Dark Fermentation of Sizing Process Waste: A Sustainable Solution for Hydrogen Production and Industrial Waste Management
Abstract
1. Introduction
2. Materials and Methods
2.1. Inoculum and Substrate
2.2. Experimental Setup
- -
- I connected to a tube with a partition, which allowed for gas sampling;
- -
- II connected to a tube that linked the reactors to bottles containing saline solution, used for measuring the volume of gas produced during the process. This volume was measured using the displacement method.
2.3. Analysis Procedures
3. Results and Discussion
3.1. pH of the Process Environment
3.2. Degradation of Organic Substances
3.3. Stability of Total Nitrogen
3.4. Volatile Fatty Acids
3.5. Hydrogen and Carbon Dioxide
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schneider, U.A.; Havlík, P.; Schmid, E.; Valin, H.; Mosnier, A.; Obersteiner, M.; Böttcher, H.; Skalský, R.; Balkovič, J.; Sauer, T.; et al. Impacts of Population Growth, Economic Development, and Technical Change on Global Food Production and Consumption. Agric. Syst. 2011, 104, 204–215. [Google Scholar] [CrossRef]
- Harte, J. Human Population as a Dynamic Factor in Environmental Degradation. Popul. Environ. 2007, 28, 223–236. [Google Scholar] [CrossRef]
- Raj Kandel, D.; Kwak, D.; Lee, S.; Jie Lim, Y.; Subedi, S.; Lee, J. Harnessing Natural Antifouling Agents for Enhancing Water and Wastewater Treatment Membranes. Sep. Purif. Technol. 2025, 359, 130254. [Google Scholar] [CrossRef]
- Pimentel, D.; Pimentel, M. Global Environmental Resources versus World Population Growth. Ecol. Econ. 2006, 59, 195–198. [Google Scholar] [CrossRef]
- United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development; United Nations: New York, NY, USA, 2015. [Google Scholar]
- Borkowski, A. Akademia Marynarki Wojennej w Gdyni. Przyczyny I Skutki Globalnego Ocieplenia Klimatu. 2021. Available online: https://nawigacja.gdynia.pl/wp-content/uploads/2021/03/PRZYCZYNY-I-SKUTKI-GLOBALNEGO-OCIEPLENIA-KLIMATU.pdf (accessed on 27 January 2025).
- Malucha, M. Przyczyny Zmian Klimatu. Próba Eksplikacji Głównych Problemów. Pr. Nauk. Uniw. Ekon. We Wrocławiu 2010, 140, 53–67. [Google Scholar]
- European Commission. Causes of Climate Change. Available online: https://climate.ec.europa.eu/climate-change/causes-climate-change_en (accessed on 27 January 2025).
- Logan, B.E.; Oh, S.-E.; Kim, I.S.; Van Ginkel, S. Biological Hydrogen Production Measured in Batch Anaerobic Respirometers. Environ. Sci. Technol. 2002, 36, 2530–2535. [Google Scholar] [CrossRef]
