Water Demand and Photosynthetic Performance of Tomatoes Grown Hydroponically Under Increasing Nitrogen Concentrations
Abstract
1. Introduction
2. Materials and Methods
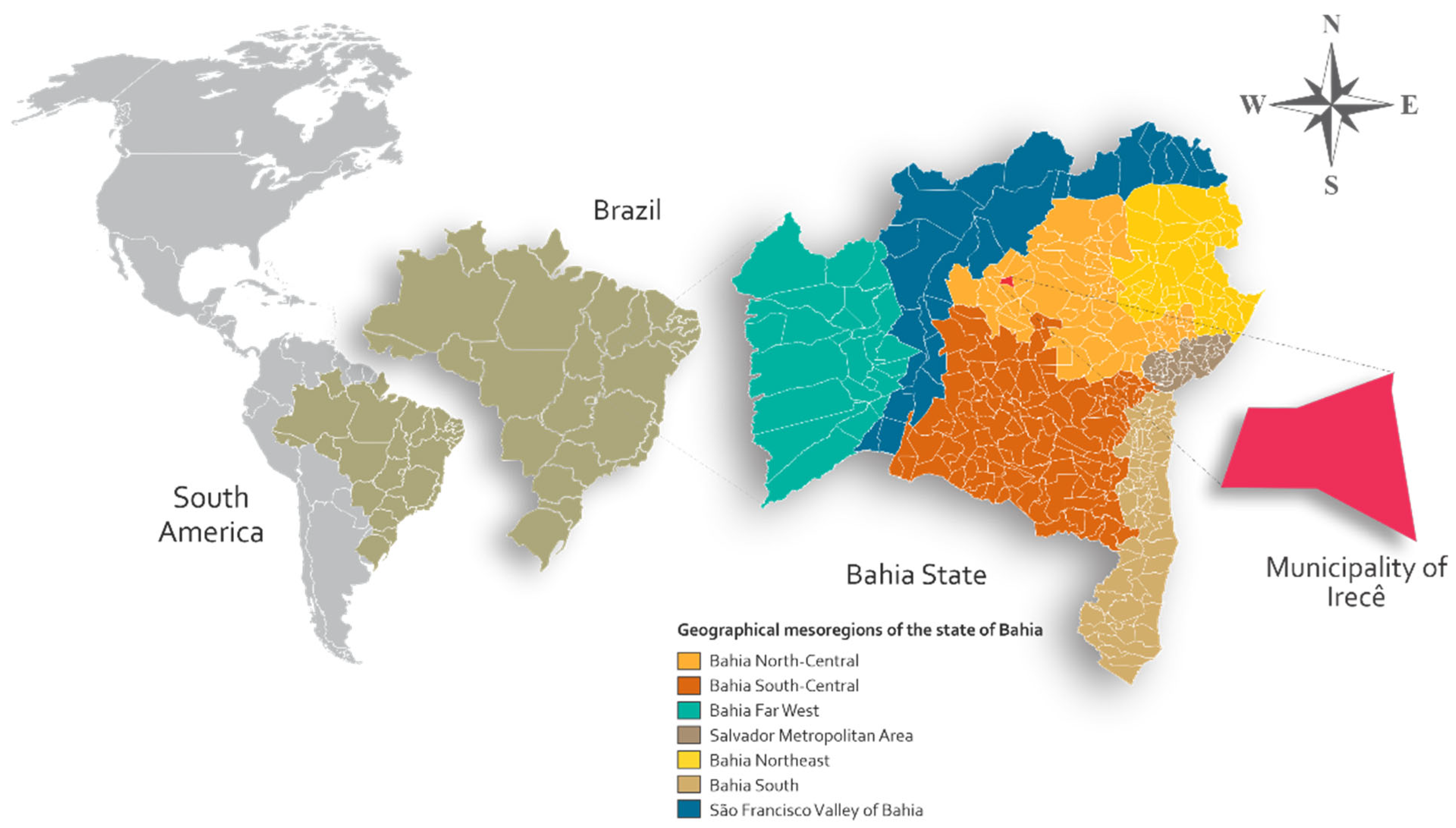
2.1. Location and Experimental Characterization
2.2. Treatment Definition
2.3. Experimental Design and Statistics
2.4. Measurements
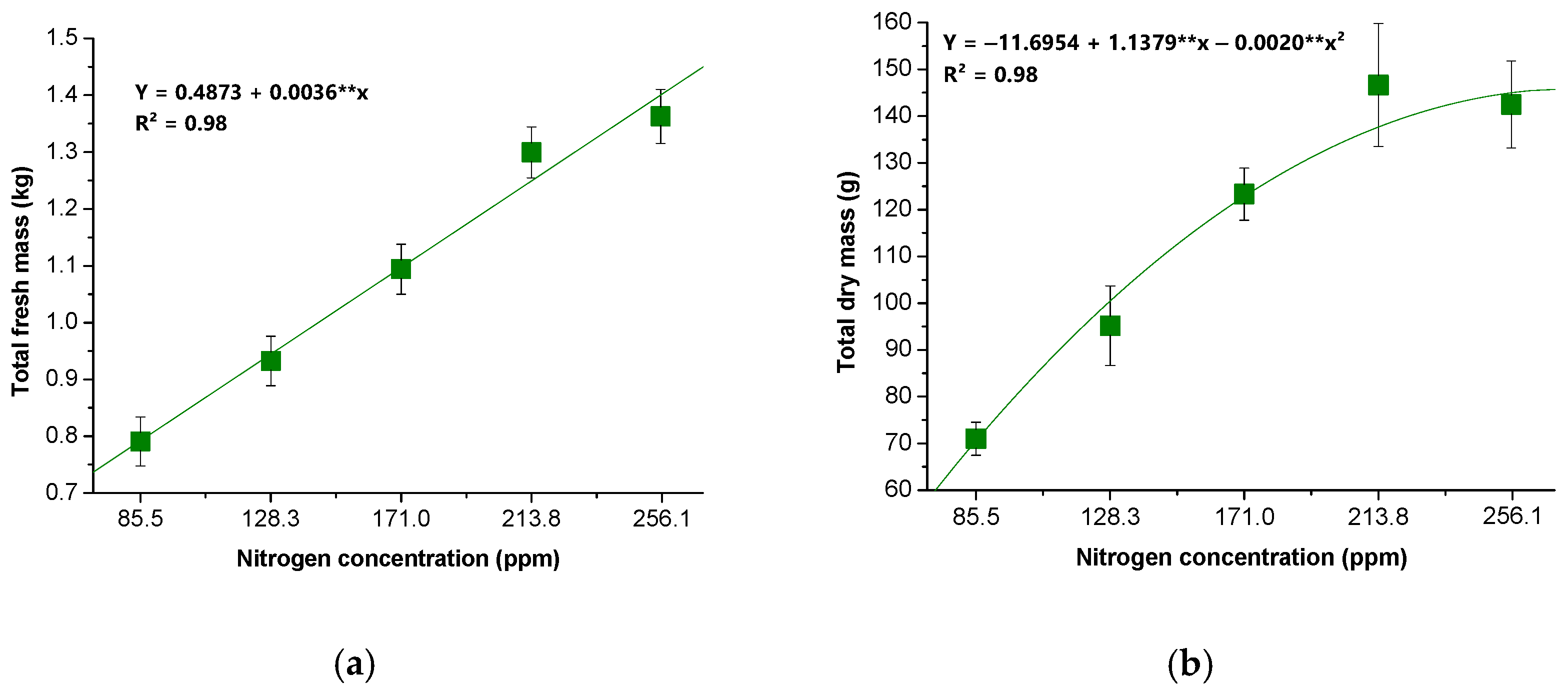
2.4.1. Biomass Production
2.4.2. Leaf Chlorophyll Index
2.4.3. Chlorophyll Fluorescence
2.4.4. Leaf N Content
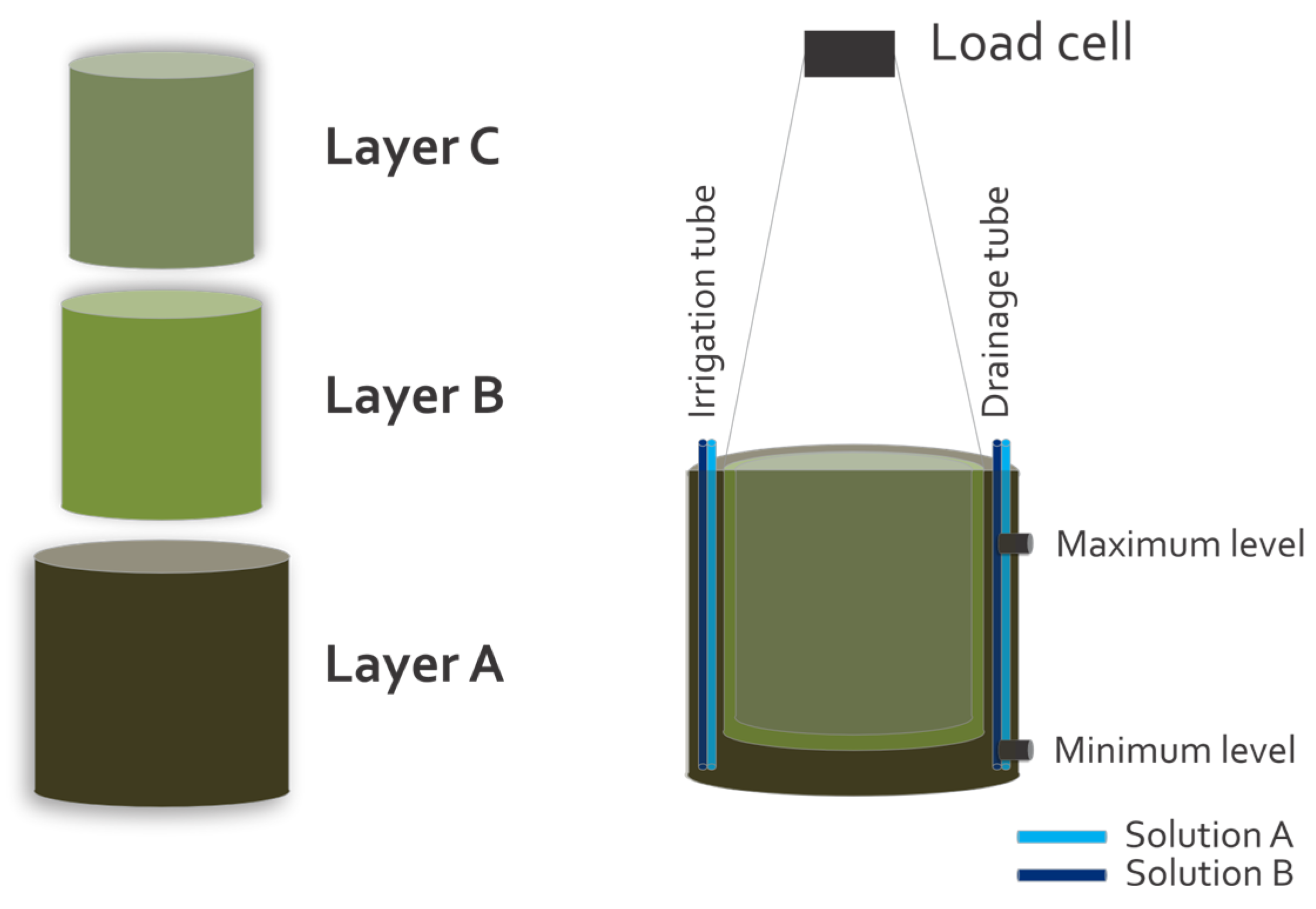
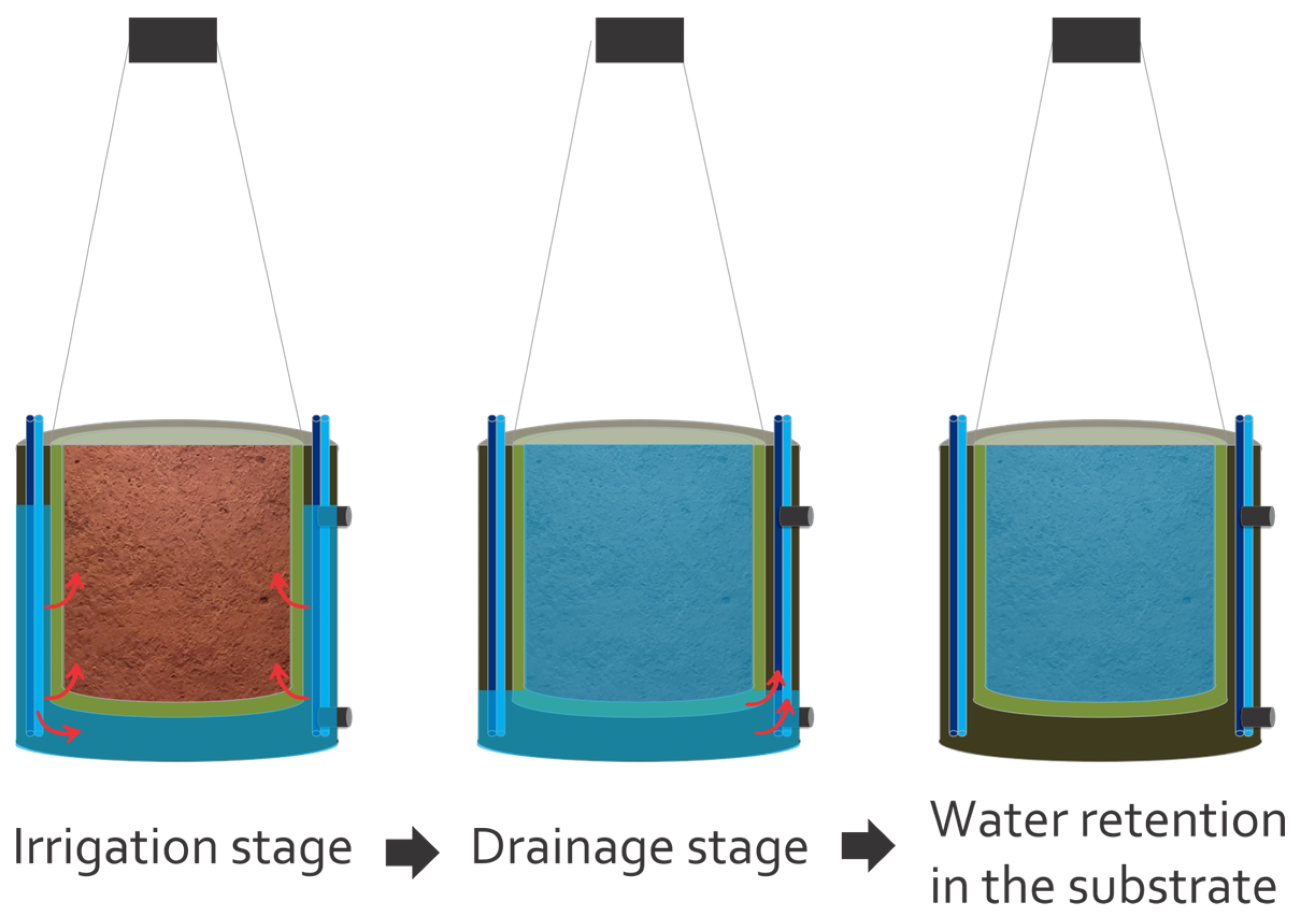
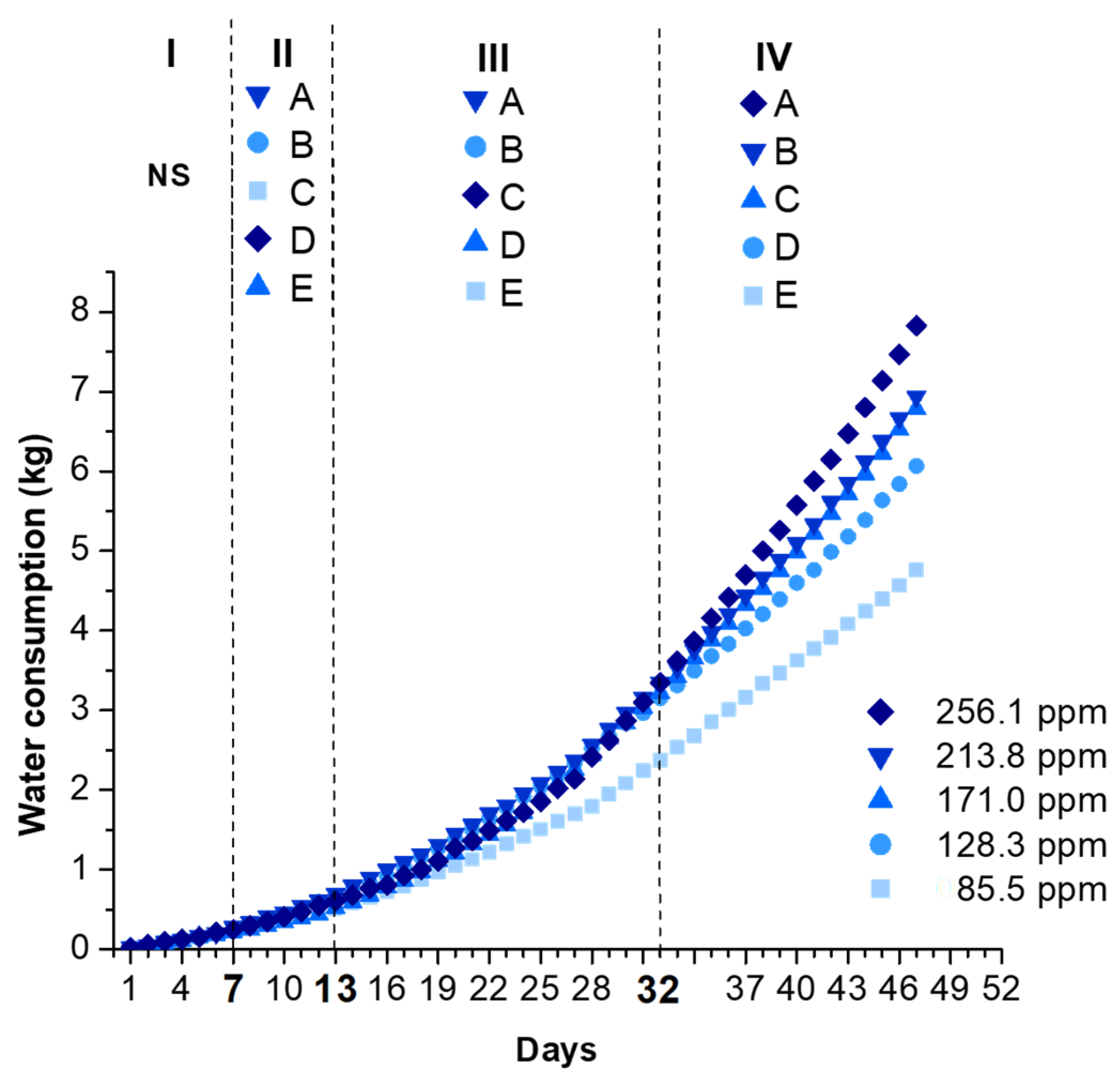
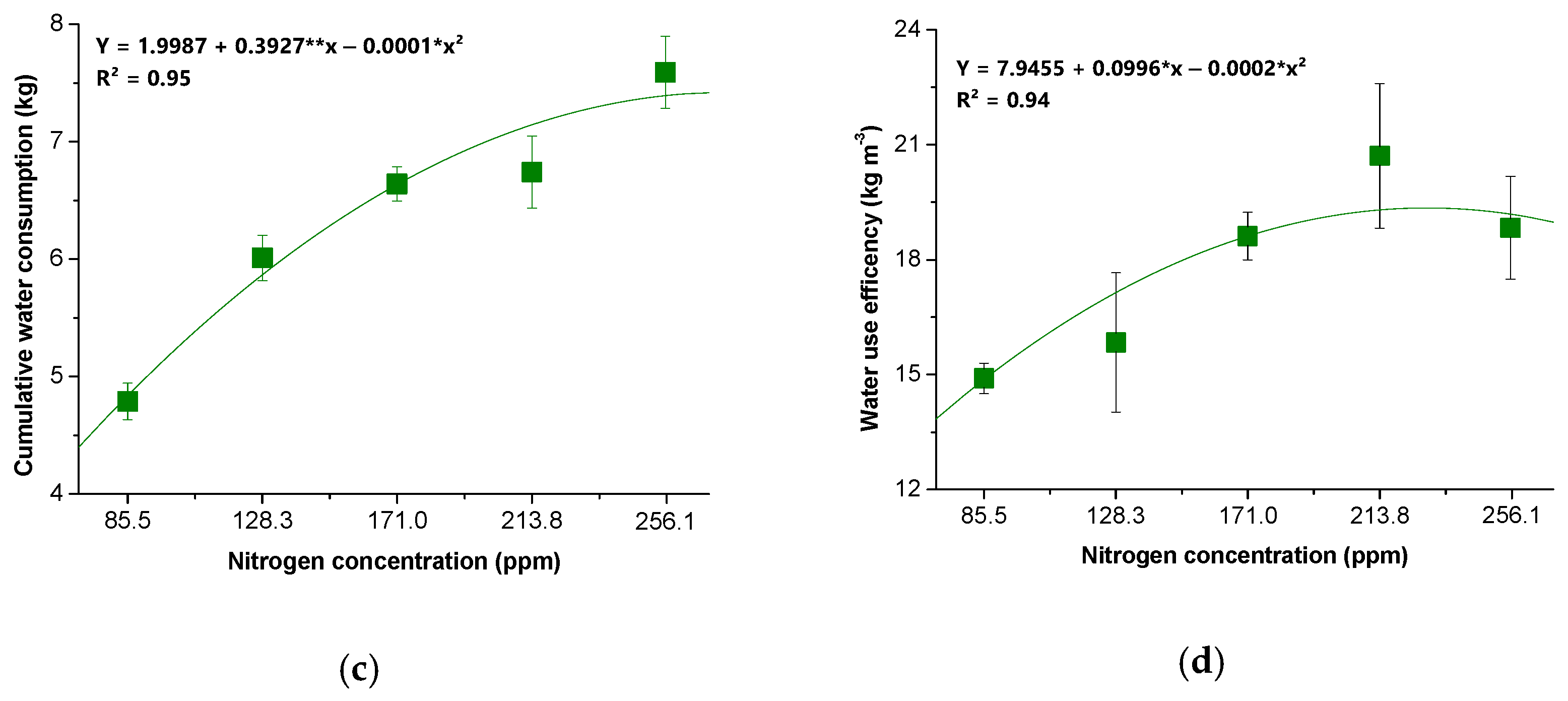
2.4.5. Water Consumption and Water Use Efficiency (WUE)
3. Results
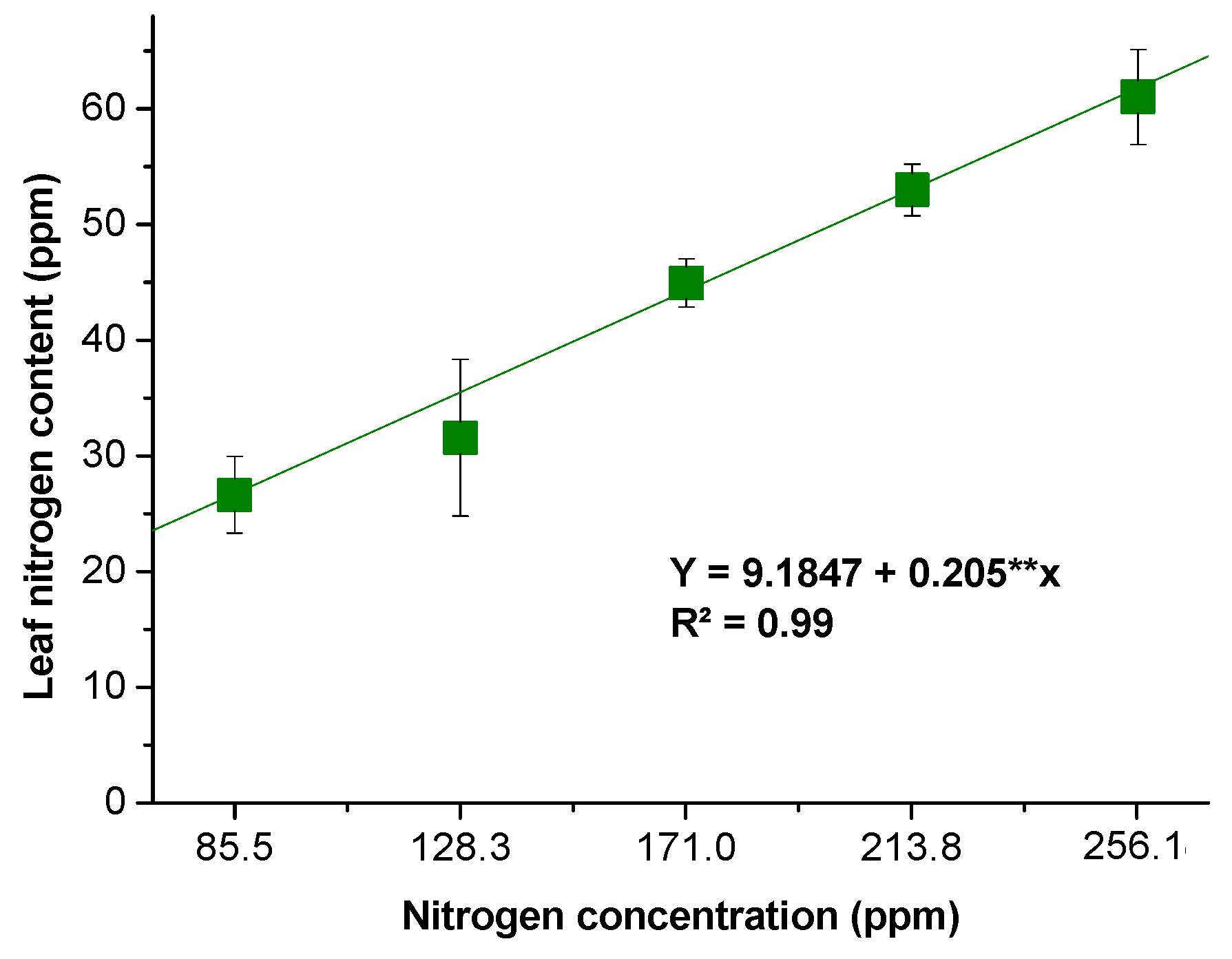
Leaf N Content and Photosynthetic Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| LNC | Leaf Nitrogen Content |
| FM | Fresh Mass |
| DM | Dry Mass |
| Fv/Fm | Maximum Quantum Efficiency of Photosystem II |
| NPQ | Non-Photochemical Quenching |
| SPAD | Soil Plant Analysis Development (Índice relativo de clorofila) |
| WUE | Water Use Efficiency |
| SOD | Superoxide Dismutase |
| ABA | Abscisic Acid |
References
- Ponsioen, T.; Helmes, R. Environmental Footprint of Tomatoes: Summary of the Representative Product Study; Wageningen Economic Research: Den Haag, The Netherlands, 2020; Report 2020-041b. [Google Scholar]
- Hasan, M.; Sabir, N.; Singh, A.K.; Singh, M.C.; Patel, N.; Khanna, M.; Rai, T.; Pragnya, P. Hydroponics Technology for Horticultural Crops; ICAR IARI: New Delhi, India, 2018; Volume 188, p. 30. [Google Scholar]
- Singh, A. Salinization of agricultural lands due to poor drainage: A viewpoint. Ecol. Indic. 2018, 95, 127–130. [Google Scholar] [CrossRef]
- Singh, A. Salinization and drainage problems of agricultural land. Irrig. Drain. 2020, 69, 844–853. [Google Scholar] [CrossRef]
- Chanda, S.; Bhat, M.; Shetty, K.G.; Jayachandran, K. Technology, Policy, and Market Adaptation Mechanisms for Sustainable Fresh Produce Industry: The Case of Tomato Production in Florida, USA. Sustainability 2021, 13, 5933. [Google Scholar] [CrossRef]
