1. Introduction
Electrochemical water disinfection involves the elimination of microorganisms by applying an electric current through the water being treated using appropriate electrodes. At the interface between the electrodes and the water, this current facilitates the electrochemical generation of disinfecting agents, either directly from the water itself (through the generation of so-called reactive oxygen species, ROS, which include hydroxyl radicals, singlet oxygen, hydrogen peroxide and even ozone [
1]) or from dissolved substances (such as the oxidation of chloride ions to active chlorine [
2]).
Despite numerous studies on electrochemical disinfection, its real-world applications remain limited. Many research efforts suffer from fundamental shortcomings, including unrealistic operating conditions, inappropriate material selection, and non-compliance with regulatory standards [
3].
The efficiency of electrochemical disinfection varies depending on the method used. Techniques relying on hydrogen peroxide [
4,
5,
6] or electroporation [
7,
8,
9,
10] do not provide a residual disinfection effect, allowing microorganisms to regrow within the distribution network, ultimately reducing the process’ effectiveness. Similarly, filtration-based methods, even when combined with short-lived ROS [
11] or bacteriostatic ions like silver [
12], fail to ensure long-term disinfection. The same limitation applies to physical approaches such as UV disinfection [
13,
14] or heat treatment. Therefore, the generation of active chlorine remains the most practical and effective approach to date.
However, several critical challenges remain. As outlined in [
3], “a drinking water disinfection device must be safe for contact with water intended for human consumption”, significantly limiting material selection. It is crucial to ensure that no harmful substances leach into the treated water. This is particularly true for electrodes, which must be stable, preferably catalytic, and capable of withstanding periodic polarity reversals to maintain performance in water containing moderate levels of calcium and magnesium ions.
One notable electrochemical disinfection system, the
Aquades-EL, has been produced and distributed by the German companies AquaRotter GmbH and GERUS mbH since 1998 [
15]. Over 400 systems were installed in various facilities, including hotels, hospitals, and retirement homes. However, despite regular polarity reversal, the formation of calcareous deposits could not be completely prevented. Depending on water hardness and operating conditions, maintenance—including acid washing to remove calcareous deposits—was required every few weeks or months. The
Aquades-EL systems were sold as packages that included both the device and ongoing maintenance, providing consistent revenue for the companies. Maintenance tasks involved cleaning the electrode stack, recalibrating the free chlorine sensor, and occasionally replacing the electrode stack. However, these costs proved burdensome for customers, ultimately leading to the discontinuation of the product [
16].
Since the early 2000s, electrochemical reactors equipped with conductive diamond electrodes have been explored [
3,
17]. However, despite nearly 2 decades of research, large-scale, real-world applications remain scarce. Condias GmbH [
18] claims that its
Condiapure® technology is well-suited for large-scale disinfection, generating up to 6 g/h of ozone directly from water, enabling chlorine-free disinfection. Unfortunately, the system is limited to treating up to 15 m
3/h (250 L/min), while the instability of ozone in water [
19] and its corrosive nature [
20] present additional challenges. Maintaining a residual disinfectant effect, which is essential to ensure the safety of drinking water at the point of use, remains difficult.
Hand and Cusick examined how water composition affects oxidant demand and disinfectant dosage across different water types, including drinking water, centralized wastewater, and distributed wastewater [
21]. Their analysis covered both dimensionally stable anodes and boron-doped diamond electrodes, revealing that the type of oxidizer and the anode material significantly affect the overall energy consumption. Notably, free chlorine-based systems produce a disinfectant dose up to three orders of magnitude higher than hydroxyl radical-based systems at comparable energy levels. This discrepancy in energy efficiency arises from the different standard potentials required to generate each oxidant. Specifically, free chlorine generation requires a minimum cell voltage of 1.4 V vs. SHE, whereas hydroxyl radical generation requires a higher minimum cell voltage of 2.73 V vs. SHE.
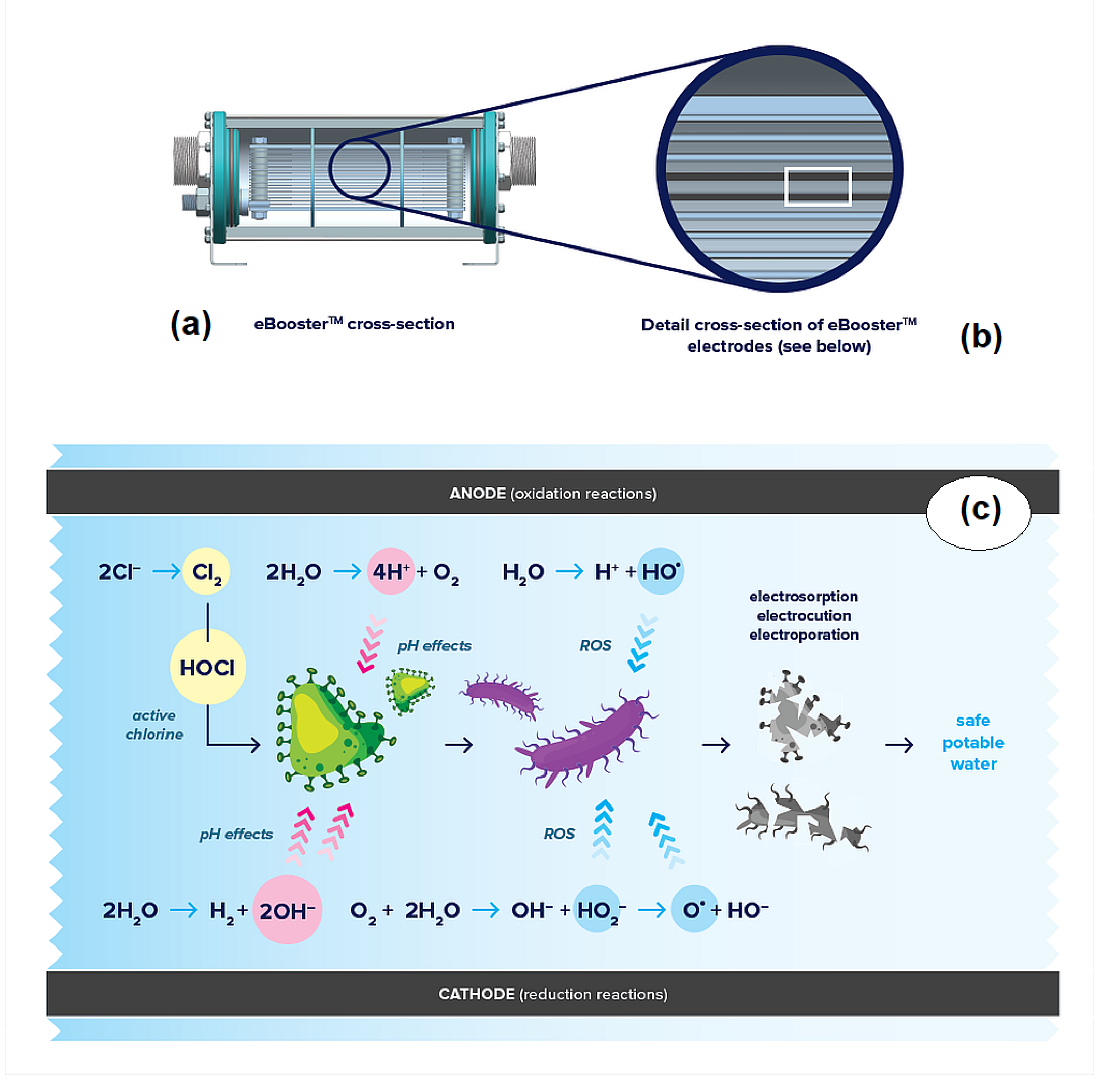
Electrochemical disinfection is not solely dependent on oxidizing species. As partially illustrated in