- Chen, W.; Chen, S.; Kumarkhanal, S.; Sung, S. Kinetic Study of Biological Hydrogen Production by Anaerobic Fermentation. Int. J. Hydrogen Energy 2006, 31, 2170–2178. [Google Scholar] [CrossRef]
- Sun, J.; Hopkins, R.C.; Jenney, F.E.; McTernan, P.M.; Adams, M.W.W. Heterologous Expression and Maturation of an NADP-Dependent [NiFe]-Hydrogenase: A Key Enzyme in Biofuel Production. PLoS ONE 2010, 5, e10526. [Google Scholar] [CrossRef]
- Das, D. Hydrogen Production by Biological Processes: A Survey of Literature. Int. J. Hydrogen Energy 2001, 26, 13–28. [Google Scholar] [CrossRef]
- Gopalakrishnan, B.; Khanna, N.; Das, D. Dark-Fermentative Biohydrogen Production. In Biomass, Biofuels, Biochemicals: Biohydrogen, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 79–122. ISBN 9780444642035. [Google Scholar]
- Albuquerque, M.M.; Sartor, G.D.; Martinez-Burgos, W.J.; Scapini, T.; Edwiges, T.; Soccol, C.R.; Medeiros, A.B. Biohydrogen Produced via Dark Fermentation: A Review. Methane 2024, 3, 500–532. [Google Scholar] [CrossRef]
- Guo, X.M.; Trably, E.; Latrille, E.; Carrère, H.; Steyer, J.-P. Hydrogen Production from Agricultural Waste by Dark Fermentation: A Review. Int. J. Hydrogen Energy 2010, 35, 10660–10673. [Google Scholar] [CrossRef]
- Chalima, A.; Oliver, L.; Fernández de Castro, L.; Karnaouri, A.; Dietrich, T.; Topakas, E. Utilization of Volatile Fatty Acids from Microalgae for the Production of High Added Value Compounds. Fermentation 2017, 3, 54. [Google Scholar] [CrossRef]
- Trancone, G.; Policastro, G.; Spasiano, D.; Race, M.; Parrino, F.; Fratino, U.; Fabbricino, M.; Pirozzi, F. Treatment of Concrete Waste from Construction and Demolition Activities: Application of Organic Acids from Continuous Dark Fermentation in Moving Bed Biofilm Reactors. Chem. Eng. J. 2025, 505, 159536. [Google Scholar] [CrossRef]
- Lee, W.S.; Chua, A.S.M.; Yeoh, H.K.; Ngoh, G.C. A Review of the Production and Applications of Waste-Derived Volatile Fatty Acids. Chem. Eng. J. 2014, 235, 83–99. [Google Scholar] [CrossRef]
- Guilayn, F.; Jimenez, J.; Monlau, F.; Vaneeckhaute, C. Valorisation of Anaerobic Digestate: Towards Value-Added Products. In Renewable Energy Technologies for Energy Efficient Sustainable Development; Springer: Berlin/Heidelberg, Germany, 2022; pp. 227–262. [Google Scholar]
- Kumar, S. Hydrothermal Processing of Biomass for Biofuels. Biofuel Res. J. 2014, 1, 43. [Google Scholar] [CrossRef]
