Screening and Application of High-Efficiency Ammonia Nitrogen Degrading Bacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Screening of Pseudomonas Scp1
2.2. Screening of Rhodococcus Scr1
2.3. Identification and Preservation of Microbial Strains
2.4. Laboratory Pilot Experiment
2.5. Specific Experimental Steps
2.6. Shrimp Pond Experiment
3. Results
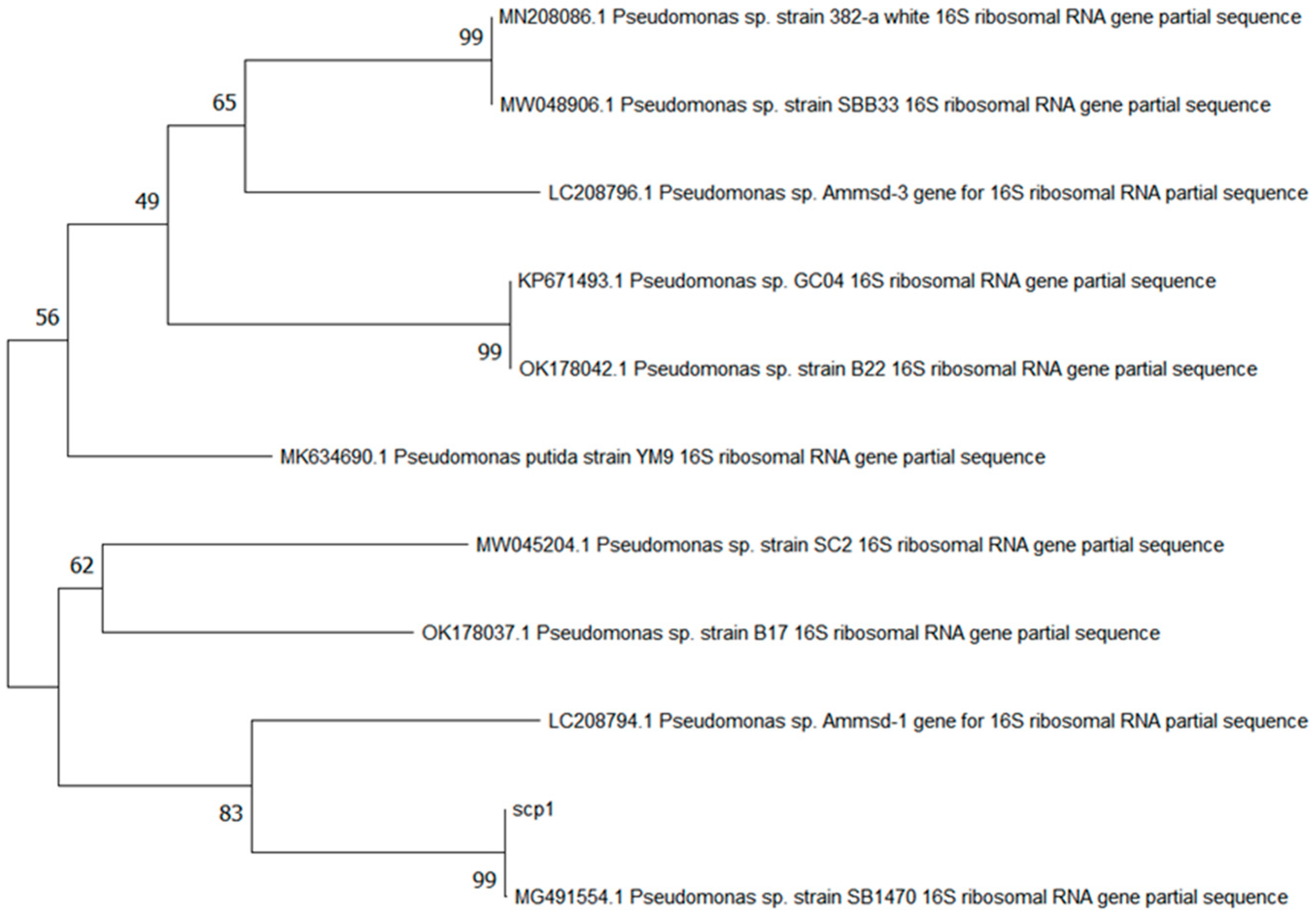
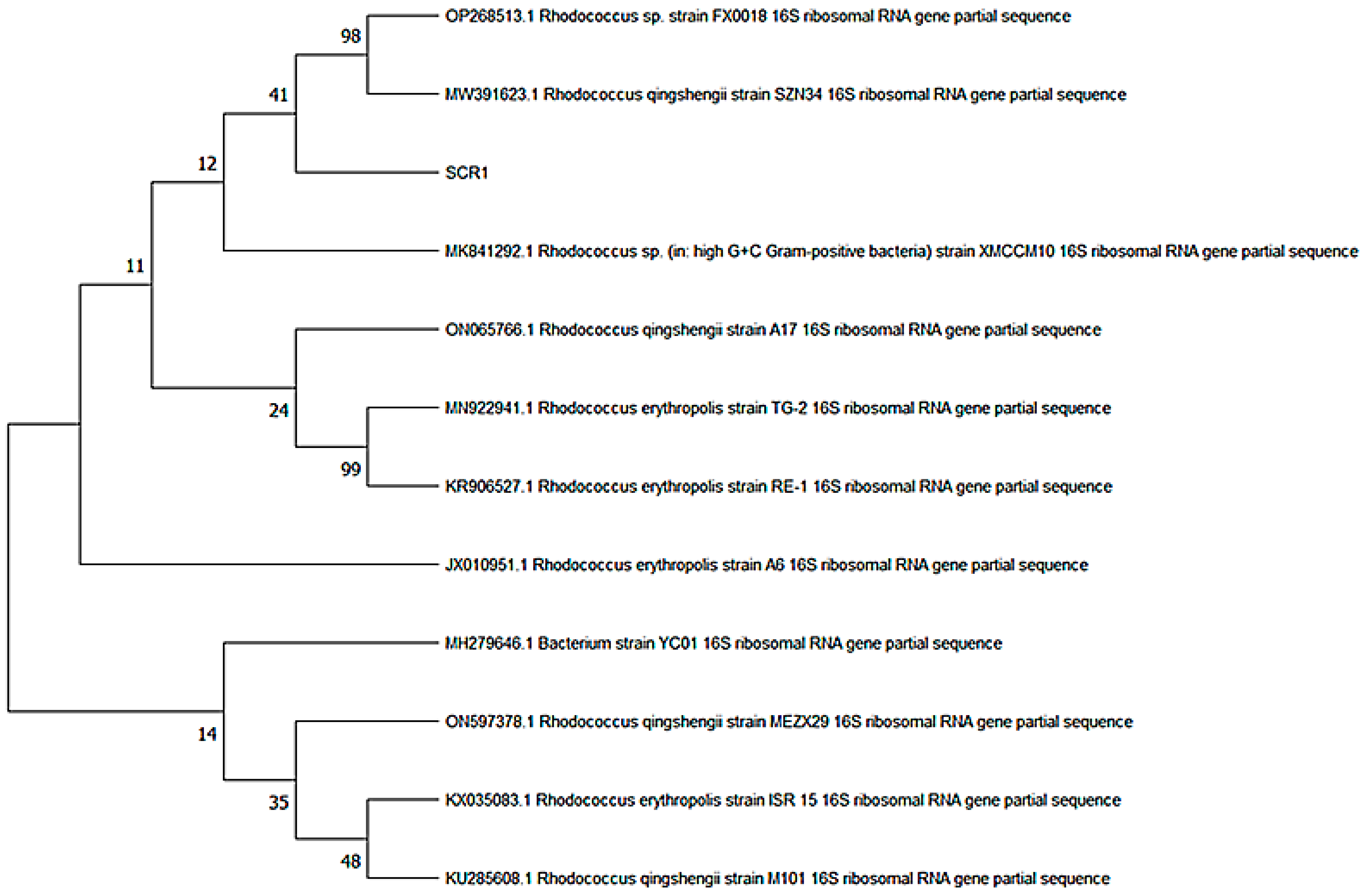
3.1. Screening and Identification Results
3.2. Results of Ammonia Nitrogen Degradation Experiment in Polluted River Water
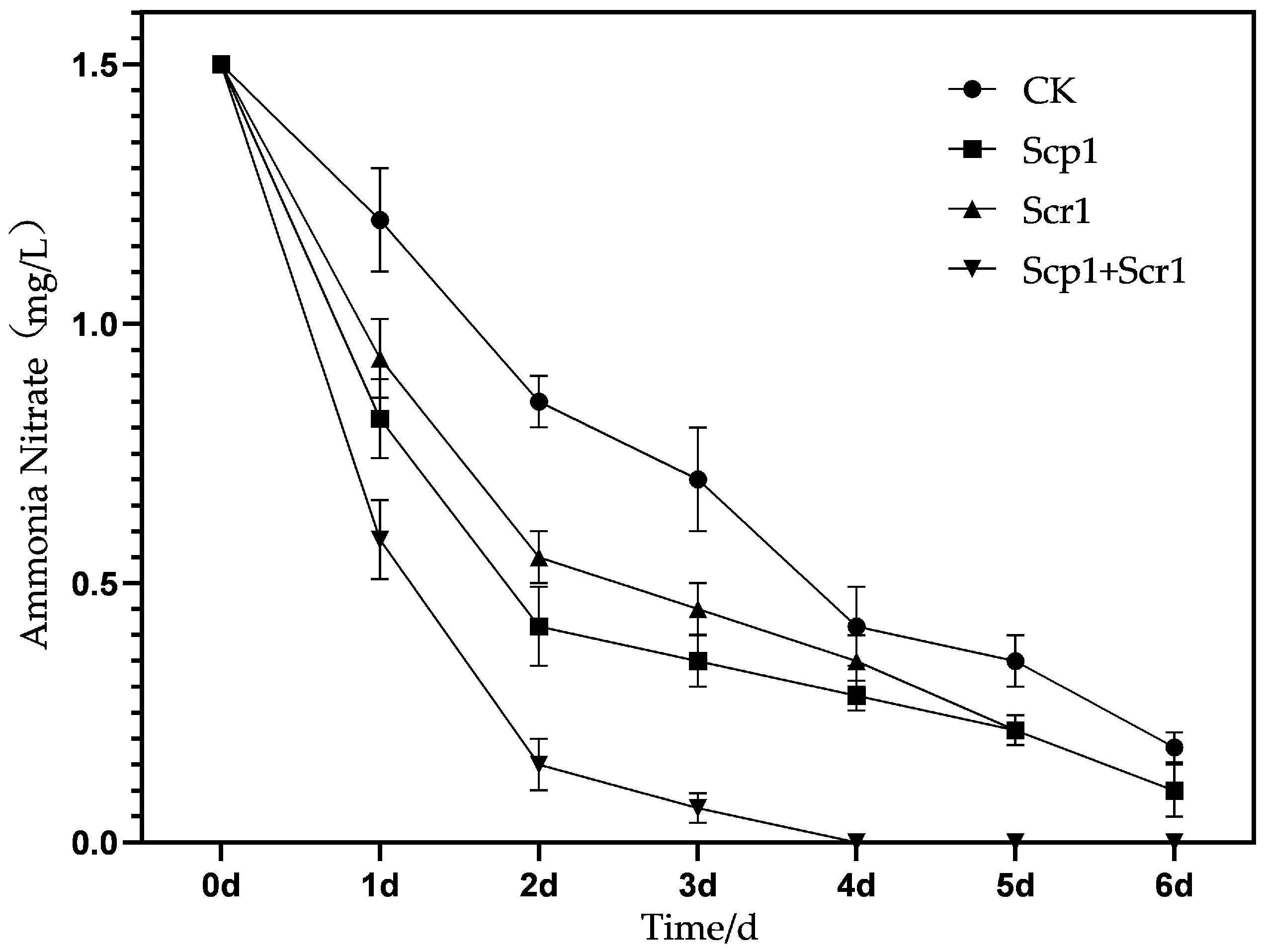
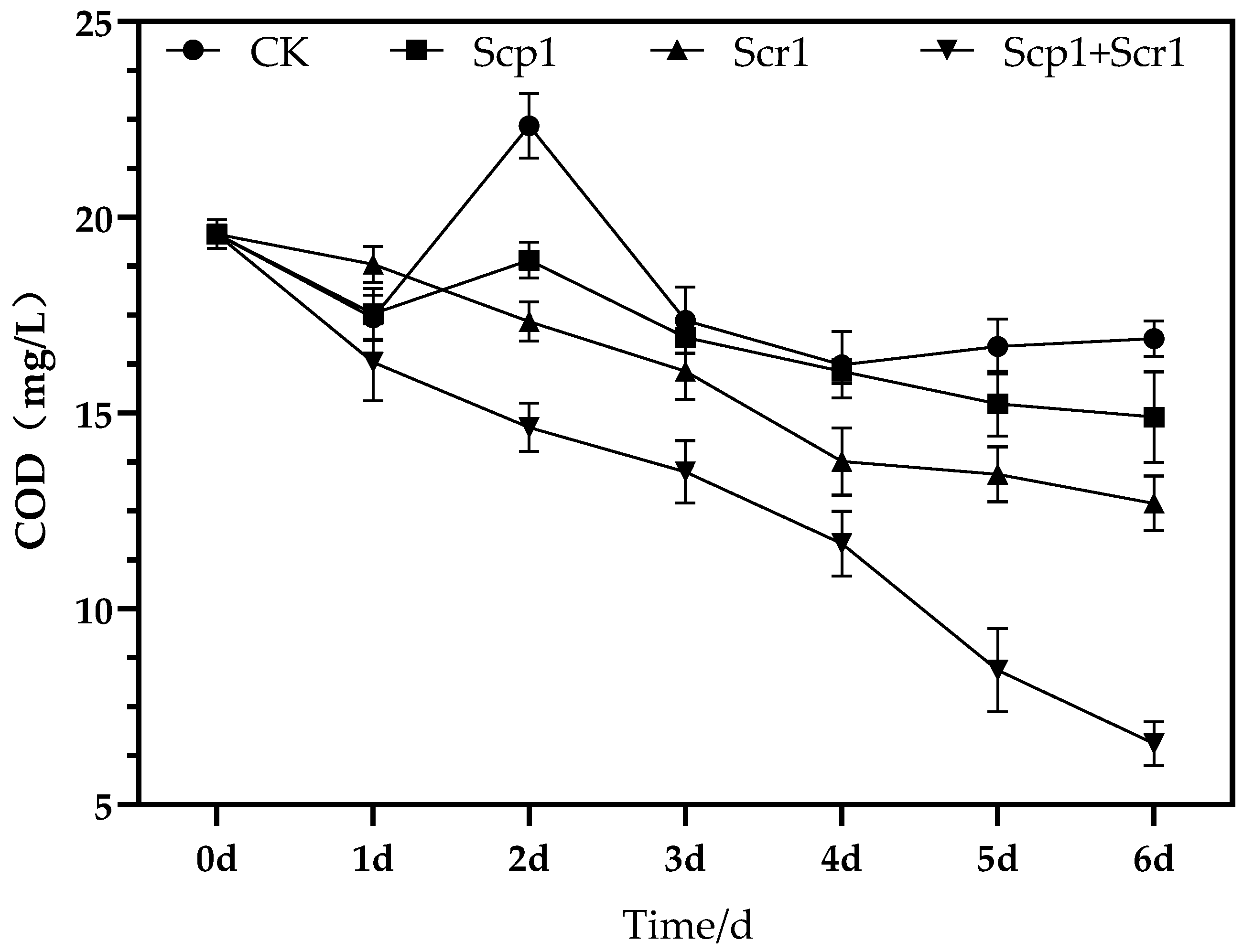
3.3. Results of Laboratory Nitrogen Degradation Experiment
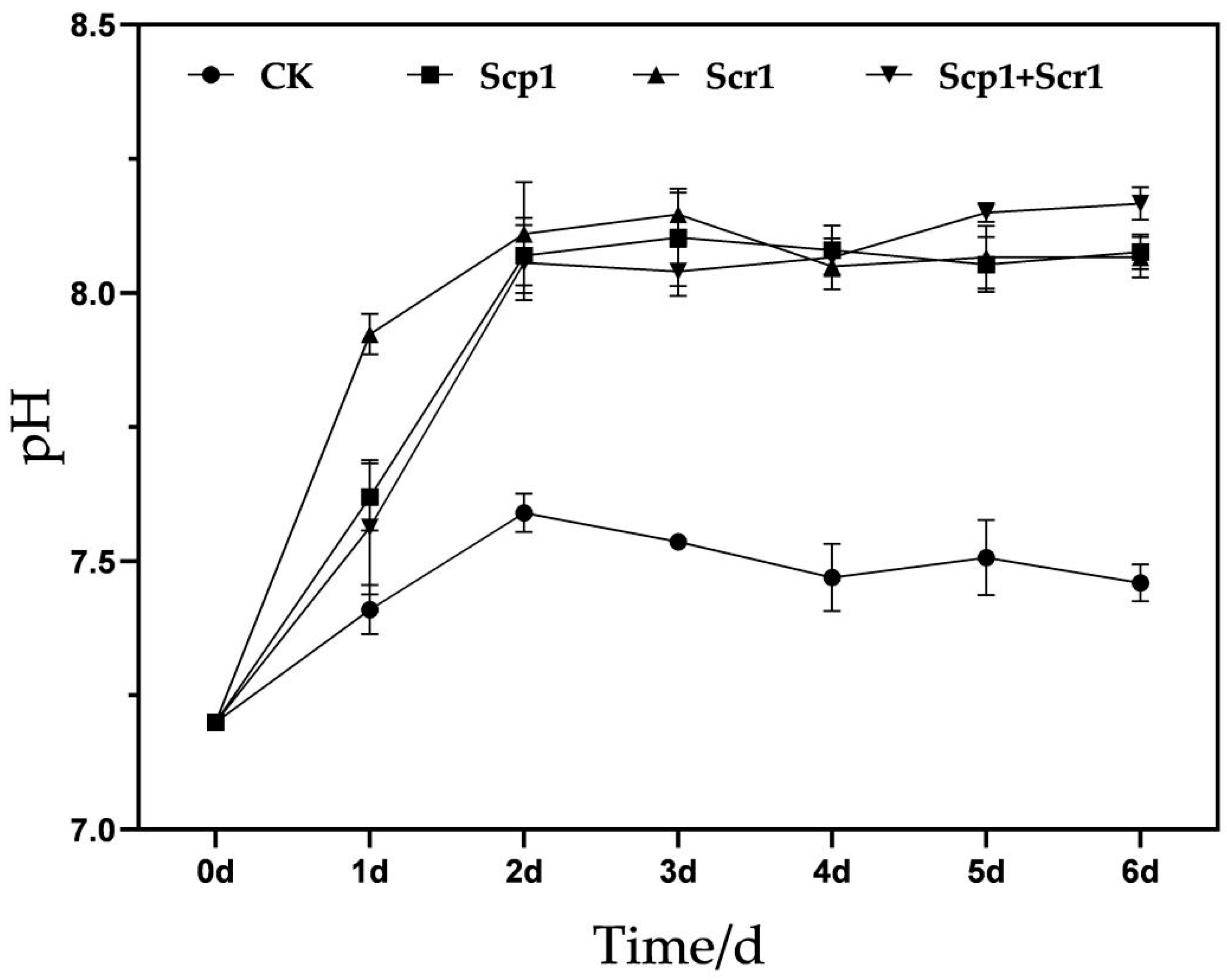
3.4. Results of Laboratory Vial pH Change Experiment
3.5. Results of COD in Laboratory Vials
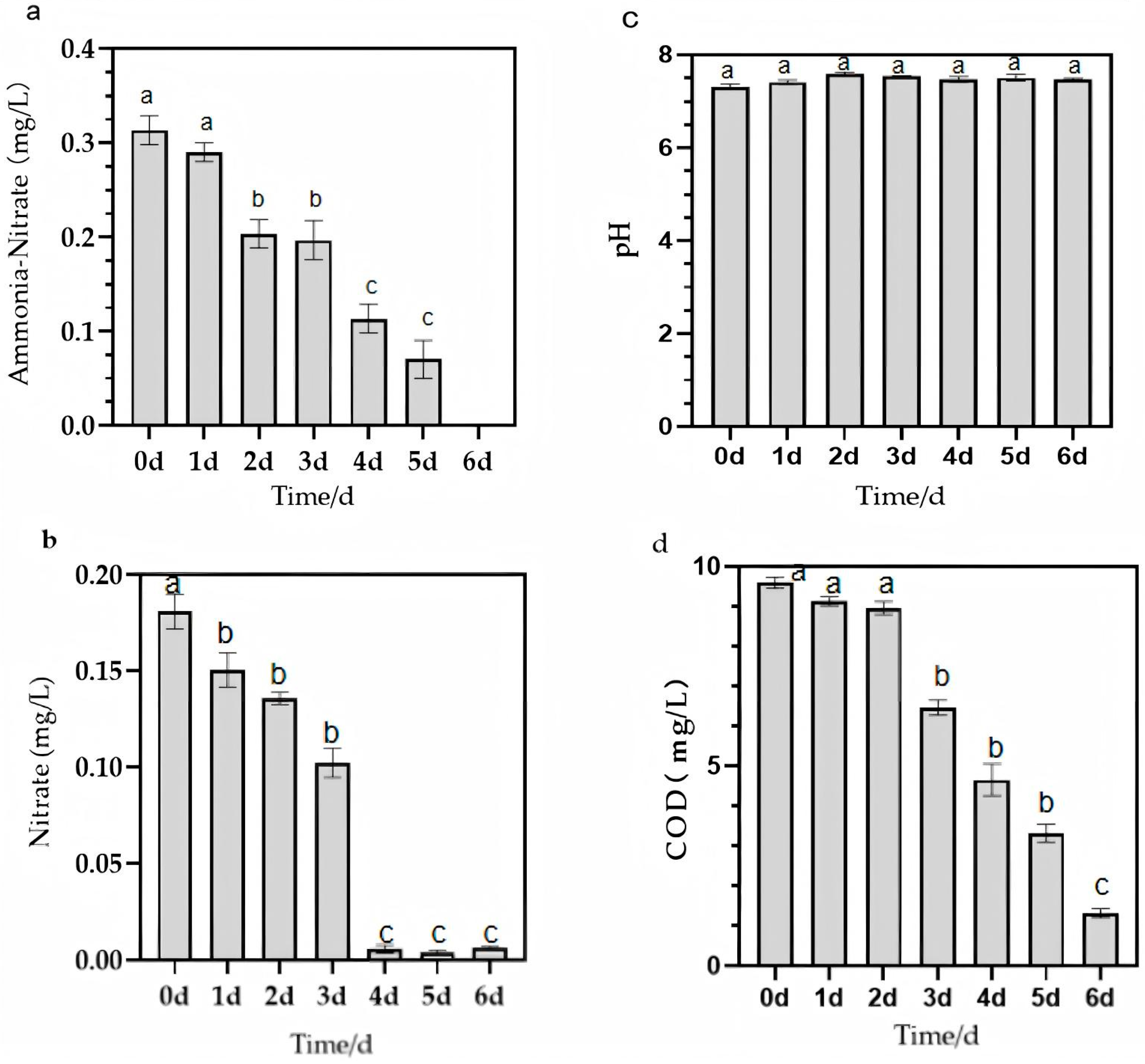
3.6. Results of Penaeus Vannamei Aquaculture Water Test
4. Discussion
4.1. Comparison of Ammonia Nitrogen Degradation Performance
4.2. Metabolic Mechanism of Ammonia Nitrogen Degradation
4.3. Limitations and Challenges
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qin, X.; Li, T.; Zhu, L. Research progress of aquaculture pollution prevention and control technology. Environ. Prot. Front. 2021, 11, 1118–1123. [Google Scholar]
