Evaluation of Water Quality in the Production of Rainbow Trout (Oncorhynchus mykiss) in a Recirculating Aquaculture System (RAS) in the Precordilleran Region of Northern Chile
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Recirculating Aquaculture System Characteristics
2.2. Water Quality Monitoring
2.2.1. Fundamental Water Quality Parameters in a SAR System
2.2.2. Specific Water Quality Parameters in a SAR System
2.3. Juvenile Growth Assessment
2.4. Data Analyses
3. Results
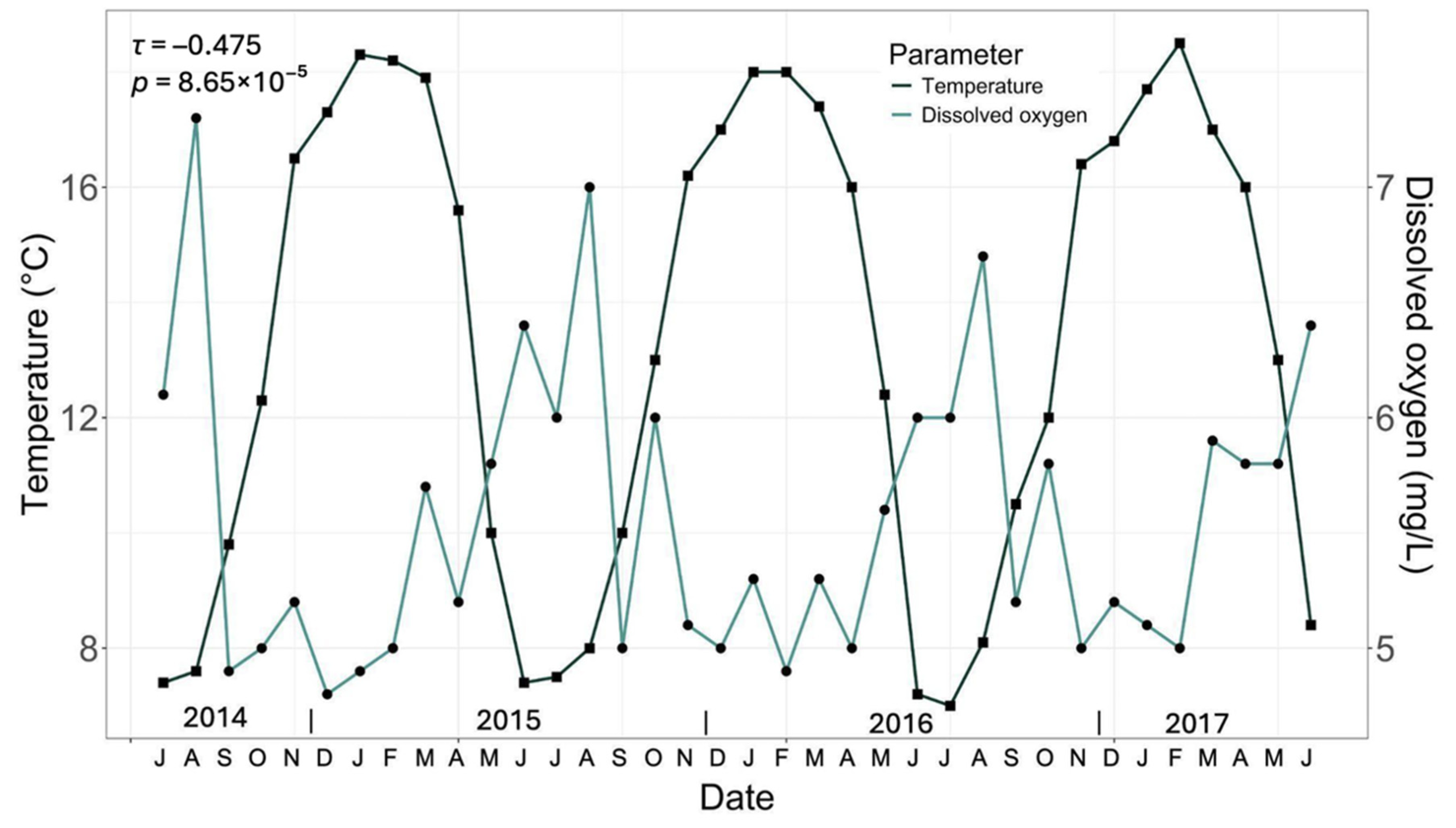
3.1. General Water Quality Parameters
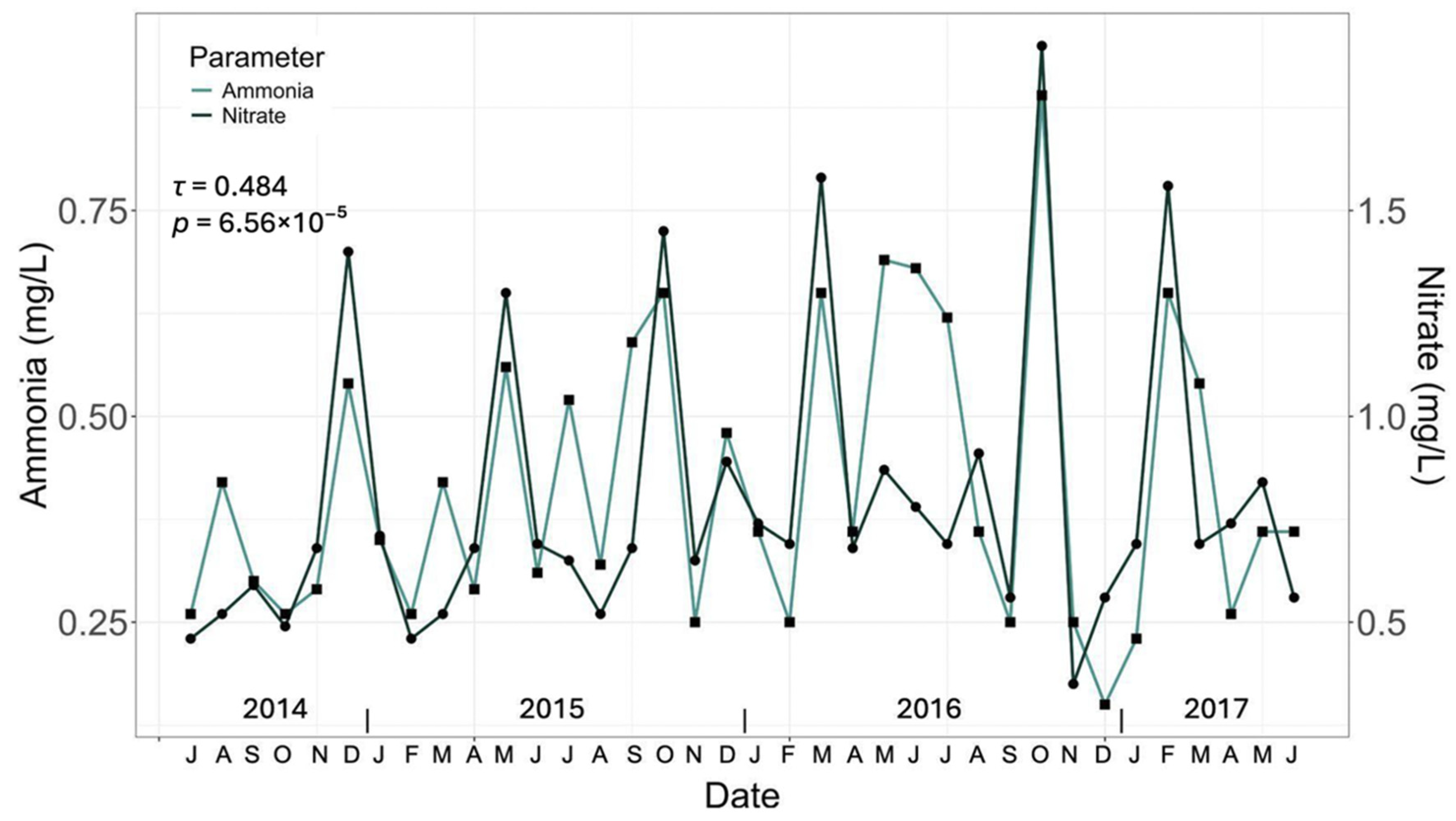
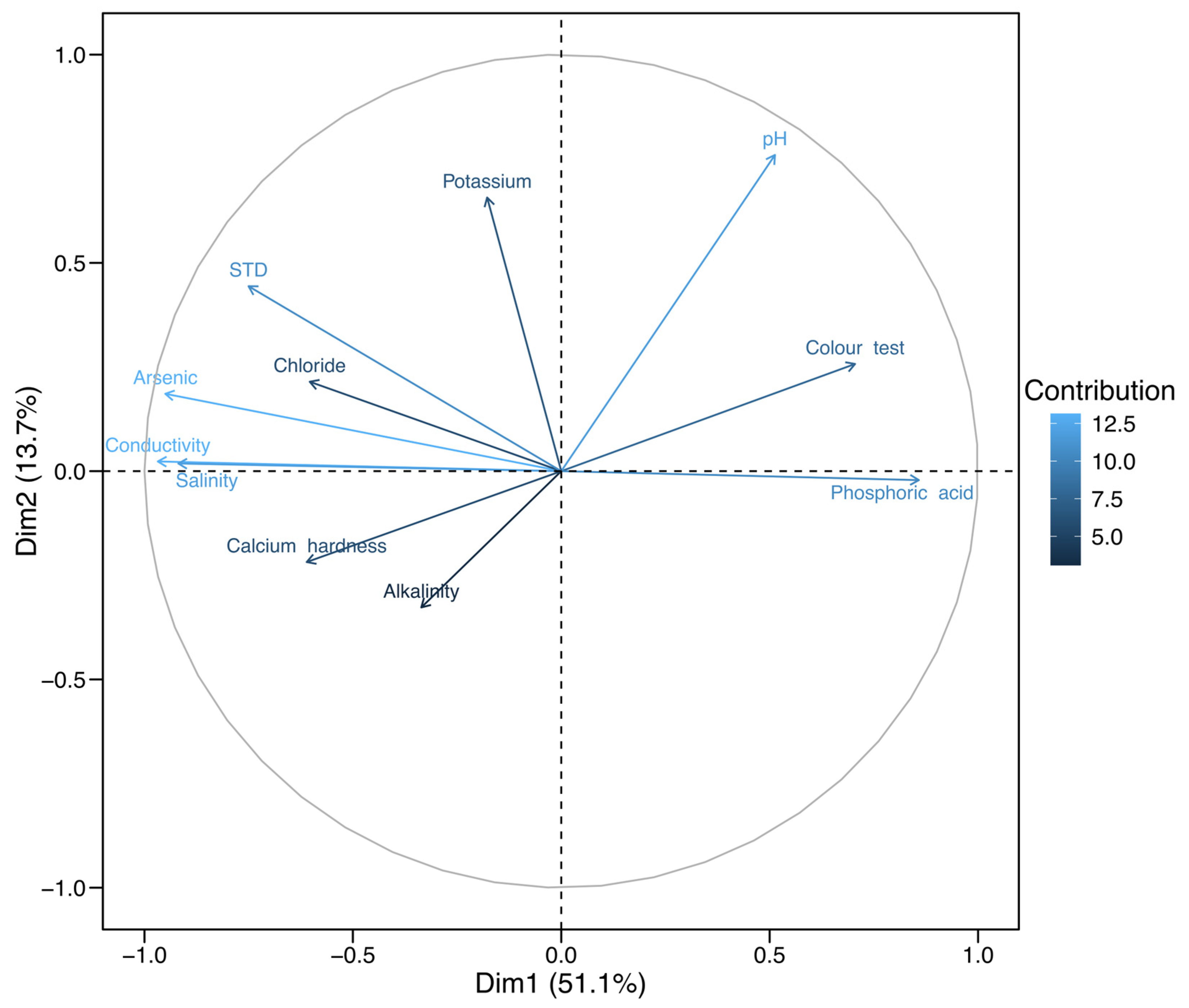
3.2. Specific Water Quality Parameters
3.3. Juvenile Growth Assessment
4. Discussion
4.1. Water Quality in High-Altitude Aquaculture
4.2. Key Water Quality Parameters in a RAS System
4.3. Specific Water Quality Parameters in a RAS System
4.4. Impact of Fish Presence on Water Quality in the Culture System
4.5. Variations in Water Quality Within the Recirculating System
4.6. Growth
4.7. Regulatory Standards for Water Quality in Fish Farming
4.8. Sustainability of the Culture
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pepe-Victoriano, R.; Pepe-Vargas, P.; Yañez-Valenzuela, M.; Aravena-Ambrosetti, H.; Olivares-Cantillano, G.; Méndez-Abarca, F.; Huanacuni, J.I.; Méndez, S.; Espinoza-Ramos, L. Growth of Oncorhynchus mykiss (Rainbow Trout) through a Recirculation System in the Foothills of the Extreme North of Chile. Animals 2024, 14, 2567. [Google Scholar] [CrossRef] [PubMed]
- Pepe-Victoriano, R.; Pepe-Vargas, P.; Huanacuni, J.I.; Aravena-Ambrosetti, H.; Olivares-Cantillano, G.; Méndez-Abarca, F.; Méndez, S.; Espinoza-Ramos, L. Conditioning of Rainbow Trout (Oncorhynchus mykiss) Broodstock in a High-Altitude Recirculating Aquaculture System: First Spawning at 3000 m.a.s.l. in Northern Chile. Animals 2025, 15, 1506. [Google Scholar] [CrossRef]
- Pepe-Victoriano, R.; Espinoza-Ramos, L.; Aravena-Ambrosetti, H.; Huanacuni, J.I. Cultivo de Truchas Arcoíris (Oncorhynchus mykiss, Walbaum 1792) En El Norte de Chile y Sur Del Perú. In Tópicos en Biodiversidad Transfronteriza; Valladares, P., Aragón, G., Gazritano, A., Eds.; Ril Editores: Santiago, Chile, 2021; p. 260. [Google Scholar]
- Pepe-Victoriano, R.; Araya, M.; Wurmann, C.; Mery, J.; Vélez, A.; Oxa, P.; León, C.; Fuentealba, S.; Canáles, R. Estrategia Para El Desarrollo de La Acuicultura En La Región de Arica y Parinacota 2015–2024. Informe Final; Corfo: Santiago, Chile, 2015. [Google Scholar]
- Pepe-Victoriano, R.; Aravena-Ambrosetti, H. Primer Centro de Cultivo de truchas Arcoíris bajo un Sistema de Recirculación. En el sector de Copaquilla, región de Arica y Parinacota. Rev. Versión Difer. Salmón-Acuícola 2018, 29, 58–62. [Google Scholar]
- Cornejo-Ponce, L.; Vilca-Salinas, P.; Arenas-Herrera, M.; Lienqueo-Aburto, H.; Tapia-Caroca, H.; Kukulis-Martínez, S. Uso Eficiente de Los Recursos Hídricos En Zonas Áridas y Semiáridas: Soluciones Integrales Basadas En La Energía Solar Para Promover El Desarrollo. Rev. Versión Difer. Versión Espec. Segundo Semest. 2022, 19, 49–53. [Google Scholar]