- Tapia-Venegas, E.; Ramirez-Morales, J.E.; Silva-Illanes, F.; Toledo-Alarcón, J.; Paillet, F.; Escudie, R.; Lay, C.-H.; Chu, C.-Y.; Leu, H.-J.; Marone, A.; et al. Biohydrogen Production by Dark Fermentation: Scaling-up and Technologies Integration for a Sustainable System. Rev. Environ. Sci. Bio/Technol. 2015, 14, 761–785. [Google Scholar] [CrossRef]
- Li, Y.-C.; Chu, C.-Y.; Wu, S.-Y.; Tsai, C.-Y.; Wang, C.-C.; Hung, C.-H.; Lin, C.-Y. Feasible Pretreatment of Textile Wastewater for Dark Fermentative Hydrogen Production. Int. J. Hydrogen Energy 2012, 37, 15511–15517. [Google Scholar] [CrossRef]
- Alqahtani, H.S. Lower-Carbon Hydrogen Production from Wastewater: A Comprehensive Review. Sustainability 2024, 16, 8659. [Google Scholar] [CrossRef]
- Rosa e Silva, G.O.; Carpanez, T.G.; Dos Santos, C.R.; Casella, G.S.; Moreira, V.R.; de Paula, E.C.; Amaral, M.C.S. Biohydrogen Production from Wastewater: Production Technologies, Environmental and Economic Aspects. J. Environ. Chem. Eng. 2024, 12, 114104. [Google Scholar] [CrossRef]
- Casanova-Mina, A.A.; Suárez-Vázquez, S.I.; Acuña-Askar, K.; Alfaro-Barbosa, J.M.; Cruz-López, A. Continuous Dark Fermentation by Industrial Food Wastewater: The Effect of Hydraulic Retention Time on Hydrogen Production and Microbial Variation. Biomass Convers. Biorefinery 2024, 14, 23909–23920. [Google Scholar] [CrossRef]
- Elsayad, R.M.; Sharshir, S.W.; Khalil, A.; Basha, A.M. Recent Advancements in Wastewater Treatment via Anaerobic Fermentation Process: A Systematic Review. J. Environ. Manag. 2024, 366, 121724. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, A.; Zhou, J.L.; Altaee, A.; Li, D. Machine Learning Modeling and Analysis of Biohydrogen Production from Wastewater by Dark Fermentation Process. Bioresour. Technol. 2022, 343, 126111. [Google Scholar] [CrossRef] [PubMed]
- Dzulkarnain, E.L.N.; Audu, J.O.; Wan Dagang, W.R.Z.; Abdul-Wahab, M.F. Microbiomes of Biohydrogen Production from Dark Fermentation of Industrial Wastes: Current Trends, Advanced Tools and Future Outlook. Bioresour. Bioprocess. 2022, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Barca, C.; Soric, A.; Ranava, D.; Giudici-Orticoni, M.-T.; Ferrasse, J.-H. Anaerobic Biofilm Reactors for Dark Fermentative Hydrogen Production from Wastewater: A Review. Bioresour. Technol. 2015, 185, 386–398. [Google Scholar] [CrossRef]
- Li, X.; Guo, L.; Liu, Y.; Wang, Y.; She, Z.; Gao, M.; Zhao, Y. Effect of Salinity and PH on Dark Fermentation with Thermophilic Bacteria Pretreated Swine Wastewater. J. Environ. Manage. 2020, 271, 111023. [Google Scholar] [CrossRef]
