Comparison of the Influences of Fresh and Corroded Carbon Steels on the Decay Law of Sodium Hypochlorite in Reclaimed Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Materials
2.3. Experimental Methods
3. Results and Discussion
3.1. Comparison of the Effects of Sodium Hypochlorite Under the Influences of Fresh and Corroded Carbon Steels
3.1.1. Comparison of Microbiological Control Effects
3.1.2. Comparison of Fresh and Corroded Carbon Steel Corrosion Rates
3.2. Comparison of Residual Chlorine Decay Models Under the Influences of Fresh and Corroded Carbon Steels
3.3. Comparison of the Effects of Wall Substances on Residual Chlorine Decay
3.3.1. The Effects of Fe0 and Corrosion Products on the Decay Process of Residual Chlorine
3.3.2. The Effects of Fe0 and Corrosion Products on Microbial Control Effectiveness
3.3.3. Discussion on the Mechanisms of Fe0 and Corrosion Products Affecting the Decay of Residual Chlorine
3.4. Discussion of the Effects of Fe0 and Corrosion Products on the Consumption of Sodium Hypochlorite and Decay Coefficients
3.4.1. The Effects of Fe0 and Corrosion Products on the Consumption of Sodium Hypochlorite
3.4.2. The Effects of Fe0 and Corrosion Products on the Wall Decay Coefficients
4. Conclusions
- (1)
- Under the conditions of fresh and corroded carbon steels, adding sodium hypochlorite to achieve initial free chlorine concentrations of 5 mg/L and 9 mg/L can relatively more effectively achieve disinfection effects, while the corrosion rate is the lowest. Under the conditions of the fresh carbon steel, it has a good control effect on microorganisms, and the effect of sodium hypochlorite can last for about 48 h. Under the conditions of the corroded carbon steel, the microbial activity is relatively high, and the recommended dosing frequency is once every 8 h.
- (2)
- The decay law of residual chlorine in both fresh and corroded carbon steel conditions conforms to the restricted first-order decay model. The decay coefficient of residual chlorine under the corroded carbon steel conditions is 2.61~6.94 times that of the fresh carbon steel. Compared with the fresh carbon steel, the average consumption of sodium hypochlorite under the corroded carbon steel conditions will increase by 13.91% to 26.57%. The corrosion products and high microbial content in the corroded carbon steel are the main reasons for accelerating the consumption of sodium hypochlorite.
- (3)
- Fe0 accelerates the consumption of sodium hypochlorite in the first 4 h, with the highest consumption rate reaching 42.7%. The corrosion products rapidly consume sodium hypochlorite in the first 12 h, with a consumption rate of up to 25.7%. As time goes on, the consumption of sodium hypochlorite by Fe0 and corrosion products decreases. Under experimental conditions, the average consumption increase rates of sodium hypochlorite by Fe0 and corrosion products are 18.9% and 17.4%, respectively. The results also show that the bulk decay coefficient is 0.073. Additionally, the wall decay coefficient represented by Fe0 is 0.204, which is higher than the wall decay coefficient represented by corrosion products, which is 0.077.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Caicedo, C.; Rosenwinkel, K.-H.; Exner, M.; Verstraete, W.; Suchenwirth, R.; Hartemann, P.; Nogueira, R. Legionella Occurrence in Municipal and Industrial Wastewater Treatment Plants and Risks of Reclaimed Wastewater Reuse: Review. Water Res. 2019, 149, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Al-Jassim, N.; Ansari, M.I.; Harb, M.; Hong, P.-Y. Removal of Bacterial Contaminants and Antibiotic Resistance Genes by Conventional Wastewater Treatment Processes in Saudi Arabia: Is the Treated Wastewater Safe to Reuse for Agricultural Irrigation? Water Res. 2015, 73, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, Y.; Wu, Y.; Hu, H. Effect of Ultraviolet Disinfection on Growth and Secretion Characteristics of Microorganism in Reclaimed Water. Environ. Eng. 2020, 38, 28–34. [Google Scholar] [CrossRef]