- Timmons, M.B.; Ebeling, J.M.; Piedrahita, R.H. Acuicultura En Sistemas de Recirculación; Cayuga Aqua Ventures: Ithaca, NY, USA, 2009. [Google Scholar]
- Badiola, M.; Mendiola, D.; Bostock, J. Recirculating Aquaculture Systems (RAS) Analysis: Main Issues on Management and Future Challenges. Aquac. Eng. 2012, 51, 26–35. [Google Scholar] [CrossRef]
- Pepe-Victoriano, R.; Miranda, L.; Ortega, A.; Merino, G. First Natural Spawning of Wild-Caught Premature South Pacific Bonito (Sarda Chiliensis Chiliensis, Cuvier 1832) Conditioned in Recirculating Aquaculture System and a Descriptive Characterization of Their Eggs Embryonic Development. Aquac. Rep. 2021, 19, 100563. [Google Scholar] [CrossRef]
- Buřič, M.; Bláhovec, J.; Kouřil, J. Feasibility of Rearing Brook Char Fingerlings in an Intensive Recirculating Hatchery as a Complementary Species to Rainbow Trout. Czech J. Anim. Sci. 2016, 61, 8–14. [Google Scholar] [CrossRef]
- Betanzo-Torres, E.A.; Piñar-Álvarez, M.d.l.Á.; Sierra-Carmona, C.G.; Santamaria, L.E.G.; Loeza-Mejía, C.I.; Marín-Muñiz, J.L.; Herazo, L.C.S. Proposal of Ecotechnologies for Tilapia (Oreochromis niloticus) Production in Mexico: Economic, Environmental, and Social Implications. Sustainability 2021, 13, 6853. [Google Scholar] [CrossRef]
- Li, H.; Cui, Z.; Cui, H.; Bai, Y.; Yin, Z.; Qu, K. A Review of Influencing Factors on a Recirculating Aquaculture System: Environmental Conditions, Feeding Strategies, and Disinfection Methods. J. World Aquac. Soc. 2023, 54, 566–602. [Google Scholar] [CrossRef]
- Taufik, M.; Tuan Ismail, T.I.; Manan, H.; Ikhwanuddin, M.; Abdul Salam, A.I.; Abdul Rahim, A.I.; Ishak, A.N.; Kamaruzzan, A.S.; Draman, A.S.; Kasan, N.A. Synergistic Effects of Recirculating Aquaculture System (RAS) with Combination of Clear Water, Probiotic and Biofloc Technology: A Review. Aquac. Fish. 2023, 9, 883–892. [Google Scholar] [CrossRef]
- Bregnballe, J. A Guide to Recirculation Aquaculture: An Introduction to the New Environmentally Friendly and Highly Productive Closed Fish Farming Systems; FAO and Eurofish International Organisation: Rome, Italy, 2015; ISBN 9789251087763. [Google Scholar]
- Galli, O.; Sal, F. Sistemas de Recirculación y Tratamiento de Agua; Secretaría de Agricultura, Ganadería, Pesca y Alimentos CENADAC: Santa Ana-Corrientes, Argentina, 2007. [Google Scholar]
- Bautista, J.C.; Ruíz-Velazco, J.M. Calidad de Agua Para El Cultivo de Tilapia En Tanques de Geomembrana. Rev. Fuente 2011, 3, 10–14. [Google Scholar]
- Rodríguez-Gómez, H.; Ánzola-Escobar, E. La Calidad Del Agua y La Productividad de Un Estanque En Acuicultura. In Fundamentos de Acuicultura Continental; Rodriguez, H., Victoria, P., Carrillo, M., Eds.; Instituto Nacional de Pesca y Acuicultura: Bogotá, Colombia, 2001; pp. 43–71. [Google Scholar]
- Acosta-Angulo, O.; Pepe-Victoriano, R.; Cornejo-Ponce, L.; Huanacuni, J.I.; Silva, Y.Y.C. Aquatic Quantification of Toxic Heavy Metals Due to Physical-Chemical Factors in Meat Production of Oncorhynchus mykiss (Rainbow Trout) in Controlled Open Systems Case: Lagunillas-Lampa, Puno. In Proceedings of the 8th Brazilian Technology Symposium (BTSym’22). BTSym 2022. Smart Innovation, Systems and Technologies; Springer: Cham, Switzerland, 2023; pp. 461–471. [Google Scholar]
