Hybrid Coagulation–Membrane Filtration Techniques for Sustainable Soap Wastewater Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Source of Wastewater
2.2. Coagulation—Floculation
- Coagulation: Rapid mixing was applied at 150 rpm for 5 min immediately following the addition of the FeCl3 coagulant.
- Flocculation: Slow mixing was then carried out at 30 RPM for 20 min to promote floc growth.
- Sedimentation: The samples were allowed to settle without mixing for 30 min.
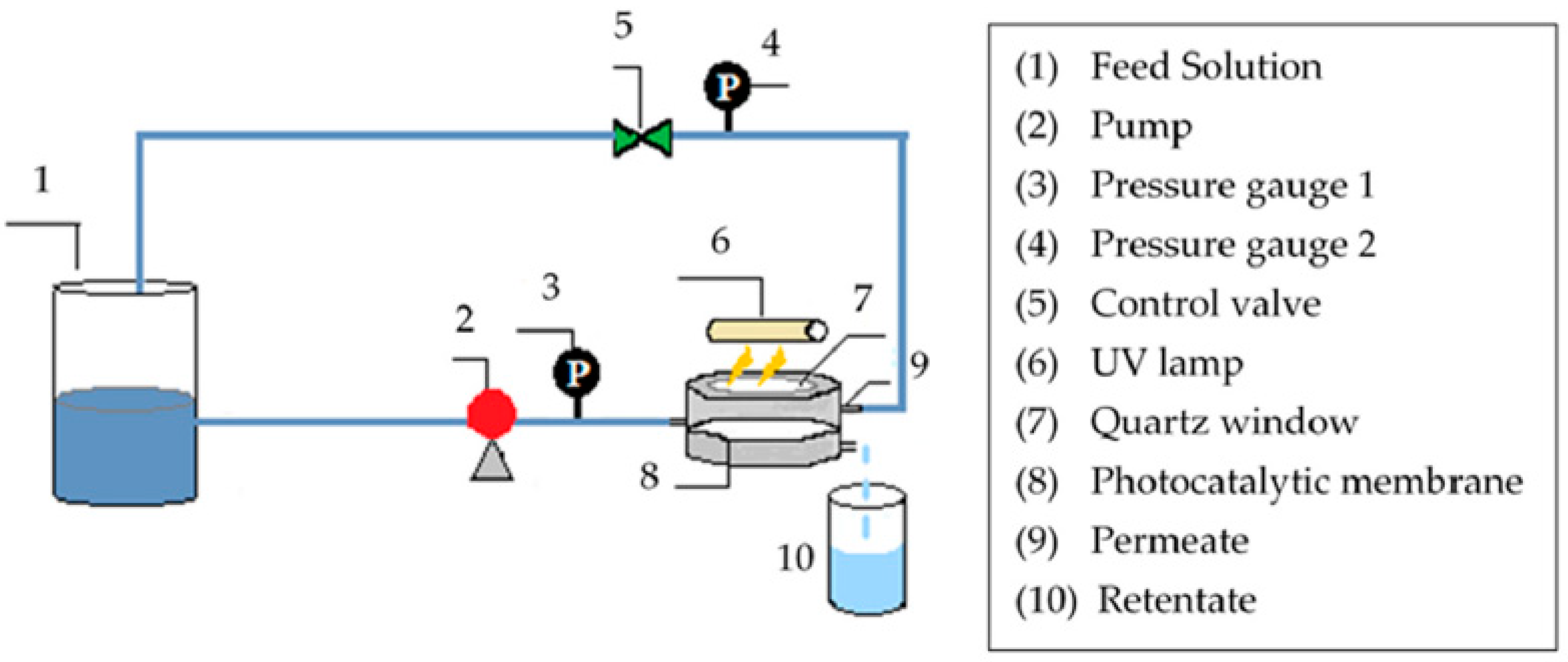
2.3. Membrane Filtration
3. Results and Discussion