- García-Ávila, F.; Sánchez-Alvarracín, C.; Cadme-Galabay, M.; Conchado-Martínez, J.; García-Mera, G.; Zhindón-Arévalo, C. Relationship between Chlorine Decay and Temperature in the Drinking Water. MethodsX 2020, 7, 101002. [Google Scholar] [CrossRef]
- Li, C.; Yang, Y.J.; Yu, J.; Zhang, T.; Mao, X.; Shao, W. Second-Order Chlorine Decay and Trihalomethanes Formation in a Pilot-Scale Water Distribution Systems. Water Environ. Res. 2012, 84, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Blokker, M.; Vreeburg, J.; Speight, V. Residual Chlorine in the Extremities of the Drinking Water Distribution System: The Influence of Stochastic Water Demands. Procedia Eng. 2014, 70, 172–180. [Google Scholar] [CrossRef]
- Liu, C.; Von Gunten, U.; Croué, J.-P. Enhanced Chlorine Dioxide Decay in the Presence of Metal Oxides: Relevance to Drinking Water Distribution Systems. Environ. Sci. Technol. 2013, 47, 8365–8372. [Google Scholar] [CrossRef]
- Monteiro, L.; Viegas, R.M.C.; Covas, D.I.C.; Menaia, J. Modelling Chlorine Residual Decay as Influenced by Temperature. Water Environ. J. 2015, 29, 331–337. [Google Scholar] [CrossRef]
- Liu, J.Q.; Jiang, W.; Wu, J.M.; Li, C. The Influence of Total Hardness on Chlorine Decay in Water Distribution Systems. Appl. Mech. Mater. 2014, 535, 776–784. [Google Scholar] [CrossRef]
- Hua, F.; West, J.R.; Barker, R.A.; Forster, C.F. Modelling of Chlorine Decay in Municipal Water Supplies. Water Res. 1999, 33, 2735–2746. [Google Scholar] [CrossRef]
- Hallam, N.B.; West, J.R.; Forster, C.F.; Powell, J.C.; Spencer, I. The Decay of Chlorine Associated with the Pipe Wall in Water Distribution Systems. Water Res. 2002, 36, 3479–3488. [Google Scholar] [CrossRef]
- Pandey, S.; Lemmons, D.; de Alwis, C.; Mackie, R.; Kolodge, D.; Perrine, K.A. Reactions of Disinfectants at Solution/Iron Interfaces: Modeling Pipeline Surface Chemistry. Appl. Surf. Sci. 2025, 684, 161876. [Google Scholar] [CrossRef]
- Baig, S.A.; Lou, Z.; Baig, M.A.; Qasim, M.; Shams, D.F.; Mahmood, Q.; Xu, X. Assessment of Tap Water Quality and Corrosion Scales from the Selected Distribution Systems in Northern Pakistan. Environ. Monit. Assess. 2017, 189, 194. [Google Scholar] [CrossRef]
- Safiur Rahman, M.; Whalen, M.; Gagnon, G.A. Adsorption of Dissolved Organic Matter (DOM) onto the Synthetic Iron Pipe Corrosion Scales (Goethite and Magnetite): Effect of pH. Chem. Eng. J. 2013, 234, 149–157. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, D.; Zhao, L.; Wang, J.; Xie, S.; Liu, S.; Lin, P.; Zhang, X.; Chen, C. Review on Corrosion and Corrosion Scale Formation upon Unlined Cast Iron Pipes in Drinking Water Distribution Systems. J. Environ. Sci. 2022, 117, 173–189. [Google Scholar] [CrossRef] [PubMed]
- Li, R.A.; McDonald, J.A.; Sathasivan, A.; Khan, S.J. Disinfectant Residual Stability Leading to Disinfectant Decay and By-Product Formation in Drinking Water Distribution Systems: A Systematic Review. Water Res. 2019, 153, 335–348. [Google Scholar] [CrossRef] [PubMed]
- HG/T 3523-2008; Technical Conditions of Standard Corrosion Spool of Chemical Treatment in Cooling Water. The Standardization Administration of the People’s Republic of China: Beijing, China, 2008.