Figure 1, oxidation [
22,
23] and electric field effects [
24] also play key roles in the disinfection process.
Oxidation contributes to bacterial inactivation through two main pathways: direct and indirect oxidation. In direct oxidation, bacteria come into direct contact with the anode surface, triggering multi-electron reactions with proteins or functional groups in the cell membrane [
22]. This interaction creates sites for free radical activity and localized electrolytic oxidation, ultimately disrupting the membrane. Studies show that lower voltages cause mild cellular compression and dehydration, while higher voltages intensify lipid peroxidation, leading to membrane rupture and cell death [
23].
Indirect oxidation involves the inactivation of microorganisms through oxidizing species generated at the electrodes. In addition to the well-documented anodic electro-generation of reactive oxygen species (ROS) and active chlorine, cathodic oxygen reduction can produce hydrogen peroxide-related species, which also contribute to disinfection. At the anode, water and chloride ions are oxidized, producing hydroxyl radicals, nascent oxygen, protons, and chlorine. The latter then reacts with water to form hypochlorous acid. At the cathode, in addition to the reduction of oxygen, water is reduced, generating hydroxyl ions and hydrogen. Interestingly, in aqueous media with limited chloride concentrations, the formation of hydroxyl radicals, precursors of the oxygen evolution reaction, can occur at more positive potentials [
25]. This shift enhances the system’s overall disinfection efficiency.
Additionally, pH variations resulting from water electrolysis can significantly impact microbial inactivation. Protons are produced at the anode, while hydroxyl ions form at the cathode. Proteins, which are particularly sensitive to pH changes, can undergo alteration in the ionization of amino acid functional groups, disrupting hydrogen bonding and protein folding [
26]. Even moderate changes in pH can promote denaturation, ultimately compromising protein function and leading to microorganism destruction.
To fully leverage these mechanisms, it is essential that all water undergoes electrochemical treatment. This requires directing the entire water flow through the interelectrode space, ensuring no water bypasses the treatment process. This is a distinctive feature of the eBoosterTM technology developed by Ecas4 Australia, which also utilizes electrodes specifically designed for drinking water treatment and capable of functioning under conditions of periodic polarity reversal.
The combination of these various aspects results in a system that enables the electrochemical disinfection of water without the need for external interventions. Additionally, since most water sources contain measurable concentrations of chlorides, the system can produce active chlorine in situ, providing a residual protective effect downstream of treatment.
Unlike the
Aquades-EL system, which was commented on previously, the
eBoosterTM technology does not require periodic maintenance of the electrochemical cell. This has been confirmed in real-world applications, where the technology has been implemented to ensure high-quality water for several hospitals in the Darling Downs region (Queensland, Australia). In 2022, the Queensland Government announced an investment of AU
$ 9.78 billion (approximately US
$ 6.1 billion) over six years to build new hospitals and expand capacity [
27]. Additionally, more than AU
$ 1 billion has been allocated to the
Building Rural and Remote Health Program, which aims to improve healthcare delivery in regional areas by upgrading or replacing aging infrastructure [
28]. Projects funded by this program include the new Tara Hospital and the new Millmerran Multipurpose Health Facility. Due to the regional and rural locations of many of these services,
eBoosterTM technology was seen as an appealing alternative to traditional hypochlorite dosing, which requires regular replenishment of reagents and presents logistical challenges. As a result,
eBoosterTM systems were implemented in most of these healthcare facilities.
For some facilities, such as the new Tara and Millmerran hospitals, the water supply is from storage tanks. In these cases, the electrochemical disinfection system is installed in a recirculating loop to treat the water and maintain a consistent level of active chlorine in the tanks. In other cases, such as at Dalby Hospital, the system has been installed at the water meters upstream of the facility’s distribution system. As recurring treatments are not possible in an in-line system, unlike recirculating configuration, the system must be highly reliable and effective. This is the focus of this communication.