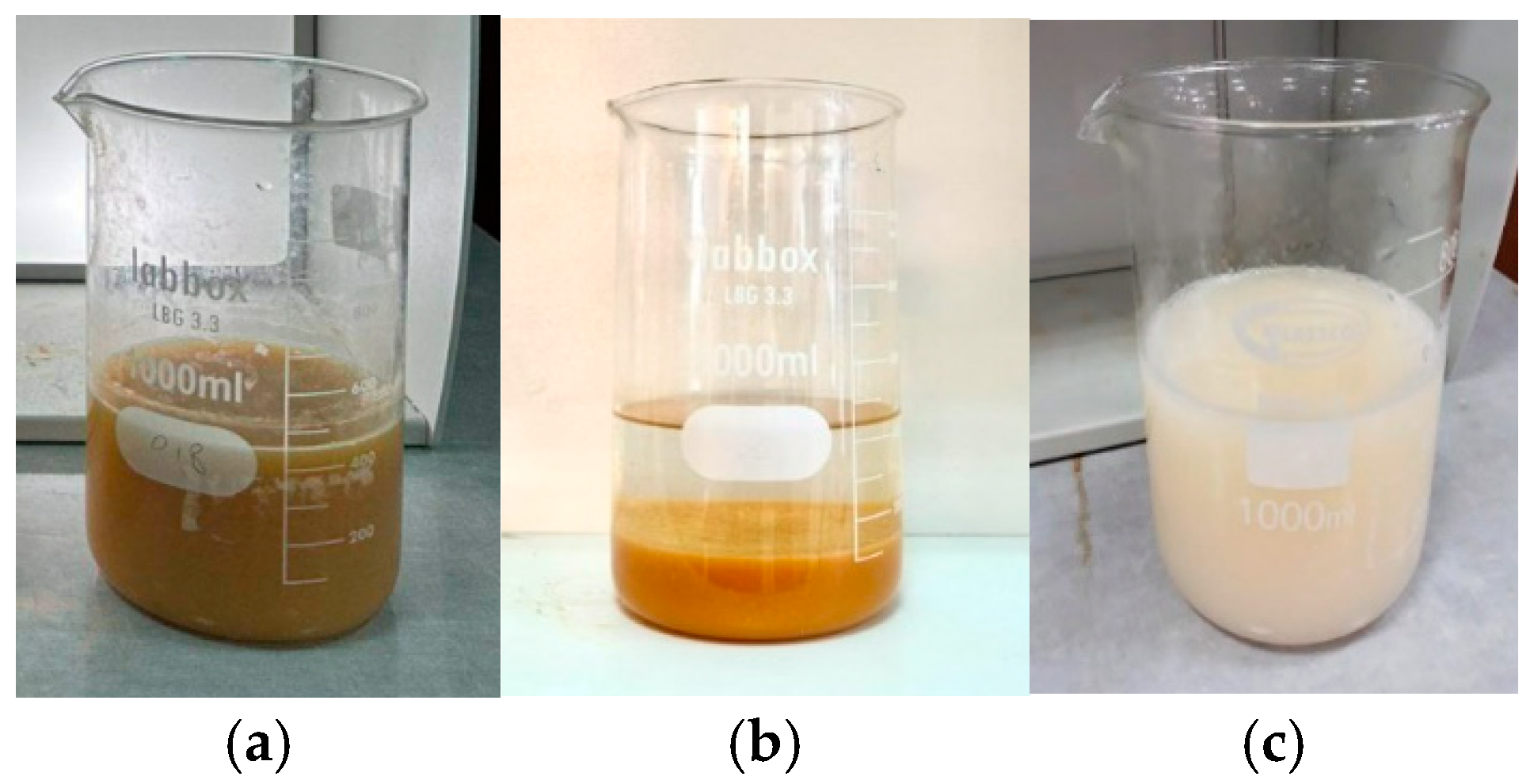
3.1. Coagulation Flocculation Treatment
3.1.1. Selection of the Appropriate Coagulant
- Coagulation speed: 150 rpm, coagulation time: 5 min;
- Flocculation speed: 30 rpm, flocculation time: 20 min;
- Settling time: 30 min.
3.1.2. Parametric Study of Coagulation–Flocculation
- Coagulant dose
- Coagulation time and speed
- Coagulation speed: 150 rpm;
- Flocculation speed: 30 rpm;
- Flocculation time: 20 min;
- Settling time was held constant at 30 min.
- Flocculation speed: 30 rpm;
- Flocculation time: 20 min;
- Settling time: 30 min.