- Ziara, R.M.M.; Miller, D.N.; Subbiah, J.; Dvorak, B.I. Lactate Wastewater Dark Fermentation: The Effect of Temperature and Initial PH on Biohydrogen Production and Microbial Community. Int. J. Hydrogen Energy 2019, 44, 661–673. [Google Scholar] [CrossRef]
- Wicher, E.; Seifert, K.; Zagrodnik, R.; Pietrzyk, B.; Laniecki, M. Hydrogen Gas Production from Distillery Wastewater by Dark Fermentation. Int. J. Hydrogen Energy 2013, 38, 7767–7773. [Google Scholar] [CrossRef]
- Sinbuathong, N.; Sillapacharoenkul, B. Dark Fermentation of Starch Factory Wastewater with Acid- and Base-Treated Mixed Microorganisms for Biohydrogen Production. Int. J. Hydrogen Energy 2021, 46, 16622–16630. [Google Scholar] [CrossRef]
- Chen, S.; Sheu, D.; Chen, W.; Lo, Y.; Huang, T.; Lin, C.; Chang, J. Dark Hydrogen Fermentation from Hydrolyzed Starch Treated with Recombinant Amylase Originating from Caldimonas Taiwanensis On1. Biotechnol. Prog. 2007, 23, 1312–1320. [Google Scholar] [CrossRef]
- Masset, J.; Calusinska, M.; Hamilton, C.; Hiligsmann, S.; Joris, B.; Wilmotte, A.; Thonart, P. Fermentative Hydrogen Production from Glucose and Starch Using Pure Strains and Artificial Co-Cultures of Clostridium spp. Biotechnol. Biofuels 2012, 5, 35. [Google Scholar] [CrossRef]
- Tagrida, M.; Gulzar, S.; Martín-Belloso, O.; Elez-Martínez, P.; Soliva-Fortuny, R. Ultrasound and Freeze-Thaw Modifications of Cassava Starch: Microstructure, Functionality, and 3D Printing Potential. Food Hydrocoll. 2025, 162, 110963. [Google Scholar] [CrossRef]
- Parker, R.; Ring, S.G. Aspects of the Physical Chemistry of Starch. J. Cereal Sci. 2001, 34, 1–17. [Google Scholar] [CrossRef]
- Fortuna, T. Skrobie Modyfikowane w Produkcji Żywności. Żywność. Technol. Jakość 1995, 1, 3–7. [Google Scholar]
- Adewale, P.; Yancheshmeh, M.S.; Lam, E. Starch Modification for Non-Food, Industrial Applications: Market Intelligence and Critical Review. Carbohydr. Polym. 2022, 291, 119590. [Google Scholar] [CrossRef]
- Bangar, S.P.; Balakrishnan, G.; Navaf, M.; Sunooj, K.V. Recent Advancements on Barnyard Millet Starch: A Sustainable Alternative to Conventional Starch. Starch-Stärke 2024, 76, 2300232. [Google Scholar] [CrossRef]
- Makroo, H.A.; Naqash, S.; Saxena, J.; Sharma, S.; Majid, D.; Dar, B.N. Recovery and Characteristics of Starches from Unconventional Sources and Their Potential Applications: A Review. Appl. Food Res. 2021, 1, 100001. [Google Scholar] [CrossRef]
- Barbhuiya, R.I.; Wroblewski, C.; Ravikumar, S.P.; Kaur, G.; Routray, W.; Subramanian, J.; Elsayed, A.; Singh, A. Upcycling of Industrial Pea Starch by Rapid Spray Nanoprecipitation to Develop Plant-Derived Oil Encapsulated Starch Nanoparticles for Potential Agricultural Applications. Carbohydr. Polym. 2024, 346, 122618. [Google Scholar] [CrossRef]
