Effects of Three Antibiotics on Nitrogen-Cycling Bacteria in Sediment of Aquaculture Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Batch Treatment
2.3. Determination of the Water Quality Index
2.4. Determination of Functional Genes of the Nitrogen Cycle
2.5. High-Throughput Sequencing
2.6. Data Analysis
3. Results
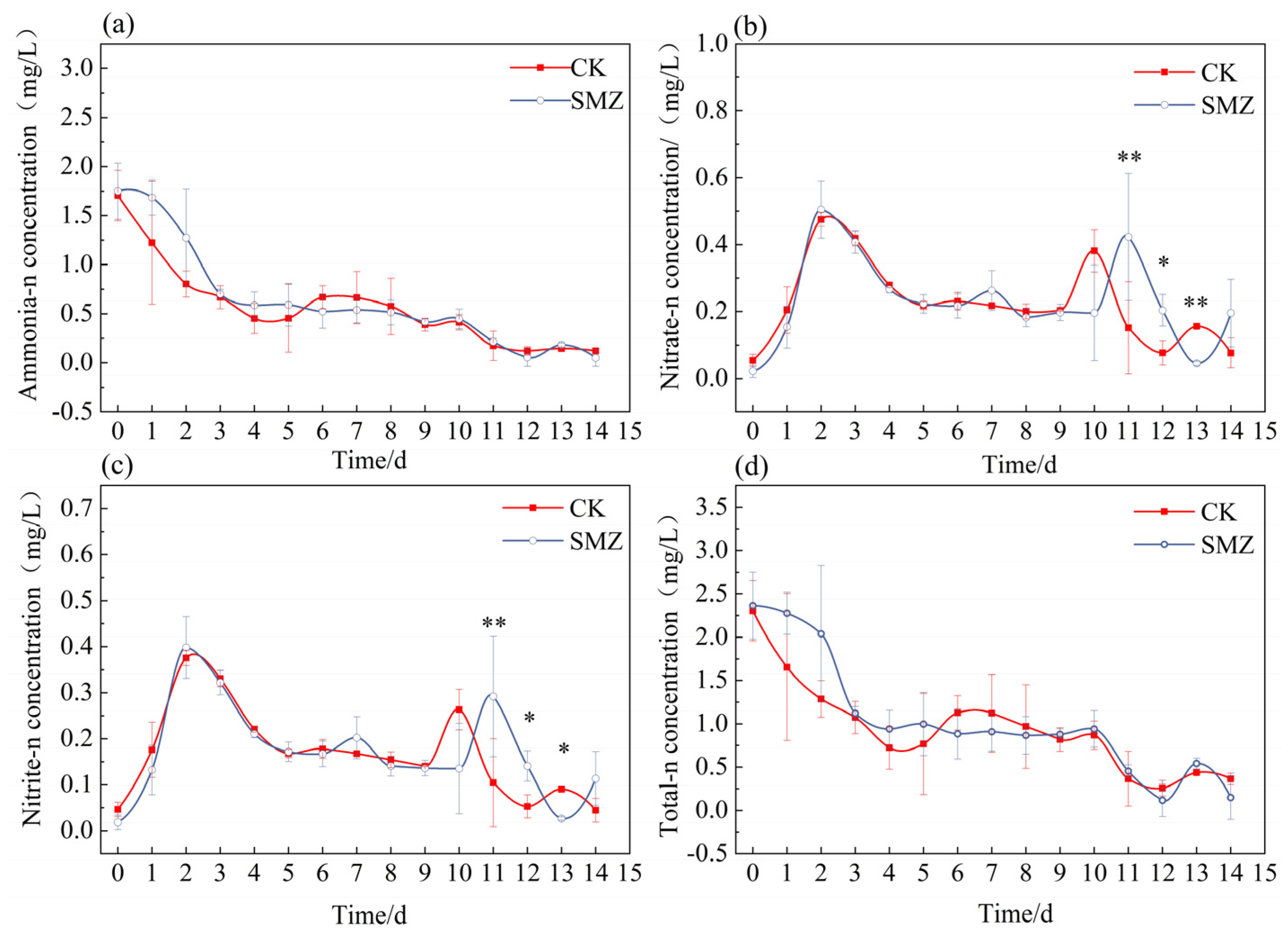
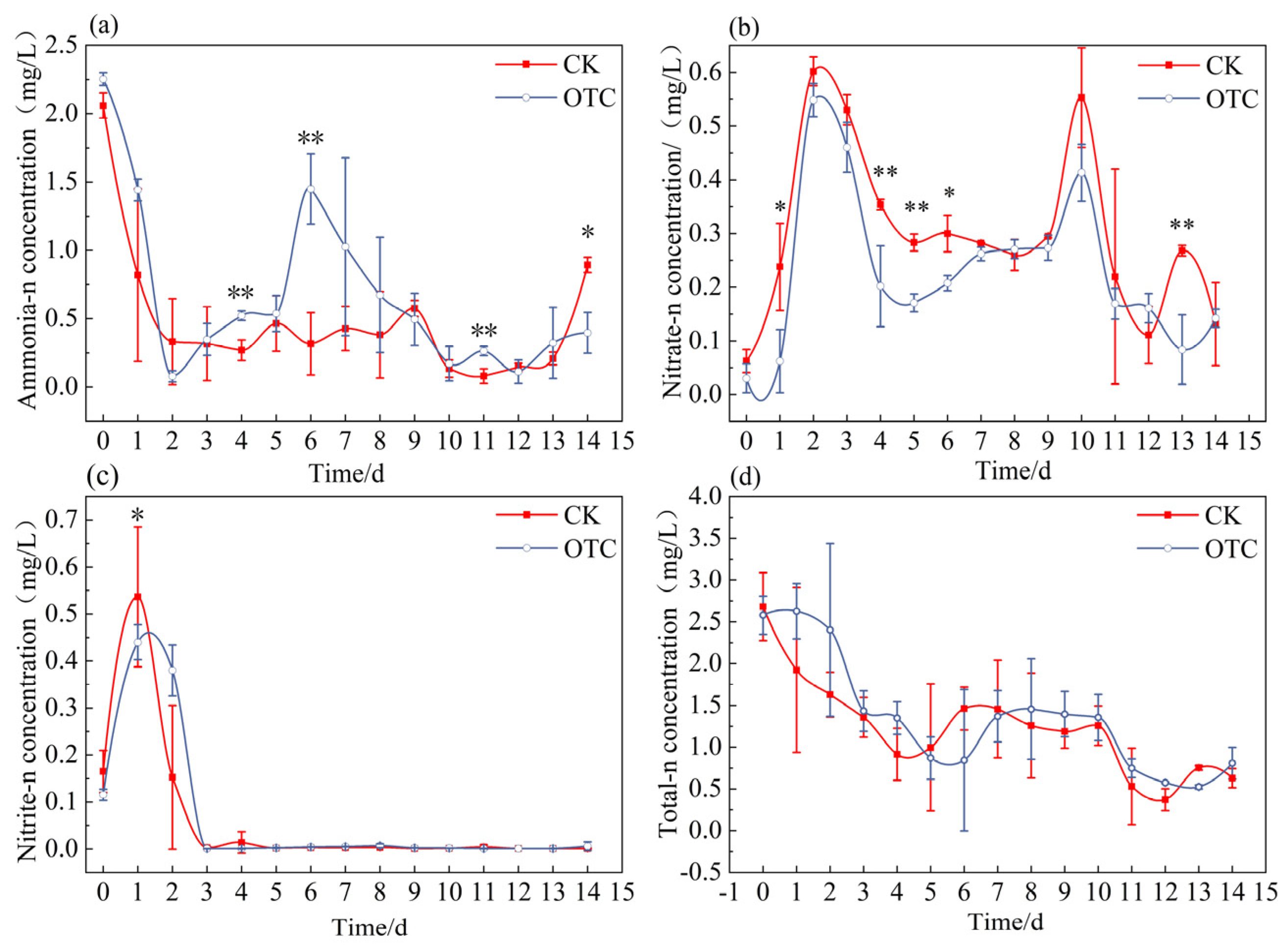
3.1. Changes in Various Nitrogen Compounds in Water
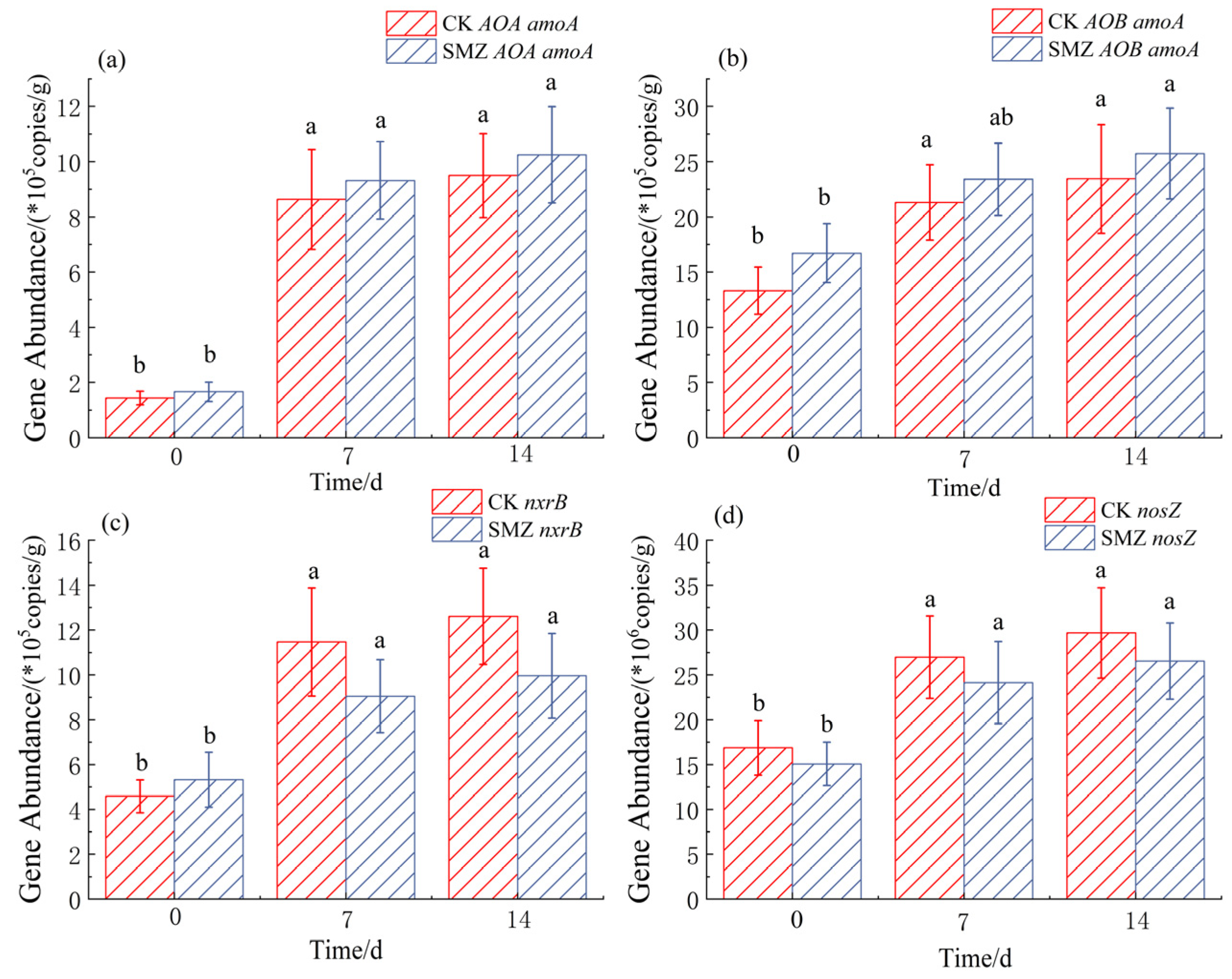
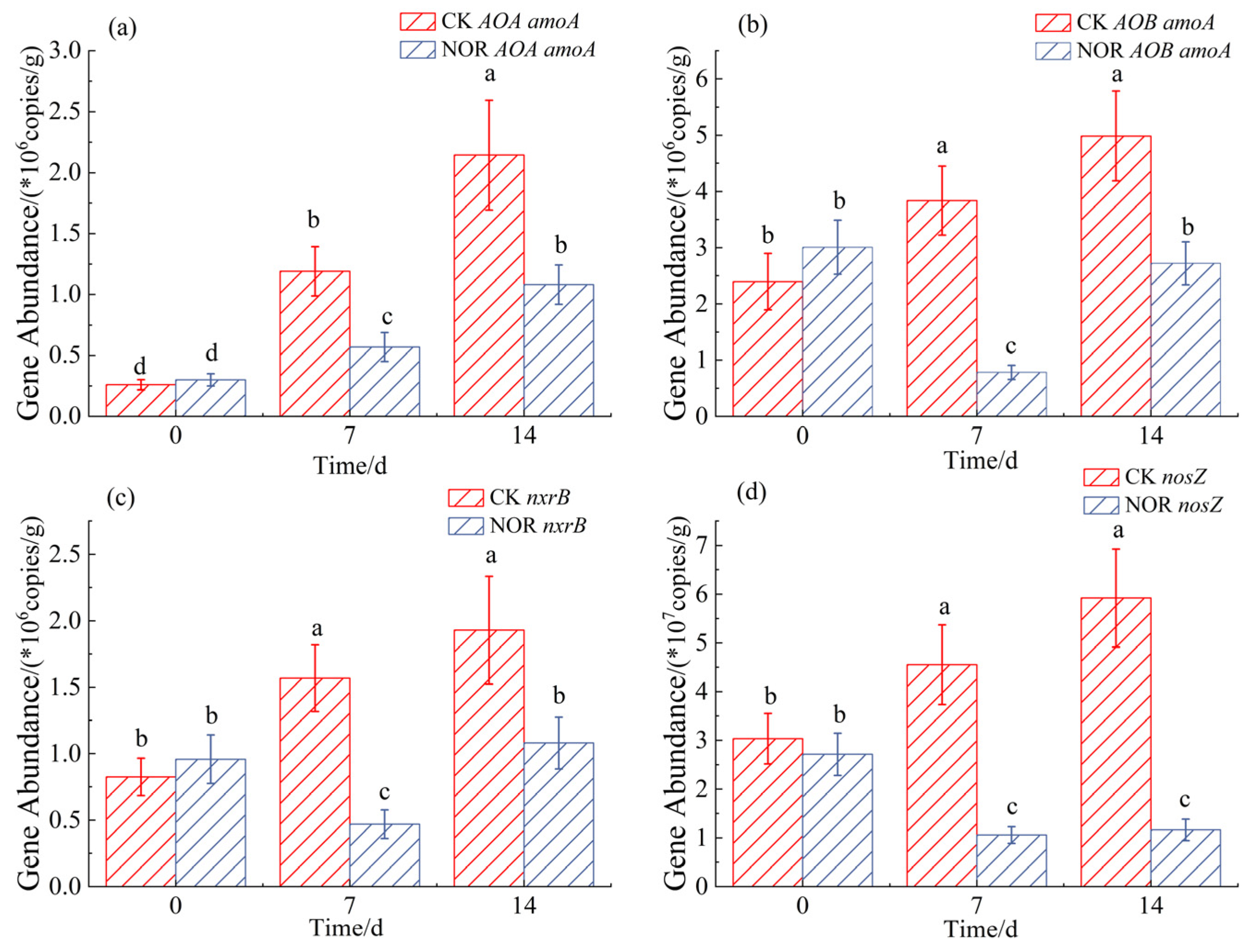
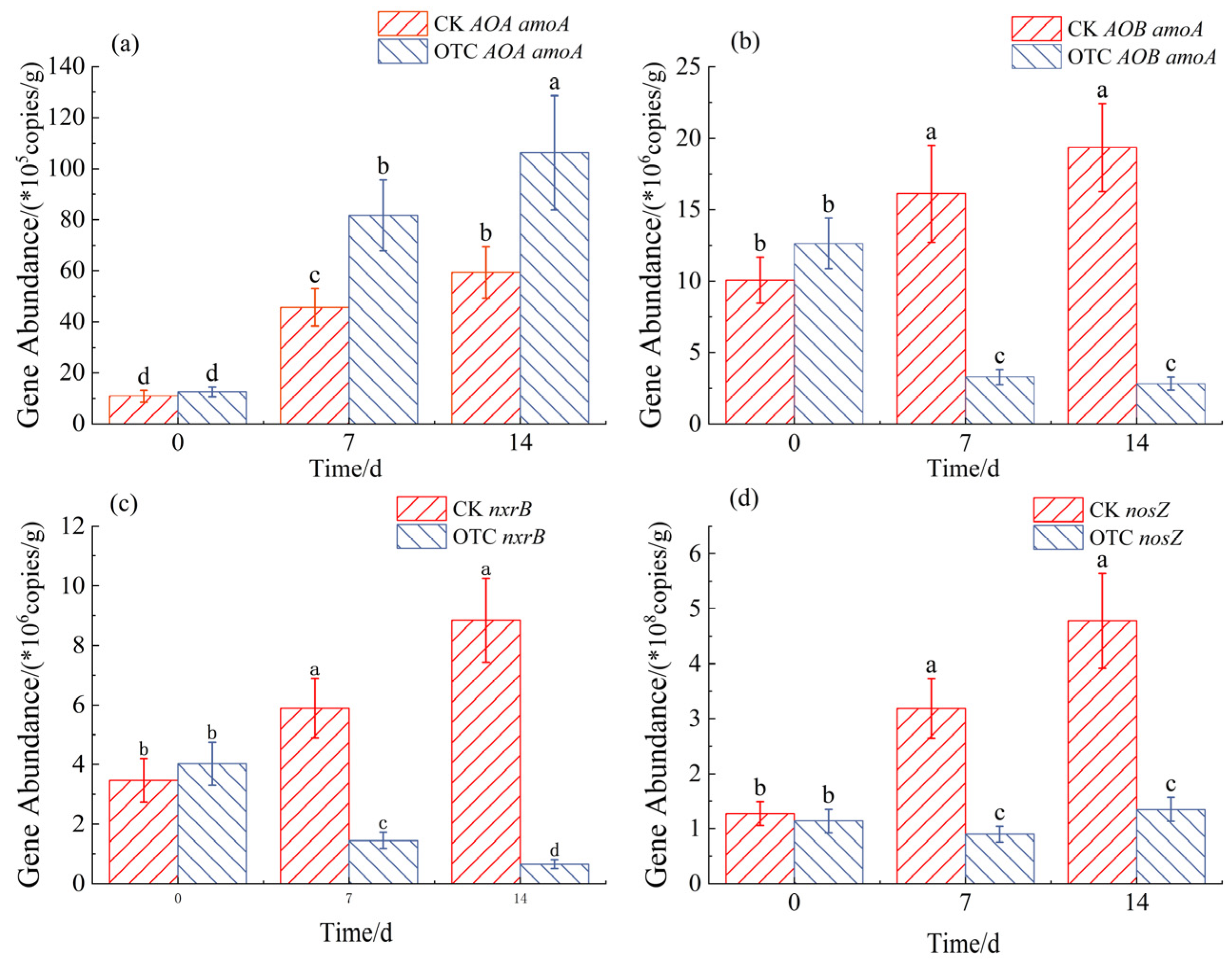
3.2. Nitrogen-Cycling Gene Abundance
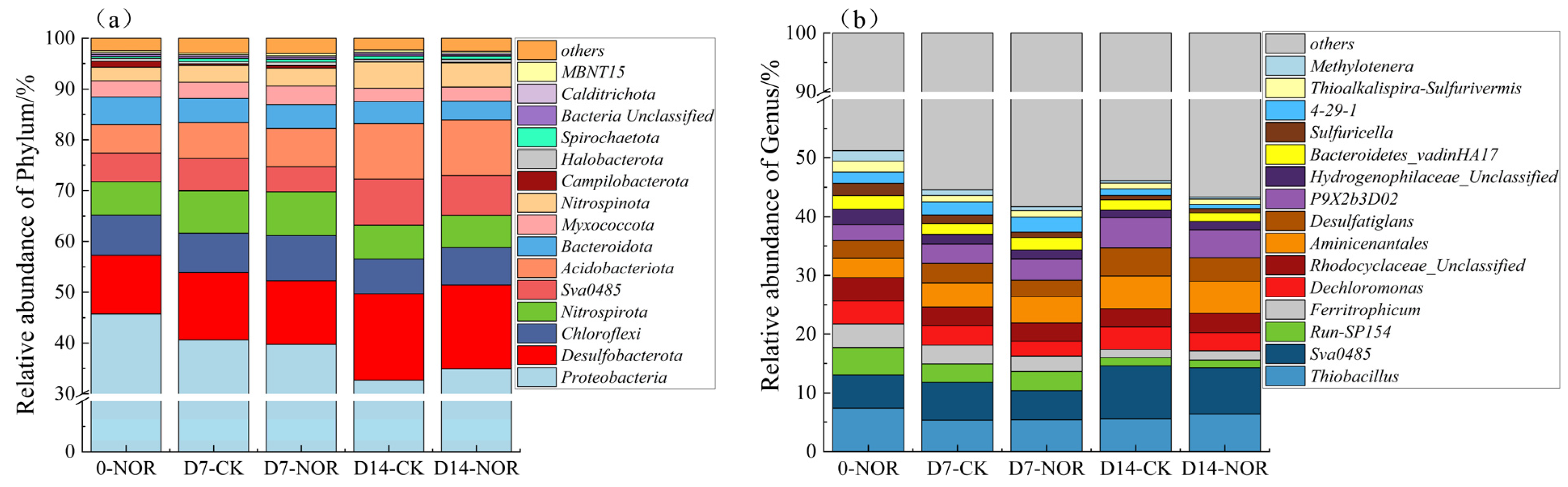
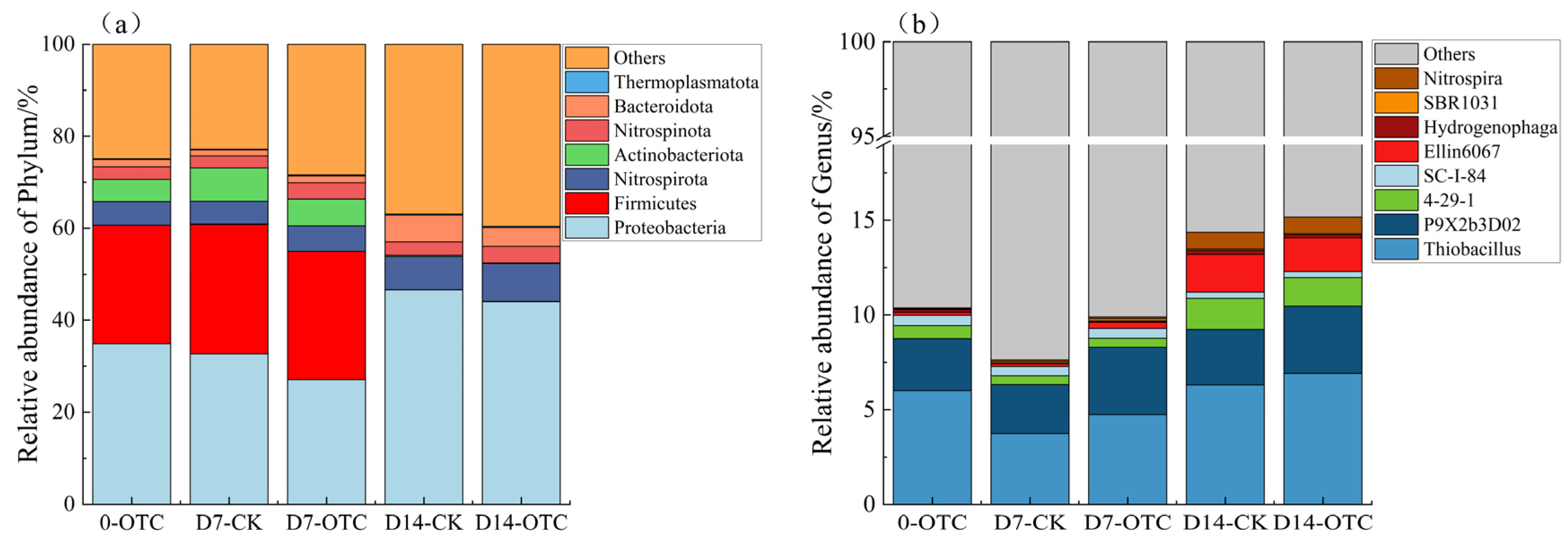
3.3. Composition of Microbiome Communities
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, W.H.; Zhang, G.; Zou, S.C.; Li, X.D.; Liu, Y.C. Determination of selected antibiotics in the Victoria Harbour and the Pearl River, South China using high-performance liquid chromatography-electrospray ionization tandem mass spectrometry. Environ. Pollut. 2007, 145, 672–679. [Google Scholar] [CrossRef]
