Water Quality Assessment: Endotoxin Brings Real-Time Measurements and Non-Faecally Transmitted Bacteria to the Table
Abstract
1. Introduction
2. Materials and Methods
2.1. Water Sampling
2.2. Bacterial Culture Identification (ISO Methods)
2.3. Bacterisk Assay
2.4. Sequencing Analysis
2.5. qPCR
2.6. Determination of Uncertainty of Measurement (UoM)
2.7. Statistical Analysis
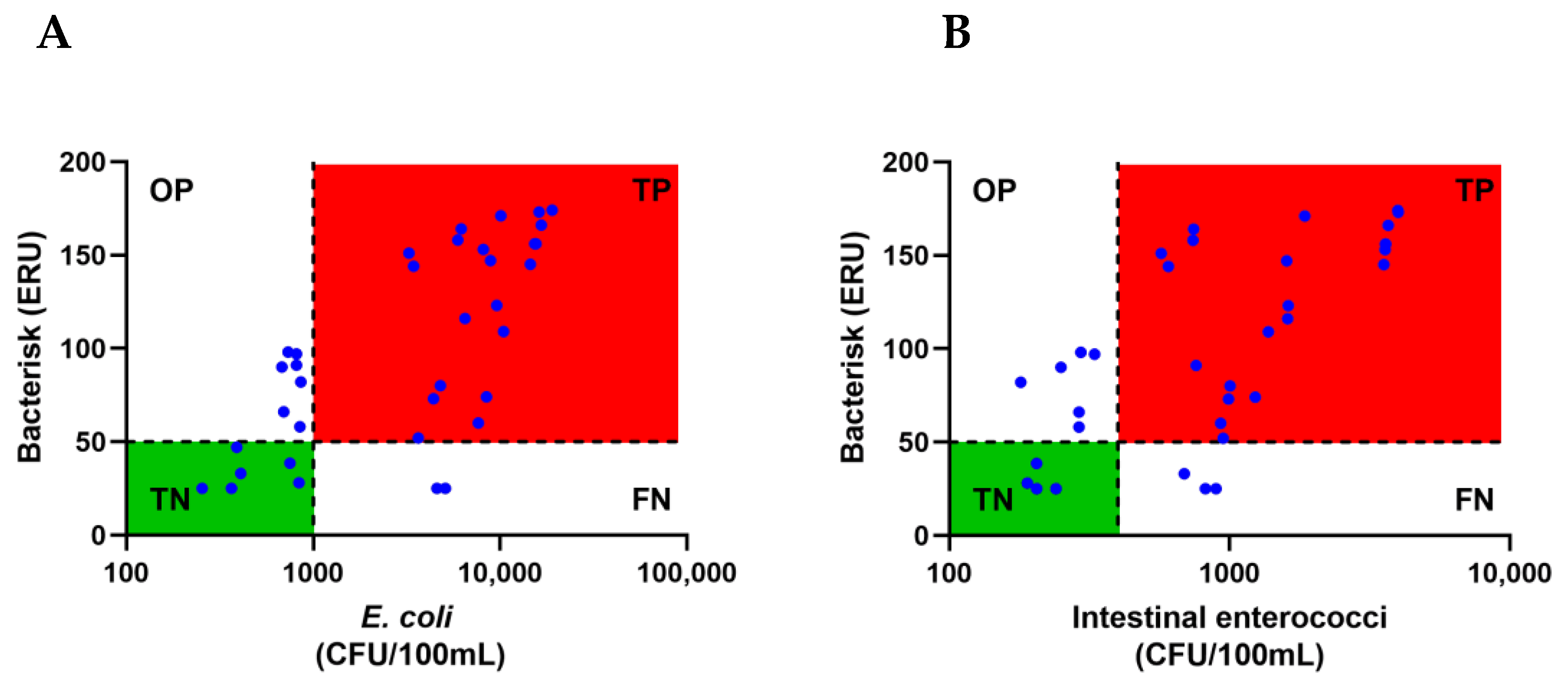
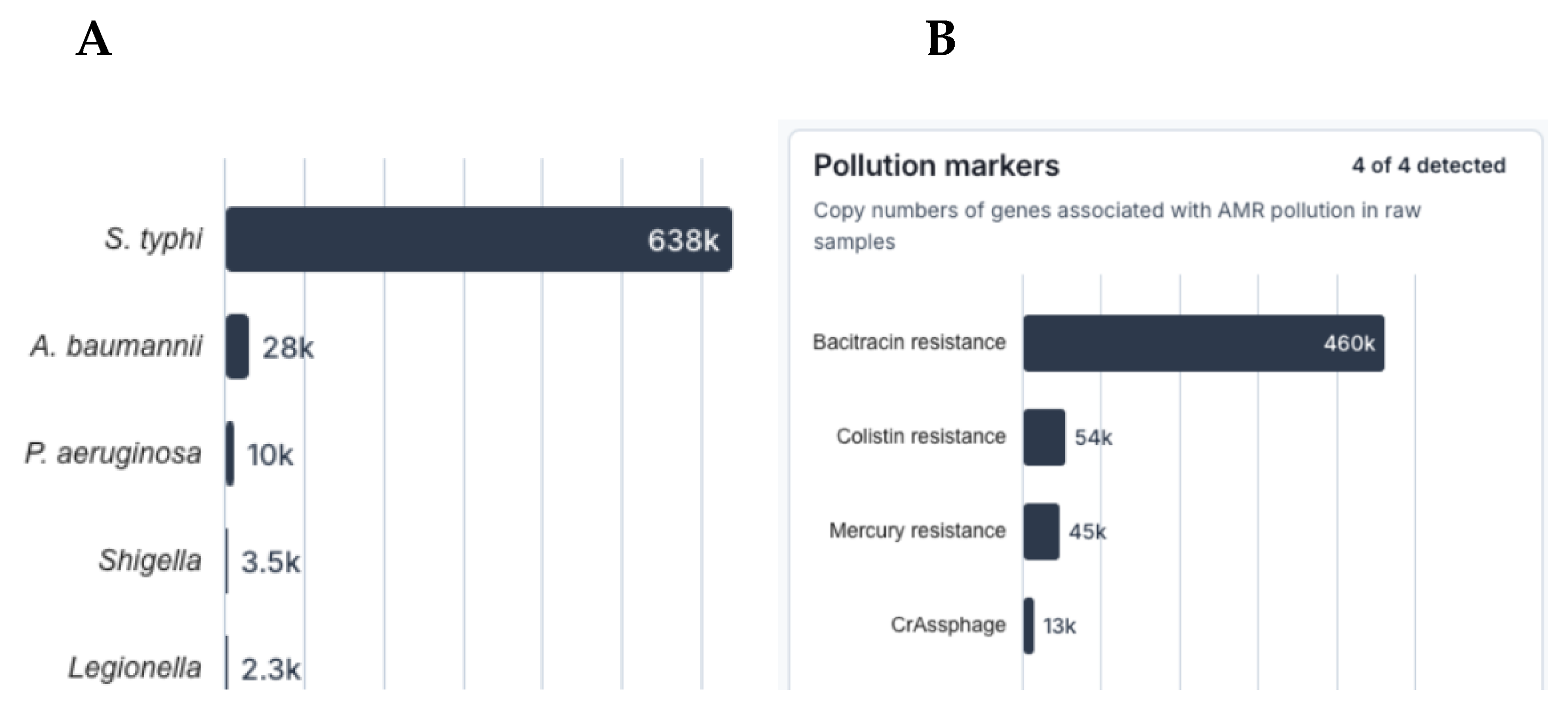
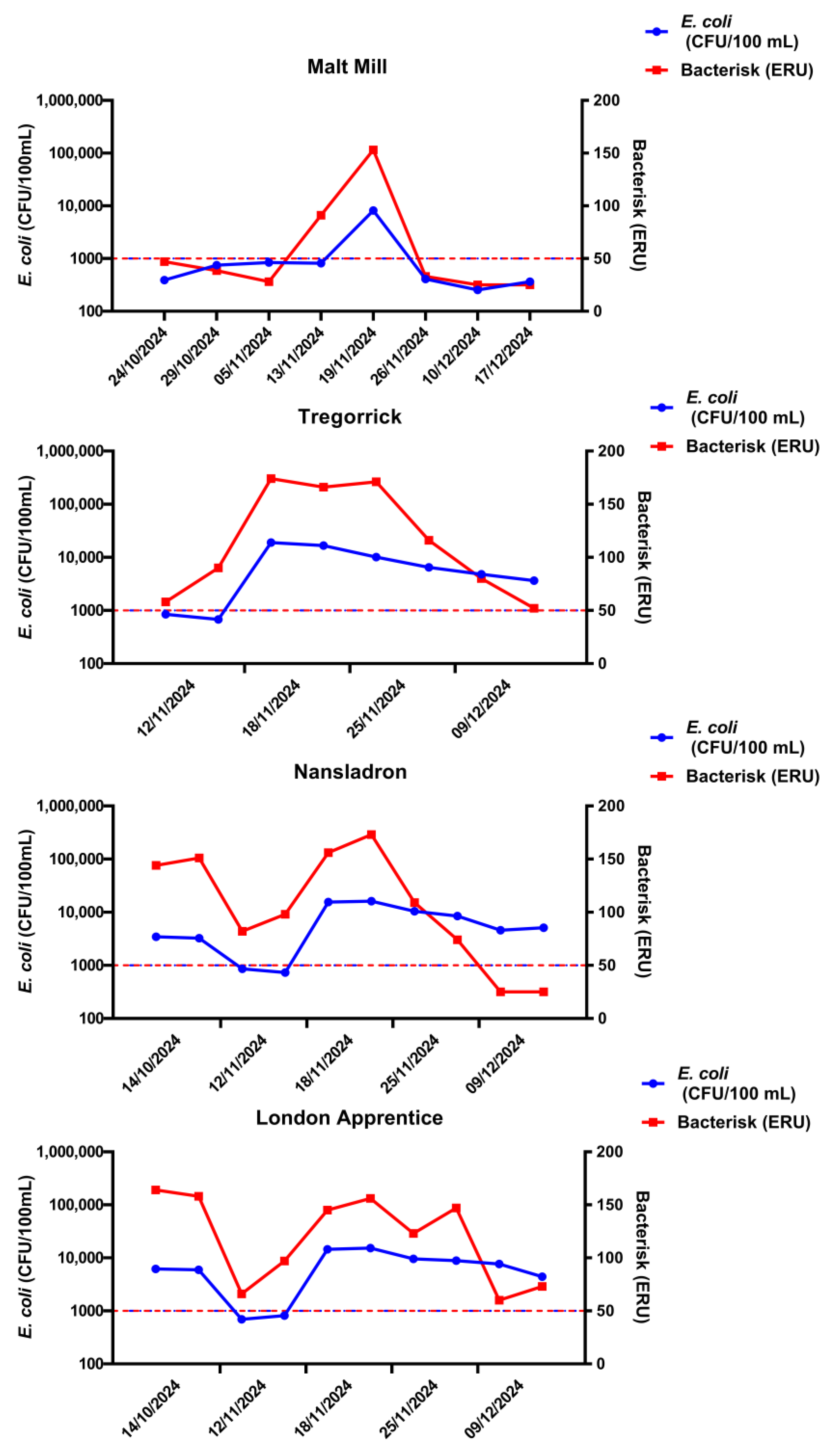
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holcomb, D.A.; Stewart, J.R. Microbial Indicators of Faecal Pollution: Recent Progress and Challenges in Assessing Water Quality. Curr. Environ. Health Rep. 2020, 7, 311–324. [Google Scholar] [CrossRef] [PubMed]
- WHO Guidelines on Recreational Water Quality; Volume 1: Coastal and fresh waters; World Health Organization: Geneva, Switzerland, 2021.