- Schreck, C.; Hiram, W. Performance Capacity of Fish: Stress and Water Quality. In Aquaculture and Water Quality; Brune, D., Tomasso, J., Eds.; The World Aquaculture Society: Baton Rouge, LA, USA, 1991. [Google Scholar]
- Boyd, C.E.; D’Abramo, L.R.; Glencross, B.D.; Huyben, D.C.; Juarez, L.M.; Lockwood, G.S.; McNevin, A.A.; Tacon, A.G.J.; Teletchea, F.; Tomasso, J.R.; et al. Achieving Sustainable Aquaculture: Historical and Current Perspectives and Future Needs and Challenges. J. World Aquac. Soc. 2020, 51, 578–633. [Google Scholar] [CrossRef]
- Alabaste, J.; Lloyd, R. Water Quality Criteria for Freshwater Fish, 2nd ed.; Elsevier: Harlow, UK, 1982; ISBN 9780408108492. [Google Scholar]
- Gutiérrez, X.A.; Kolarevic, J.; Takle, H.; Baeverfjord, G.; Ytteborg, E.; Fyhn Terjesen, B. Effects of Chronic Sub-lethal Nitrite Exposure at High Water Chloride Concentration on Atlantic Salmon (Salmo salar, Linnaeus 1758) Parr. Aquac. Res. 2019, 50, 2687–2697. [Google Scholar] [CrossRef]
- Perry, S.F. THE CHLORIDE CELL:Structure and Function in the Gills of Freshwater Fishes. Annu. Rev. Physiol. 1997, 59, 325–347. [Google Scholar] [CrossRef]
- Rosso, L.; Giannuzzi, L. Factores Ambientales y Antropogénicos Que Afectan La Formación de Floraciones de Cianobacterias y Cianotoxinas. In Cianobacterias Como Determinantes Ambientales de la Salud; Ministerio de Salud de la Nación: Buenos Aires, Argentina, 2017; pp. 79–93. [Google Scholar]
- Wedemeyer, G.A. Physiology of Fish in Intensive Culture Systems, 1st ed.; Springer: Boston, MA, USA, 1996; ISBN 978-1-4613-7754-2. [Google Scholar]
- Rahmaningsih, S.; Ari, A.I. Pakan Dan Pertumbuhan IKan Kerapu Cantang (Epinephelus Fuscoguttatuslanceolatus). J. Ekol. 2013, 13, 25–30. [Google Scholar]
- Pepe-Victoriano, R.; Aravena-Ambrosetti, H.; Pepe-Vargas, P. Integrated Culture of Oncorhynchus mykiss (Rainbow Trout) in Pre-Cordilleran Sector under a Recirculation System in Northern Chile. In Salmon Aquaculture; IntechOpen: London, UK, 2021. [Google Scholar]
- Matulić, D.; Andabaka, Ž.; Radman, S.; Fruk, G.; Leto, J.; Rošin, J.; Rastija, M.; Varga, I.; Tomljanović, T.; Čeprnja, H.; et al. Agrivoltaics and Aquavoltaics: Potential of Solar Energy Use in Agriculture and Freshwater Aquaculture in Croatia. Agriculture 2023, 13, 1447. [Google Scholar] [CrossRef]
- Rice, E.; Bridgewater, L. Standard Methods for the Examination of Water and Wastewater; American Public Health Association, Ed.; American Public Health Association: Washington, DC, USA, 2012; Volume 10. [Google Scholar]
- Ricker, W.E. Growth Rates and Models. Fish Physiol. 1979, VII, 677–743. [Google Scholar]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2010; ISBN 9780131008465. [Google Scholar]
- Phillips, V.; Tschida, R.; Hernandez, M.; Aquino, M. Manual Básico Para El Cultivo de Trucha Arcoiris (Oncorhynchus mykiis). 2017. Available online: https://rnia.produce.gob.pe/wp-content/uploads/2022/09/Manual-de-Trucha-1.pdf (accessed on 15 July 2024).