- Fortuna, T.; Rożnowski, J. Skrobie Modyfikowane Chemicznie, Ich Właściwości i Zastosowanie. Żywność 2002, 2, 16–29. [Google Scholar]
- Wang, J.; Wang, C.; Yu, J.; Yang, Y.; Copeland, L.; Wang, S. A Novel Composite Resistant Starch with Improved Prebiotic Functions. Food Hydrocoll. 2025, 162, 111015. [Google Scholar] [CrossRef]
- Carvalho, H.J.; Barcia, M.T.; Schmiele, M. Non-Conventional Starches: Properties and Potential Applications in Food and Non-Food Products. Macromol 2024, 4, 886–909. [Google Scholar] [CrossRef]
- Shoukat, L.; Javed, S.; Afzaal, M.; Akhter, N.; Shah, Y.A. Starch-Based Encapsulation to Enhance Probiotic Viability in Simulated Digestion Conditions. Int. J. Biol. Macromol. 2024, 283, 137606. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Guo, F.; Li, J.; Wang, Z.; Liang, Z.; Yi, C. Development of a Novel Energy Saving and Environmentally Friendly Starch via a Graft Copolymerization Strategy for Efficient Warp Sizing and Easy Removal. Polymers 2024, 16, 182. [Google Scholar] [CrossRef] [PubMed]
- Szosland, J. Podstawy Budowy i Technologii Kanin; Wydawnictwo Naukowo-Techniczne: Warsaw, Poland, 1974. [Google Scholar]
- Islam, M.M.; Mahmud, K.; Faruk, O.; Billah, S. Assessment of Environmental Impacts for Textile Dyeing Industries in Bangladesh. In Proceedings of the International Conference on Green Technology and Environmental Conservation (GTEC-2011), Chennai, India, 15–17 December 2011; IEEE: Piscataway, NJ, USA, 2011; pp. 173–181. [Google Scholar]
- Drumond Chequer, F.M.; de Oliveira, G.A.R.; Anastacio Ferraz, E.R.; Carvalho, J.; Boldrin Zanoni, M.V.; de Oliveir, D.P. Textile Dyes: Dyeing Process and Environmental Impact. In Eco-Friendly Textile Dyeing and Finishing; InTech: London, UK, 2013. [Google Scholar]
- Khattab, T.A.; Abdelrahman, M.S.; Rehan, M. Textile Dyeing Industry: Environmental Impacts and Remediation. Environ. Sci. Pollut. Res. 2020, 27, 3803–3818. [Google Scholar] [CrossRef] [PubMed]
- Kant, R. Textile Dyeing Industry an Environmental Hazard. Nat. Sci. 2012, 4, 22–26. [Google Scholar] [CrossRef]
- Zeeshan, M.H.; Ruman, U.E.; He, G.; Sabir, A.; Shafiq, M.; Zubair, M. Environmental Issues Concerned with Poly (Vinyl Alcohol) (PVA) in Textile Wastewater. In Polymer Technology in Dye-containing Wastewater. Sustainable Textiles: Production, Processing, Manufacturing & Chemistry; Springer: Singapore, 2022; pp. 225–236. [Google Scholar]