- Boehm, A.B.; Soller, J.A. Recreational Water Risk: Pathogens and Faecal Indicators. In Environmental Toxicology; Laws, E., Ed.; Springer: New York, NY, USA, 2013. [Google Scholar]
- Fewtrell, L.; Kay, D. Recreational Water and Infection: A Review of Recent Findings. Curr. Environ. Health Rep. 2015, 2, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.; Cunha, M.Â. Assessment of the microbiological quality of recreational waters: Indicators and methods. Euro-Mediterr. J. Environ. Integr. 2017, 2, 25. [Google Scholar] [CrossRef]
- Hlavsa, M.C.; Aluko, S.K.; Miller, A.D.; Person, J.; Gerdes, M.E.; Lee, S.; Laco, J.P.; Hannapel, E.J.; Hill, V.R. Outbreaks Associated with Treated Recreational Water—United States, 2015–2019. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 733–738. [Google Scholar] [CrossRef]
- Sampaio, A.; Silva, V.; Poeta, P.; Aonofriesei, F. Vibrio spp.: Life Strategies, Ecology, and Risks in a Changing Environment. Diversity 2022, 14, 97. [Google Scholar] [CrossRef]
- João, B.; Weiskerger, C.; Valério, E.; Pitkänen, T.; Meriläinen, P.; Avolio, L.; Heaney, C.D.; Sadowsky, M.J. Climate Change Impacts on Microbiota in Beach Sand and Water: Looking Ahead. Int. J. Environ. Res. Public Health 2022, 19, 1444. [Google Scholar]
- Ayi, B. Infections Acquired via Fresh Water: From Lakes to Hot Tubs. Microbiol Spectr. 2015, 3, 55–81. [Google Scholar] [CrossRef]
- Guida, M.; Di Onofrio, V.; Gallè, F.; Gesuele, R.; Valeriani, F.; Liguori, R.; Romano Spica, V.; Liguori, G. Pseudomonas aeruginosa in Swimming Pool Water: Evidences and Perspectives for a New Control Strategy. Int. J. Environ. Res. Public Health 2016, 13, 919. [Google Scholar] [CrossRef]
- Pantazidou, G.; Dimitrakopoulou, M.E.; Kotsalou, C.; Velissari, J.; Vantarakis, A. Risk analysis of otitis externa (swimmer’s ear) in children pool swimmers: A case study from Greece. Water 2022, 14, 1983. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Division on Earth and Life Studies; Board on Population Health and Public Health Practice; Board on Life Sciences; Water Science and Technology Board; Committee on Management of Legionella in Water Systems. Management of Legionella in Water Systems; National Academies Press: Cambridge, MA, USA, 2019. [Google Scholar]
- European Parliament and Council. Directive 2006/7/EC concerning the management of bathing water quality and repealing Directive 76/160/EEC. Off. J. Eur. Union. L 2006, 64, 37–51. [Google Scholar]
- Wade, T.J.; Sams, E.; Brenner, K.P.; Haugland, R.; Chern, E.; Beach, M.; Wymer, L.; Rankin, C.C.; Love, D.; Li, Q.; et al. Rapidly measured indicators of recreational water quality and swimming-associated illness at marine beaches: A prospective cohort study. Environ. Health A Glob. Access Sci. Source 2010, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Evans, T.M.; Schillinger, J.E.; Stuart, D.G. Rapid determination of bacteriological water quality by using imulus lysate. Appl. Environ. Microbiol. 1978, 35, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, J.H.; Lee, J.C.; Alexander, G.A.; Wolf, H.W. Comparison of Limulus assay, standard plate count, and total coliform count for microbiological assessment of renovated wastewater. Appl. Environ. Microbiol. 1979, 37, 928–931. [Google Scholar] [CrossRef]
- Haas, C.N.; Meyer, M.A.; Paller, M.S.; Zapkin, M.A. The utility of endotoxins as a surrogate indicator in potable water microbiology. Water Res. 1983, 17, 803–807. [Google Scholar] [CrossRef]
- Sattar, A.A.; Jackson, S.K.; Bradley, G. The potential of lipopolysaccharide as a real-time biomarker of bacterial contamination in marine bathing water. J. Water Health 2014, 12, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Good, C.R.; White, A.; Brandao, J.; Jackson, S. Endotoxin, a novel biomarker for the rapid risk assessment of faecal contamination of coastal and transitional waters. J. Water Health 2024, 22, 1044–1052. [Google Scholar] [CrossRef]