- Liñan-Giraldo, W.E. Crianza de Truchas; Macro: Lima, Peru, 2007; p. 104. [Google Scholar]
- Camacho, B. Guía Para El Cultivo de Trucha: Oncorhynchus mykiss; Recursos Naturales y Pesca: México, México, 2000. [Google Scholar]
- Papadopoulos, D.K.; Lattos, A.; Chatzigeorgiou, I.; Tsaballa, A.; Ntinas, G.K.; Giantsis, I.A. The Influence of Water Nitrate Concentration Combined with Elevated Temperature on Rainbow Trout Oncorhynchus mykiss in an Experimental Aquaponic Setup. Fishes 2024, 9, 74. [Google Scholar] [CrossRef]
- García-Ortega, A.; Calvario-Martínez, O. Manual de Buenas Prácticas de Producción Acuícola de Trucha para la Inocuidad Alimentaria; SENASICA/SAGARPA: México, México, 2003. [Google Scholar]
- Boyd, C.E. Water Quality; Springer International Publishing: Cham, Switzerland, 2015; ISBN 978-3-319-17445-7. [Google Scholar]
- Blanco, M. La Trucha. Cría En Cautividad; Mundi-Prensa: Madrid, Spain, 1994. [Google Scholar]
- Sánchez, I.; Sanguino, W.; Gómez, A.; García, R. Evaluación de Un Sistema de Recirculación de Agua Para Levante de Trucha Arcoiris (Oncorhynchus mikyss). Rev. MVZ Córdoba 2014, 19, 4226–4241. [Google Scholar]
- Timmons, M.B.; Ebeling, J.M. Recirculating Aquaculture; Ithaca Publishing Company. LLC: Ithaca, NY, USA, 2013. [Google Scholar]
- Tomasso, J.R.; Brune, D.E. Aquacultural Water Quality: The Emergence of an Applied Discipline. In Aquaculture and Water Quality; The World Aquaculture Society: Baton Rouge, LA, USA, 1991. [Google Scholar]
- Cornejo-Ponce, L.; Vilca-Salinas, P.; Lienqueo-Aburto, H.; Arenas, M.J.; Pepe-Victoriano, R.; Carpio, E.; Rodríguez, J. Integrated Aquaculture Recirculation System (Iars) Supported by Solar Energy as a Circular Economy Alternative for Resilient Communities in Arid/Semi-Arid Zones in Southern South America: A Case Study in the Camarones Town. Water 2020, 12, 3469. [Google Scholar] [CrossRef]
- Boyd, C.E.; Tucker, C.S. Pond Aquaculture Water Quality Management, 1st ed.; Springer: Boston, MA, USA, 1998; ISBN 978-1-4613-7469-5. [Google Scholar]
- Llanes-Cárdenas, O.; Campos, M.N.; Sevilla, P.M.; Guerrero, R.R. Agua Subterránea: Alternativa Acuícola En El Noroeste de México. AquaTIC 2013, 38, 10–20. [Google Scholar]
- Sahuquillo, A. La Utilizacion Conjunta de Aguas Superficiales y Subterraneas En Las Sequias. Rev. Real Acad. Cienc. Exactas Fis. Nat. 2000, 94, 183–196. [Google Scholar]
- Kinsman-Costello, L.E.; O’Brien, J.M.; Hamilton, S.K. Natural Stressors in Uncontaminated Sediments of Shallow Freshwaters: The Prevalence of Sulfide, Ammonia, and Reduced Iron. Environ. Toxicol. Chem. 2015, 34, 467–479. [Google Scholar] [CrossRef]
- Vásquez-Quispesivana, W.; Talavera Núñez, M.; Inga Guevara, M. Evaluación Del Impacto En La Calidad de Agua Debido a La Producción Semi Intensiva de Trucha (Oncorhynchus mykiss) En Jaulas Flotantes. Rev. Soc. Química Perú 2016, 82, 15–28. [Google Scholar] [CrossRef]
- Geng, C.Y.; Sun, Y.C.; Li, B.L.; Ding, L.; Liu, Y.J.; Wei, X.F.; Liu, W.Z.; Han, L.; Yuan, F.Y.; Wang, P.; et al. Physiological Responses to Heat Stress in the Gill of Rainbow Trout (Oncorhynchus mykiss) Revealed Based on Uplc-q-Tof/Ms Metabolomics. Acta Hydrobiol. Sin. 2023, 47, 1185–1194. [Google Scholar] [CrossRef]
- Barnes, R.; King, H.; Carter, C.G. Hypoxia Tolerance and Oxygen Regulation in Atlantic Salmon, Salmo Salar from a Tasmanian Population. Aquaculture 2011, 318, 397–401. [Google Scholar] [CrossRef]
- Bergsson, H.; Svendsen, M.B.S.; Steffensen, J.F. Model of Oxygen Conditions within Aquaculture Sea Cages. Biology 2023, 12, 1408. [Google Scholar] [CrossRef] [PubMed]
- Sandblom, E.; Axelsson, M. Venous Hemodynamic Responses to Acute Temperature Increase in the Rainbow Trout (Oncorhynchus mykiss). Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R2292–R2298. [Google Scholar] [CrossRef]
- Ruiz, N.; García-Meilán, I.; Khansari, A.R.; Teles, M.; Pastor, J.; Tort, L. Repeated Hypoxic Episodes Allow Hematological and Physiological Habituation in Rainbow Trout. Front. Physiol. 2024, 15, 1289903. [Google Scholar] [CrossRef]
- Mehrim, A.I.; Refaey, M.M. An Overview of the Implication of Climate Change on Fish Farming in Egypt. Sustainability 2023, 15, 1679. [Google Scholar] [CrossRef]
- Supraba, I.; Istiarto; Triatmadja, R.; Legono, D. Water Temperature Simulation of Rivers in Indonesia. In Proceedings of the 22nd Congress of the International Association for Hydro-Environment Engineering and Research-Asia Pacific Division, IAHR-APD 2020: “Creating Resilience to Water-Related Challenges”, Sapporo, Japan, 14–17 September 2020. [Google Scholar]
- Suhr, K.I.; Pedersen, P.B. Nitrification in Moving Bed and Fixed Bed Biofilters Treating Effluent Water from a Large Commercial Outdoor Rainbow Trout RAS. Aquac. Eng. 2010, 42, 31–37. [Google Scholar] [CrossRef]
- Cabello, P.; Luque-Almagro, V.M.; Roldán, M.D.; Moreno-Vivián, C. Nitrogen Cycle. In Encyclopedia of Microbiology; Academic Press: Cambridge, MA, USA, 2019; ISBN 9780128117378. [Google Scholar]