- Zhou, X.; Zhao, J.; Li, Z.; Song, J.; Li, X.; Yang, X.; Wang, D. Enhancement Effects of Ultrasound on Secondary Wastewater Effluent Disinfection by Sodium Hypochlorite and Disinfection By-Products Analysis. Ultrason. Sonochem. 2016, 29, 60–66. [Google Scholar] [CrossRef]
- Douterelo, I.; Husband, S.; Boxall, J.B. The Bacteriological Composition of Biomass Recovered by Flushing an Operational Drinking Water Distribution System. Water Res. 2014, 54, 100–114. [Google Scholar] [CrossRef]
- Garner, E.; McLain, J.; Bowers, J.; Engelthaler, D.M.; Edwards, M.A.; Pruden, A. Microbial Ecology and Water Chemistry Impact Regrowth of Opportunistic Pathogens in Full-Scale Reclaimed Water Distribution Systems. Environ. Sci. Technol. 2018, 52, 9056–9068. [Google Scholar] [CrossRef]
- Li, D.; Zeng, S.; Gu, A.Z.; He, M.; Shi, H. Inactivation, Reactivation and Regrowth of Indigenous Bacteria in Reclaimed Water after Chlorine Disinfection of a Municipal Wastewater Treatment Plant. J. Environ. Sci. 2013, 25, 1319–1325. [Google Scholar] [CrossRef]
- Li, W.; Tian, Y.; Chen, J.; Wang, X.; Zhou, Y.; Shi, N. Synergistic Effects of Sodium Hypochlorite Disinfection and Iron-Oxidizing Bacteria on Early Corrosion in Cast Iron Pipes. Front. Environ. Sci. Eng. 2021, 16, 72. [Google Scholar] [CrossRef]
- Liu, T.; Cheng, Y.F.; Sharma, M.; Voordouw, G. Effect of Fluid Flow on Biofilm Formation and Microbiologically Influenced Corrosion of Pipelines in Oilfield Produced Water. J. Pet. Sci. Eng. 2017, 156, 451–459. [Google Scholar] [CrossRef]