- Sharma, N.; Acharya, S.; Kumar, K.; Singh, N.; Chaurasia, O.P. Hydroponics as an advanced technique for vegetable production: An overview. J. Soil Water Conserv. 2018, 17, 364–371. [Google Scholar] [CrossRef]
- Wang, X.; Yun, J.; Shi, P.; Li, Z.; Li, P.; Xing, Y. Root growth, fruit yield and water use efficiency of greenhouse grown tomato under different irrigation regimes and nitrogen levels. J. Plant Growth Regul. 2019, 38, 400–415. [Google Scholar] [CrossRef]
- Du, Y.D.; Cao, H.X.; Liu, S.Q.; Gu, X.B.; Cao, Y.X. Response of yield, quality, water and nitrogen use efficiency of tomato to different levels of water and nitrogen under drip irrigation in Northwestern China. J. Integr. Agric. 2017, 16, 1153–1161. [Google Scholar] [CrossRef]
- Wei, Z.; Du, T.; Li, X.; Fang, L.; Liu, F. Interactive effects of CO2 concentration elevation and nitrogen fertilization on water and nitrogen use efficiency of tomato grown under reduced irrigation regimes. Agric. Water Manag. 2018, 202, 174–182. [Google Scholar] [CrossRef]
- Zhou, H.; Kang, S.; Li, F.; Du, T.; Shukla, M.K.; Li, X. Nitrogen application modified the effect of deficit irrigation on tomato transpiration, and water use efficiency in different growth stages. Sci. Hortic. 2020, 263, 109112. [Google Scholar] [CrossRef]
- Ding, L.; Li, Y.; Gao, L.; Lu, Z.; Wang, M.; Ling, N.; Guo, S. Aquaporin expression and water transport pathways inside leaves are affected by nitrogen supply through transpiration in rice plants. Int. J. Mol. Sci. 2018, 19, 256. [Google Scholar] [CrossRef]
- Gloser, V.; Dvorackova, M.; Mota, D.H.; Petrovic, B.; Gonzalez, P.; Geilfus, C.M. Early changes in nitrate uptake and assimilation under drought in relation to transpiration. Front. Plant Sci. 2020, 11, 602065. [Google Scholar] [CrossRef]
- Ullah, H.; Santiago-Arenas, R.; Ferdous, Z.; Attia, A.; Datta, A. Improving water use efficiency, nitrogen use efficiency, and radiation use efficiency in field crops under drought stress: A review. Adv. Agron. 2019, 156, 109–157. [Google Scholar] [CrossRef]
- Kunrath, T.R.; Lemaire, G.; Sadras, V.O.; Gastal, F. Water use efficiency in perennial forage species: Interactions between nitrogen nutrition and water deficit. Field Crops Res. 2018, 222, 1–11. [Google Scholar] [CrossRef]