- White, L.J.; Rose, M.; Lawson, M.; Joyce, D.; Smith, A.M.; Thomas, G.H.; Dasmahapatra, K.K.; Pownall, M.E. Two Closely Related Ureotelic Fish Species of the Genus Alcolapia Express Different Levels of Ammonium Transporters in Gills. Biol. Open 2022, 11, bio059575. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, A.M. Ammonia Excretion by the Fish Gill: Discoveries and Ideas That Shaped Our Current Understanding. J. Comp. Physiol. B 2024, 194, 697–715. [Google Scholar] [CrossRef]
- van Kessel, M.A.H.J.; Mesman, R.J.; Arshad, A.; Metz, J.R.; Spanings, F.A.T.; van Dalen, S.C.M.; van Niftrik, L.; Flik, G.; Wendelaar Bonga, S.E.; Jetten, M.S.M.; et al. Branchial Nitrogen Cycle Symbionts Can Remove Ammonia in Fish Gills. Environ. Microbiol. Rep. 2016, 8, 590–594. [Google Scholar] [CrossRef]
- Balm, P.H.M.; Pottinger, T.G. Acclimation of Rainbow Trout (Oncorhynchus mykiss) to Low Environmental PH Does Not Involve an Activation of the Pituitary-Interrenal Axis, but Evokes Adjustments in Branchial Ultrastructure. Can. J. Fish. Aquat. Sci. 1993, 50, 2532–2541. [Google Scholar] [CrossRef]
- Fivelstad, S.; Haavik, H.; Løvik, G.; Olsen, A.B. Sublethal Effects and Safe Levels of Carbon Dioxide in Seawater for Atlantic Salmon Postsmolts (Salmo salar L.): Ion Regulation and Growth. Aquaculture 1998, 160, 305–316. [Google Scholar] [CrossRef]
- Witschi, W.A.; Ziebell, C.D. Evaluation of Ph Shock on Hatchery-Reared Rainbow Trout. Progress. Fish-Cult. 1979, 41, 3–5. [Google Scholar] [CrossRef]
- Fivelstad, S. Long-Term Carbon Dioxide Experiments with Salmonids. Aquac. Eng. 2013, 53, 40–48. [Google Scholar] [CrossRef]
- Leduc, A.O.H.C.; Kelly, J.M.; E. Brown, G. Detection of Conspecific Alarm Cues by Juvenile Salmonids under Neutral and Weakly Acidic Conditions: Laboratory and Field Tests. Oecologia 2004, 139, 318–324. [Google Scholar] [CrossRef][Green Version]
- Kırkağaç, M.U.; Pulatsu, S.; Topcu, A. Trout Farm Effluent Effects on Water Sediment Quality and Benthos. Clean 2009, 37, 386–391. [Google Scholar] [CrossRef]
- Lapenkov, A.; Guzeva, A.; Zaripova, K.; Slukovskii, Z. The Seasonal Dynamics of Geochemical Characteristics of Sediments in the Impact Zone of the Fish Farm (Lake Ladoga, Russia). Aquac. Fish. 2023, 8, 654–660. [Google Scholar] [CrossRef]
- Loyless, J.C.; Malone, R.F. A Sodium Bicarbonate Dosing Methodology for PH Management in Freshwater-Recirculating Aquaculture Systems. Progress. Fish-Cult. 1997, 59, 198–205. [Google Scholar] [CrossRef]
- Wenzel, L.C.; Strauch, S.M.; Eding, E.; Presas-Basalo, F.X.; Wasenitz, B.; Palm, H.W. Effects of Dissolved Potassium on Growth Performance, Body Composition, and Welfare of Juvenile African Catfish (Clarias gariepinus). Fishes 2021, 6, 11. [Google Scholar] [CrossRef]
- Boyd, C.E.; McNevin, A.A. Aquaculture, Resource Use, and the Environment; Wiley: Hoboken, NJ, USA, 2015; ISBN 9780470959190. [Google Scholar]
- Chatvijitkul, S.; Boyd, C.E.; Davis, D.A.; McNevin, A.A. Pollution Potential Indicators for Feed-Based Fish and Shrimp Culture. Aquaculture 2017, 477, 43–49. [Google Scholar] [CrossRef]
- d’Orbcastel, E.R.; Blancheton, J.P.; Belaud, A. Water Quality and Rainbow Trout Performance in a Danish Model Farm Recirculating System: Comparison with a Flow through System. Aquac. Eng. 2009, 40, 135–143. [Google Scholar] [CrossRef]
- Sugiura, S.H. Phosphorus, Aquaculture, and the Environment. Rev. Fish. Sci. Aquac. 2018, 26, 515–521. [Google Scholar] [CrossRef]
- Becke, C.; Steinhagen, D.; Schumann, M.; Brinker, A. Physiological Consequences for Rainbow Trout (Oncorhynchus mykiss) of Short-Term Exposure to Increased Suspended Solid Load. Aquac. Eng. 2017, 78, 63–74. [Google Scholar] [CrossRef]
- Becke, C.; Schumann, M.; Steinhagen, D.; Rojas-Tirado, P.; Geist, J.; Brinker, A. Effects of Unionized Ammonia and Suspended Solids on Rainbow Trout (Oncorhynchus mykiss) in Recirculating Aquaculture Systems. Aquaculture 2019, 499, 348–357. [Google Scholar] [CrossRef]
- Mojiri, A.; Razmi, E.; KarimiDermani, B.; Rezania, S.; Kasmuri, N.; Vakili, M.; Farraji, H. Adsorption Methods for Arsenic Removal in Water Bodies: A Critical Evaluation of Effectiveness and Limitations. Front. Water 2024, 6, 1301648. [Google Scholar] [CrossRef]
- Zhang, N.; Wei, C.; Yang, L. Occurrence of Arsenic in Two Large Shallow Freshwater Lakes in China and a Comparison to Other Lakes around the World. Microchem. J. 2013, 110, 169–177. [Google Scholar] [CrossRef]
- Cornejo-Ponce, L.; Lienqueo, H.H.; Arriaza, B.T. Levels of Total Arsenic in Edible Fish and Shellfish Obtained from Two Coastal Sectors of the Atacama Desert in the North of Chile: Use of Non-Migratory Marine Species as Bioindicators of Sea Environmental Pollution. J. Environ. Sci. Health Part A 2011, 46, 1274–1282. [Google Scholar] [CrossRef]