- Jia, Y.; Qi, B.; Zhao, J.; Guo, X.; Dong, W.; Lv, X.; Dai, Z.; Yuan, Y. Recent Challenges and Research Progress in Microbiologically Influenced Corrosion in Drinking Water Distribution Systems. Environ. Technol. Innov. 2025, 38, 104054. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Song, Y.; Liu, W.; Zhang, J.; Li, N.; Dong, K.; Cai, Y.; Han, E.-H. The Respective Roles of Sulfate-Reducing Bacteria (SRB) and Iron-Oxidizing Bacteria (IOB) in the Mixed Microbial Corrosion Process of Carbon Steel Pipelines. Corros. Sci. 2024, 240, 112479. [Google Scholar] [CrossRef]
- Emerson, D. The Role of Iron-Oxidizing Bacteria in Biocorrosion: A Review. Biofouling 2018, 34, 989–1000. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Tian, Y.; Peng, S. The Influence of Sodium Hypochlorite Biocide on the Corrosion of Carbon Steel in Reclaimed Water Used as Circulating Cooling Water. Appl. Surf. Sci. 2014, 315, 95–103. [Google Scholar] [CrossRef]
- Fukuzaki, S. Mechanisms of Actions of Sodium Hypochlorite in Cleaning and Disinfection Processes. Biocontrol Sci. 2006, 11, 147–157. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Xu, P.; Lu, L.; Sun, L. Effect of NaClO Addition on Bacteriostasis of Iron Bacteria and Corrosion Control of Pipe Line Steel for Water Distribution Network in Urban Reclaimed Water. Corros. Sci. Prot. Technol. 2015, 27, 165–170. (In Chinese) [Google Scholar] [CrossRef]
- Feben, D.; Taras, M.J.; Faber, H.A.; Hedgepeth, L.L. Studies on Chlorine Demand Constants [with Discussion]. J. (Am. Water Work. Assoc.) 1951, 43, 922–932. [Google Scholar] [CrossRef]
- Clark, R.M.; Sivaganesan, M. Predicting Chlorine Residuals and Formation of TTHMs in Drinking Water. J. Environ. Eng. 1998, 124, 1203–1210. [Google Scholar] [CrossRef]
- Kastl, G.J.; Fisher, I.H.; Jegatheesan, V. Evaluation of Chlorine Decay Kinetics Expressions for Drinking Water Distribution Systems Modelling. J. Water Supply Res. Technol.-Aqua 1999, 48, 219–226. [Google Scholar] [CrossRef]