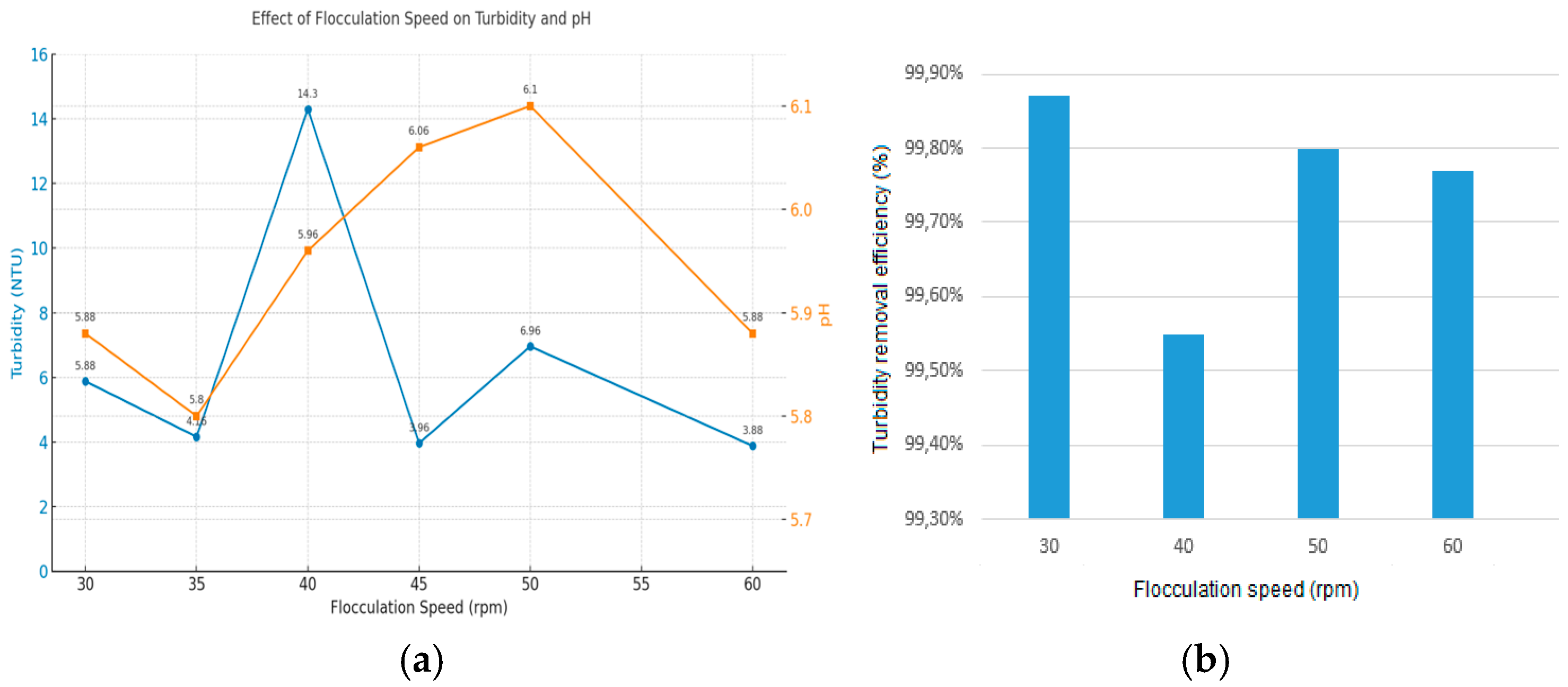
- Flocculation time and speed
- Coagulant dosage (1.2 g/L);
- Coagulation speed (150 rpm, 5 min);
- Flocculation speed (30 rpm);
- Settling time (30 min).