- Sattar, A.A.; Abate, W.; Fejer, G.; Bradley, G.; Jackson, S.K. Evaluation of the proinflammatory effects of contaminated bathing water. J. Toxicol. Environ. Health A 2019, 82, 1076–1087. [Google Scholar] [CrossRef]
- Anwar, M.A.; Choi, S. Gram-negative marine bacteria: Structural features of lipopolysaccharides and their relevance for economically important diseases. Mar. Drugs 2014, 12, 2485–2514. [Google Scholar] [CrossRef]
- Leonard, A.F.; Zhang, L.; Balfour, A.J.; Garside, R.; Gaze, W.H. Human recreational exposure to antibiotic resistant bacteria in coastal bathing waters. Environ. Int. 2015, 82, 92–100. [Google Scholar] [CrossRef]
- Farrell, M.L.; Chueiri, A.; O’Connor, L.; Duane, S.; Maguire, M.; Miliotis, G.; Cormican, M.; Hooban, B.; Leonard, A.; Gaze, W.H.; et al. Assessing the impact of recreational water use on carriage of antimicrobial resistant organisms. Sci. Total Environ. 2023, 888, 164201. [Google Scholar] [CrossRef]
- Singh, A.; Srivastava, A.; Pandey, S.; Shukla, R. Antimicrobials and antimicrobial resistance genes in the environment: A review on their occurrence and spread through wastewater and natural water. Front. Environ. Sci. 2022, 10, 830861. [Google Scholar]
- Han, Q.; Wang, Y.; Li, Y.; Qiao, W.; Wu, X. Antimicrobial resistance genes and bacterial communities in recreational coastal waters and adjacent estuaries: Evidence from Qinhuangdao, China. Front. Mar. Sci. 2022, 9, 976438. [Google Scholar]
- Li, Y.; Zhang, C.; Mou, X.; Zhang, P.; Liang, J.; Wang, Z. Distribution characteristics of antibiotic-resistant bacteria and related genes in urban recreational lakes replenished by different supplementary water sources. Water Sci. Technol. 2022, 85, 1176–1190. [Google Scholar] [CrossRef] [PubMed]
- ISO 9308–2, 2012; Water Quality-Enumeration of Escherichia coli and Coliform bacteria, Part 2: Most Probable Number Method. International Organization for Standardization: Geneva, Switzerland, 2012.
- ISO 7899–2, 2000; Water Quality- Detection and Enumeration of Intestinal Enterococci—Part 2: Membrane Filtration Method. International Organization for Standardization: Geneva, Switzerland, 2000.
- Magnusson, B.; Näykki, T.; Hovind, H.; Krysell, M.; Sahlin, E. Handbook for Calculation of Measurement Uncertainty in Environmental Laboratories; Nordtest Report TR 537 (ed. 4); NORDTEST: Serravalle Scrivia, Italy, 2017. [Google Scholar]
- Nahm, F.S. Receiver operating characteristic curve: Overview and practical use for clinicians. Korean J. Anesthesiol. 2022, 75, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kabir, F.; Manneh, J.; Lertsethtakarm, P.; Begum, S.; Gratz, J.; Becker, S.M.; Operario, D.J.; Taniuchi, M.; Janaki, L.; et al. Development and assessment of molecular diagnostic tests for 15 enteropathogens causing childhood diarrhoea: A multicentre study. Lancet Infect. Dis. 2014, 14, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Stec, J.; Kosikowska, U.; Mendrycka, M.; Stępień-Pyśniak, D.; Niedźwiedzka-Rystwej, P.; Bębnowska, D.; Hrynkiewicz, R.; Ziętara-Wysocka, J.; Grywalska, E. Opportunistic Pathogens of Recreational Waters with Emphasis on Antimicrobial Resistance-A Possible Subject of Human Health Concern. Int. J. Environ. Res. Public Health 2022, 19, 7308. [Google Scholar] [CrossRef]
- Available online: http://data.europa.eu/eli/dir/2024/3019/oj (accessed on 12 December 2024).
- Sattar, A.A.; Good, C.R.; Saletes, M.; Brandao, J.; Jackson, S.K. Endotoxin as a Marker for Water Quality. Int. J. Environ. Res. Public Health 2022, 19, 16528. [Google Scholar] [CrossRef]
- Mena, K.D.; Gerba, C.P. Risk assessment of Pseudomonas aeruginosa in water. Rev. Environ. Contam. Toxicol. 2009, 201, 71–115. [Google Scholar]
- WHO Bacterial Priority Pathogens List; WHO: Geneva, Switzerland, 2024.