- Assefa, A.; Abunna, F. Maintenance of Fish Health in Aquaculture: Review of Epidemiological Approaches for Prevention and Control of Infectious Disease of Fish. Vet. Med. Int. 2018, 2018, 5432497. [Google Scholar] [CrossRef]
- Kümmerer, K. Antibiotics in the aquatic environment—A review—Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef]
- Fahrenfeld, N.; Knowlton, K.; Krometis, L.A.; Hession, W.C.; Xia, K.; Lipscomb, E.; Libuit, K.; Green, B.L.; Pruden, A. Effect of manure application on abundance of antibiotic resistance genes and their attenuation rates in soil: Field-scale mass balance approach. Environ. Sci. Technol. 2014, 48, 2643–2650. [Google Scholar] [CrossRef]
- Białk-Bielińska, A.; Stolte, S.; Arning, J.; Uebers, U.; Böschen, A.; Stepnowski, P.; Matzke, M. Ecotoxicity evaluation of selected sulfonamides. Chemosphere 2011, 85, 928–933. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Ying, G.G.; Pan, C.G.; Liu, Y.S.; Zhao, J.L. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: Source analysis, multimedia modeling, and linkage to bacterial resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef]
- Grenni, P.; Ancona, V.; Caracciolo, A.B. Ecological effects of antibiotics on natural ecosystems: A review. Microchem. J. 2018, 136, 25–39. [Google Scholar] [CrossRef]
- Wen, L.-L.; Song, J.-M.; Li, X.-G.; Ma, J.; Dai, J.-J.; Yuan, H.-M.; Duan, L.-Q.; Wang, Q.-D. Environmental pollution of fluoroquinolones and its relationship with nitrogen cycling mediated by microorganisms. Ying Yong Sheng Tai Xue Bao 2023, 34, 3114–3126. [Google Scholar]
- Wang, M.J.; Huang, Z.Q.; Zhang, B.B.; Shi, X.Z. Soil nitrification and denitrification in Cunninghamia lanceolata plantations with different stand ages. Ying Yong Sheng Tai Xue Bao 2023, 34, 18–24. [Google Scholar]
- Yin, G.; Hou, L.; Liu, M.; Zheng, Y.; Li, X.; Lin, X.; Gao, J.; Jiang, X. Effects of thiamphenicol on nitrate reduction and N2O release in estuarine and coastal sediments. Environ. Pollut. 2016, 214, 265–272. [Google Scholar] [CrossRef]