- Maldonado-Reyes, A.; Montero-Ocampo, C.; Medina-Garcia, J.; Bolado-Rodríguez, S.; Alvárez-Benedí, J.; Herrera-Vazquez, A.; Castaño, V.M. Electro Coagulation Removal of As from Water: The Role of Phases Formation. Water Air Soil Pollut. 2015, 226, 249. [Google Scholar] [CrossRef]
- Jiménez-Cedillo, M.J.; Olguín, M.T.; Fall, C.; Colín, A. Adsorption Capacity of Iron- or Iron-Manganese-Modified Zeolite-Rich Tuffs for As(III) and As(V) Water Pollutants. Appl. Clay Sci. 2011, 54, 206–216. [Google Scholar] [CrossRef]
- Singh, T.S.; Pant, K.K. Kinetics and Mass Transfer Studies on the Adsorption of Arsenic onto Activated Alumina and Iron Oxide Impregnated Activated Alumina. Water Qual. Res. J. Can. 2006, 41, 147–156. [Google Scholar] [CrossRef]
- Kasagi, S.; Miura, M.; Okazaki, T.; Mizusawa, K.; Takahashi, A. Effects of Tank Color Brightness on the Body Color, Somatic Growth, and Endocrine Systems of Rainbow Trout Oncorhynchus mykiss. Gen. Comp. Endocrinol. 2020, 298, 113581. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, A.H.; Saad, F.S.A.; Sudin, S.; Ahmad, Z.A.; Ahmad, I.; Abu Bakar, N.; Omar, S.; Sulaiman, S.F.; Che Mat, M.H.; Umoruddin, N.A.; et al. Development of Aquaculture Water Quality Real-Time Monitoring Using Multi-Sensory System and Internet of Things. J. Phys. Conf. Ser. 2021, 2107, 012011. [Google Scholar] [CrossRef]
- Chong, R.S.-M. Ammonia Toxicosis. In Aquaculture Pathophysiology; Academic Press: Cambridge, MA, USA, 2022; pp. 705–711. [Google Scholar]
- Roalkvam, I.; Drønen, K.; Dahle, H.; Wergeland, H.I. A Case Study of Biofilter Activation and Microbial Nitrification in a Marine Recirculation Aquaculture System for Rearing Atlantic Salmon (Salmo salar L.). Aquac. Res. 2021, 52, 94–104. [Google Scholar] [CrossRef]
- Boyd, C. Water Quality in Warmwater Fish Ponds, 2nd ed.; Craft Master Printers, Inc.: Auburn, AL, USA, 1981. [Google Scholar]
- Garduño-Paz, M.V.; Méndez-Sánchez, J.F.; Burggren, W.; García-Martínez, J.L.A. Metabolic Rate and Hypoxia Tolerance in Girardinichthys multiradiatus (Pisces: Goodeidae), an Endemic Fish at High Altitude in Tropical Mexico. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2020, 239, 110576. [Google Scholar] [CrossRef]
- Tong, C.; Li, M.; Tang, Y.; Zhao, K. Genomic Signature of Shifts in Selection and Alkaline Adaptation in Highland Fish. Genome Biol. Evol. 2021, 13, evab086. [Google Scholar] [CrossRef]
- Liu, M.; Xu, W.; Zhu, F.; Duan, X.; Liu, S.; Chen, D. Length–Weight Relationship and Spatiotemporal Distribution Pattern of Three Schizothoracinae Fishes Along the Nujiang River in the Qinghai–Tibetan Plateau, China. Fishes 2024, 9, 465. [Google Scholar] [CrossRef]
- Yapuchura-Saico, C.R.; Mamani, E.; Pari-Quispe, D.; Mamani, E. Importance of the Curves of Growth and Efficiency in the Food of Trout Arcoiris (Oncorhynchus mikyss) in the Cost of Production. Comuni Cción Rev. Investig. Comun. Desarro. 2018, 9, 68–77. [Google Scholar]
- Morales, G. Crecimiento y Eficiencia Alimentaria de Trucha Arco Iris (Oncorhynchus mykiss) En Jaulas Bajo Diferentes Regímenes de Alimentación. Undergraduate Thesis, Universidad de Buenos Aires, Buenos Aires, Argentina, 2004. [Google Scholar]
- Lu, J.; Bo, Y.; Wang, Y.; Yuan, H.; Xu, Y. Effect of Water Quality Ununiformity on Production of Marine Medaka. Aquaculture 2023, 565, 739114. [Google Scholar] [CrossRef]
- Chamorro-Montes, P. Comparación Productiva de Dos Sistemas de Cultivo, Estanques Rectangulares y Circulares, En Alevinos de Trucha Arco Iris En La Piscigranja “Los Retoños”—Jauja. Undergraduate Thesis, Universidad Nacional del Centro del Perú, Huancayo, Perú, 2021. [Google Scholar]
- Leiva, C. Manejo Reproductivo, Crecimiento y Sobrevivencia de Alevinos de Oncorhynchus mykiss Centro Piscícola de Motil: La Libertad—Perú. Undergraduate Thesis, Universidad Nacional de Trujillo, Trujillo, Perú, 2019. [Google Scholar]
- Hernández-Gallegos, O.; Pan, T.-C.F.; Hunt von Herbing, I.; Méndez-Sánchez, J.F.; Rodríguez-Vargas, G.; Garduño Paz, M.V.; Ruiz-Gómez, M. de L. Environmental Sustainability at High Altitude in Mexico. Int. J. Environ. Sustain. 2013, 8, 19–34. [Google Scholar] [CrossRef]
- Verleih, M.; Borchel, A.; Krasnov, A.; Rebl, A.; Korytář, T.; Kühn, C.; Goldammer, T. Impact of Thermal Stress on Kidney-Specific Gene Expression in Farmed Regional and Imported Rainbow Trout. Mar. Biotechnol. 2015, 17, 576–592. [Google Scholar] [CrossRef] [PubMed]
- Trenzado, C.E.; Morales, A.E.; de la Higuera, M. Physiological Effects of Crowding in Rainbow Trout, Oncorhynchus mykiss, Selected for Low and High Stress Responsiveness. Aquaculture 2006, 258, 583–593. [Google Scholar] [CrossRef]
- Fivelstad, S.; Waagbø, R.; Zeitz, S.F.; Hosfeld, A.C.D.; Olsen, A.B.; Stefansson, S. A Major Water Quality Problem in Smolt Farms: Combined Effects of Carbon Dioxide, Reduced PH and Aluminium on Atlantic Salmon (Salmo salar L.) Smolts: Physiology and Growth. Aquaculture 2003, 215, 339–357. [Google Scholar] [CrossRef]