2. Materials and Methods
2.1. Site Information
Dalby, a town in Queensland’s Western Downs region, is located approximately 82 km west of Toowoomba and 208 km northwest of Brisbane. It serves as the center of Australia’s richest cereal and cotton-growing area, with a population of 12,758 and a median age of 35, according to the 2021 census [
29].
Dalby Hospital, a 43-bed facility in the northwest of the city, spans approximately 115,000 m
2 and comprises multiple buildings. It provides maternity, surgical, emergency, palliative care, and renal dialysis services, along with specialized clinics, such as fracture, pediatric, surgical outpatients, and medical imaging. The hospital’s drinking water is supplied by the Western Downs Regional Council, which distributes over 4000 ML of water annually across the region. Raw water sources include surface supplies (dams, weirs, and rivers—44.8%), groundwater (40%), treated seawater, and infrastructure from other providers. Additionally, 15.2% of the total supply consists of recycled water (such as treated sewage), primarily used for park and garden irrigation. In 2023, 3254 ML of water were treated to drinking water standards at a water treatment plant; however, not all drinking water is produced through such facilities [
30]. The Dalby potable water system, which includes 181 km of water mains, sourced 1641 ML of water (35% from surface sources, 65% from groundwater) and produced 1538 ML of potable water [
31]. While no comprehensive online data is available on water quality, the “Queensland Water Quality Guidelines 2009” document [
32], prepared by the Queensland Department of Environment and Heritage Protection and republished in 2013, provides an overview of salinity ranges in Queensland’s watercourses. In the Dalby area, water has moderate to high salinity and is primarily composed of sodium, magnesium, chloride, and bicarbonate.
The hospital is served by two water meters, one off of Swan Street, which supplies most of the facility (estimated peak flow rate of ~10 L/s), and another near Hospital Road, primarily serving the maternity ward (estimated peak flow rate of ~6 L/s). Tap water sample measurements suggest a chloride concentration of approximately 120 mg/L.
2.2. System Description
In mid-2023, Aquastream Water Solutions installed two eBoosterTM systems, each consisting of a B10k unit and a dedicated switchboard, at the hospital’s water meters. These systems operate in-line (without recirculation), automatically adjusting the current to the electrolytic units based on the water flow rate. Their primary function is to maintain a residual chlorine level of approximately 1.5 mg/L in the hospital’s feed water.
Each
eBoosterTM system consists of two main components, the Control Panel and the
eBoosterTM electrochemical device (Australian patent, PCT patent pending), which are available in three sizes. The smallest model, the B250, is designed for localized applications such as homes and plumbing lines with flow rates of up to 25 L/min. The B2k model is suitable for flow rates of up to 200 L/min, while the B10k model can treat up to 800 L/min, depending on water conditions. All
eBoosterTM devices are WaterMark “Lead Free” certified [
33,
34], ensuring compliance with the Lead-Free requirements of the National Construction Code (volume three—Plumbing Code of Australia) [
35] and relevant Australian Standards for product quality, health, and safety [
36]. This certification confirms that
eBoosterTM devices are safe for drinking water treatment and capable of handling pressures up to 20 bar and temperatures up to 50 °C. According to Australian standards, the installation of the system requires the intervention of a licensed plumber.
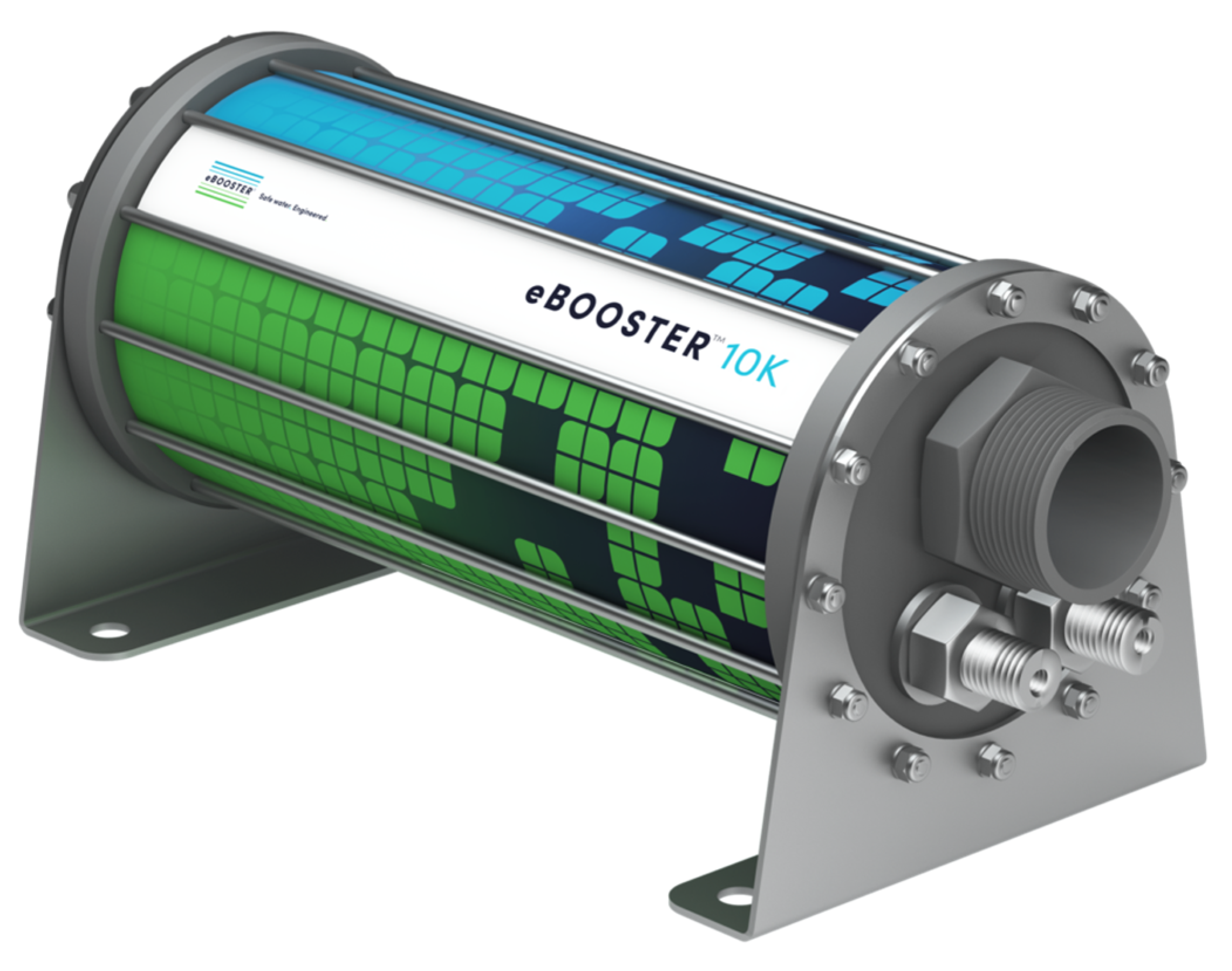
The B10k
eBoosterTM electrochemical unit (
Figure 2) features 40 planar electrodes arranged in an interdigitated configuration, providing a total anode surface area of approximately 1.3 m
2. It uses DSA-type electrodes with a proprietary coating designed to withstand periodic polarity reversal (see [
37]) and to enable the treatment of water intended for human consumption [
3].