- Powell, J.C.; West, J.R.; Hallam, N.B.; Forster, C.F.; Simms, J. Performance of Various Kinetic Models for Chlorine Decay. J. Water Resour. Plan. Manag. 2000, 126, 13–20. [Google Scholar] [CrossRef]
- Jonkergouw, P.M.R.; Khu, S.-T.; Savic, D.A.; Zhong, D.; Hou, X.Q.; Zhao, H.-B. A Variable Rate Coefficient Chlorine Decay Model. Environ. Sci. Technol. 2009, 43, 408–414. [Google Scholar] [CrossRef]
- Clark, R.M. Chlorine Demand and TTHM Formation Kinetics: A Second-Order Model. J. Environ. Eng. 1998, 124, 16–24. [Google Scholar] [CrossRef]
- Al-Jasser, A.O. Chlorine Decay in Drinking-Water Transmission and Distribution Systems: Pipe Service Age Effect. Water Res. 2007, 41, 387–396. [Google Scholar] [CrossRef]
- Norton, C.D.; LeChevallier, M.W. A Pilot Study of Bacteriological Population Changes through Potable Water Treatment and Distribution. Appl. Environ. Microbiol. 2000, 66, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Yang, X.; Cizmas, L.; McDonald, T.J.; Luque, R.; Sayes, C.M.; Yuan, B.; Dionysiou, D.D. Impact of Metal Ions, Metal Oxides, and Nanoparticles on the Formation of Disinfection Byproducts during Chlorination. Chem. Eng. J. 2017, 317, 777–792. [Google Scholar] [CrossRef]
- Chen, W.; Chen, J. Formation and Prevention of Pipe Scale in Water Supply Pipelines with Anti-Corrosion Lining. Water Supply 2022, 22, 4006–4014. [Google Scholar] [CrossRef]
- Galleguillos Madrid, F.M.; Soliz, A.; Cáceres, L.; Bergendahl, M.; Leiva-Guajardo, S.; Portillo, C.; Olivares, D.; Toro, N.; Jimenez-Arevalo, V.; Páez, M. Green Corrosion Inhibitors for Metal and Alloys Protection in Contact with Aqueous Saline. Materials 2024, 17, 3996. [Google Scholar] [CrossRef]
- Zhang, Z.; Stout, J.E.; Yu, V.L.; Vidic, R. Effect of Pipe Corrosion Scales on Chlorine Dioxide Consumption in Drinking Water Distribution Systems. Water Res. 2008, 42, 129–136. [Google Scholar] [CrossRef]