- Toth, J.D.; Feng, Y.C.; Dou, Z.X. Veterinary antibiotics at environmentally relevant concentrations inhibit soil iron reduction and nitrification. Soil Biol. Biochem. 2011, 43, 2470–2472. [Google Scholar] [CrossRef]
- Guo, H.; Chen, Z.; Lu, C.; Guo, J.; Li, H.; Song, Y.; Han, Y.; Hou, Y. Effect and ameliorative mechanisms of polyoxometalates on the denitrification under sulfonamide antibiotics stress. Bioresour. Technol. 2020, 305, 123073. [Google Scholar] [CrossRef]
- Edwards, P. Aquaculture environment interactions: Past, present and likely future trends. Aquaculture 2015, 447, 2–14. [Google Scholar] [CrossRef]
- Zou, Y.; Lin, M.; Xiong, W.; Wang, M.; Zhang, J.; Wang, M.; Sun, Y. Metagenomic insights into the effect of oxytetracycline on microbial structures, functions and functional genes in sediment denitrification. Ecotox Environ. Safe 2018, 161, 85–91. [Google Scholar] [CrossRef]
- Lebeaux, D.; Ghigo, J.M.; Beloin, C. Biofilm-related infections: Bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol. Mol. Biol. Rev. 2014, 78, 510–543. [Google Scholar] [CrossRef]