- Liu, G. Hazards and prevention of ammonia nitrogen and nitrite nitrogen in aquaculture. Aquac. Feed. 2018, 5, 37–38. [Google Scholar]
- Lu, J.; Zhang, Y.; Wu, J.; Wang, J. Nitrogen removal in recirculating aquaculture water with high dissolved oxygen conditions using the simultaneous partial nitrification, anammox and denitrification system. Bioresour. Technol. 2020, 305, 123037. [Google Scholar] [CrossRef] [PubMed]
- Zadinelo, I.V.; Dos Santos, L.D.; Cagol, L.; de Muniz, G.I.B.; de Souza Neves Ellendersen, L.; Alves, H.J.; Bombardelli, R.A. Adsorption of aquaculture pollutants using a sustainable biopolymer. Environ. Sci. Pollut. Res. Int. 2018, 25, 4361–4370. [Google Scholar] [CrossRef]
- Cerda, Á.; González, M.; Rodríguez, C.; Serrano, J.; Leiva, E. Feammox Bacterial Biofilms as an Alternative Biological Process for the Removal of Nitrogen from Agricultural Wastewater. Agriculture 2023, 13, 728. [Google Scholar] [CrossRef]
- Ciji, A.; Akhtar, M.S. Nitrite implications and its management strategies in aquaculture: A review. Rev. Aquac. 2020, 12, 878–908. [Google Scholar] [CrossRef]
- John, E.M.; Krishnapriya, K.; Sankar, T.V. Treatment of ammonia and nitrite in aquaculture wastewater by an assembled bacterial consortium. Aquaculture 2020, 526, 735390. [Google Scholar] [CrossRef]