The eBoosterTM Control Panel typically features a custom IoT-based DIN-rail-mounted controller, whose operation is managed via dedicated cloud software (eBoosterTM controller WebApp – “station mode” operation) or via direct Wi-Fi access to the controller itself (“access point” operation). For users who are less inclined to adopt new technologies, the Control Panel can alternatively be equipped with a PLC and touchscreen, although this option offers less functionality than the standard DIN-rail-mounted controller.
The IoT-based control system consists of several key components. At its core is a single ESP32 System-on-Chip processor from Espressif Systems (Shanghai, China), which hosts the control firmware. Communication between the control system and the power supplies is handled via an I2C bus, which enables the transmission of voltage and current setpoints and the real-time reception of process voltage and current values. MOSFETs and relays play a crucial role in controlling the DC power supply to the eBoosterTM electrochemical unit. Additionally, the system periodically transmits telemetry data via Wi-Fi to a custom cloud-based web application for monitoring and analysis.
The Control Panel also supports a number of optional components, including a flow switch, a flow meter (either Pulse or Analog Output), and a Chlorine meter. It also features solid-state relays (SSRs), which can be used to control equipment with a power consumption of up to 1000 VA for resistive loads (such as heating elements) or 790 VA for inductive loads (including electric motors, solenoids, contactor coils, and transformers). Alternatively, these SSRs can be configured to send alarms to a Building Management System (BMS).
2.3. System Efficacy
As explained previously, the eBoosterTM system allows in-line water sanitization through a dual disinfection mechanism. Inside the device, microorganisms present in the water are eliminated by electrolytic treatment. At the same time, chlorides naturally present in the water are converted into active chlorine, ensuring continuous disinfection through a residual effect that goes beyond the device itself.
Laboratory tests conducted at both the University of South Australia (UniSA) and the Federal University of Rio Grande do Norte (UFRN) in Brazil using a model B250 reactor have demonstrated that the effectiveness of direct oxidation in chloride-free solutions is primarily influenced by current density and flow rate, the latter determining the residence time in the unit.
At UFRN, experiments on
Escherichia coli using a 50 mM Na
2SO
4 solution containing 1.2 × 10
8 CFU/mL, operated at a current density of 10 mA/cm
2, achieved microbial reductions ranging from 0.4 to 2.3 Log (equivalent to 58% to 99.5%). These results varied with flow rates between 2.5 and 15 L/min, with lower flow rates producing greater disinfection effects due to longer residence times [
38]. Under comparable operating conditions, the larger B10k reactor would operate at 130 A with flow rates ranging from 130 to 780 L/min.
At UniSA, tests targeting bioluminescent
Pseudomonas aeruginosa XEN41 in a 30 mM Na
2SO
4 solution (1.15 × 10
8 CFU/mL), run at 23.5 mA/cm
2 and a flow rate of 6.15 L/min, resulted in a 1.14 Log reduction, or approximately 92% inactivation. When chloride ions were introduced—using a 10 mM NaCl solution (equivalent to 355 mg/L of chloride)—the disinfection efficiency increased dramatically, yielding a 5.37 Log reduction, corresponding to 99.99966% inactivation [
39]. In this case, the B10k reactor would require a current of 310 A and a flow rate of 320 L/min to achieve similar conditions.
The two scenarios explored represent extremes, one with no chloride (a condition rarely encountered in practice) and the other with a relatively high chloride concentration (335 mg/L, slightly above the 250 mg/L drinking water limit). In a real-world application, disinfection efficiency would likely fall somewhere in between.
To maintain consistent performance, the eBoosterTM system is equipped with a self-cleaning electrochemical process that periodically reverses the current supplied to the electrolytic cell. This prevents the accumulation of deposits and prolongs the life of the electrodes. The polarity reversal time can be adjusted based on water hardness. When the cathodes temporarily switch to anode mode, any accumulated calcium carbonate and magnesium hydroxide deposits dissolve, ensuring the electrodes remain clean and fully operational.
