Tarigan, N.B.R.; David, L.H.; Portella, M.C.; Keesman, K.J. Modelling FLOCponics systems: Towards improved water and nitrogen use efficiency in biofloc-based fish culture. Biosyst. Eng. 2023, 229, 96–115. [Google Scholar] [CrossRef]
- Pinho, S.M.; David, L.H.; Garcia, F.; Portella, M.C.; Keesman, K.J. Sustainability assessment of FLOCponics compared to stand-alone hydroponic and biofloc systems using emergy synthesis. Ecol. Indic. 2022, 141, 109092. [Google Scholar] [CrossRef]
- Yu, Y.B.; Choi, J.H.; Lee, J.H.; Jo, A.H.; Han, S.W.; Han, S.H.; Choi, H.J.; Choi, C.Y.; Kang, J.C.; Min, E.Y.; et al. Biofloc Application Using Aquaponics and Vertical Aquaculture Technology in Aquaculture: Review. Fishes 2023, 8, 543. [Google Scholar] [CrossRef]
- Deswati, D.; Yani, E.; Safni, S.; Norita Tetra, O.; Pardi, H. Development methods in aquaponics systems using biofloc to improve water quality (ammonia, nitrite, nitrate) and growth of tilapia and samhong mustard. Int. J. Environ. Anal. Chem. 2022, 102, 7824–7834. [Google Scholar] [CrossRef]
- Pinho, S.M.; Molinari, D.; de Mello, G.L.; Fitzsimmons, K.M.; Coelho Emerenciano, M.G. Effluent from a biofloc technology (BFT) tilapia culture on the aquaponics production of different lettuce varieties. Ecol. Eng. 2017, 103, 146–153. [Google Scholar] [CrossRef]
- Pinheiro, I.; Arantes, R.; do Espirito Santo, C.M.; do Nascimento Vieira, F.; Lapa, K.R.; Gonzaga, L.V.; Fett, R.; Barcelos-Oliveira, J.L.; Seiffert, W.Q. Production of the halophyte Sarcocornia ambigua and Pacific white shrimp in an aquaponic system with biofloc technology. Ecol. Eng. 2017, 100, 261–267. [Google Scholar] [CrossRef]
- Pinho, S.M.; David, L.H.C.; Goddek, S.; Emerenciano, M.G.C.; Portella, M.C. Integrated production of Nile tilapia juveniles and lettuce using biofloc technology. Aquac. Int. 2021, 29, 37–56. [Google Scholar] [CrossRef]
- Lee, D.-H.; Dae, K.J. Comparative study on growth of leafy vegetables grown in a hybrid BFT-aquaponics using Japanese eel, Anguilla japonica and hydroponics. Fish. Aquat. Sci. 2021, 24, 260–275. [Google Scholar] [CrossRef]
- Pinho, S.M.; de Lima, J.P.; David, L.H.; Emerenciano, M.G.C.; Goddek, S.; Verdegem, M.C.J.; Keesman, K.J.; Portella, M.C. FLOCponics: The integration of biofloc technology with plant production. Rev. Aquac. 2022, 14, 647–675. [Google Scholar] [CrossRef]
- Rodrigues, A.B.; Pinheiro, I.C.; Nonato, T.C.M.; Ferenhof, E.A.; Sens, M.L. Valorization of reverse osmosis concentrate in the production of Litopenaeus vannamei and New Zealand spinach in an aquaponic system with biofloc. Desalination 2023, 549, 116330. [Google Scholar] [CrossRef]
- Nadia, Z.M.; Akhi, A.R.; Roy, P.; Farhad, F.B.; Hossain, M.M.; Salam, M.A. Yielding of aquaponics using probiotics to grow tomatoes with tilapia. Aquac. Rep. 2023, 33, 101799. [Google Scholar] [CrossRef]
- Legarda, E.C.; da Silva, D.; Miranda, C.S.; Pereira, P.K.M.; Martins, M.A.; Machado, C.; de Lorenzo, M.A.; Hayashi, L.; Vieira, F.D. Sea lettuce integrated with Pacific white shrimp and mullet cultivation in biofloc impact system performance and the sea lettuce nutritional composition. Aquaculture 2021, 534, 736265. [Google Scholar] [CrossRef]