- Emenike, E.C.; Iwuozor, K.O.; Anidiobi, S.U. Heavy Metal Pollution in Aquaculture: Sources, Impacts and Mitigation Techniques. Biol. Trace Elem. Res. 2022, 200, 4476–4492. [Google Scholar] [CrossRef]
- Hejazy, M.; Norouzi, R.; Abdi, F.; Javid, F. The impact of aquaculture activities on nitrogenous and phosphorous pollution of water resources in northern Iran. Arab. J. Geosci. 2023, 16, 255. [Google Scholar] [CrossRef]
- Zhai, X.; Li, S.; Wang, Y.; Cao, S.; Sun, W.; Liu, M.; Mao, G.; Cao, B.; Wang, H. A magnet-renewable electroanalysis strategy for hydrogen sulfide in aquaculture freshwater using magnetic silver metal-organic frameworks. Anal. Chim. Acta 2022, 1195, 339450. [Google Scholar] [CrossRef]
- Huang, S.; Fu, Y.; Zhang, H.; Wang, C.; Zou, C.; Lu, X. Research progress of novel bio-denitrification technology in deep wastewater treatment. Front. Microbiol. 2023, 14, 1284369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, M.; Qu, J.; Zhang, Y.; Liu, H. Characterization and mechanism analysis of tylosin biodegradation and simultaneous ammonia nitrogen removal with strain Klebsiella pneumoniae TN-1. Bioresour. Technol. 2021, 336, 125342. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Chen, W.; Cai, M.; Hei, L. The screening of high-efficiency ammonia-nitrogen degrading bacteria and their influencing factors were studied. IOP Conf. Ser. Earth Environ. Sci. 2022, 983, 012103. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, H.; Cui, T. Identification of a Strain Degrading Ammonia Nitrogen, Optimization of Ammonia Nitrogen Degradation Conditions, and Gene Expression of Key Degrading Enzyme Nitrite Reductase. Fermentation 2023, 9, 397. [Google Scholar] [CrossRef]
- Gutiérrez-Salazar, G.J.; Molina-Garza, Z.J.; Hernández-Acosta, M.; García-Salas, J.A.; Mercado-Hernández, R.; Galaviz-Silva, L. Pathogens in Pacific white shrimp (Litopenaeus vannamei Boone, 1931) and their relationship with physicochemical parameters in three different culture systems in Tamaulipas, Mexico. Aquaculture 2011, 321, 34–40. [Google Scholar] [CrossRef]
- Aguilar-Torrejón, J.A.; Balderas-Hernández, P.; Roa-Morales, G.; Barrera-Díaz, C.E.; Rodríguez-Torres, I.; Torres-Blancas, T. Relationship, importance, and development of analytical techniques: COD, BOD, and, TOC in water—An overview through time. SN Appl. Sci. 2023, 5, 118. [Google Scholar] [CrossRef]
- Chatziantoniou, A.; Charalampis Spondylidis, S.; Stavrakidis-Zachou, O.; Papandroulakis, N.; Topouzelis, K. Dissolved oxygen estimation in aquaculture sites using remote sensing and machine learning. Remote Sens. Appl. Soc. Environ. 2022, 28, 100865. [Google Scholar] [CrossRef]