3. Project Report
Prior to approving the eBoosterTM system for use in other hospitals, Queensland Health oversaw a trial at the existing medical block at Toowoomba Hospital (the new hospital is currently under construction). This trial commenced in August 2021. The system was installed in a recirculation loop to treat water in two 15 kL storage tanks. In January 2023, an additional eBoosterTM system was implemented in the hospital’s surgical block. After verifying the effectiveness and reliability of the system, approval was granted for the technology to be implemented in other healthcare facilities, particularly those in remote locations, where maintaining a consistent supply of reagents for hypochlorite dosing was challenging.
With peak water flow rates estimated between 6 and 10 L/s and the need to treat the water in-line (without recirculation), the large B10k electrochemical unit was deemed the most suitable option for Dalby. For the water meter with the largest flow rate, the system was designed with a control panel featuring four 125 A/12 V power supplies, with the total current limited by software to 400 A. For the second meter, which handles lower flows, three 125 A/12 V power supplies were used, with the total current limited to 300 A. Using multiple power supplies in parallel helps to spread the load and provides redundancy, which is beneficial in the event of a power supply failure.
As mentioned above, the eBoosterTM device is an electrochemical unit that uses low-voltage electricity to generate multiple effects. One of these is the electrolysis of water, which occurs at both the anodes and cathodes (the electrodes inside the unit), resulting in the production of oxygen and hydrogen. Although there is no risk of reaction between these gases, their stagnation inside the eBoosterTM device can reduce the effectiveness of the treatment and cause uneven wear in some areas of the electrodes. To mitigate this problem, the eBoosterTM device should always be installed in an upright position, allowing the gas to escape effectively. If necessary, a gas release valve can be installed downstream of the device at an appropriate point.
To facilitate maintenance without interrupting the main water supply, it is recommended that the eBoosterTM unit be installed within a bypass line. The bypass line should be constructed of PVC, with PVC pressure valves on both the inlet and outlet sides of the eBoosterTM device. Additionally, when adding the bypass line, the installer should also install a valve in the main water line to divert water through the bypass line and direct it to the eBoosterTM device. It is also recommended that a flow control mechanism (such as a flow switch or flowmeter) be installed within the bypass section upstream of the eBoosterTM device. This will ensure proper monitoring of water flow through the device, allowing the current to be shut off if there is no flow.