- Wang, J.; Chadwick, D.R.; Cheng, Y.; Yan, X. Global analysis of agricultural soil denitrification in response to fertilizer nitrogen. Sci. Total Environ. 2018, 616, 908–917. [Google Scholar] [CrossRef]
- Cheng, M.; Wang, H.; Fan, J.; Xiang, Y.; Tang, Z.; Pei, S.; Zhang, F. Effects of nitrogen supply on tomato yield, water use efficiency and fruit quality: A global meta-analysis. Sci. Hortic. 2021, 290, 110553. [Google Scholar] [CrossRef]
- Qu, Z.; Qi, X.; Shi, R.; Zhao, Y.; Hu, Z.; Chen, Q.; Li, C. Reduced N fertilizer application with optimal blend of controlled-release urea and urea improves tomato yield and quality in greenhouse production system. J. Soil Sci. Plant Nutr. 2020, 20, 1741–1750. [Google Scholar] [CrossRef]
- Ronga, D.; Parisi, M.; Pentangelo, A.; Mori, M.; Di Mola, I. Effects of nitrogen management on biomass production and dry matter distribution of processing tomato cropped in southern Italy. Agronomy 2019, 9, 855. [Google Scholar] [CrossRef]
- Padilla, F.M.; Farneselli, M.; Gianquinto, G.; Tei, F.; Thompson, R.B. Monitoring nitrogen status of vegetable crops and soils for optimal nitrogen management. Agric. Water Manag. 2020, 241, 106356. [Google Scholar] [CrossRef]
- Lins, C.M.T.; de Souza, E.R.; dos Santos Souza, T.E.M.; Paulino, M.K.S.S.; Monteiro, D.R.; de Souza Júnior, V.S.; Schaffer, B. Influence of vegetation cover and rainfall intensity on soil attributes in an area undergoing desertification in Brazil. Catena 2023, 221, 106751. [Google Scholar] [CrossRef]
- Kanan, A.; Allahham, A.; Bouleau, C.; Sayara, T.; Qurie, M.; Awad, L. Improving water use efficiency using sensors and communication system for irrigation of greenhouse tomato in Tulkarm, Palestine. Agric. Res. 2021, 11, 728–736. [Google Scholar] [CrossRef]
- Mhadhbi, H. Plant Hydroponic cultivation: A support for biology research. In Hydroponics—A Standard Methodology for Plant Biological Researches; Asao, T., Ed.; IntechOpen: London, UK, 2012; Volume 1, 256p. [Google Scholar]
- Treftz, C.; Omaye, T. Comparision between hydroponic and soil systems for growing strawberries in a greenhouse. Int. J. Agric. Ext. 2016, 3, 195–200. [Google Scholar]