- Var, C.; Palamutcu, S. Sustainable Approaches in Textile-Sizing Process. In Sustainable Manufacturing Practices in the Textiles and Fashion Sector; Springer: Cham, Switzerland, 2024; pp. 55–74. [Google Scholar]
- Sarkodie, B.; Feng, Q.; Xu, C.; Xu, Z. Desizability and Biodegradability of Textile Warp Sizing Materials and Their Mechanism: A Review. J. Polym. Environ. 2023, 31, 3317–3337. [Google Scholar] [CrossRef]
- Xue, Y.; Chew, J.W. Sustainable Carbonaceous Materials-Based Catalytic Membranes for Organic Wastewater Treatment: Progress and Prospects. Sep. Purif. Technol. 2025, 360, 131119. [Google Scholar] [CrossRef]
- Goswami, B.C.; Anandjiwala, R.D.; Hall, D. Textile Sizing; CRC Press: Boca Raton, FL, USA, 2004; ISBN 9780429223945. [Google Scholar]
- Madhav, S.; Ahamad, A.; Singh, P.; Mishra, P.K. A Review of Textile Industry: Wet Processing, Environmental Impacts, and Effluent Treatment Methods. Environ. Qual. Manag. 2018, 27, 31–41. [Google Scholar] [CrossRef]
- Biyada, S.; Urbonavičius, J. Circularity in Textile Waste: Challenges and Pathways to Sustainability. Clean. Eng. Technol. 2025, 24, 100905. [Google Scholar] [CrossRef]
- Trancone, G.; Spasiano, D.; Race, M.; Luongo, V.; Petrella, A.; Pirozzi, F.; Fratino, U.; Piccinni, A.F. A Combined System for Asbestos-Cement Waste Degradation by Dark Fermentation and Resulting Supernatant Valorization in Anaerobic Digestion. Chemosphere 2022, 300, 134500. [Google Scholar] [CrossRef]
- Domińska, M.; Paździor, K.; Ślęzak, R.; Ledakowicz, S. The Influence of Inoculum Source and Pretreatment on the Biohydrogen Production in the Dark Fermentation Process. Chem. Process Eng. New Front. 2024, 45, e63. [Google Scholar] [CrossRef]
- Broekaert, J.A.C. Daniel C. Harris: Quantitative Chemical Analysis, 9th Ed. Anal. Bioanal. Chem. 2015, 407, 8943–8944. [Google Scholar] [CrossRef]
- Magrel, L. Metodyka Oceny Efektywności Procesu Fermentacji Metanowej Wybranych Osadów Ściekowych; Wydawnictwo PB: Białystok, Poland, 2002. [Google Scholar]
- Boruszko, D.; Butarewicz, A.; Dąbrowski, W.; Magrel, L. Badania Nad Ostatecznym Wykorzystaniem Odwodnionych Osadów Ściekowych Do Nieprzemysłowego Wykorzystania; Wydawnictwo PB: Białystok, Poland, 2005. [Google Scholar]
- Stevenson, F.J. Humus Chemistry: Genesis, Composition, Reactions; John Wiley & Sons: Hoboken, NJ, USA, 1994; ISBN 978-0-471-59474-1. [Google Scholar]
- Jain, R.; Panwar, N.L.; Jain, S.K.; Gupta, T.; Agarwal, C.; Meena, S.S. Bio-Hydrogen Production through Dark Fermentation: An Overview. Biomass Convers. Biorefinery 2024, 14, 12699–12724. [Google Scholar] [CrossRef]