Two electromagnetic flow sensors (Siemens AG, Berlin, Germany; model Sitrans FM MAG 5100 W) were used in Dalby and connected to the Control Panels installed at the respective water meters via the 4–20 mA signal.
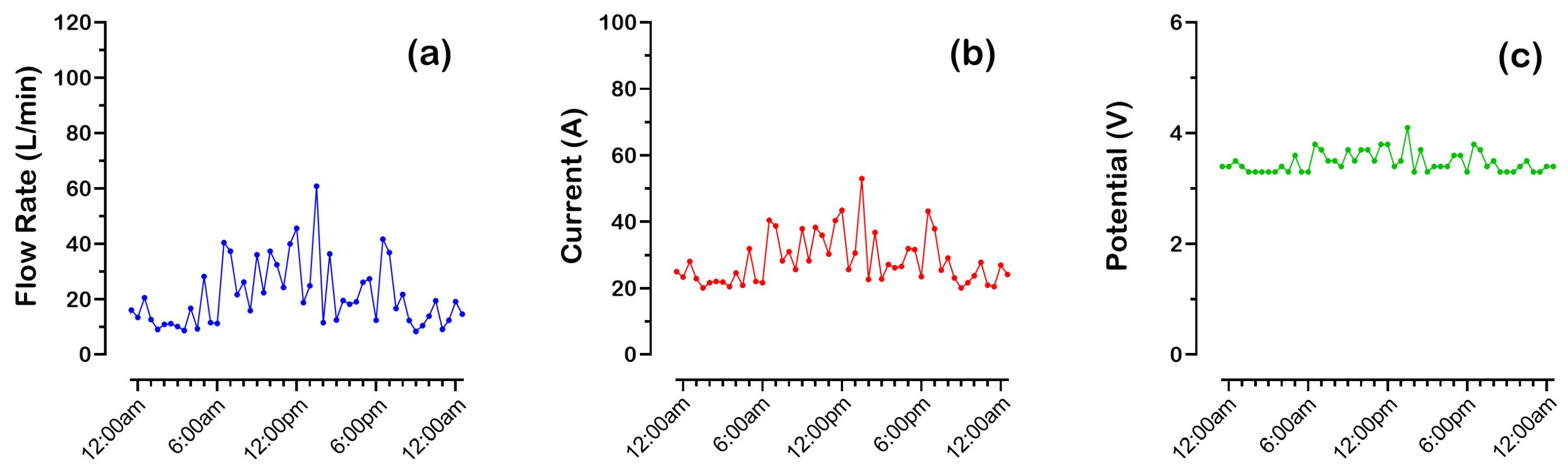
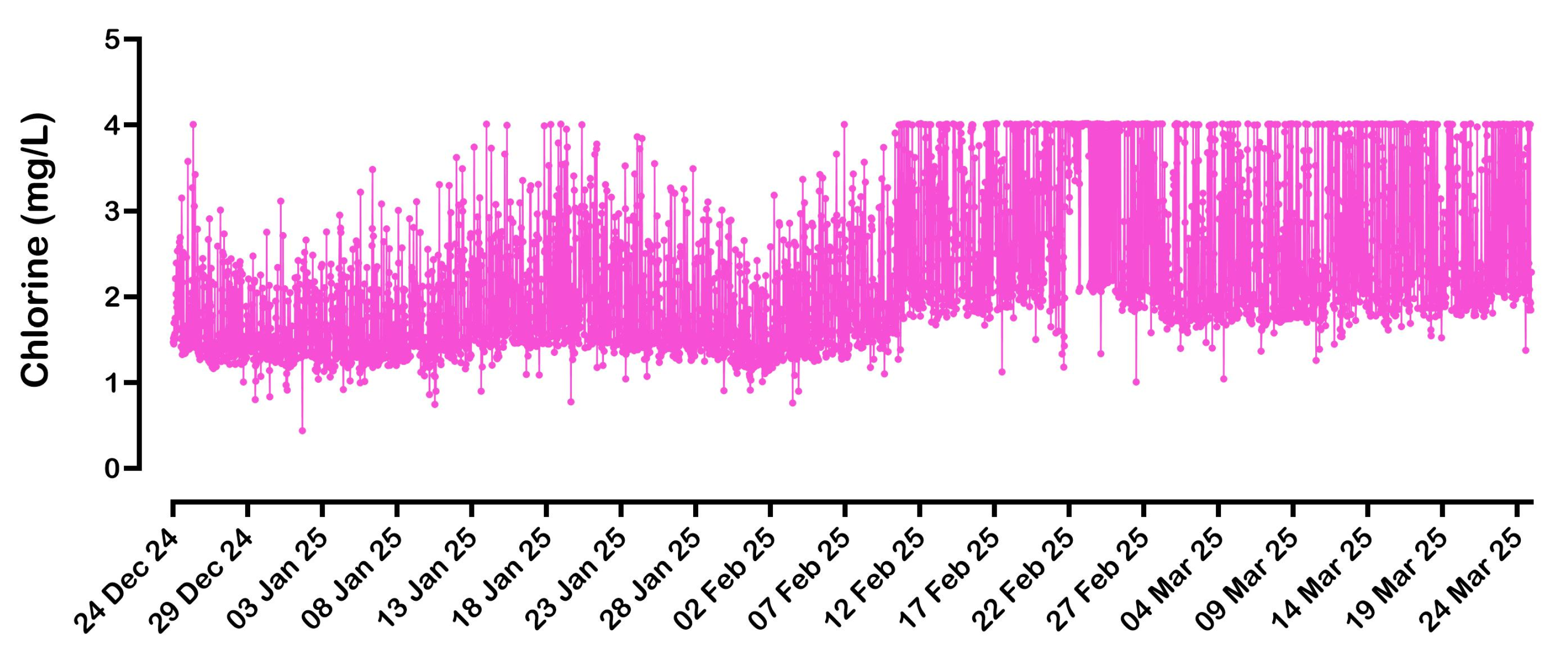
Figure 3 shows the flow, current, and potential trends for the
eBoosterTM system installed at the water meter near Swan Street over the last 3 months (from 24 December 2024 to 24 March 2025). This meter serves the largest part of the hospital and, therefore, has the highest water consumption (24,860 kL over 584 days; average flow rate of 29.6 L/min).
The DIN-rail-mounted controller transmits data to the cloud every 30 min. The data is acquired just before transmission, which means that the sampling may not accurately reflect the conditions of the previous 30 min. For example, as shown in
Figure 3, it cannot be ruled out that the flow rate never exceeded 120 L/min. The
X-axis in
Figure 3 represents different monitoring days; since 48 points are sent every single day, the tick interval was arbitrarily chosen as 240 points (5 days). A close-up of the data acquired in one day (on 3 January 2025) is shown in
Figure 4.
The hospital’s water consumption fluctuates unpredictably, with flow rates apparently reaching 120 L/min. Analysis of the collected data also shows that water consumption is not seasonal. In the three months from 15 September 2024 to 15 December 2024, flow rates remained between 0 and 120 L/min, and the same was true for the previous 12 months (from 15 August 2023 to 15 August 2024). However, in the period from 15 August 2024 to 15 September 2024, consumption was slightly higher, with peak flow rates reaching 200 L/min.
In all cases, the eBoosterTM system automatically adjusts the current supplied to the electrochemical unit based on the water flow rate. This is achieved by a built-in algorithm that ensures a relatively constant chlorine concentration in the water. As the flow rate increases, the system increases the current, maintaining an adequate chlorine level for effective residual protection.
Since the electrochemical unit behaves primarily as a resistor when current is passed through it (with the capacitive component of the electrical double layer at the electrodes having no measurable effects in direct current), increasing the current requires an increase in the potential difference across the device’s electrical contacts. As shown in
Figure 3b,c, current values ranged from 0 to 90 A, with corresponding potentials reaching up to 5 V.