- Cho, W.J.; Kim, H.J.; Jung, D.H.; Kim, D.W.; Ahn, T.I.; Son, J.E. On-site ion monitoring system for precision hydroponic nutrient management. Comput. Electron. Agric. 2018, 146, 51–58. [Google Scholar] [CrossRef]
- Pant, T.; Agarwal, A.; Bhoj, A.S.; Joshi, R.P.; Prakash, O.; Dwivedi, S.K. Vegetable cultivation under hydroponics in Himalayas: Challenges and opportunities. Def. Life Sci. J. 2018, 3, 111–119. [Google Scholar] [CrossRef]
- Silva, F.J.D.; Santos, J.A.; Silva, M.M.D.; Silva, Ê.F.F.; Souza, E.R.D. Water relations of chives in function of salinity and circulation frequency of nutrient solutions. Rev. Bras. Eng. Agric. Ambient. 2019, 23, 359–365. [Google Scholar] [CrossRef]
- Nicola, S.; Pignata, G.; Ferrante, A.; Bulgari, R.; Cocetta, G.; Ertani, A. Water use efficiency in greenhouse systems and its application in horticulture. AgroLife Sci. J 2020, 9, 248–262. [Google Scholar]
- Phene, C.J.; Mccormick, R.L.; Davis, K.R.; Pierre, J.D.; Meek, D.W. A lysimeter feedback irrigation controller system for evapotranspiration measurements and real time irrigation scheduling. Trans. ASAE 1989, 32, 477–484. [Google Scholar] [CrossRef]
- Medrano, E.; Alonso, F.J.; Sánchez-Guerrero, M.C.; Lorenzo, P.; Marhuenda, A.; Briones, P.A. A Simplified P-M model for improving irrigation management of strawberries in a semi-closed hydroponic system. Int. Hortic. Congr. Sci. Hortic. People 2010, 927, 361–366. [Google Scholar] [CrossRef]
- Liang, L.; Ridoutt, B.G.; Lal, R.; Wang, D.; Wu, W.; Peng, P.; Zhao, G. Nitrogen footprint and nitrogen use efficiency of greenhouse tomato production in north China. J. Clean. Prod. 2019, 208, 285–296. [Google Scholar] [CrossRef]
- Jones, J.B., Jr. Tomato Plant Culture: In the Field, Greenhouse, and Home Garden, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007; 420p. [Google Scholar]
- Macwan, J.; Pandya, D. Review on soilless method of cultivation: Hydroponics. Int. J. Recent Sci. Res. 2019, 11, 37122–37127. [Google Scholar] [CrossRef]
- Yang, T.; Altland, J.E.; Samarakoon, U. Evaluation of organic substrates as an alternative to perlite for cucumber production in the dutch bucket hydroponic system. In International Symposium on Growing Media, Soilless Cultivation, and Compost Utilization in Horticulture; ISHS: Portland, OR, USA, 2021; Volume 1317, pp. 319–326. [Google Scholar] [CrossRef]