- Marques, C.N.; Morozov, A.; Planzos, P.; Zelaya, H.M. The fatty acid signaling molecule cis-2-decenoic acid increases metabolic activity and reverts persister cells to an antimicrobial-susceptible state. Appl. Environ. Microb. 2014, 80, 6976–6991. [Google Scholar] [CrossRef]
- Dai, Q.; Liu, R.; Liang, Y.-T.; Shu, X.-M.; Xu, C.-C.; Chen, L.-J. Influence of Ciprofloxacin on the Microbial Community and Antibiotics Resistance Genes in a Membrane Bioreactor. Environ. Sci. 2018, 39, 1333–1341. [Google Scholar]
- Li, S.; Ma, B.; She, Z.; Guo, L.; Zhao, Y.; Jin, C.; Gao, M. Effect of norfloxacin on performance, microbial enzymatic activity and microbial community of a sequencing batch reactor. Environ. Technol. Inno. 2020, 18, 100726. [Google Scholar] [CrossRef]
- Bondarczuk, K.; Piotrowska-Seget, Z. Microbial diversity and antibiotic resistance in a final effluent-receiving lake. Sci. Total Environ. 2019, 650, 2951–2961. [Google Scholar] [CrossRef]
- Xi, X.; Wang, M.; Chen, Y.; Yu, S.; Hong, Y.; Ma, J.; Wu, Q.; Lin, Q.; Xu, X. Adaption of the microbial community to continuous exposures of multiple residual antibiotics in sediments from a salt-water aquacultural farm. J. Hazard. Mater. 2015, 290, 96–105. [Google Scholar] [CrossRef]
- Feng, Y.; Hu, J.; Chen, Y.; Xu, J.; Yang, B.; Jiang, J. Ecological effects of antibiotics on aquaculture ecosystems based on microbial community in sediments. Ocean. Coast. Manag. 2022, 224, 106173. [Google Scholar] [CrossRef]
- Tong, X.N.; Wang, X.Z.; He, X.J.; Wang, Z.; Li, W.X. Effects of antibiotics on microbial community structure and microbial functions in constructed wetlands treated with artificial root exudates. Environ. Sci. Process. Impacts 2020, 22, 217–226. [Google Scholar] [CrossRef]
- Cui, H.; Wang, S.-P.; Fu, J.; Zhou, Z.-Q.; Zhang, N.; Guo, L. Influence of ciprofloxacin on microbial community structure and function in soils. Biol. Fert. Soils 2014, 50, 939–947. [Google Scholar] [CrossRef]