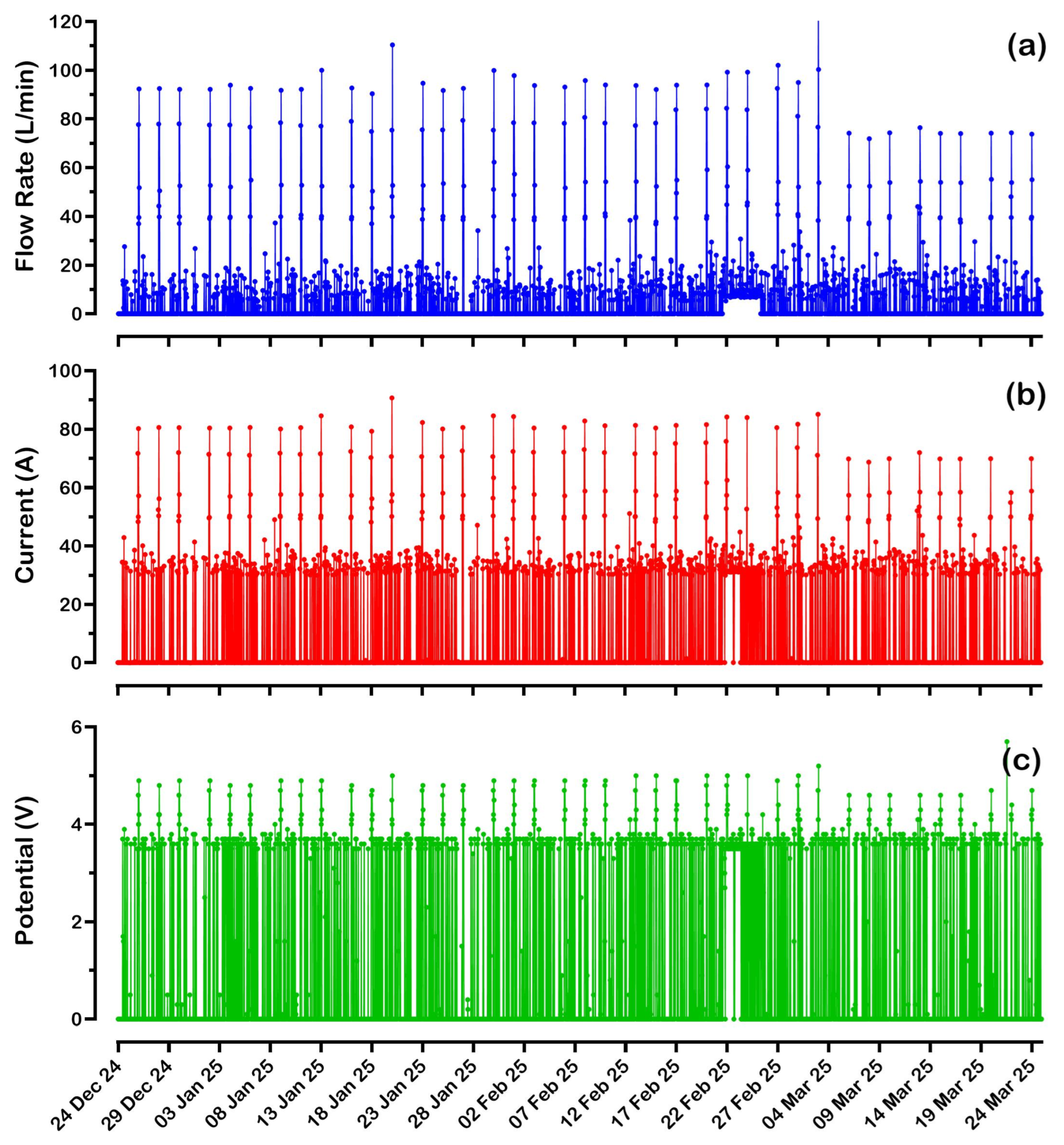
The second
eBoosterTM system, installed at the water meter near Hospital Road, recorded significantly lower water consumption, treating only 7850 kL in 645 days of operation (
Figure 5). This low consumption is directly related to an average water flow rate of less than 20 L/min, with periodic flow peaks reaching around 90 L/min approximately every three days. Given the low water consumption of the maternity ward, it seems likely that the hospital periodically flushes the ward’s outlets to prevent stagnation and maintain residual disinfectant protection.
At the second water meter, the hospital requested the installation of a chlorine meter (Lutz-Jesco GmbH, Wedemark, Germany; model Topax L1, equipped with a CS120 amperometric probe) to facilitate the monitoring of chlorine levels in the water supplied to the maternity ward.
Figure 6 shows the residual chlorine values recorded downstream of the
eBoosterTM system in the pipeline leading toward the hospital.
Although the chlorine meter is connected to the Control Panel, in this installation, it does not directly control the operation of the system. However, the controller firmware includes an algorithm that, when enabled, adjusts the current based on the measured chlorine level. This can also work in conjunction with the flow rate algorithm. In practice, when the flow varies, the estimated current value serves as a reference (pivot current) for the chlorine algorithm. The proportional-integral-derivative (PID) controller then optimizes the current to maintain the desired chlorine concentration in the water.
As shown in
Figure 6, consistently higher chlorine levels were observed starting from the second week of February for both the maximum and minimum recorded values. Since the hospital did not request any operational changes during this period, and routine internal checks did not indicate any system issues, the likely cause is a calibration drift in the chlorine probe. It appears the probe is overestimating chlorine concentrations, highlighting the need for recalibration. This issue reflects a known limitation of residual chlorine probes, which require frequent maintenance and recalibration to maintain accuracy. In contrast, the
eBoosterTM system, which has been operating continuously since early June 2023 (specifically, the unit installed near Hospital Road), has not required any electrode cleaning to date.
It is important to note that while high levels of chlorine are effective in inactivating pathogens, they can also lead to the formation of halogenated disinfection by-products (DBPs), which may have adverse effects on human health and/or the aquatic ecosystem [
40]. The presence of organic material is a key factor in this. In drinking water, dissolved organic material is generally limited, as purification processes are specifically designed to remove these components. However, this does not mean that water supplied by the municipal system is entirely “clean”, as contamination can still arise from issues such as pipe breaks or bacterial infiltration. When organic material is present, the formation of DBPs is likely unavoidable. This issue would be even more pronounced if sodium hypochlorite were used as the disinfectant, as its inherent instability leads to the formation of additional unwanted by-products (e.g., chlorates), while its high pH reduces effectiveness, often requiring higher dosages.
4. Cost Analysis
The DIN-rail-mounted controller transmits data to the cloud every 30 min. Since the system began operating at the main water meter (off Swan Street) in early August 2023, approximately 25,000 kL of water have been treated, with a total consumption of 308,500 Ah. Dividing this total consumption by the number of operating hours (the system runs 24/7), the average current can be estimated at 22 A, with a required potential of less than 3.5 V. With these values, the daily energy consumption of the system is estimated to be approximately 1.8 kWh, resulting in a daily treatment cost of approximately AUD 0.70 (USD 0.40), assuming an energy cost of AUD 0.40/kWh (USD 0.24/kWh).
Regarding energy consumption and daily cost of water treatment at the second water meter (near Hospital Road), with the available data, an average current of about 8.5 A is estimated. This value appears low due to the rather discontinuous operation of this system, resulting in intermittent usage. This current can be obtained with a potential lower than 2.5 V. The daily energy consumption, therefore, does not exceed 500 Wh (0.5 kWh), which plausibly results in a cost to the hospital of less than AUD 0.20 (USD 0.12) per day.