- Kuo, D.H. Light-driven nitrite reduction by Rhodobacter capsulatus. Bioresour. Technol. 2015, 190, 522–528. [Google Scholar]
- Lu, H. Synergistic ammonia oxidation by Pseudomonas putida and anoxygenic phototrophs. Environ. Sci. Technol. 2018, 52, 8523–8532. [Google Scholar]
- Madigan, M.T.; Aiyer, J.; Buckley, D.; Sattley, W.; Stahl, D. Brock Biology of Microorganisms, 16th ed.; Pearson: London, UK, 2022. [Google Scholar]
- Chen, J. Cross-feeding of oxygen and carbon between bacteria for nitrogen removal. Water Res. 2020, 178, 115846. [Google Scholar]
- Li, Y. Engineering consortia for sustainable ammonia removal. Appl. Microbiol. Biotechnol. 2021, 105, 3721–3734. [Google Scholar]
- Valencia-Castañeda, G.; Frías-Espericueta, M.G.; Vanegas-Pérez, R.C.; Pérez-Ramírez, J.A.; Chávez-Sánchez, M.C.; Páez-Osuna, F. Acute Toxicity of Ammonia, Nitrite and Nitrate to Shrimp Litopenaeus vannamei Postlarvae in Low-Salinity Water. Bull. Environ. Contam. Toxicol. 2018, 101, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Deng, X.; Hou, D.; Zeng, S.; Deng, Z.; Zhou, R.; Zhang, L.; Hou, Q.; Chen, Q.; Weng, S. Effects of water ammonia nitrogen on hemolymph and intestinal microbiota of Litopenaeus vannamei. Adv. Biotechnol. 2024, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- Tu, P.T.; Hai, V.H.; Lien, N.T.; Diep, D.X. Evaluation of short-term toxicity of ammonia and nitrite on the survival of whiteleg shrimp, Litopenaeus vannamei juveniles. Isr. J. Aquac. 2022, 74. [Google Scholar] [CrossRef]