- Snyder, R.G. Greenhouse Tomato Handbook; Mississippi State University, Cooperative Extension Service (Department of Information Services, Division of Agriculture, Forestry, and Veterinary Medicine): Starkville, MS, USA, 1994; p. 24. [Google Scholar]
- Miyazawa, M.; Pavan, M.A.; Muraoka, T.; do Carmo, C.A.F.S.; de Melo, W.J. Análise química de tecido vegetal. In Manual de Análises Químicas de Solos, Plantas e Fertilizantes; da Silva, F.C., Ed.; Embrapa Informação Tecnológica: Brasília, DF, Brasil, 2009; 627p. [Google Scholar]
- Luo, J.; Yang, Z.; Zhang, F.; Li, C. Effect of nitrogen application on enhancing high-temperature stress tolerance of tomato plants during the flowering and fruiting stage. Front. Plant Sci. 2023, 14, 1172078. [Google Scholar] [CrossRef]
- Pérez-Molina, J.P. Chlorophyll fluorescence and biomass partitioning within light and nitrogen deficiency: An example of the use of the R programming language for teaching. UNED Res. J. 2020, 12, 92–105. [Google Scholar] [CrossRef][Green Version]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Jiménez, M.; Piñero, M.C.; Del Amor, F.M. Heat shock, high CO2 and nitrogen fertilization effects in pepper plants submitted to elevated temperatures. Sci. Hortic. 2019, 244, 322–329. [Google Scholar] [CrossRef]
- Banks, J.M. Chlorophyll fluorescence as a tool to identify drought stress in Acer genotypes. Environ. Exp. Bot. 2018, 155, 118–127. [Google Scholar] [CrossRef]
- Ali, K.A.; Noraldeen, S.S.; Yaseen, A.A. An evaluation study for chlorophyll estimation techniques. Sarhad J. Agric. 2021, 37, 1458–1465. [Google Scholar] [CrossRef]
- Song, Y.; Wan, G.Y.; Wang, J.X.; Zhang, Z.S.; Xia, J.Q.; Sun, L.Q.; Lu, J.; Ma, C.X.; Yu, L.H.; Xiang, C.B.; et al. Balanced nitrogen–iron sufficiency boosts grain yield and nitrogen use efficiency by promoting tillering. Mol. Plant 2023, 16, 1661–1677. [Google Scholar] [CrossRef]
- Larsen, A.; Dale, K.; Eek, M. Radiographic evaluation of rheumatoid arthritis and related conditions by standard reference films. Acta Radiol. Diagn. 1977, 18, 481–491. [Google Scholar] [CrossRef]