Supplying chemicals in regional and rural areas can be challenging. At Dalby Hospital, this challenge is further complicated by the location of the water meters, which are housed in metal sheds within the facility’s green spaces. As a result, alternative disinfection methods—such as sodium hypochlorite (NaClO) dosing, which the hospital has ruled out but is worth considering for cost comparison—would be problematic due to exposure to adverse weather conditions.
Dalby has long, hot, and partly cloudy summers, while winters are short, cold, dry, and mostly clear. The hospital’s two water meters take an average of 42.6 kL and 12.2 kL per day. Assuming a sodium hypochlorite dosage of 2 mg/L, this would require approximately 0.7 L and 0.2 L of 12% NaClO per day, respectively. However, considering the effects of transport and storage on product stability, it would be more realistic to assume a lower concentration, say 8% NaClO, which increases the daily consumption to approximately 1.1 L and 0.3 L.
NaClO is available locally, for example, from Bunnings Warehouse, in 15 L containers at a price of AUD 27.60 (USD 16.60). This approach would require frequent personnel intervention to supply and replace containers. Even without these additional costs, the hospital’s costs would be nearly double those of the eBoosterTM system, despite offering inferior performance. As explained in the introduction, electrochemical disinfection offers benefits beyond the simple generation of active chlorine.
Ozone is another potential alternative; however, it is more corrosive to piping materials than chlorine [
20] and does not provide a long-lasting residual disinfection effect. Electrochemical drinking water treatment devices typically incorporate a solid polymer electrolyte membrane, also known as a Proton Exchange Membrane (PEM), placed between two boron-doped diamond (BDD) electrodes [
41]. This configuration reduces inter-electrode resistance, thereby lowering energy consumption. However, this also means that water flows around the electrode package rather than through it, limiting electrolytic treatment to the production of oxidants for indirect oxidation.
As previously mentioned,
Condiapure® technology from Condias GmbH can treat water flow rates of up to 250 L/min [
18]. Interestingly, other devices, such as
CabECO® and
Mikrozon®, appear to be 4–5 times more efficient but were designed for smaller applications [
42]. The
Condiapure® device consumes approximately 11.4 Wh of energy to generate 1 mg of ozone [
42]. The ozone concentration required to disinfect drinking water depends on several factors, including water quality, temperature, pH, and contact time. Typically, an ozone dose of 0.2 to 1.0 mg/L is sufficient for disinfection, with contact times ranging from 1 to 10 min. Even assuming the lowest dose of just 0.2 mg/L, the daily water consumption of Dalby Hospital (~55 kL) would require approximately 125 kWh of energy, with a daily cost of AUD 50 (USD 30).
5. Conclusions
The implementation of the eBoosterTM electrochemical disinfection system at Dalby Hospital and other healthcare facilities in Queensland has demonstrated its effectiveness, reliability, and sustainability as a long-term solution for microbial control in drinking water distribution networks. Unlike earlier electrochemical disinfection technologies, which required frequent maintenance due to scale buildup and performance degradation, the eBoosterTM system leverages periodic polarity reversal to maintain electrode performance, ensuring long-term operational stability.
Performance data collected over nearly 2 years at Dalby Hospital confirmed that the systems effectively maintained a reasonably stable residual chlorine concentration while adapting to fluctuating flow rates. Energy consumption remained minimal, with an estimated daily operating cost of less than AUD 1.00 (USD 0.60) for the entire system (serving two water meters). This makes it a cost-effective alternative to traditional chemical disinfection. The eBoosterTM system also stands out for its flexibility, operating effectively in an in-line, non-recirculating configuration, which broadens its applicability across various water distribution scenarios.
Beyond operational benefits, the system offers a sustainable and environmentally friendly solution by eliminating the need to transport, store, and handle hazardous chemicals. By generating disinfectants in situ, using only the naturally occurring chloride ions present in water, it significantly reduces the environmental and logistical burdens of chemical dosing.
A comparative summary of key performances and cost metrics is provided below.
Energy Use:
eBoosterTM: ~1.8 kWh/day for the main system (AUD 0.70/day)
Ozone systems: ≥125 kWh/day for comparable disinfection (AUD 50/day)
Cost per Day:
eBoosterTM: <AUD 1.00/day (energy only)
Hypochlorite dosing: ~AUD 2.00/day (chemical cost only, without labor/logistics)
Summary of Benefits and Drawbacks:
eBoosterTM: low ongoing cost, no chemical handling, maintenance-free electrodes
Ozone: high energy consumption, complex maintenance, no residual effect
Hypochlorite: moderate chemical cost, ongoing labor, chemical degradation over time.
The successful deployment at Dalby Hospital highlights the wider potential of electrochemical disinfection, especially in healthcare and remote settings where traditional chemical methods face logistical challenges. There is a significant opportunity to further develop this technology by refining the system design for various water compositions, expanding its use in municipal-scale water treatment, and exploring integration with real-time water quality monitoring technologies to enhance control and responsiveness. Future studies will assess the efficacy of the system against chlorine-resistant pathogens, such as Cryptosporidium, to further validate its potential applications.
As highlighted by university studies (
Section 2.3), indirect oxidation driven by electrochemically generated oxidizing agents plays a central role in the disinfection process. While microbial reductions can occur without chloride ions, their presence significantly enhances sanitization efficiency and provides a critical residual disinfection effect. In water with very low chloride concentrations (typically 50 mg/L or less), a modest salt addition may be advisable. This introduces minimal maintenance requirements and is far simpler than hypochlorite handling. Sodium chloride is chemically stable, widely available, and does not degrade over time, making it a manageable and low-risk additive. In some regions—particularly within the European Community—this approach may also be necessary to meet regulatory requirements regarding drinking water treatment.