- Trifonova, T.; Kosmacheva, A.G.; Chesnokova, S.M. Effect of antibiotics on the cellulolytic and nitrification activity of gray forest soil. South. Russ. Ecol. Dev. 2020, 15, 52–62. [Google Scholar] [CrossRef]
- Kotzerke, A.; Sharma, S.; Schauss, K.; Heuer, H.; Thiele-Bruhn, S.; Smalla, K.; Wilke, B.-M.; Schloter, M. Alterations in soil microbial activity and N-transformation processes due to sulfadiazine loads in pig-manure. Environ. Pollut. 2008, 153, 315–322. [Google Scholar] [CrossRef]
- Ollivier, J.; Schacht, D.; Kindler, R.; Groeneweg, J.; Engel, M.; Wilke, B.-M.; Kleineidam, K.; Schloter, M. Effects of repeated application of sulfadiazine-contaminated pig manure on the abundance and diversity of ammonia and nitrite oxidizers in the root-rhizosphere complex of pasture plants under field conditions. Front. Microbiol. 2013, 4, 22. [Google Scholar] [CrossRef]
- Gentle, B.S.; Ellis, P.S.; Grace, M.R.; McKelvie, I.D. Flow analysis methods for the direct ultra-violet spectrophotometric measurement of nitrate and total nitrogen in freshwaters. Anal. Chim. Acta 2011, 704, 116–122. [Google Scholar] [CrossRef]