- Saseendran, S.; Dube, K.; Chandrakant, M.H.; Rani, A.M.B. Enhanced growth response and stress mitigation of genetically improved farmed Tilapia in a biofloc integrated aquaponic system with bell pepper. Aquaculture 2021, 533, 736200. [Google Scholar] [CrossRef]
- Barbosa, P.T.L.; Povh, J.A.; Farias, K.N.N.; da Silva, T.V.; Teodoro, G.C.; Ribeiro, J.S.; Stringhetta, G.R.; dos Santos Fernandes, C.E.; Filho, R.A.C.C. Nile tilapia production in polyculture with freshwater shrimp using an aquaponic system and biofloc technology. Aquaculture 2022, 551, 737916. [Google Scholar] [CrossRef]
- Pinho, S.M.; Lima, J.P.; David, L.H.; Oliveira, M.S.; Goddek, S.; Carneiro, D.J.; Keesman, K.J.; Portella, M.C. Decoupled FLOCponics systems as an alternative approach to reduce the protein level of tilapia juveniles’ diet in integrated agri-aquaculture production. Aquaculture 2021, 543, 736932. [Google Scholar] [CrossRef]
- Pinheiro, I.; Carneiro, R.F.S.; Vieira, F.d.N.; Gonzaga, L.V.; Fett, R.; Costa, A.C.d.O.; Magallón-Barajas, F.J.; Seiffert, W.Q. Aquaponic production of Sarcocornia ambigua and Pacific white shrimp in biofloc system at different salinities. Aquaculture 2020, 519, 734918. [Google Scholar] [CrossRef]
- Yuan, Q.; Meng, F.; Liu, Y.; de Oliveira, J.A.P.; Zhang, L.; Cai, W.; Yang, Z. Shaping Resilient Edible Cities: Innovative Aquaponics for Sustainable Food–Water–Energy Nexus. Engineering 2025, in press. [CrossRef]
- Pinho, S.M.M.; Flores, R.; David, L.H.K.; Emerenciano, M.G.C.; Quagrainie, K.K.; Portella, M.C. Economic comparison between conventional aquaponics and FLOCponics systems. Aquaculture 2022, 552, 737987. [Google Scholar] [CrossRef]
- Padeniya, U.M.; Davis, D.A.; Wells, D.E.; Harrison, C.E.; LaFrentz, B.R.; Beck, B.H.; Roy, L.A.; Farmer, M.; Bruce, T.J. Influence of dietary fermented yeast products (Saccharomyces cerevisiae) on performance, health and microbiome of Nile tilapia (Oreochromis niloticus) and the influence of discharge water in the production of romaine lettuce (Lactuca sativa). Anim. Feed Sci. Technol. 2025, 325, 116348. [Google Scholar] [CrossRef]
- Murshid, S.; Antonysamy, A.; Dhakshinamoorthy, G.; Jayaseelan, A.; Pugazhendhi, A. A review on biofilm-based reactors for wastewater treatment: Recent advancements in biofilm carriers, kinetics, reactors, economics, and future perspectives. Sci. Total Environ. 2023, 892, 164796. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.C.; Lai, Y.Y.; Ke, Y.Q.; Huang, Y.N.; Tao, Y.W.; Han, X.M.; Ma, J.X. Recent advances in biofilm technologies for breeding wastewater treatment: Fundamentals, performance and impacts of antibiotics. J. Water Process Eng. 2024, 62, 105429. [Google Scholar] [CrossRef]
- de Lara, G.R.; Poersch, L.H.; Wasielesky, W., Jr. The quantity of artificial substrates influences the nitrogen cycle in the biofloc culture system of Litopenaeus vannamei. Aquac. Eng. 2021, 94, 102171. [Google Scholar] [CrossRef]