- Brailo, M.; Schreier, H.J.; McDonald, R.; Maršić-Lučić, J.; Gavrilović, A.; Pećarević, M.; Jug-Dujaković, J. Bacterial community analysis of marine recirculating aquaculture system bioreactors for complete nitrogen removal established from a commercial inoculum. Aquaculture 2019, 503, 198–206. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, B.; Sun, Z.; Wang, T.; Tan, S.; Fan, X.; Zou, D.; Zhuang, Y.; Liu, X.; Wang, Y.; et al. Comparision of nitrogen removal characteristic and microbial community in freshwater and marine recirculating aquaculture systems. Sci. Total Environ. 2023, 878, 162870. [Google Scholar] [CrossRef]
- Li, R.; Xiao, X.; Zhao, Y.; Tu, B.; Zhang, Y. Screening of efficient ammonia–nitrogen degrading bacteria and its application in livestock wastewater. Biomass Convers. Biorefinery 2024, 14, 8513–8521. [Google Scholar] [CrossRef]
- Wu, Y.; Ren, X.; Kou, Y. Strengthening effect of compound microbial inoculum on the treatment of high concentration ammonia-nitrogen wastewater. Sci. Technol. Eng. 2020, 20, 10544–10549. [Google Scholar]
- Ding, Q.; Xie, M.; Chen, F. Isolation and identification of a strain with high efficiency of removing ammonia-nitrogen and nitrate nitrogen and its application in biological floc shrimp culture. China Fish. Sci. 2019, 26, 959–970. [Google Scholar]
- Dewangan, S.K.; Toppo, D.N.; Kujur, A. Investigating the impact of pH levels on water quality: An experimental approach. Int. J. Res. Appl. Sci. Eng. Technol. 2023, 11, 756–759. [Google Scholar] [CrossRef]
- Kochhar, N.; Kaiya, I.K.; Shrivastava, S.; Ghosh, A.; Rawat, V.S.; Sodhi, K.K.; Kumar, M. Perspectives on the microorganism of extreme environments and their applications. Curr. Res. Microb. Sci. 2022, 3, 100134. [Google Scholar] [CrossRef] [PubMed]
- Tom, A.P.; Jayakumar, J.S.; Biju, M.; Somarajan, J.; Ibrahim, M.A. Aquaculture wastewater treatment technologies and their sustainability: A review. Energy Nexus 2021, 4, 100022. [Google Scholar] [CrossRef]
- Lu, T.; Yang, Y.; Feng, W.J.; Jin, Q.C.; Wu, Z.G.; Jin, Z.H. Effect of the compound bacterial agent on microbial community of the aerobic compost of food waste. Lett. Appl. Microbiol. 2022, 74, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yu, E.; Zhang, K.; Gong, W.; Xia, Y.; Tian, J.; Wang, G.; Xie, J. Water Treatment Effect, Microbial Community Structure, and Metabolic Characteristics in a Field-Scale Aquaculture Wastewater Treatment System. Front. Microbiol. 2020, 11, 930. [Google Scholar] [CrossRef]