- Sedimentation time
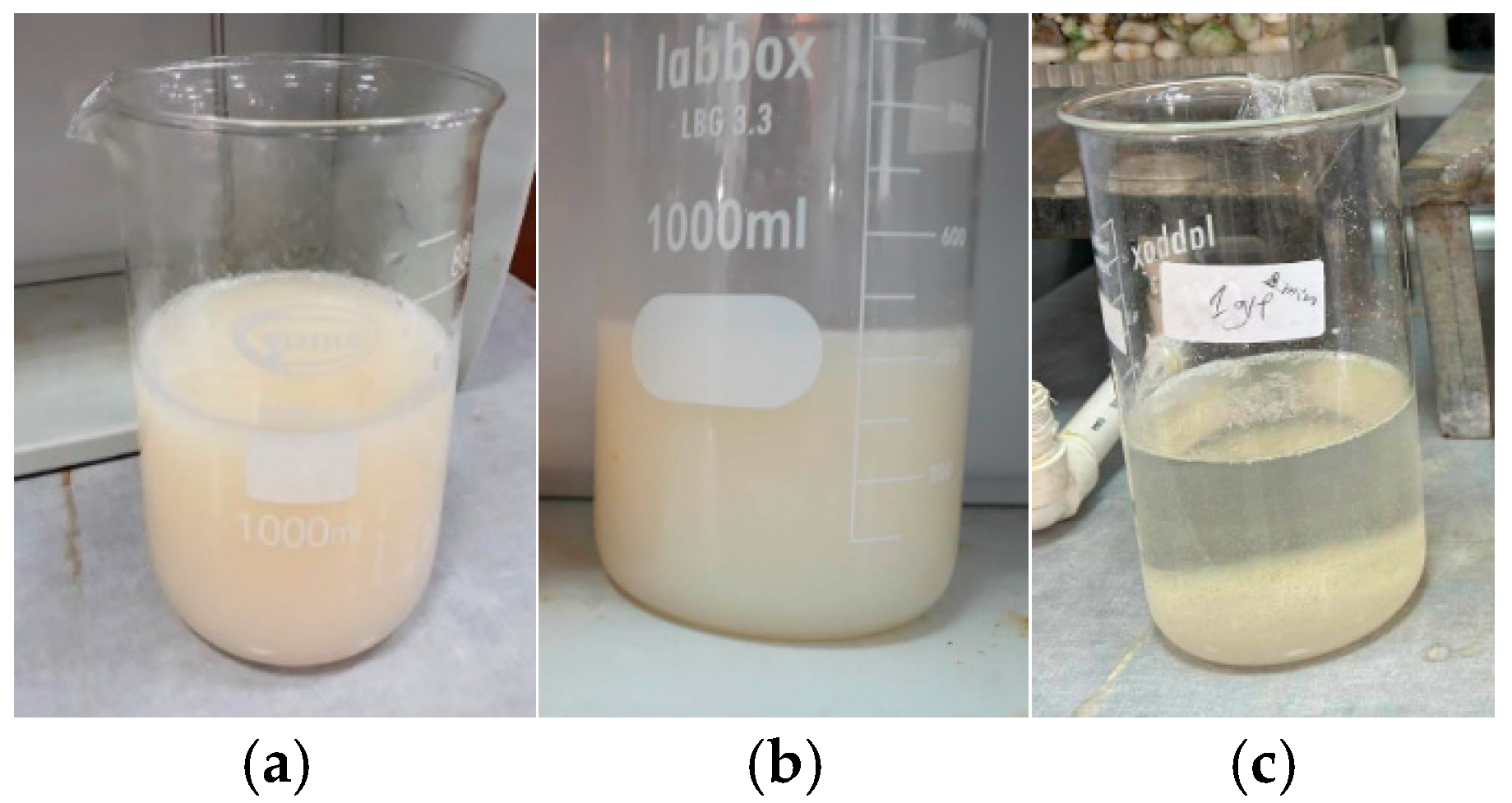
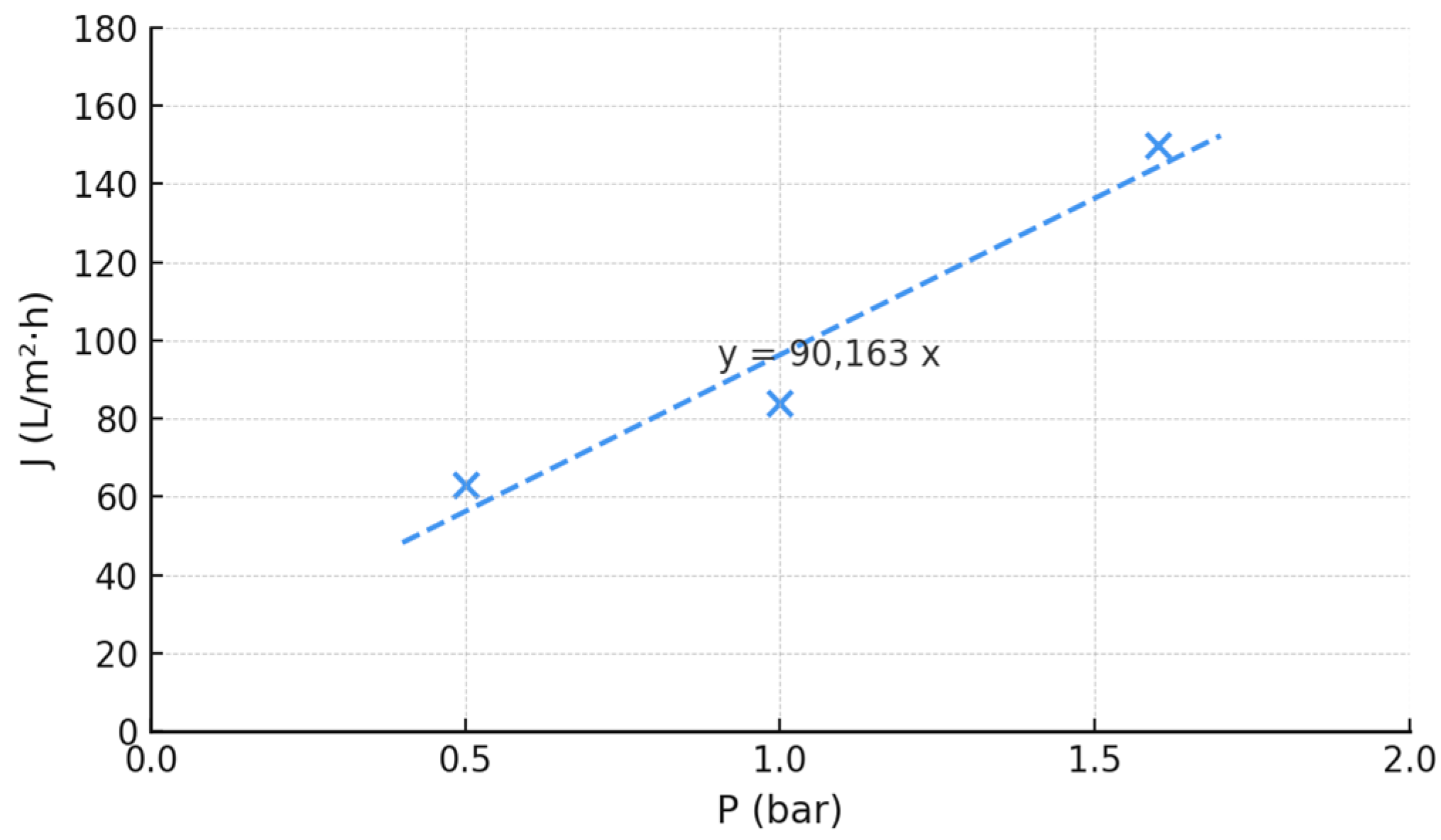
3.2. Membrane Filtration Treatment
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- National Sanitation Office (ONA). Environmental Manual, Environmental Management System. 2012. Available online: https://ona-dz.org/IMG/pdf/MANUEL_JUIN_2012.pdf (accessed on 1 March 2025).