- Rahman, M.S.; Gagnon, G.A. Bench-Scale Evaluation of Drinking Water Treatment Parameters on Iron Particles and Water Quality. Water Res. 2014, 48, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, L.; Liu, D.; Wang, J.; Zhang, X.; Chen, C. Early Period Corrosion and Scaling Characteristics of Ductile Iron Pipe for Ground Water Supply with Sodium Hypochlorite Disinfection. Water Res. 2020, 176, 115742. [Google Scholar] [CrossRef] [PubMed]
- Sancy, M.; Gourbeyre, Y.; Sutter, E.M.M.; Tribollet, B. Mechanism of Corrosion of Cast Iron Covered by Aged Corrosion Products: Application of Electrochemical Impedance Spectrometry. Corros. Sci. 2010, 52, 1222–1227. [Google Scholar] [CrossRef]
- Li, M.; Liu, Z.; Chen, Y.; Hai, Y. Characteristics of Iron Corrosion Scales and Water Quality Variations in Drinking Water Distribution Systems of Different Pipe Materials. Water Res. 2016, 106, 593–603. [Google Scholar] [CrossRef]
- Little, B.J.; Gerke, T.L.; Lee, J.S. Mini-Review: The Morphology, Mineralogy and Microbiology of Accumulated Iron Corrosion Products. Biofouling 2014, 30, 941–948. [Google Scholar] [CrossRef]
- Hassan, K.Z.A.; Bower, K.C.; Miller, C.M. Iron Oxide Enhanced Chlorine Decay and Disinfection By-Product Formation. J. Environ. Eng. 2006, 132, 1609–1616. [Google Scholar] [CrossRef]
- Hu, C.-Y.; Zhang, J.; Xu, B.; Lin, Y.-L.; Zhang, T.-Y.; Tian, F.-X. Effect of Pipe Corrosion Product–Goethite–on the Formation of Disinfection by-Products during Chlorination. Desalination Water Treat. 2016, 57, 553–561. [Google Scholar] [CrossRef]
- Hua, P.; Gao, Q.; Wang, Z.; Jiang, S.; de Oliveira, K.R.F.; Macedo, D.O. Modeling and Elucidation the Effects of Iron Deposits on Chlorine Decay and Trihalomethane Formation in Drinking Water Distribution System. Water Res. 2021, 207, 117804. [Google Scholar] [CrossRef]