- Marschner, B.; Kalbitz, K. Controls of Bioavailability and Biodegradability of Dissolved Organic Matter in Soils. Geoderma 2003, 113, 211–235. [Google Scholar] [CrossRef]
- Fog, K. The Effect of Added Nitrogen on The Rate of Decomposition of Organic Matter. Biol. Rev. 1988, 63, 433–462. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Formation of Propionate and Butyrate by the Human Colonic Microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef]
- Macfarlane, S.; Macfarlane, G.T. Regulation of Short-Chain Fatty Acid Production. Proc. Nutr. Soc. 2003, 62, 67–72. [Google Scholar] [CrossRef]
- Mead, G.C. The Amino Acid-Fermenting Clostridia. J. Gen. Microbiol. 1971, 67, 47–56. [Google Scholar] [CrossRef]
- Smith, E.A.; Macfarlane, G.T. Formation of Phenolic and Indolic Compounds by Anaerobic Bacteria in the Human Large Intestine. Microb. Ecol. 1997, 33, 180–188. [Google Scholar] [CrossRef]
- Elsden, S.R.; Hilton, M.G. Volatile Acid Production from Threonine, Valine, Leucine and Isoleucine by Clostridia. Arch. Microbiol. 1978, 117, 165–172. [Google Scholar] [CrossRef]
- Huang, X.-Y.; Liu, C.-G.; Lin, Y.-H. A Novel Explainable Kinetic Model for Two-Stage Fermentation Profile. Chem. Eng. J. 2024, 493, 152745. [Google Scholar] [CrossRef]
- Detman, A.; Laubitz, D.; Chojnacka, A.; Kiela, P.R.; Salamon, A.; Barberan, A.; Chen, Y.; Yang, F.; Błaszczyk, M.K.; Sikora, A. Dynamics of Dark Fermentation Microbial Communities in the Light of Lactate and Butyrate Production. Microbiome 2021, 9, 1–21. [Google Scholar] [CrossRef]
- Nzeteu, C.o.; Coelho, F.; Trego, A.C.; Abram, F.; Ramiro-Garcia, J.; Paulo, L.; O’Flaherty, V. Development of an Enhanced Chain Elongation Process for Caproic Acid Production from Waste-Derived Lactic Acid and Butyric Acid. J. Clean. Prod. 2022, 338, 130655. [Google Scholar] [CrossRef]
- Ding, H.-B.; Tan, G.-Y.A.; Wang, J.-Y. Caproate Formation in Mixed-Culture Fermentative Hydrogen Production. Bioresour. Technol. 2010, 101, 9550–9559. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yin, Y. Clostridium Species for Fermentative Hydrogen Production: An Overview. Int. J. Hydrogen Energy 2021, 46, 34599–34625. [Google Scholar] [CrossRef]
- Cheng, C.-H.; Hung, C.-H.; Lee, K.-S.; Liau, P.-Y.; Liang, C.-M.; Yang, L.-H.; Lin, P.-J.; Lin, C.-Y. Microbial Community Structure of a Starch-Feeding Fermentative Hydrogen Production Reactor Operated under Different Incubation Conditions. Int. J. Hydrogen Energy 2008, 33, 5242–5249. [Google Scholar] [CrossRef]