- Steiner, I.D. Models for inferring relationships between group size and potential group productivity. Behav. Sci. 1966, 11, 273–283. [Google Scholar] [CrossRef]
- Rosa-Rodríguez, R.D.L.; Lara-Herrera, A.; Trejo-Téllez, L.I.; Padilla-Bernal, L.E.; Solis-Sánchez, L.O.; Ortiz-Rodríguez, J.M. Water and fertilizers use efficiency in two hydroponic systems for tomato production. Hortic. Bras. 2020, 38, 47–52. [Google Scholar] [CrossRef]
- Yang, X.; Wang, S.; Liu, W.; Huang, S.; Xie, Y.; Meng, X.; Li, Z.; Jin, N.; Jin, L.; Lyu, J.; et al. Different spatial configurations of LED light sources enhance growth in tomato seedlings by influencing photosynthesis, CO2 assimilation, and endogenous hormones. Plants 2025, 14, 1369. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.I. Photoprotection and other responses of plants to high light stress. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992, 43, 599–626. [Google Scholar] [CrossRef]
- Tietz, S.; Hall, C.C.; Cruz, J.A.; Kramer, D.M. NPQ (T): A chlorophyll fluorescence parameter for rapid estimation and imaging of non-photochemical quenching of excitons in photosystem-II-associated antenna complexes. Plant Cell Environ. 2017, 40, 1243–1255. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Chu, Y.; Ma, G.; Zhang, Y.; Zhang, X.; Wang, M.; Lu, H.; Wang, L.; Kang, G.; Ma, D.; et al. Physiological mechanisms underlying reduced photosynthesis in wheat leaves grown in the field under conditions of nitrogen and water deficiency. Crop J. 2023, 11, 638–650. [Google Scholar] [CrossRef]
- Niu, J.; Chen, F.; Mi, G.; Li, C.; Zhang, F. Transpiration, and nitrogen uptake and flow in two maize (Zea mays L.) inbred lines as affected by nitrogen supply. Ann. Bot. 2007, 99, 153–160. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Fernandes, Â.; Xyrafis, E.; Polyzos, N.; Antoniadis, V.; Barros, L.; CFR Ferreira, I. The optimization of nitrogen fertilization regulates crop performance and quality of processing tomato (Solanum lycopersicum L. cv. Heinz 3402). Agronomy 2020, 10, 715. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, S.; Li, Z.; Chang, T.; Xu, Q.; Xu, H.; Zhang, J. Effects of excessive nitrogen fertilizer and soil moisture deficiency on antioxidant enzyme system and osmotic adjustment in tomato seedlings. Int. J. Agric. Biol. Eng. 2022, 15, 127–134. [Google Scholar]