- Chebeir, M.; Liu, H. Oxidation of Cr(III)–Fe(III) Mixed-Phase Hydroxides by Chlorine: Implications on the Control of Hexavalent Chromium in Drinking Water. Environ. Sci. Technol. 2018, 52, 7663–7670. [Google Scholar] [CrossRef]
- Lee, H.D.; Park, J.H.; Kang, S.W.; Kong, M.S. A Study on Evaluation of the Pipe Wall Decay Constants of Residual Chlorine and Affecting Factors in Reclaimed Water Supply System. Desalination Water Treat. 2015, 53, 2378–2387. [Google Scholar] [CrossRef]
- Vasconcelos, J.J. Characteristics and Modeling of Chlorine Decay in Distribution Systems; AWWA Research Foundation: Denver, CO, USA, 1996. [Google Scholar]
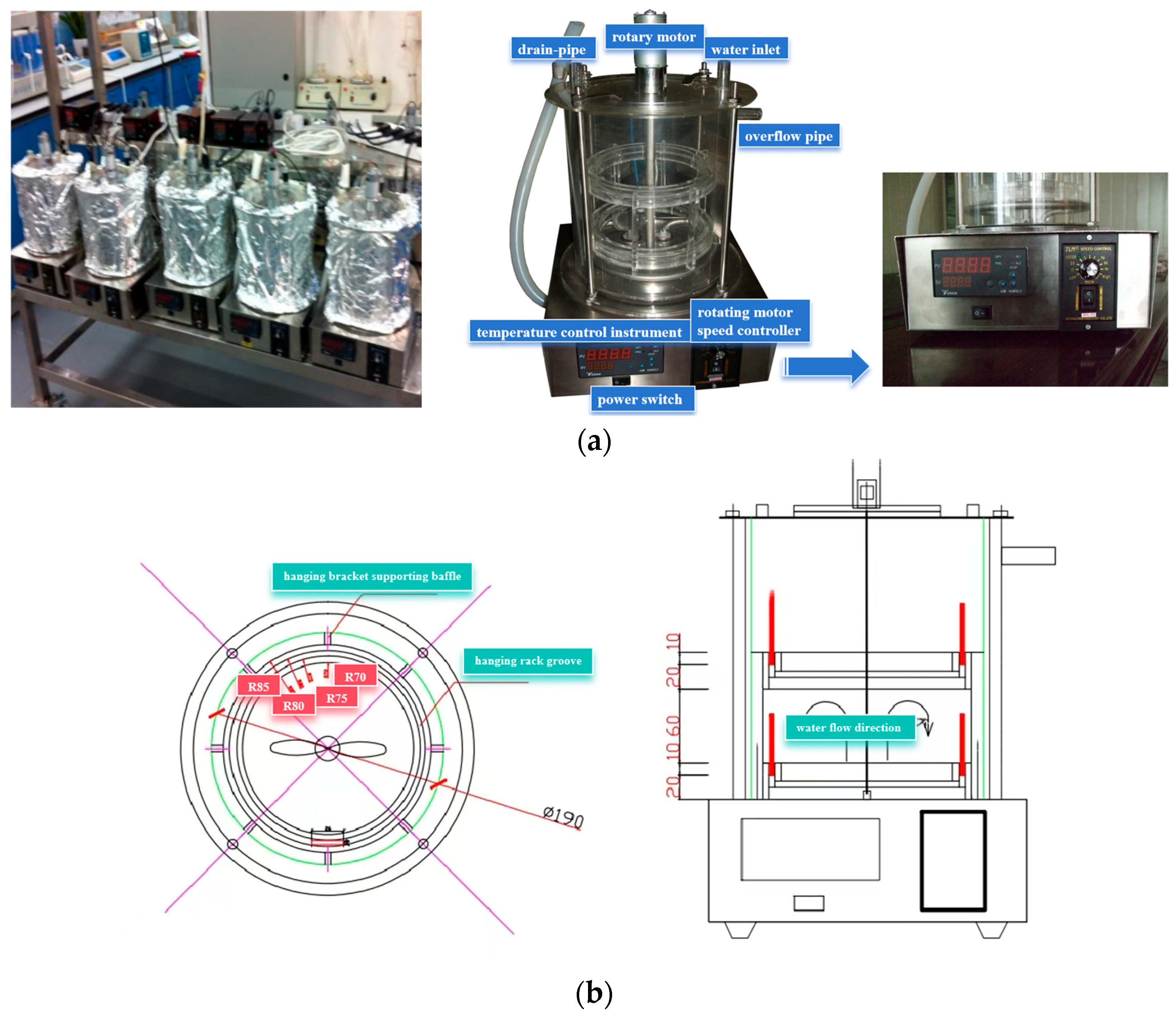

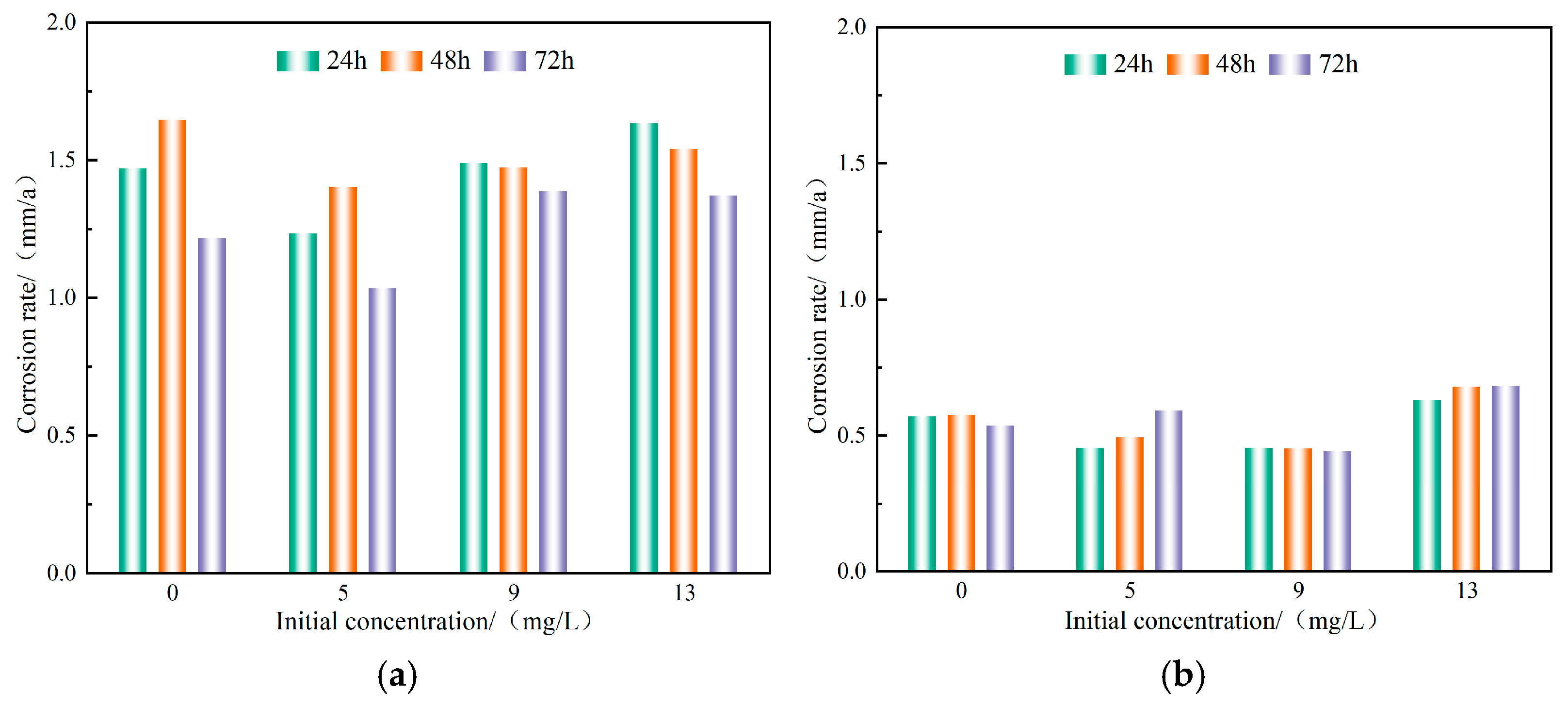
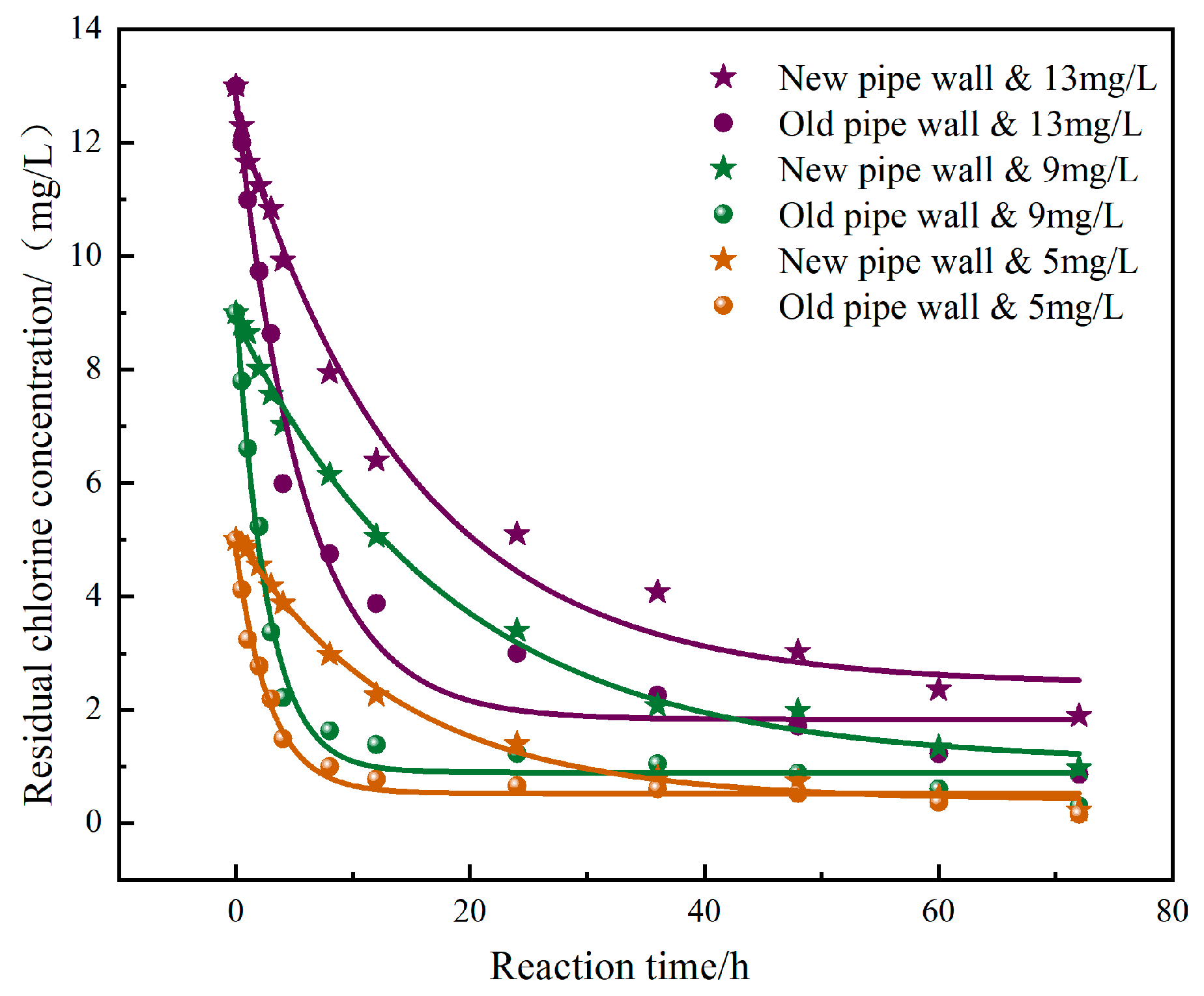

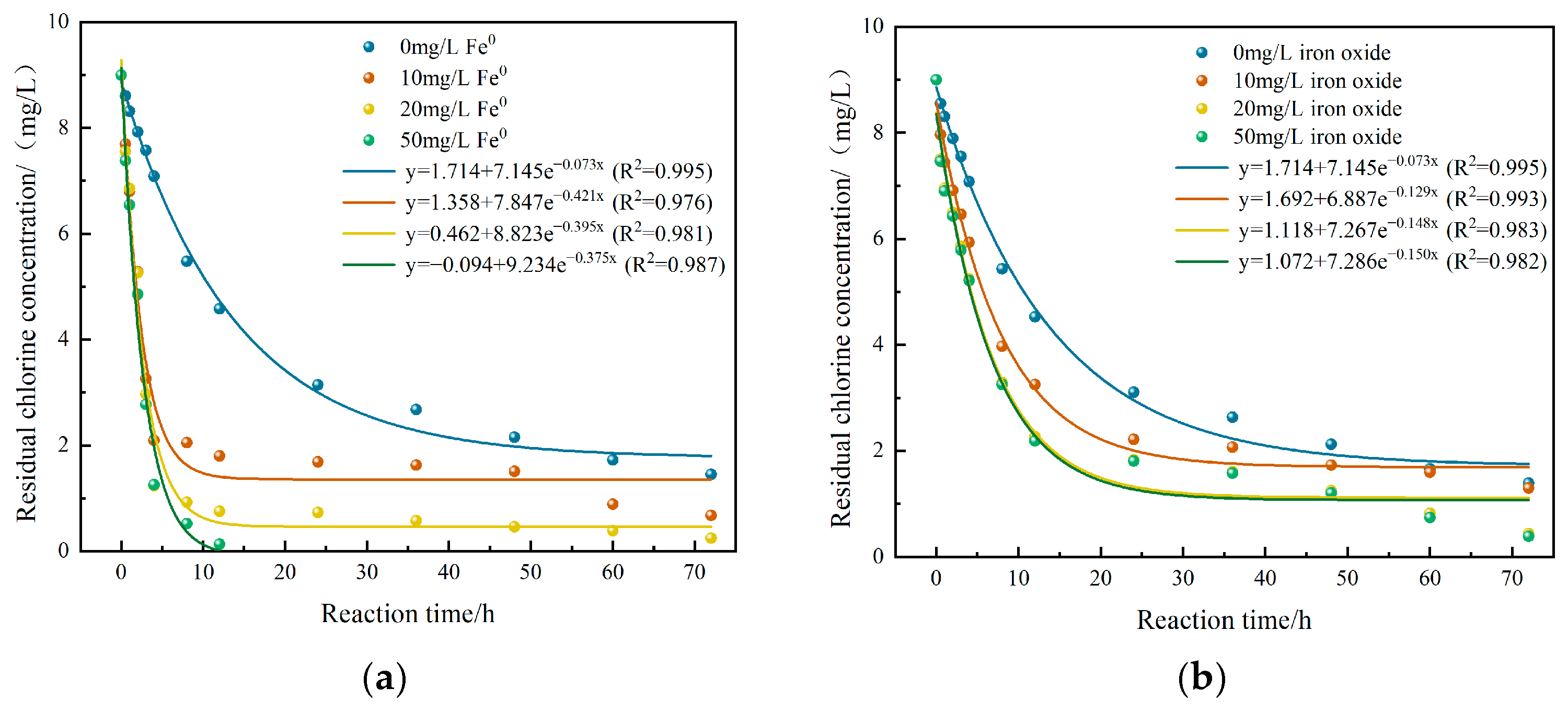



| Working Condition | a | b | k | R2 |
|---|---|---|---|---|
| Fresh carbon steel and 13 mg/L | 2.444 | 10.093 | 0.067 | 0.989 |
| Corroded carbon steel and 13 mg/L | 1.831 | 10.998 | 0.175 | 0.979 |
| Fresh carbon steel and 9 mg/L | 1.071 | 7.816 | 0.054 | 0.996 |
| Corroded carbon steel and 9 mg/L | 0.898 | 8.241 | 0.375 | 0.987 |
| Fresh carbon steel and 5 mg/L | 0.413 | 4.666 | 0.071 | 0.996 |
| Corroded carbon steel and 5 mg/L | 0.525 | 4.297 | 0.343 | 0.985 |
| Working Condition | C0 (1st) | k | R2 (1st) | C0 (2nd) | R | u | R2 (2nd) |
|---|---|---|---|---|---|---|---|
| Fresh carbon steel and 13 mg/L | 11.895 | 0.033 | 0.962 | 12.814 | 0.971 | 0.002 | 0.995 |
| Corroded carbon steel and 13 mg/L | 12.067 | 0.102 | 0.920 | 13.235 | 0.933 | 0.016 | 0.988 |
| Fresh carbon steel and 9 mg/L | 8.663 | 0.038 | 0.987 | 9.215 | 0.999 | 0.000 | 0.993 |
| Corroded carbon steel and 9 mg/L | 8.897 | 0.286 | 0.942 | 9.388 | 0.948 | 0.029 | 0.979 |