- Brauner, C.J.; Randall, D.J. The Interaction between Oxygen and Carbon Dioxide Movements in Fishes. Comp. Biochem. Physiol. A Physiol. 1996, 113, 83–90. [Google Scholar] [CrossRef]
- Bahabadi, M.N.; Soltani, M. Effect Dietary Energy Levels and Feeding Rates on Growth and Body Composition of Fingerling Rainbow Trout (Oncorhynchus mykiss). Iran. J. Fish. Sci. 2008, 7, 171–186. [Google Scholar]
- Montaña, A. Crecimiento y Sobrevivencia En El Levante de Alevinos de Trucha Arcoiris (Oncorhynchus mykiss) En Sistemas Cerrados de Recirculación de Agua. Undergraduate Thesis, Universidad Militar Nueva Granada, Facultad de Ciencias, Biología Aplicada, 2009. [Google Scholar]
- Flores-Mendoza, M.; Ortega B., R.; del Pilar Blanco E., M.; Araníbar, M.J. Respuesta Productiva de Truchas Arcoíris (Oncorhynchus mykiss) al Régimen Alimenticio Con Alimentos Comerciales Bajo Condiciones de Crianza Intensiva En El Lago Titicaca. Rev. Investig. Vet. Perú 2023, 34, e25131. [Google Scholar] [CrossRef]
- Badiola, M.; Basurko, O.C.; Piedrahita, R.; Hundley, P.; Mendiola, D. Energy Use in Recirculating Aquaculture Systems (RAS): A Review. Aquac. Eng. 2018, 81, 57–70. [Google Scholar] [CrossRef]
- Almeida, P.; Dewasme, L.; Wouwer, A. Vande Denitrification Control in a Recirculating Aquaculture System—A Simulation Study. Processes 2020, 8, 1306. [Google Scholar] [CrossRef]
- Lim, C.; Webster, C.D.; Lee, C.S. Alternative Protein Sources in Aquaculture Diets; CRC Press: Boca Raton, FL, USA, 2023. [Google Scholar]
- Hall, J.R.; Martin, G. Filtration of Dissolved Organic Nutrients from Fish Farm Wastewater Using a Macroalgae Biofilter. In WIT Transactions on Ecology and the Environment; WIT Press: Ashurst, Southampton, UK, 2021; Volume 250. [Google Scholar]
- Zhang, K.; Ye, Z.; Qi, M.; Cai, W.; Saraiva, J.L.; Wen, Y.; Liu, G.; Zhu, Z.; Zhu, S.; Zhao, J. Water Quality Impact on Fish Behavior: A Review From an Aquaculture Perspective. Rev. Aquac. 2025, 17, e12985. [Google Scholar] [CrossRef]
- Martins, C.I.M.; Eding, E.H.; Verdegem, M.C.J.; Heinsbroek, L.T.N.; Schneider, O.; Blancheton, J.P.; d’Orbcastel, E.R.; Verreth, J.A.J. New Developments in Recirculating Aquaculture Systems in Europe: A Perspective on Environmental Sustainability. Aquac. Eng. 2010, 43, 83–93. [Google Scholar] [CrossRef]

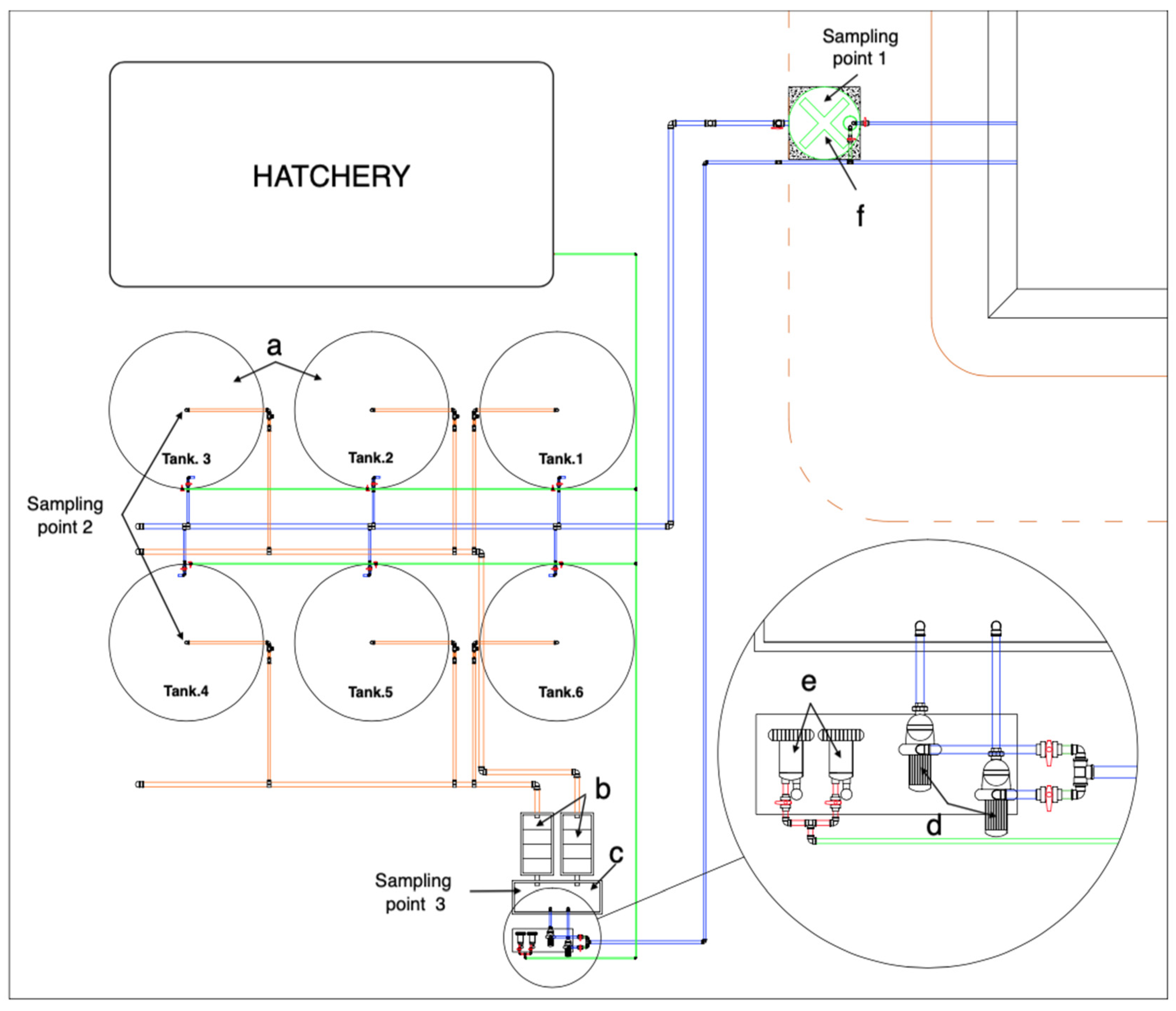

| Parameters | Unit | Methodology |
|---|---|---|
| Alkalinity | mg CaCO3/L | SMWW 2320B |
| Arsenic | mg/L | SMWW 3114C |
| Chloride | mg/L | SMWW 4500Cl- |
| True Color | Pt-Co | SMWW 2120C |
| Conductivity | µS/cm | SMWW 2510B |
| Hardness | mg CaCO3/L | SMWW 2340C |
| Phosphorus (P-H2PO4) | mg/L | SMWW 4500P-C |
| pH | SMWW 4500B-H*B | |
| Potassium | mg/L | SMWW 3111B |
| Total dissolved solids | mg/L | SMWW 2540B |
| Salinity | PSU | SMWW 2520B |
| Water Quality Parameters | Sampling Point | Without Fish | With Fish | Normal Value | |||
|---|---|---|---|---|---|---|---|
| Sep | Oct | Nov | Dec | Jan | |||
| Temperature (°C) | HT | 11.00 ± 0.37 | 14.00 ± 0.33 | 15.00 ± 0.31 | 17.00 ± 0.89 | 21.00 ± 1.29 | 9 to17°C [32] |
| CT3 | 8.00 ± 0.29 b | 9.00 ± 0.55 c | 12.98 ± 1.66 b | 17.00 ± 0.89 a | 20.03 ± 0.74 ab | ||
| CT4 | 7.00 ± 0.16 c | 8.00 ± 0.12 d | 13.98 ± 0.15 b | 17.03 ± 0.48 a | 19.00 ± 0.72 b | ||
| DT | 8.03 ± 0.36 b | 9.95 ± 0.75 b | 14.00 ± 0.14 b | 17.03 ± 0.24 a | 19.03 ± 0.23 b | ||
| Dissolved oxygen (mg/L) | HT | 6.31 ± 0.08 c | 3.43 ± 0.17 b | 4.45 ± 0.10 a | 3.14 ± 0.17 b | 6.80 ± 0.57 a | 7.5 to 12 mg/L [33] |
| CT3 | 7.48 ± 0.08 a | 4.90 ± 0.12 a | 2.73 ± 0.11 d | 1.78 ± 0.01 c | 3.52 ± 0.23 d | ||
| CT4 | 7.48 ± 0.07 a | 2.96 ± 0.13 c | 3.09 ± 0.12 c | 1.82 ± 0.06 c | 6.03 ± 0.74 b | ||
| DT | 6.75 ± 0.13 b | 2.90 ± 0.01 c | 3.40 ± 0.03 b | 4.84 ± 0.35 a | 4.71 ± 0.09 c | ||
| Ammonium (mg/L) | HT | <0.1 | <0.1 | 0.12 ± 0.01 c | 0.29 ± 0.02 b | 0.63 ± 0.02 a | <0.012 mg/L [34] |
| CT3 | <0.1 | <0.1 | 0.32 ± 0.02 a | 0.48 ± 0.02 a | 0.41 ± 0.03 b | ||
| CT4 | <0.1 | <0.1 | 0.28 ± 0.03 b | 0.21 ± 0.01 c | 0.20 ± 0.01 c | ||
| DT | <0.1 | <0.1 | 0.27 ± 0.01 b | 0.14 ± 0.01 d | 0.19 ± 0.01 d | ||
| Nitrate (mg/L) | HT | 3.83 ± 0.26 a | 1.63 ± 0.11 c | 111.00 ± 2.24 c | 115.00 ± 2.55 d | 52.00 ± 1.87 c | <110 mg/L [35] |
| CT3 | 2.63 ± 0.17 b | 3.01 ± 0.16 a | 121.00 ± 2.41 a | 131.00 ± 3.30 b | 43.00 ± 1.73 d | ||
| CT4 | 3.59 ± 0.11 a | 2.89 ± 0.16 a | 116.00 ± 2.23 b | 121.00 ± 1.87 c | 62.00 ± 4.05 a | ||
| DT | 2.01 ± 0.04 c | 2.69 ± 0.10 b | 118.00 ± 1.57 b | 135.00 ± 1.80 a | 58.00 ± 1.30 b | ||
| Water Quality Parameters | Sampling Point | Without Fish | With Fish | Normal Values | |||
|---|---|---|---|---|---|---|---|
| Sep | Oct | Nov | Dec | Jan | |||
| Alkalinity mg CaCO3/L | HT | 38.00 | 37.00 | 36.00 | 36.00 | 38.00 | 75–150 mg/L [7] |
| CT3 | 41.00 | 38.00 | 37.00 | 38.00 | 38.00 | ||
| CT4 | 43.00 | 35.00 | 36.00 | 36.00 | 36.00 | ||
| DT | 36.00 | 36.00 | 38.00 | 36.00 | 38.00 | ||