- Jiao, X.; Yu, X.; Ding, J.; Du, Q.; Zhang, J.; Song, X.; Bai, P.; Li, J. Effects of rising VPD on the nutrient uptake, water status and photosynthetic system of tomato plants at different nitrogen applications under low temperature. Sci. Hortic. 2022, 304, 111335. [Google Scholar] [CrossRef]
- Yue, W.; Liu, L.; Chen, S.; Bai, Y.; Li, N. Effects of water and nitrogen coupling on growth, yield and quality of greenhouse tomato. Water 2022, 14, 3665. [Google Scholar] [CrossRef]
- Li, H.; Liu, H.; Gong, X.; Li, S.; Pang, J.; Chen, Z.; Sun, J. Optimizing irrigation and nitrogen management strategy to trade off yield, crop water productivity, nitrogen use efficiency and fruit quality of greenhouse grown tomato. Agric. Water Manag. 2021, 245, 106570. [Google Scholar] [CrossRef]



| N Treatment | N-NO3 | N-NH4 | P | K | Ca | Mg | Na | S-SO4 | Cl | Fe | B | Cu | Zn | Mn | Mo | CE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ppm | dS m−1 | |||||||||||||||
| 85.5 | 78.5 | 7.0 | 48.0 | 303.8 | 180.0 | 47.9 | 1.1 | 145.5 | 177.9 | 3.00 | 1.07 | 0.20 | 0.40 | 1.50 | 0.10 | 2.22 |
| 128.3 | 117.8 | 10.5 | 48.0 | 303.8 | 180.0 | 47.9 | 1.1 | 149.0 | 79.5 | 3.00 | 1.07 | 0.20 | 0.40 | 1.50 | 0.10 | 2.23 |
| 171 | 157.0 | 14.0 | 48.0 | 303.8 | 180.0 | 47.9 | 1.1 | 144.1 | 0.0 | 3.00 | 1.07 | 0.20 | 0.40 | 1.50 | 0.10 | 2.26 |
| 213.8 | 178.5 | 35.3 | 48.0 | 303.8 | 180.0 | 47.9 | 1.1 | 143.9 | 0.0 | 3.00 | 1.07 | 0.20 | 0.40 | 1.50 | 0.10 | 2.4 |
| 256.1 | 200.7 | 55.4 | 48.0 | 303.8 | 180.0 | 47.9 | 1.1 | 141.4 | 0.0 | 3.00 | 1.07 | 0.20 | 0.40 | 1.50 | 0.10 | 2.54 |
| N Treatment | MgSO4 | Mg(NO3)2 | (NH4)2SO4 | KH2PO4 | KNO3 | K2SO4 | KCl | Borax | CuS4 | ZnSO4 | MnSO4 | (NH4)6 Mo7O24 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ppm | g | |||||||||||
| 85.5 | 50.00 | - | - | 20.00 | - | 50.00 | 0.01 | 0.95 | 0.08 | 0.18 | 0.46 | 0.02 |
| 128.3 | 50.00 | - | - | 20.00 | - | 50.00 | 0.01 | 0.95 | 0.08 | 0.18 | 0.46 | 0.02 |
| 171 | 50.00 | - | - | 20.00 | 10.00 | 50.00 | - | 0.95 | 0.08 | 0.18 | 0.46 | 0.02 |