- Farn, R.J. Chemestry and Technology of Surfactants; Blackwell Publishing Ltd.: Oxford, UK, 2007. [Google Scholar]
- Ho, Y.C.; Chua, S.C.; Chong, F.K. Coagulation-Flocculation Technology in Water and Wastewater Treatment. In Handbook of Research on Resource Management for Pollution and Waste Treatment; IGI Global: Hershey, PA, USA, 2020; pp. 432–457. [Google Scholar]
- Kumar, R.N.; Sadaf, S.; Verma, M.; Chakraborty, S.; Kumari, S.; Polisetti, V.; Kallem, P.; Iqbal, J.; Banat, F. Old Landfill Leachate and Municipal Wastewater Co-Treatment by Sequencing Batch Reactor Combined with Coagulation–Flocculation Using Novel Flocculant. Sustainability 2023, 15, 8205. [Google Scholar] [CrossRef]
- Vasiljević, S.; Vujić, M.; Agbaba, J.; Federici, S.; Ducoli, S.; Tomić, R.; Tubić, A. Efficiency of Coagulation/Flocculation for the Removal of Complex Mixture of Textile Fibers from Water. Processes 2023, 11, 820. [Google Scholar] [CrossRef]
- Tsoutsa, E.K.; Tolkou, A.K.; Kyzas, G.Z.; Katsoyiannis, I.A. New Trends in Composite Coagulants for Water and Wastewater Treatment. Macromol 2024, 4, 509–532. [Google Scholar] [CrossRef]
- Zaharia, C.; Musteret, C.-P.; Afrasinei, M.-A. The Use of Coagulation–Flocculation for Industrial Colored Wastewater Treatment—(I) The Application of Hybrid Materials. Appl. Sci. 2024, 14, 2184. [Google Scholar] [CrossRef]
- Khadidi, J.; Al-Shorgani, N.; Ali, E.; Abdulhamid, A.; Sahaid, M. A New Flocculant-Coagulant with Potential Use for Industrial Wastewater Treatment. In Proceedings of the 2nd International Conference on Environment, Energy and Biotechnology, Kuala Lumpur, Malaysia, 8–9 June 2013; Volume 51. [Google Scholar]
- Jiang, J.Q. The role of coagulation in water treatment. Curr. Opin. Chem. Eng. 2015, 8, 36–44. [Google Scholar] [CrossRef]
- Bratby, J. Coagulation and Flocculation in Water and Wastewater Treatment, 3rd ed.; IWA Publishing: London, UK, 2016. [Google Scholar]
- Prakash, N.B.; Sockan, V.; Jayakaran, P. Waste Water Treatment by Coagulation and Flocculation. Int. J. Eng. Sci. Innov. Technol. (IJESIT) 2014, 3, 479–484. [Google Scholar]
- Dunn, P.J. The importance of Green Chemistry in Process Research and Development. Chem. Soc. Rev. 2012, 41, 1452–1461. [Google Scholar] [CrossRef]
- Liv, F.T.; Torove, L. The effect of coagulation with MF/UF membrane filtration for the removal of virus in drinking water. J. Membr. Sci. 2006, 279, 364–371. [Google Scholar]
- Yinghua, L.; Yiyan, W.; Mengxi, L.; Fei, S.; Yue, Z.; Linlin, P. Effects of electroflocculation/oxidation pretreatment on the fouling characteristics of ultrafiltration membranes. Water Sci. Technol. 2022, 85, 1079–1089. [Google Scholar]
- Baños, R.; Manzano-Agugliaro, F.; Montoya, F.G.; Gil, C.; Alcayde, A.; Gómez, J. Optimization methods pplied to renewable and sustainable energy: A review. Renew. Sustain. Energy Rev. 2011, 15, 1753–1766. [Google Scholar] [CrossRef]
- Aboulhassan, M.A.; Souabi, S.; Yaacoubi, A.; Baudu, M. Removal of surfactant from industrial wastewaters by coagulation flocculation process. Int. J. Environ. Sci. Tech. 2006, 3, 327–332. [Google Scholar] [CrossRef]
- Sharghi, E.A.; Davarpanah, L. Optimization of chemical coagulation–flocculation process of detergent manufacturing plant wastewater treatment for full-scale applications: A case study. Desalination Water Treat. 2022, 262, 38–53. [Google Scholar] [CrossRef]
- Maung, S.S.; Htwe, T.T. Proposal of Wastewater Treatment Process and Design for Soap Industry. Int. J. Sci. Eng. Technol. Res. (IJSETR) 2014, 1, 2–3. [Google Scholar]
- Ali, M.N.; El-Hazek, A.N.; Neinaa, A.M. Using Polymers as Coagulants for Treatment of Soap Industry Wastewater. Des. Eng. 2022, 1, 2840–2849. [Google Scholar]