| Arsenic mg/L | HT | 0.24 | 0.25 | 0.16 | 0.15 | 0.08 | 0.05 mg/L [36] |
| CT3 | 0.29 | 0.29 | 0.16 | 0.13 | 0.07 | ||
| CT4 | 0.24 | 0.24 | 0.16 | 0.13 | 0.07 | ||
| DT | 0.27 | 0.27 | 0.16 | 0.14 | 0.07 | ||
| Chloride mg/L | HT | 65.00 | 85.00 | 55.00 | 55.00 | 28.00 | 10–50 mg/L [37] |
| CT3 | 100.00 | 90.00 | 73.00 | 55.00 | 20.00 | ||
| CT4 | 0.24 | 0.24 | 0.16 | 0.13 | 0.07 | ||
| DT | 110.00 | 70.00 | 70.00 | 58.00 | 10.00 | ||
| Colour test Pt-Co | HT | 5.00 | 6.00 | 11.00 | 27.00 | 52.00 | 30–50 TCU [37] |
| CT3 | 8.00 | 7.00 | 11.00 | 24.00 | 13.00 | ||
| CT4 | 6.00 | 5.00 | 11.00 | 28.00 | 13.00 | ||
| DT | 7.00 | 7.00 | 12.00 | 25.00 | 18.00 | ||
| Conductivity µS/cm | HT | 551.00 | 568.00 | 477.00 | 415.00 | 447.00 | 20–500 µS/cm (20 °C) [38] |
| CT3 | 621.00 | 590.00 | 513.00 | 412.00 | 421.00 | ||
| CT4 | 572.00 | 550.00 | 489.00 | 414.00 | 418.00 | ||
| DT | 631.00 | 570.00 | 491.00 | 418.00 | 417.00 | ||
| Calcium hardness mg CaCO3/L | HT | 163.00 | 161.00 | 181.00 | 99.00 | 137.00 | 20–100 mg/L [39] |
| CT3 | 185.00 | 157.00 | 125.00 | 107.00 | 133.00 | ||
| CT4 | 572.00 | 148.00 | 156.00 | 101.00 | 129.00 | ||
| DT | 631.00 | 161.00 | 142.00 | 101.00 | 134.00 | ||
| Phosphoric acid mg/L | HT | 8.70 | 8.70 | 16.00 | 20.00 | 41.59 | <0.1 mg/L [40] |
| CT3 | 7.25 | 8.41 | 14.00 | 21.00 | 40.72 | ||
| CT4 | 8.41 | 8.99 | 18.00 | 21.00 | 43.19 | ||
| DT | 9.28 | 11.16 | 19.00 | 23.00 | 41.74 | ||
| pH | HT | 6.80 | 8.59 | 6.90 | 8.30 | 9.19 | 6.7–9 [36] |
| CT3 | 6.60 | 7.92 | 5.90 | 8.10 | 7.95 | ||
| CT4 | 6.50 | 7.60 | 6.10 | 8.20 | 8.44 | ||
| DT | 6.50 | 8.65 | 6.80 | 8.60 | 8.11 | ||
| Potassium mg/L | HT | 8.25 | 7.00 | 7.10 | 8.26 | 8.25 | 5–20 mg/L [37] |
| CT3 | 7.50 | 7.50 | 6.90 | 8.21 | 8.19 | ||
| CT4 | 9.00 | 9.00 | 7.70 | 7.67 | 7.98 | ||
| DT | 8.50 | 12.50 | 7.80 | 8.13 | 8.01 | ||
| STD mg/L | HT | 449.00 | 462.00 | 372.00 | 418.00 | 392.00 | <400 mg/L [36] |
| CT3 | 515.00 | 465.00 | 389.00 | 409.00 | 366.00 | ||
| CT4 | 476.00 | 453.00 | 257.00 | 409.00 | 373.00 | ||
| DT | 483.00 | 472.00 | 396.00 | 400.00 | 285.00 | ||
| Salinity PSU | HT | 0.27 | 0.28 | 0.23 | 0.20 | 0.23 | 0 to 35 PSU [36] |
| CT3 | 0.30 | 0.29 | 0.25 | 0.20 | 0.22 | ||
| CT4 | 0.28 | 0.27 | 0.24 | 0.20 | 0.23 | ||
| DT | 0.31 | 0.28 | 0.24 | 0.20 | 0.22 | ||
| Variables | Total |
|---|---|
| Food provided (kg) | 274.1 |
| Initial biomass (kg) | 60.4 |
| Final biomass (kg) | 240.6 |
| Increase in weight (g) | 180.3 |
| Initial density (kg/m3) | 1.5 |
| Final density (kg/m3) | 6 |
| Initial No. of fish | 5000 |
| Final No. of fish | 4810 |
| Feed conversion ratio (FCA) | 1.52 |
| Specific growth rate (SGR) | 1.49 |
| Weight gain (%) | 298.7 |
| Survival rate (%) | 96.2 |
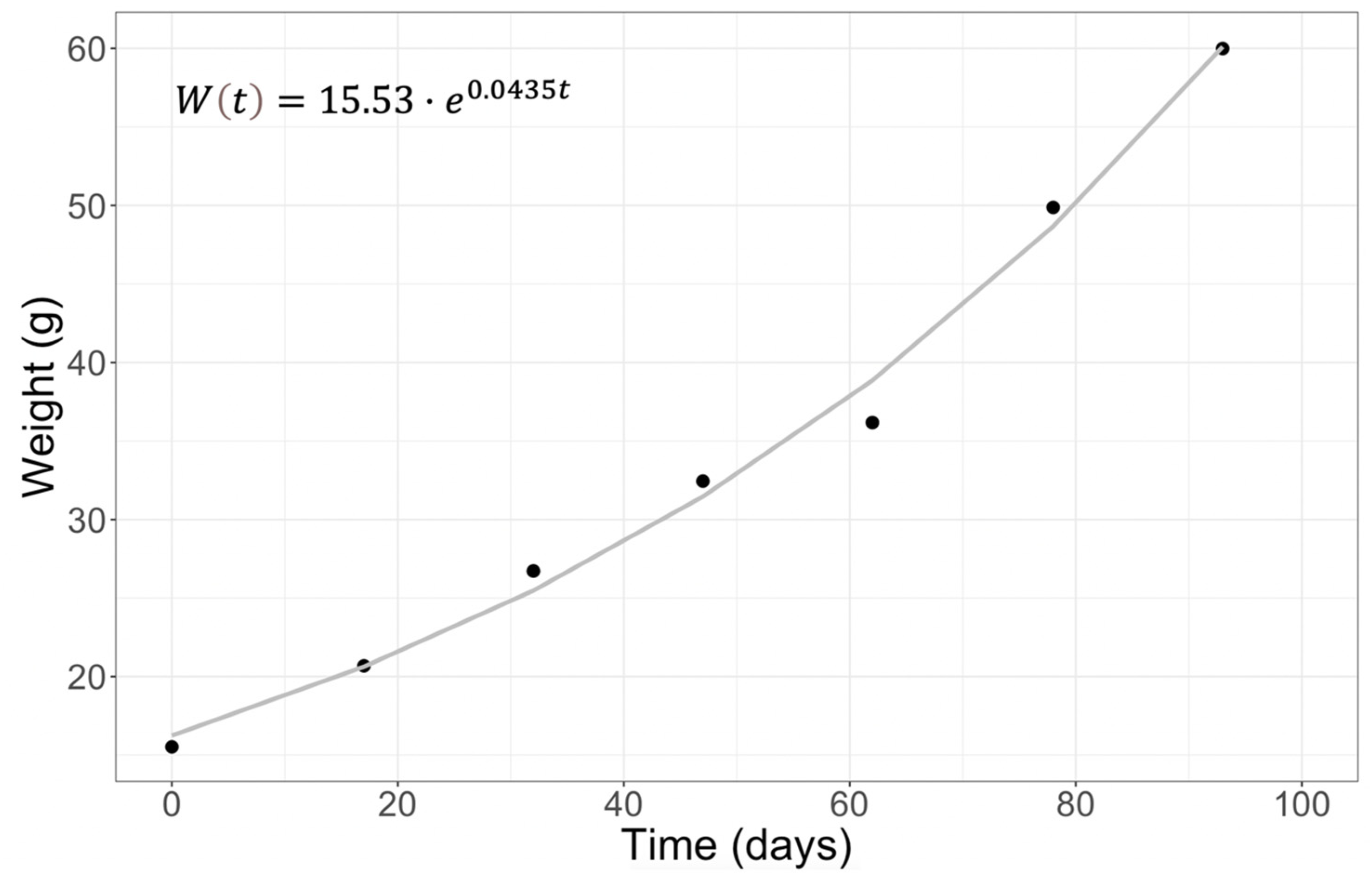
| Culture Day | Weight (g) | SGR (d−1) | CGR (d−1) | Weight Gain (%) |
|---|---|---|---|---|
| 0 | 15.52 ± 2.51 | |||
| 17 | 20.68 ± 1.61 | 0.17 | 0.17 | 24.9 |
| 32 | 26.72 ± 3.06 | 0.21 | 0.10 | 22.6 |
| 47 | 32.44 ± 3.37 | 0.22 | 0.07 | 17.6 |
| 62 | 36.17 ± 3.62 | 0.23 | 0.06 | 10.3 |
| 78 | 49.88 ± 4.80 | 0.24 | 0.05 | 27.5 |
| 93 | 59.99 ± 3.64 | 0.27 | 0.04 | 16.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pepe-Victoriano, R.; Pepe-Vargas, P.; Pérez-Aravena, A.; Aravena-Ambrosetti, H.; Huanacuni, J.I.; Méndez-Abarca, F.; Olivares-Cantillano, G.; Acosta-Angulo, O.; Espinoza-Ramos, L. Evaluation of Water Quality in the Production of Rainbow Trout (Oncorhynchus mykiss) in a Recirculating Aquaculture System (RAS) in the Precordilleran Region of Northern Chile. Water 2025, 17, 1685. https://doi.org/10.3390/w17111685
Pepe-Victoriano R, Pepe-Vargas P, Pérez-Aravena A, Aravena-Ambrosetti H, Huanacuni JI, Méndez-Abarca F, Olivares-Cantillano G, Acosta-Angulo O, Espinoza-Ramos L. Evaluation of Water Quality in the Production of Rainbow Trout (Oncorhynchus mykiss) in a Recirculating Aquaculture System (RAS) in the Precordilleran Region of Northern Chile. Water. 2025; 17(11):1685. https://doi.org/10.3390/w17111685
Chicago/Turabian StylePepe-Victoriano, Renzo, Piera Pepe-Vargas, Anahí Pérez-Aravena, Héctor Aravena-Ambrosetti, Jordan I. Huanacuni, Felipe Méndez-Abarca, Germán Olivares-Cantillano, Olger Acosta-Angulo, and Luis Espinoza-Ramos. 2025. "Evaluation of Water Quality in the Production of Rainbow Trout (Oncorhynchus mykiss) in a Recirculating Aquaculture System (RAS) in the Precordilleran Region of Northern Chile" Water 17, no. 11: 1685. https://doi.org/10.3390/w17111685
APA StylePepe-Victoriano, R., Pepe-Vargas, P., Pérez-Aravena, A., Aravena-Ambrosetti, H., Huanacuni, J. I., Méndez-Abarca, F., Olivares-Cantillano, G., Acosta-Angulo, O., & Espinoza-Ramos, L. (2025). Evaluation of Water Quality in the Production of Rainbow Trout (Oncorhynchus mykiss) in a Recirculating Aquaculture System (RAS) in the Precordilleran Region of Northern Chile. Water, 17(11), 1685. https://doi.org/10.3390/w17111685