- Field, K.G.; Samadpour, M. Faecal source tracking, the indicator paradigm, and managing water quality. Water Res. 2007, 41, 3517–3538. [Google Scholar] [CrossRef]
- Stewart, J.R.; Gast, R.J.; Fujioka, R.S.; Solo-Gabriele, H.M.; Meschke, J.S.; Amaral-Zettler, L.A.; del Castillo, E.; Polz, M.F.; Collier, T.K.; Strom, M.S.; et al. The coastal environment and human health: Microbial indicators, pathogens, sentinels and reservoirs. Environ. Health 2008, 7, S3. [Google Scholar] [CrossRef]
- Stewart, J.R.; Boehm, A.B.; Dubinsky, E.A.; Fong, T.-T.; Goodwin, K.D.; Griffith, J.F.; Noble, R.T.; Shanks, O.C.; Vijayavel, K.; Weisberg, S.B. Recommendations following a multi-laboratory comparison of microbial source tracking methods. Water Res. 2013, 47, 6829–6838. [Google Scholar] [CrossRef] [PubMed]
- Korajkic, A.; McMinn, B.; Harwood, V. Relationships between microbial indicators and pathogens in recreational water settings. Int. J. Environ. Res. Public Health 2018, 15, 2842. [Google Scholar] [CrossRef]
- Griffith, J.F.; Weisberg, S.B.; Arnold, B.F.; Cao, Y.; Schiff, K.C.; Colford, J.M. Epidemiologic evaluation of multiple alternate microbial water quality monitoring indicators at three California beaches. Water Res. 2016, 94, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Long, S.C.; Das, D.; Dorner, S.M. Are microbial indicators and pathogens correlated? A statistical analysis of 40 years of research. J. Water Health 2011, 9, 265. [Google Scholar] [CrossRef] [PubMed]
- Byappanahalli, M.; Fowler, M.; Shively, D.; Whitman, R. Ubiquity and persistence of Escherichia coli in a Midwestern coastal stream. Appl. Environ. Microbiol. 2003, 69, 4549–4555. [Google Scholar] [CrossRef]
- Oh, S.; Buddenborg, S.; Yoder-Himes, D.R.; Tiedje, J.M.; Konstantinidis, K.T. Genomic diversity of Escherichia isolates from diverse habitats. PLoS ONE 2012, 7, e47005. [Google Scholar] [CrossRef]
- Korajkic, A.; Wanjugi, P.; Brooks, L.; Cao, Y.; Harwood, V.J. Persistence and decay of faecal microbiota in aquatic habitats. Microbiol. Mol. Biol. Rev. 2019, 83, e00005-19. [Google Scholar] [CrossRef]
- Bernhard, A.E.; Field, K.G. A PCR assay to discriminate human and ruminant feces on the basis of host differences in Bacteroides-Prevotella genes encoding 16S rRNA. Appl. Environ. Microbiol. 2000, 66, 4571–4574. [Google Scholar] [CrossRef]
- Dick, L.K.; Simonich, M.T.; Field, K.G. Microplate subtractive hybridization to enrich for Bacteroidales genetic markers for faecal source identification. Appl. Environ. Microbiol. 2005, 71, 3179–3183. [Google Scholar] [CrossRef]
- Schriewer, A.; Goodwin, K.D.; Sinigalliano, C.D.; Cox, A.M.; Wanless, D.; Bartkowiak, J.; Ebentier, D.L.; Hanley, K.T.; Ervin, J.; Deering, L.A.; et al. Performance evaluation of canine associated Bacteroidales assays in a multi-laboratory comparison study. Water Res. 2023, 47, 6909–6920. [Google Scholar] [CrossRef]
- Schriewer, A.; Miller, W.A.; Byrne, B.A.; Miller, M.A.; Oates, S.; Conrad, P.A.; Hardin, D.; Yang, H.H.; Chouicha, N.; Melli, A.; et al. Presence of Bacteroidales as a predictor of pathogens in surface waters of the central California coast. Appl. Environ. Microbiol. 2010, 76, 5802–5814. [Google Scholar] [CrossRef] [PubMed]