- Ramiro, B.D.; Wasielesky, W., Jr.; Pimentel, O.; Martin, N.P.S.; Borges, L.D.; Krummenauer, D. Different management strategies for artificial substrates on nitrification, microbial composition, and growth of Penaeus vannamei in a super-intensive biofloc system. Aquaculture 2025, 596, 741853. [Google Scholar] [CrossRef]
- Zheng, H.; Luo, G.; Abakari, G.; Lv, G.; Tan, H.; Liu, W. Effect of seeding biofloc on the nitrification establishment in moving bed biofilm reactor (MBBR). Aquac. Fish. 2023, 8, 617–625. [Google Scholar] [CrossRef]
- Xu, W.J.; Xu, Y.; Su, H.C.; Hu, X.J.; Yang, K.; Wen, G.L.; Cao, Y.C. Characteristics of Ammonia Removal and Nitrifying Microbial Communities in a Hybrid Biofloc-RAS for Intensive Litopenaeus vannamei Culture: A Pilot-Scale Study. Water 2020, 12, 3000. [Google Scholar] [CrossRef]
- Lara, G.; Honda, M.; Poersch, L.; Wasielesky, W. The use of biofilm and different feeding rates in biofloc culture system: The effects in shrimp growth parameters. Aquac. Int. 2017, 25, 1959–1970. [Google Scholar] [CrossRef]
- Lara, G.; Furtado, P.S.; Hostins, B.; Poersch, L.; Wasielesky, W. Addition of sodium nitrite and biofilm in a Litopenaeus vannamei biofloc culture system. Lat. Am. J. Aquat. Res. 2016, 44, 760–768. [Google Scholar] [CrossRef]
- Ferreira, L.M.H.; Lara, G.; Wasielesky, W.; Abreu, P.C. Biofilm versus biofloc: Are artificial substrates for biofilm production necessary in the BFT system? Aquac. Int. 2016, 24, 921–930. [Google Scholar] [CrossRef]
- Jiang, X.L.; Zhang, B.Q.; Zheng, W.G.; Zeng, X.B.; Li, Z.Q.; Deng, L.F. Study on the water-saving and pollution-reducing effect of biofilm-biofloc technique in Anguilla marmorata aquaculture. Desalination Water Treat. 2019, 149, 69–75. [Google Scholar] [CrossRef]
- Pourrostami Niavol, K.; Andaluri, G.; Achary, M.P.; Suri, R.P.S. How does carbon to nitrogen ratio and carrier type affect moving bed biofilm reactor (MBBR): Performance evaluation and the fate of antibiotic resistance genes. J. Environ. Manag. 2025, 377, 124619. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Yang, S.; Hu, H.; Li, Z.; Duan, H.; Chen, X.; Liu, Y.; Sheng, G. Enhanced deep denitrification for high-salinity wastewater treatment by constructing biofilm-based technology. J. Environ. Manag. 2025, 388, 125980. [Google Scholar] [CrossRef] [PubMed]
- Buslima, F.A.; Abu Hasan, H.; Sheikh Abdullah, S.R.; Othman, A.R. Water recovery from domestic wastewater using integrated biofilm-phytoremediation technology: A review. J. Water Process Eng. 2024, 65, 105875. [Google Scholar] [CrossRef]
- Ruiz-Vanoye, J.A.; Diaz-Parra, O.; Vera, M.A.M.; Fuentes-Penna, A.; Barrera-Cámara, R.A.; Ruiz-Jaimes, M.A.; Toledo-Navarro, Y.; Bernábe-Loranca, M.B.; Simancas-Acevedo, E.; Trejo-Macotela, F.R.; et al. A Comprehensive Review of Quality of Aquaculture Services in Integrated Multi-Trophic Systems. Fishes 2025, 10, 54. [Google Scholar] [CrossRef]
- Troell, M.; Joyce, A.; Chopin, T.; Neori, A.; Buschmann, A.H.; Fang, J.G. Ecological engineering in aquaculture—Potential for integrated multi-trophic aquaculture (IMTA) in marine offshore systems. Aquaculture 2009, 297, 1–9. [Google Scholar] [CrossRef]