| Fresh carbon steel and 5 mg/L | 4.969 | 0.055 | 0.989 | 5.304 | 0.999 | 0.000 | 0.988 |
| Corroded carbon steel and 5 mg/L | 4.648 | 0.247 | 0.933 | 5.040 | 0.934 | 0.035 | 0.991 |
| Ref. Code | Score | Compound Name | Component Ratio | Chemical Formula |
|---|---|---|---|---|
| 00-022-0353 | 16 | Iron oxide hydroxide | 31.2% | γ-FeOOH |
| 01-072-0469 | 14 | Iron(III) oxide | 5.4% | Fe2O3 |
| 00-026-1136 | 13 | Iron oxide | 20.3% | Fe3O4 |
| 01-089-6096 | 14 | Iron oxide hydroxide | 43.1% | α-FeOOH |
| Working Condition | a | b | k | kb | kw | R2 |
|---|---|---|---|---|---|---|
| The bulk of the water | 1.714 | 7.145 | 0.073 | 0.073 | 0 | 0.995 |
| The bulk of the water and 5.5 mg/L Fe0 | 1.805 | 7.124 | 0.277 | 0.073 | 0.204 | 0.967 |
| The bulk of the water and 50 mg/L corrosion products | 1.072 | 7.286 | 0.150 | 0.073 | 0.077 | 0.982 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, P.; Wang, X.; Liu, B. Comparison of the Influences of Fresh and Corroded Carbon Steels on the Decay Law of Sodium Hypochlorite in Reclaimed Water. Water 2025, 17, 1428. https://doi.org/10.3390/w17101428
Xu P, Wang X, Liu B. Comparison of the Influences of Fresh and Corroded Carbon Steels on the Decay Law of Sodium Hypochlorite in Reclaimed Water. Water. 2025; 17(10):1428. https://doi.org/10.3390/w17101428
Chicago/Turabian StyleXu, Ping, Xuan Wang, and Bo Liu. 2025. "Comparison of the Influences of Fresh and Corroded Carbon Steels on the Decay Law of Sodium Hypochlorite in Reclaimed Water" Water 17, no. 10: 1428. https://doi.org/10.3390/w17101428
APA StyleXu, P., Wang, X., & Liu, B. (2025). Comparison of the Influences of Fresh and Corroded Carbon Steels on the Decay Law of Sodium Hypochlorite in Reclaimed Water. Water, 17(10), 1428. https://doi.org/10.3390/w17101428