| 213.8 | 50.00 | - | 10.00 | 20.00 | 10.00 | 30.00 | - | 0.95 | 0.08 | 0.18 | 0.46 | 0.02 |
| 256.1 | 40.00 | 10.00 | 10.00 | 20.00 | 10.00 | 40.00 | - | 0.95 | 0.08 | 0.18 | 0.46 | 0.02 |
| N Concentrations | Ca(NO3)2 | CaCl2 | KNO3 | NH4NO3 | Ferro EDTA |
|---|---|---|---|---|---|
| ppm | g | ||||
| 85.5 | 50.00 | 30.00 | - | 10.00 | 2.31 |
| 128.3 | 90.00 | 10.00 | - | 10.00 | 2.31 |
| 171 | 110.00 | - | 10.00 | 10.00 | 2.31 |
| 213.8 | 110.00 | - | 10.00 | 10.00 | 2.31 |
| 256.1 | 110.00 | - | 10.00 | 20.00 | 2.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dourado, P.R.M.; Paulino, M.K.S.S.; Leal, L.Y.d.C.; Araújo, C.A.F.; Nunes, J.A.; de Oliveira, E.C.; Santos Júnior, J.A.; de Camargo Santos, A.; de Sá Leitão, D.A.H.; Nunes, M.R.; et al. Water Demand and Photosynthetic Performance of Tomatoes Grown Hydroponically Under Increasing Nitrogen Concentrations. Water 2025, 17, 1951. https://doi.org/10.3390/w17131951
Dourado PRM, Paulino MKSS, Leal LYdC, Araújo CAF, Nunes JA, de Oliveira EC, Santos Júnior JA, de Camargo Santos A, de Sá Leitão DAH, Nunes MR, et al. Water Demand and Photosynthetic Performance of Tomatoes Grown Hydroponically Under Increasing Nitrogen Concentrations. Water. 2025; 17(13):1951. https://doi.org/10.3390/w17131951
Chicago/Turabian StyleDourado, Pablo Rugero Magalhães, Martha Katharinne Silva Souza Paulino, Lucas Yago de Carvalho Leal, Cicero Aparecido Ferreira Araújo, José Alfredo Nunes, Emidio Cantídio de Oliveira, José Amilton Santos Júnior, Aline de Camargo Santos, Diego Arruda Huggins de Sá Leitão, Márcio Renato Nunes, and et al. 2025. "Water Demand and Photosynthetic Performance of Tomatoes Grown Hydroponically Under Increasing Nitrogen Concentrations" Water 17, no. 13: 1951. https://doi.org/10.3390/w17131951
APA StyleDourado, P. R. M., Paulino, M. K. S. S., Leal, L. Y. d. C., Araújo, C. A. F., Nunes, J. A., de Oliveira, E. C., Santos Júnior, J. A., de Camargo Santos, A., de Sá Leitão, D. A. H., Nunes, M. R., Schaffer, B., & Souza, E. R. d. (2025). Water Demand and Photosynthetic Performance of Tomatoes Grown Hydroponically Under Increasing Nitrogen Concentrations. Water, 17(13), 1951. https://doi.org/10.3390/w17131951