- Sarkar, S.; Rekha, P.N.; Panigrahi, A.; Das, R.R.; Rajamanickam, S.; Balasubramanian, C.P. Integrated brackishwater farming of red seaweed Agarophyton tenuistipitatum and Pacific white leg shrimp Litopenaeus vannamei (Boone) in biofloc system: A production and bioremediation way out. Aquac. Int. 2021, 29, 2145–2159. [Google Scholar] [CrossRef]
- Poli, M.A.; Legarda, E.C.; de Lorenzo, M.A.; Pinheiro, I.; Martins, M.A.; Seiffert, W.Q.; do Nascimento Vieira, F. Integrated multitrophic aquaculture applied to shrimp rearing in a biofloc system. Aquaculture 2019, 511, 734274. [Google Scholar] [CrossRef]
- Holanda, M.; Ravagnan, E.; Lara, G.; Santana, G.; Furtado, P.; Cardozo, A.; Wasielesky; Poersch, L.H. Integrated multitrophic culture of shrimp Litopenaeus vannamei and tilapia Oreochromis niloticus in biofloc system: A pilot scale study. Front. Mar. Sci. 2023, 10, 1060846. [Google Scholar] [CrossRef]
- Costa, L.C.D.; Poersch, L.H.D.; Abreu, P.C. Biofloc removal by the oyster Crassostrea gasar as a candidate species to an Integrated Multi-Trophic Aquaculture (IMTA) system with the marine shrimp Litopenaeus vannamei. Aquaculture 2021, 540, 736731. [Google Scholar] [CrossRef]
- Holanda, M.; Besold, C.; Sempere, F.L.; Abreu, P.C.; Poersch, L. Treatment of effluents from marine shrimp culture with biofloc technology: Production of Arthrospira (Spirulina) platensis (cyanobacteria) and nutrient removal. J. World Aquac. Soc. 2022, 53, 669–680. [Google Scholar] [CrossRef]
- Libao, F.J.D.; Villaverde, O.S.M.; De Luna, N.; Comedia, V.J.G.; Luna, M.O.; Atienza, A.M.C.; Espeña, G.D. Automated Control and IoT-Based Water Quality Monitoring System for a Molobicus Tilapia Recirculating Aquaculture System (RAS). In Proceedings of the Conference on Technologies for Sustainability (SusTech), Portland, OR, USA, 14–17 April 2024; pp. 410–415. [Google Scholar]
- Gao, R.C.; Liu, L.; Monto, A.R.; Su, K.; Zhang, H.; Shi, T.; Xiong, Z.Y.; Xu, G.C.; Luo, Y.J.; Bao, Y.L.; et al. Metabolomic profile of muscles from tilapia cultured in recirculating aquaculture systems and traditional aquaculture in ponds and protein stability during freeze-thaw cycles. Food Chem. 2024, 451, 139325. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Turchini, G.M. Recirculating aquaculture systems (RAS): Environmental solution and climate change adaptation. J. Clean. Prod. 2021, 297, 126604. [Google Scholar] [CrossRef]
- Martins, C.I.M.; Eding, E.H.; Verdegem, M.C.J.; Heinsbroek, L.T.N.; Schneider, O.; Blancheton, J.P.; d’Orbcastel, E.R.; Verreth, J.A.J. New developments in recirculating aquaculture systems in Europe: A perspective on environmental sustainability. Aquac. Eng. 2010, 43, 83–93. [Google Scholar] [CrossRef]
- Pai, M.J.L.; Verma, A.K.; Krishnani, K.K.; Varghese, T.; Hittinahalli, C.M.; Verma, M.K. Stocking density optimization and its impact on growth and physiological responses of Nile tilapia (Oreochromis niloticus) reared in hybrid biofloc-RAS culture system. Aquaculture 2024, 588, 740920. [Google Scholar] [CrossRef]
- Das, R.R.; Sarkar, S.; Saranya, C.; Esakkiraj, P.; Aravind, R.; Saraswathy, R.; Rekha, P.N.; Muralidhar, M.; Panigrahi, A. Co-culture of Indian white shrimp, Penaeus indicus and seaweed, Gracilaria tenuistipitata in amended biofloc and recirculating aquaculture system (RAS). Aquaculture 2022, 548, 737432. [Google Scholar] [CrossRef]