- Nascimento, C.C.; Veit, M.T.; Palácio, S.; Fagundes-Klen, M.R. Combined Application of Coagulation/Flocculation/Sedimentation and Membrane Separation for the Treatment of Laundry Wastewater. International Journal of Chemical Engineering. 2019, 13, 324710. [Google Scholar] [CrossRef]
- Harrelkas, F.; Azizi, A.; Yaacoubi, A.; Benhammou, A.; Noelle, M. Treatment of textile dye effluents using coagulation—Flocculation coupled with membrane processes or adsorption on powdered activated carbon. Desalination 2009, 235, 330–339. [Google Scholar] [CrossRef]
- Lee, J.W.; Chun, J.I.; Jung, H.J.; Kwak, D.H.; Ramesh, T.; Shim, W.G. Comparative studies on coagulation and adsorption as a pretreatment method for the performance improvement of submerged MF membrane for secondary domestic wastewater treatment. Sep. Sci. Technol. 2005, 40, 2613–2632. [Google Scholar] [CrossRef]
- Tyler, A.M.; Bérubéb, P.R.; Andrewsa, C.R. Coagulation/flocculation prior to low pressure membranes in drinking water treatment: A review. Environ. Sci. Water Res. Technol. 2020, 6, 2993. [Google Scholar]
- Keucken, A.; Heinicke, G.; Persson, K.M.; Köhler, S.J. Combined Coagulation and Ultrafiltration Process to Counteract Increasing NOM in Brown Surface Water. Water 2017, 9, 697. [Google Scholar] [CrossRef]
- Louhichi, G.; Jammeli, L.; Ghrabi, A.; Khouni, I. Efficiency of a Coagulation/Flocculation Process Combined to Membrane Filtration for the Treatment of Heavily Polluted Soap Industry Wastewater. In Proceedings of the Euro-Mediterranean Conference for Environmental Integration, Sousse, Tunisia, 1–4 November 2022. [Google Scholar]
- Huang, A.K.; Veit, M.T.; Juchen, P.T.; Trevisani, P. Sequential process of coagulation/flocculation/sedimentation-adsorption-microfiltration for laundry effluent treatment. J. Environ. Chem. Eng. 2019, 7, 103226. [Google Scholar] [CrossRef]
- Zahran, H.A. From Fat to Foam: The Fascinating World of Soap Chemistry and Technology. Egypt. J. Chem. 2024, 67, 9–17. [Google Scholar] [CrossRef]
- Algerian reuse standards document, JORA, 2012 Journal officiel de la république Algérienne N°26. Available online: https://www.joradp.dz/FTP/JO-FRANCAIS/2014/F2014026.pdf (accessed on 1 March 2025).
- Iwuozor, K.O. Prospects and Challenges of Using Coagulation-Flocculation Method in the Treatment of Effluents. Adv. J. Chem.-Sect. A 2019, 2, 105–127. [Google Scholar] [CrossRef]
- Liew, W.L.; Kassim, M.A.; Muda, K.; Loh, S.K.; Affam, A.C. Conventional methods and emerging wastewater polishing technologies for palm oil mill effluent treatment: A review. J. Environ. Manag. 2014, 149, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Benhabiles, O.; Galiano, F.; Marino, T.; Mahmoudi, H.; Lounici, H.; Figoli, A. Preparation and Characterization of TiO2-PVDF/PMMA Blend Membranes Using an Alternative Non-Toxic Solvent for UF/MF and Photocatalytic Application. Molecules 2019, 24, 724. [Google Scholar] [CrossRef]
- Benhabiles, O. Elaboration de membranes organiques photocatalytiques, Application pour la dépollution des eaux usées. Ph.D. Thesis, Ecole Nationale Polytechnique, Algiers, Algeria, 2018. [Google Scholar]
- Errahmani, K.B.; Benhabiles, O.; Bellebia, S.; Bengharez, Z.; Goosen, M.; Mahmoudi, H. Photocatalytic Nanocomposite Polymer-TiO2 Membranes for Pollutant Removal from Wastewater. Catalysts 2021, 11, 402. [Google Scholar] [CrossRef]
- Byrne, F.P.; Jin, S.; Paggiola, G.; Petchey, T.H.M.; Clark, J.H.; Farmer, T.J.; Hunt, A.J.; McElroy, C.R.; Sherwood, J. Tools and techniques for solvent selection: Green solvent selection guides. Sustain. Chem. Process. 2016, 4, 1–24. [Google Scholar] [CrossRef]
- CDH Finechemical. Material Safety Data Sheet. Available online: https://www.cdhfinechemical.com/images/product/msds/37_1771179379_TRIETHYLPHOSPHATECASNO78-40-0MSDS.pdf (accessed on 1 March 2025).