- Nissilä, M.E.; Tähti, H.P.; Rintala, J.A.; Puhakka, J.A. Effects of Heat Treatment on Hydrogen Production Potential and Microbial Community of Thermophilic Compost Enrichment Cultures. Bioresour. Technol. 2011, 102, 4501–4506. [Google Scholar] [CrossRef]
- Punia Bangar, S.; Ashogbon, A.O.; Singh, A.; Chaudhary, V.; Whiteside, W.S. Enzymatic Modification of Starch: A Green Approach for Starch Applications. Carbohydr. Polym. 2022, 287, 119265. [Google Scholar] [CrossRef]
- Su, H.; Cheng, J.; Zhou, J.; Song, W.; Cen, K. Improving Hydrogen Production from Cassava Starch by Combination of Dark and Photo Fermentation. Int. J. Hydrogen Energy 2009, 34, 1780–1786. [Google Scholar] [CrossRef]
- Zhang, Y.-H.P.; Evans, B.R.; Mielenz, J.R.; Hopkins, R.C.; Adams, M.W.W. High-Yield Hydrogen Production from Starch and Water by a Synthetic Enzymatic Pathway. PLoS ONE 2007, 2, e456. [Google Scholar] [CrossRef]
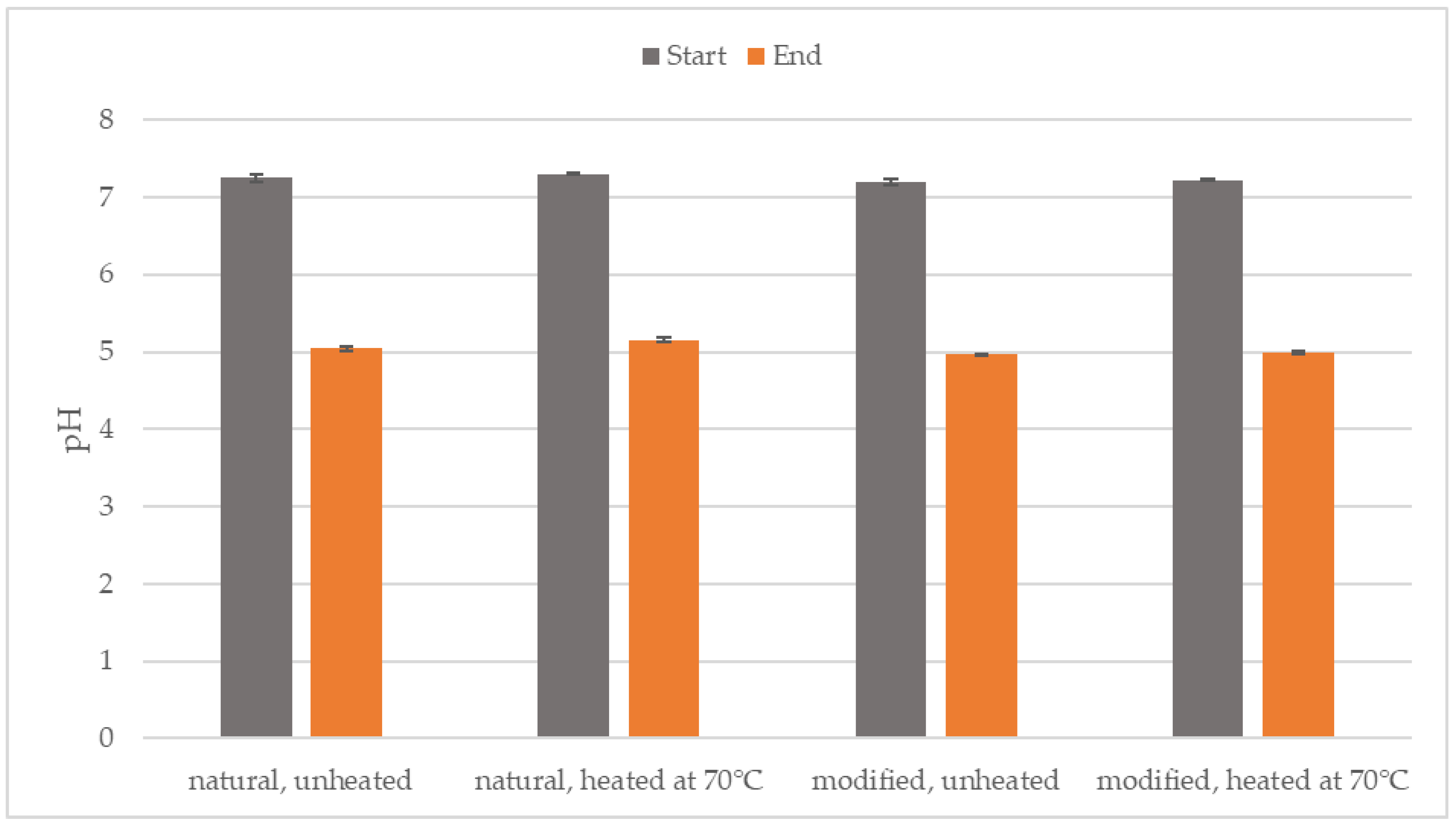
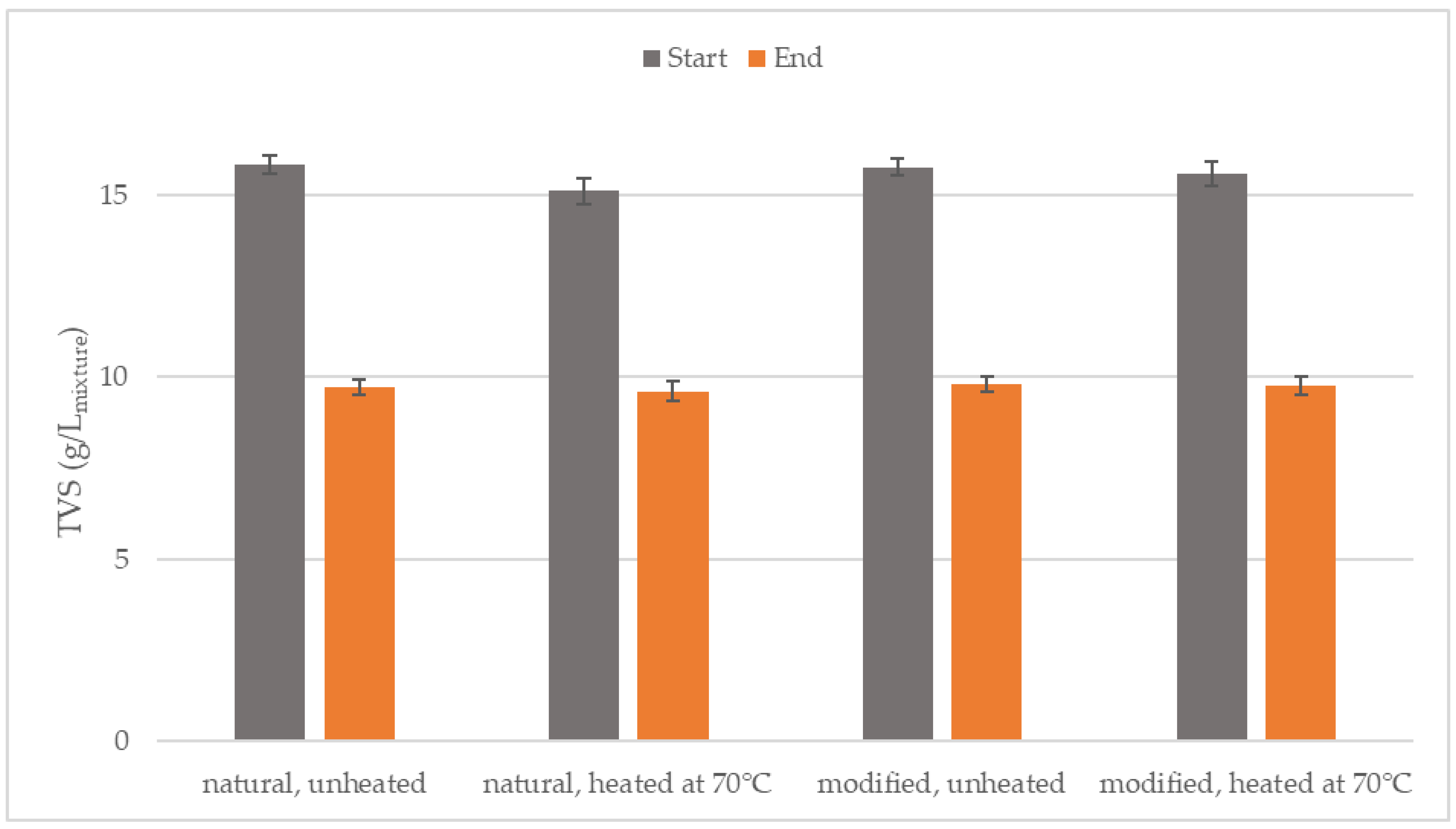
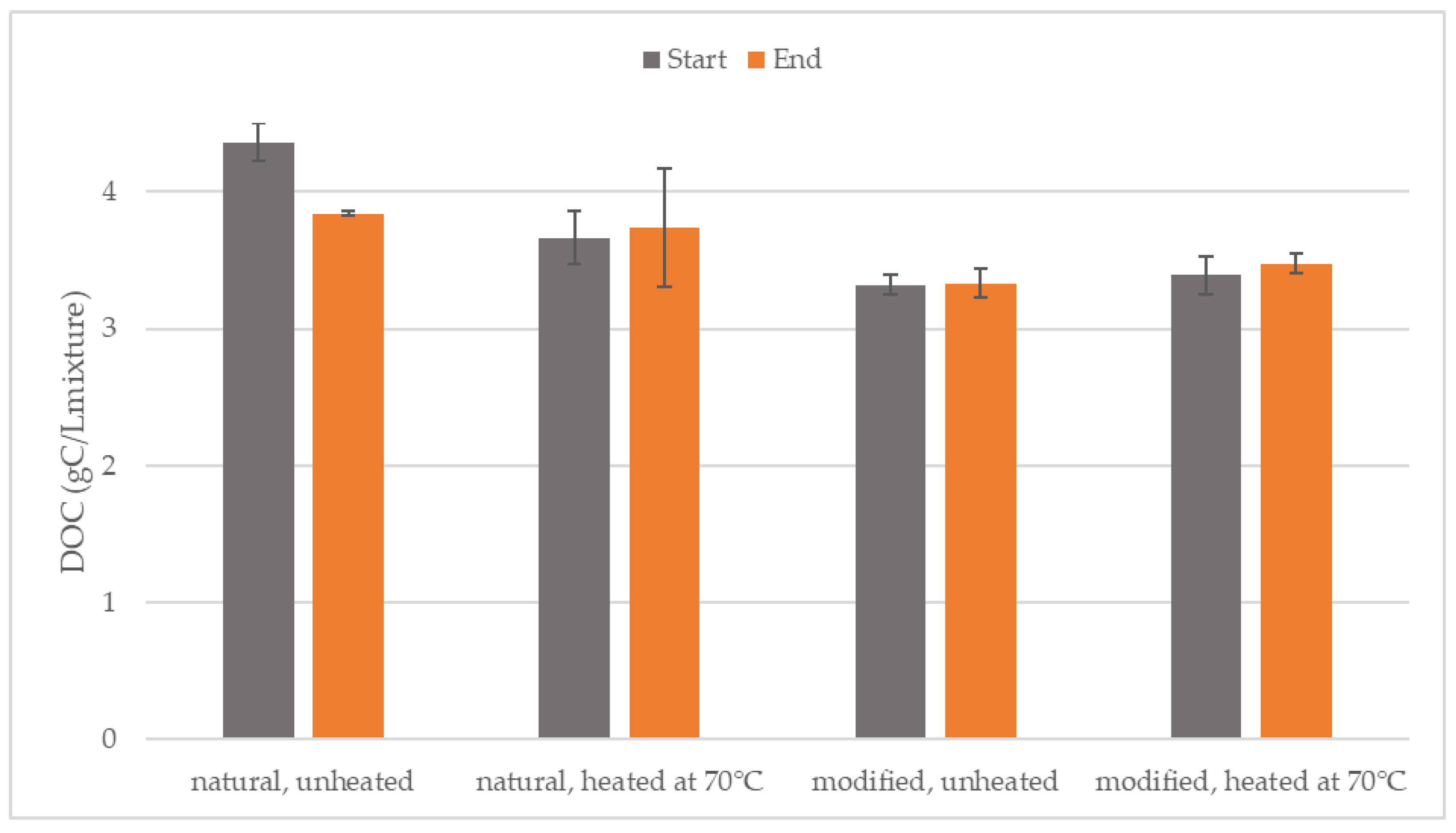
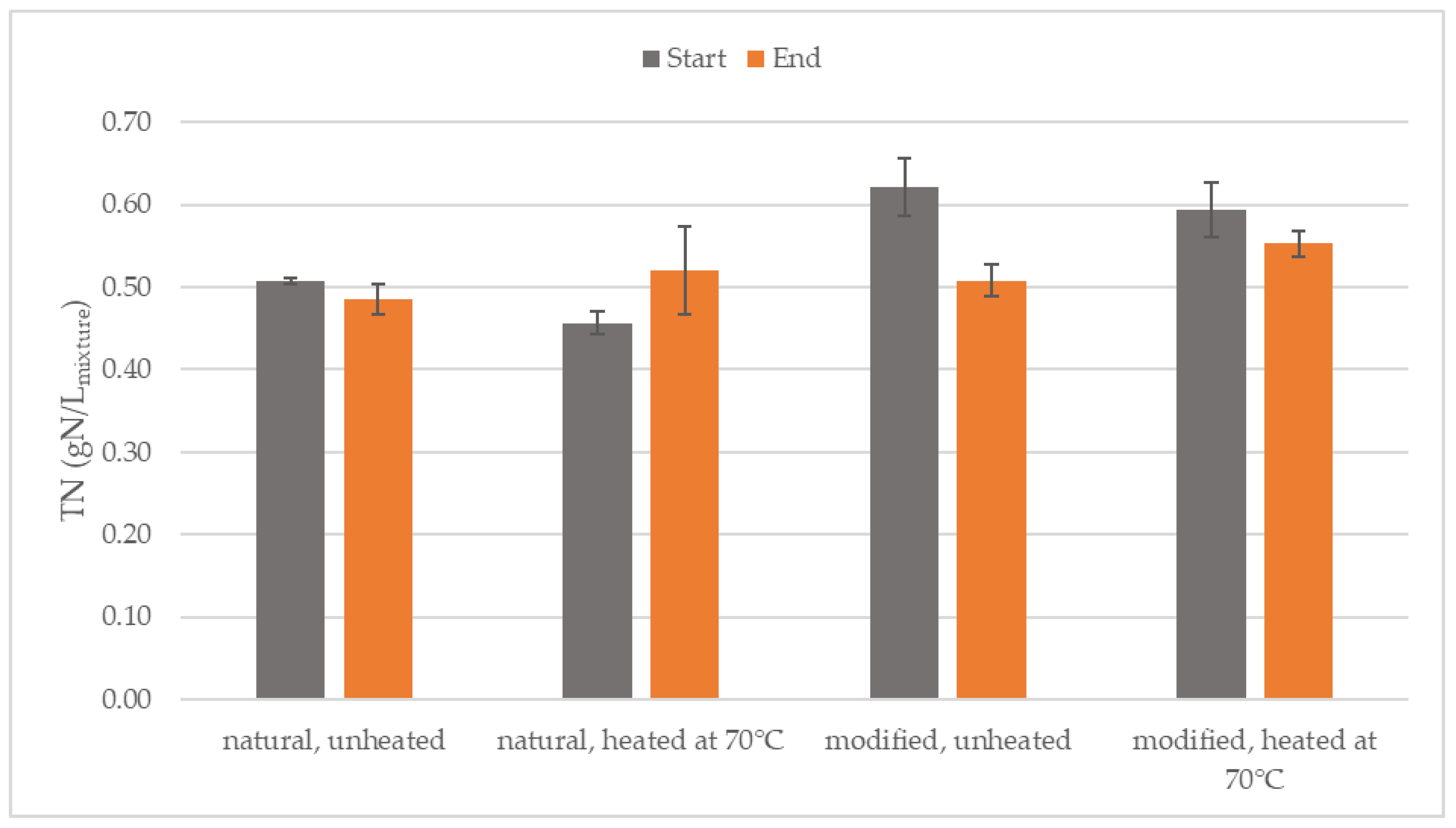
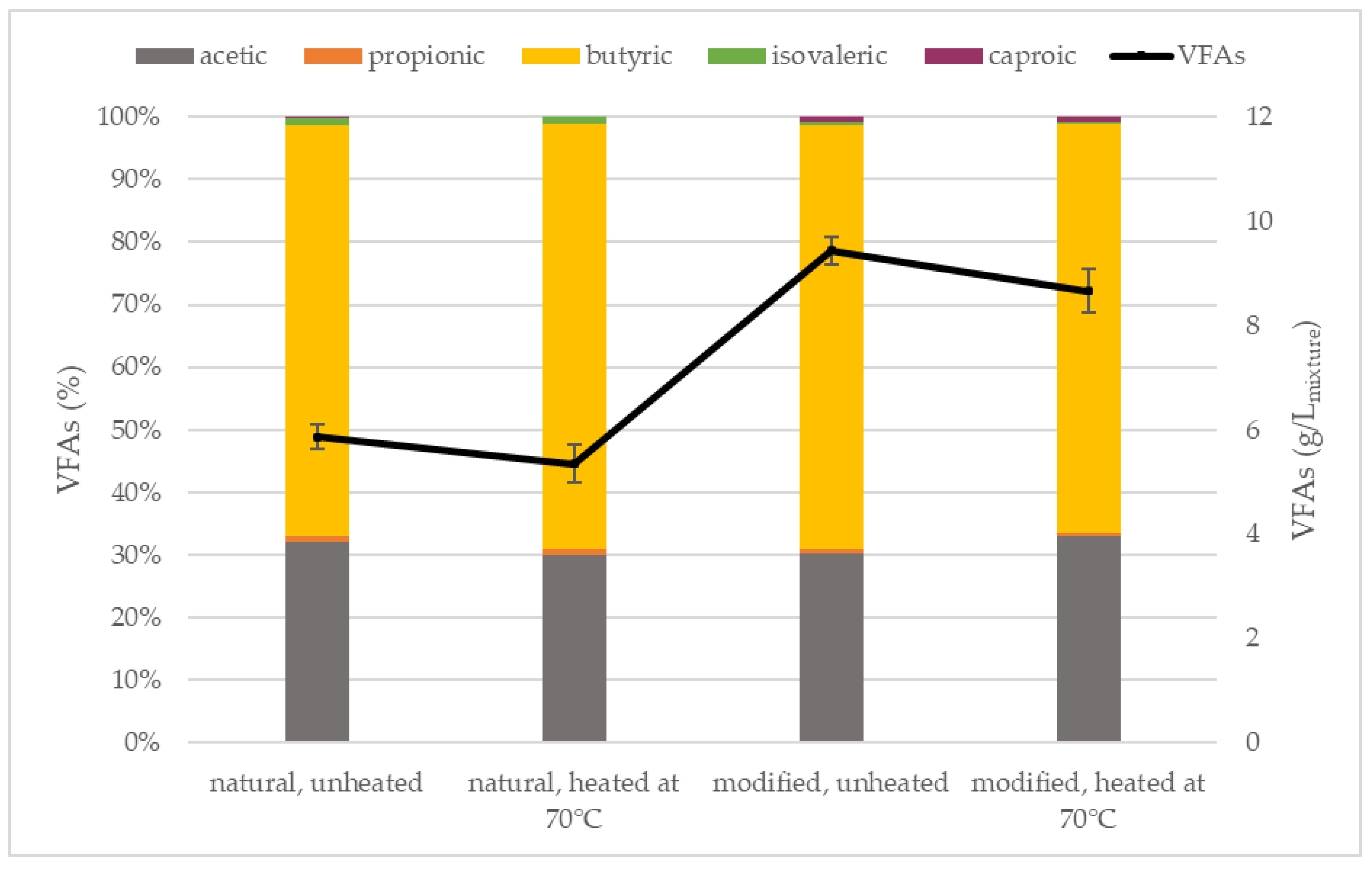
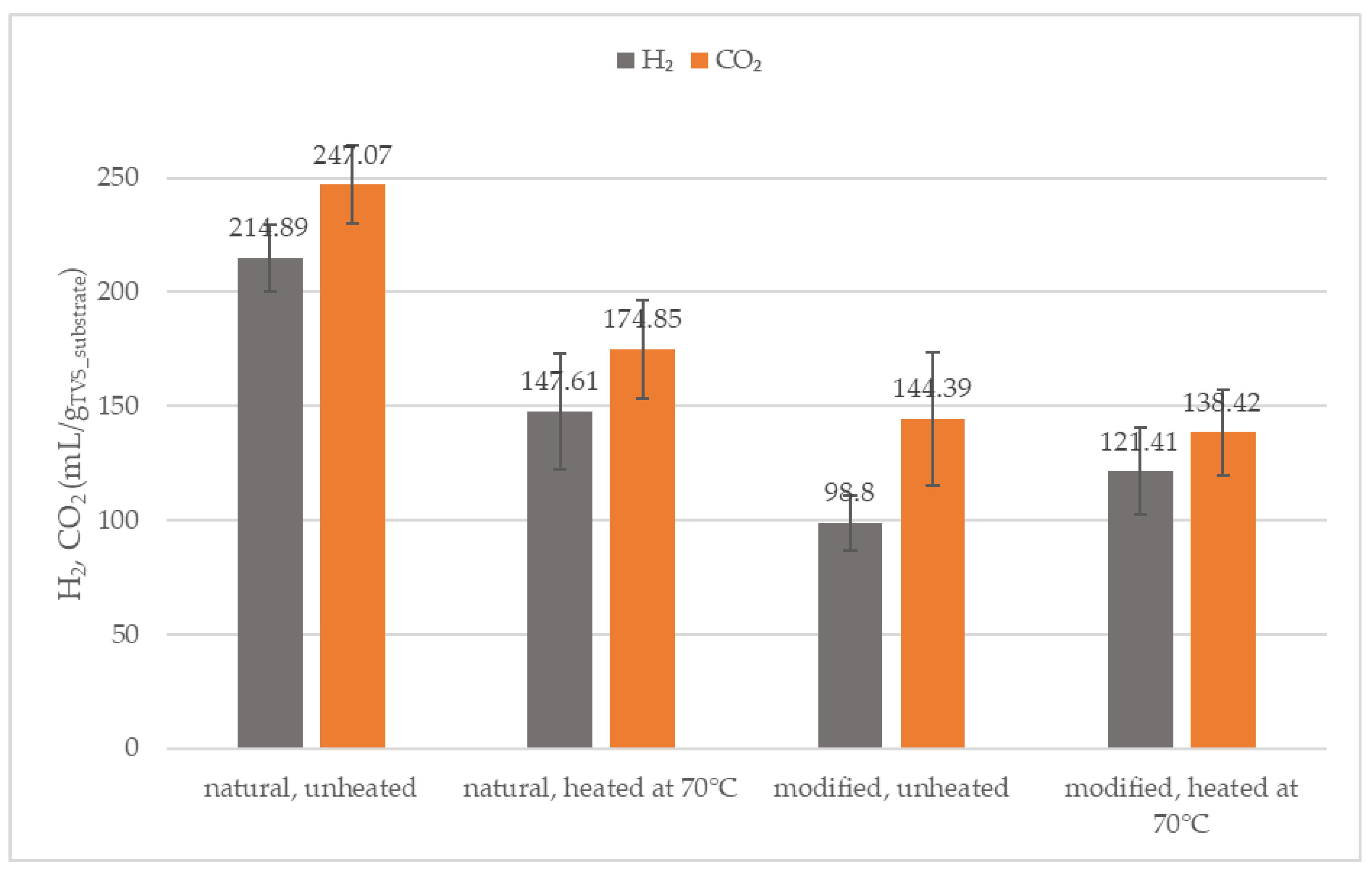
| Parameter | Inoculum (After 70 °C) | Natural Starch | Modified Starch |
|---|---|---|---|
| pH | 7.24 ± 0.02 | 7.55 ± 0.20 | 7.73 ± 0.15 |
| TS (g/L) | 33.95 ± 0.33 | 14.01 ± 0.08 | 12.05 ± 0.14 |
| TVS (g/L) | 11.75 ± 0.15 | 13.57 ± 0.20 | 11.73 ± 0.13 |
| DOC (g/L) | 1.46 ± 0.01 | 5.01 ± 0.05 | 7.85 ± 0.01 |
| TN (g/L) | 1.35 ± 0.04 | 0.011 ± 0.110 | 0.019 ± 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domińska, M.; Gloc, M.; Olak-Kucharczyk, M.; Paździor, K. Dark Fermentation of Sizing Process Waste: A Sustainable Solution for Hydrogen Production and Industrial Waste Management. Water 2025, 17, 1716. https://doi.org/10.3390/w17111716
Domińska M, Gloc M, Olak-Kucharczyk M, Paździor K. Dark Fermentation of Sizing Process Waste: A Sustainable Solution for Hydrogen Production and Industrial Waste Management. Water. 2025; 17(11):1716. https://doi.org/10.3390/w17111716
Chicago/Turabian StyleDomińska, Marlena, Martyna Gloc, Magdalena Olak-Kucharczyk, and Katarzyna Paździor. 2025. "Dark Fermentation of Sizing Process Waste: A Sustainable Solution for Hydrogen Production and Industrial Waste Management" Water 17, no. 11: 1716. https://doi.org/10.3390/w17111716
APA StyleDomińska, M., Gloc, M., Olak-Kucharczyk, M., & Paździor, K. (2025). Dark Fermentation of Sizing Process Waste: A Sustainable Solution for Hydrogen Production and Industrial Waste Management. Water, 17(11), 1716. https://doi.org/10.3390/w17111716