- Pai, M.J.L.; Verma, A.K.; Krishnani, K.K.; Varghese, T.; Hittinahalli, C.M.; John, V.C.; Debroy, S. Effects on the productivity, haematological indices, and carcass quality of Nile tilapia reared at different stocking densities in a hybrid biofloc-RAS. Aquaculture 2025, 596, 741756. [Google Scholar] [CrossRef]
- Yang, Z.W.; Zhu, A.F.; Adade, S.; Ali, S.; Chen, Q.M.; Wei, J.; Chen, X.M.; Jiao, T.H.; Chen, Q.S. Ag@Au core-shell nanoparticle-based surface-enhanced Raman scattering coupled with chemometrics for rapid determination of chloramphenicol residue in fish. Food Chem. 2024, 438, 138026. [Google Scholar] [CrossRef] [PubMed]
- Levican, A.; Fisher, J.C.; McLellan, S.L.; Avendaño-Herrera, R. Microbial Communities Associated with Farmed Genypterus chilensis: Detection in Water Prior to Bacterial Outbreaks Using Culturing and High-Throughput Sequencing. Animals 2020, 10, 1055. [Google Scholar] [CrossRef] [PubMed]
- Dowle, E.; Pochon, X.; Keeley, N.; Wood, S.A. Assessing the effects of salmon farming seabed enrichment using bacterial community diversity and high-throughput sequencing. Fems Microbiol. Ecol. 2015, 91, fiv089. [Google Scholar] [CrossRef] [PubMed]
- Nageswari, P.; Verma, A.K.; Gupta, S.; Jeyakumari, A. Finger millet as a carbon source for biofloc, improved growth performance of Pangasianodon hypophthalmus (Sauvage, 1878) fingerlings. Indian J. Fish. 2020, 67, 56–61. [Google Scholar] [CrossRef]
- Yuvarajan, P.; Cheryl, A.; Gopalakannan, A.; Mahadevi, N. Effect of Distillery Spent Wash as Carbon Source in Biofloc System on Nutrient Profile of GIFT Tilapia. Indian J. Anim. Res. 2023, 57, 249–253. [Google Scholar] [CrossRef]
- Rajeev, M.; Jung, I.; Kang, I.; Cho, J.C. Genome-centric metagenomics provides insights into the core microbial community and functional profiles of biofloc aquaculture. Msystems 2024, 9, e0078224. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Hou, H.C.; Dong, D.P.; Zhang, J.S.; Yang, X.H.; Li, X.; Song, X.F. Comparative life cycle assessment of whiteleg shrimp (Penaeus vannamei) cultured in recirculating aquaculture systems (RAS), biofloc technology (BFT) and higher-place ponds (HPP) farming systems in China. Aquaculture 2023, 574, 739625. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Ge, Z.; Dai, L.; Chen, Y. Integrated Application of Biofloc Technology in Aquaculture: A Review. Water 2025, 17, 2107. https://doi.org/10.3390/w17142107
Li C, Ge Z, Dai L, Chen Y. Integrated Application of Biofloc Technology in Aquaculture: A Review. Water. 2025; 17(14):2107. https://doi.org/10.3390/w17142107
Chicago/Turabian StyleLi, Changwei, Zhenbo Ge, Limin Dai, and Yuan Chen. 2025. "Integrated Application of Biofloc Technology in Aquaculture: A Review" Water 17, no. 14: 2107. https://doi.org/10.3390/w17142107
APA StyleLi, C., Ge, Z., Dai, L., & Chen, Y. (2025). Integrated Application of Biofloc Technology in Aquaculture: A Review. Water, 17(14), 2107. https://doi.org/10.3390/w17142107