- Flood, C.; Ufnar, J.; Wang, S.; Johnson, J.; Carr, M.; Ellender, R. Lack of correlation between enterococcal counts and the presence of human specific faecal markers in Mississippi creek and coastal waters. Water Res. 2011, 45, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.E.; Boehm, A.B. Frequent occurrence of the human-specific Bacteroides faecal marker at an open coast marine beach: Relationship to waves, tides and traditional indicators. Environ. Microbiol. 2007, 9, 2038–2049. [Google Scholar] [CrossRef] [PubMed]
- Harwood, V.J.; Staley, C.; Badgley, B.D.; Borges, K.; Korajkic, A. Microbial source tracking markers for detection of faecal contamination in environmental waters: Relationships between pathogens and human health outcomes. FEMS Microbiol. Rev. 2014, 38, 1–40. [Google Scholar] [CrossRef]
- Byappanahalli, M.N.; Nevers, M.B.; Korajkic, A.; Staley, Z.R.; Harwood, V.J. Enterococci in the environment. Microbiol. Mol. Biol. Rev. 2012, 76, 685–706. [Google Scholar] [CrossRef]
- Anderson, K.L.; Whitlock, J.E.; Valerie, J.; Harwood, V.J. Persistence and differential survival of faecal indicator bacteria in subtropical waters and sediments. Appl. Environ. Microbiol. 2005, 71, 3041–3048. [Google Scholar] [CrossRef]
- Ferguson, D.M.; Moore, D.F.; Getrich, M.A.; Zhowandai, M.H. Enumeration and speciation of enterococci found in marine and intertidal sediments and coastal water in southern California. J. Appl. Microbiol. 2005, 99, 598–608. [Google Scholar] [CrossRef]
- Sagarduy, M.; Courtois, S.; Del Campo, A.; Garmendia, J.M.; Petrau, A. Differential decay and prediction of persistence of Enterococcus spp. and Escherichia coli culturable cells and molecular markers in freshwater and seawater environments. Int. J. Hyg. Environ. Health 2019, 222, 695–704. [Google Scholar] [CrossRef]
- Shahraki, A.H.; Heath, D.; Chaganti, S.R. Recreational water monitoring: Nanofluidic qRT-PCR chip for assessing beach water safety. Environ DNA 2019, 1, 305–315. [Google Scholar] [CrossRef]

| ERU Limit | Degrees of Freedom (df) | Fixed k = 2 | Fixed k = 1.96 | Varying k Based on (df) | Expanded UoM |
|---|---|---|---|---|---|
| 20 | 287 | 31.94% | 31.30% | 1.968264 | 31.43% |
| 25 | 249 | 22.41% | 21.97% | 1.969537 | 22.07% |
| 30 | 199 | 15.87% | 15.56% | 1.971957 | 15.65% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Good, C.; White, A.; Brandão, J.; Seymour, C.; Jackson, S.K. Water Quality Assessment: Endotoxin Brings Real-Time Measurements and Non-Faecally Transmitted Bacteria to the Table. Water 2025, 17, 1674. https://doi.org/10.3390/w17111674
Good C, White A, Brandão J, Seymour C, Jackson SK. Water Quality Assessment: Endotoxin Brings Real-Time Measurements and Non-Faecally Transmitted Bacteria to the Table. Water. 2025; 17(11):1674. https://doi.org/10.3390/w17111674
Chicago/Turabian StyleGood, Christian, Alistair White, João Brandão, Christopher Seymour, and Simon K. Jackson. 2025. "Water Quality Assessment: Endotoxin Brings Real-Time Measurements and Non-Faecally Transmitted Bacteria to the Table" Water 17, no. 11: 1674. https://doi.org/10.3390/w17111674
APA StyleGood, C., White, A., Brandão, J., Seymour, C., & Jackson, S. K. (2025). Water Quality Assessment: Endotoxin Brings Real-Time Measurements and Non-Faecally Transmitted Bacteria to the Table. Water, 17(11), 1674. https://doi.org/10.3390/w17111674