- Van Den Hende, S.; Beelen, V.; Bore, G.; Boon, N.; Vervaeren, H. Up-scaling aquaculture wastewater treatment by microalgal bacterial flocs: From lab reactors to an outdoor raceway pond. Bioresour. Technol. 2014, 159, 342–354. [Google Scholar] [CrossRef]

| No | Bacterial Strain (Inoculation Amount 1%) | Water Sample |
|---|---|---|
| 1 | Scp1 | 100 mL |
| 2 | Scr1 | 100 mL |
| 3 | Scp1 and Scr1 | 100 mL |
| CK | —— | 100 mL |
| Total | —— | 100 mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Cai, R.; Wei, C.; Liu, X.; Xu, H.-L. Screening and Application of High-Efficiency Ammonia Nitrogen Degrading Bacteria. Water 2025, 17, 1952. https://doi.org/10.3390/w17131952
Song Y, Cai R, Wei C, Liu X, Xu H-L. Screening and Application of High-Efficiency Ammonia Nitrogen Degrading Bacteria. Water. 2025; 17(13):1952. https://doi.org/10.3390/w17131952
Chicago/Turabian StyleSong, Yingte, Ruitao Cai, Chuyang Wei, Xiaoyong Liu, and Hui-Lian Xu. 2025. "Screening and Application of High-Efficiency Ammonia Nitrogen Degrading Bacteria" Water 17, no. 13: 1952. https://doi.org/10.3390/w17131952
APA StyleSong, Y., Cai, R., Wei, C., Liu, X., & Xu, H.-L. (2025). Screening and Application of High-Efficiency Ammonia Nitrogen Degrading Bacteria. Water, 17(13), 1952. https://doi.org/10.3390/w17131952