- Marcos, V.S.; Matthew, E.V.; Sílvia, M.A.C.O. Removal efficiencies. In Assessment of Treatment Plant Performance and Water Quality Data: A Guide for Students, Researchers and Practitioners; IWA Publishing: London, UK; Alliance House: London, UK, 2020; pp. 183–206. [Google Scholar]












| Temperature (°C) | pH | Salinity (g/L) | Conductivity (ms/cm) | Turbidity (NTU) | COD (mg/L) | BOD5 (mg/L) |
|---|---|---|---|---|---|---|
| 27.3 °C | 12.83 | 2.6 | 5.4 | 3235 | 9200 | 662 |
| Membrane Code | Thickness (mm) | Porosity (%) | Mean Pore Diameter (μm) | Young’s Modulus (N/mm2) | Lengthening at Break (%) | Contact Angle (°) |
|---|---|---|---|---|---|---|
| M-0.12 | 0.063 ± 0.003 | 79.48 ± 1.03 | 0.35 | 114.33 ± 4.88 | 28.16 ± 1.40 | 104.6 ± 3.3 |
| Coagulants | pH Before | pH After | Turbidity Before (NTU) | Turbidity After (NTU) | Turbidity Removal Efficiency (%) |
|---|---|---|---|---|---|
| Al2(SO4)3 | 12.83 | 6.07 | 3235 | 1531 | 32.67% |
| FeCl3 | 12.83 | 8.80 | 3235 | 1025 | 68% |
| pH | Turbidity (NTU) | COD (mg/L) |
|---|---|---|
| 6.38 | 3.28 | 351 |
| Coagulant Dose (g/L) | Coagulation Time (min) | Coagulation Speed (rpm) | Floculation Time (min) | Floculation Speed (rpm) | Sedimentation Time (min) |
|---|---|---|---|---|---|
| 1.2 | 5 | 150 | 20 | 30 | 30 |
| Parameters | Raw Water | CF Treatment | MF Treatment | Standards [20] |
|---|---|---|---|---|
| Turbidity (NTU) | 3235 | 3.28 | 0.90 | - |
| Turbidity removal efficiency | - | 99.89 | 99.97 | - |
| pH | 12.83 | 6.38 | 6.10 | 6.5–8.5 |
| COD (mg/L) | 9200 | 351 | 58 | 120 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merabti, L.; Benhabiles, O.; Tigrine, Z.; Mellal, M.; Chekir, N.; Mahidine, S.; Tassalit, D.; Bentchikou, M.; Douadi, A.; Jammeli, L.; et al. Hybrid Coagulation–Membrane Filtration Techniques for Sustainable Soap Wastewater Treatment. Water 2025, 17, 1411. https://doi.org/10.3390/w17101411
Merabti L, Benhabiles O, Tigrine Z, Mellal M, Chekir N, Mahidine S, Tassalit D, Bentchikou M, Douadi A, Jammeli L, et al. Hybrid Coagulation–Membrane Filtration Techniques for Sustainable Soap Wastewater Treatment. Water. 2025; 17(10):1411. https://doi.org/10.3390/w17101411
Chicago/Turabian StyleMerabti, Leila, Ouassila Benhabiles, Zahia Tigrine, Mounir Mellal, Nadia Chekir, Sarah Mahidine, Djilali Tassalit, Merouane Bentchikou, Amira Douadi, Linda Jammeli, and et al. 2025. "Hybrid Coagulation–Membrane Filtration Techniques for Sustainable Soap Wastewater Treatment" Water 17, no. 10: 1411. https://doi.org/10.3390/w17101411
APA StyleMerabti, L., Benhabiles, O., Tigrine, Z., Mellal, M., Chekir, N., Mahidine, S., Tassalit, D., Bentchikou, M., Douadi, A., Jammeli, L., Khouni, I., & Lebouachera, S. E. I. (2025). Hybrid Coagulation–Membrane Filtration Techniques for Sustainable Soap Wastewater Treatment. Water, 17(10), 1411. https://doi.org/10.3390/w17101411