- Wang, R.; Deng, L.; Fan, X.; Li, K.; Lu, H.; Li, W. Removal of heavy metal ion cobalt (II) from wastewater via adsorption method using microcrystalline cellulose-magnesium hydroxide. Int. J. Biol. Macromol. 2021, 189, 607–617. [Google Scholar] [CrossRef]
- Chen, W.; Liao, X.; Wu, Y.; Liang, J.B.; Mi, J.; Huang, J.; Zhang, H.; Wu, Y.; Qiao, Z.; Li, X.; et al. Effects of different types of biochar on methane and ammonia mitigation during layer manure composting. Waste Manag. 2017, 61, 506–515. [Google Scholar] [CrossRef]
- Wen, G.Q.; Li, J.; Liu, X.H.; Zhang, Y.S.; Wen, S.S. Extraction of total DNA and optimization of the RAPD reaction system in Dioscorea opposita Thunb. Genet. Mol. Res. 2014, 13, 1339–1347. [Google Scholar] [CrossRef]
- Tourna, M.; Freitag, T.E.; Nicol, G.W.; Prosser, J.I. Growth, activity and temperature responses of ammonia-oxidizing archaea and bacteria in soil microcosms. Environ. Microbiol. 2008, 10, 1357–1364. [Google Scholar] [CrossRef]
- Ojuederie, O.B.; Babalola, O.O. Microbial and Plant-Assisted Bioremediation of Heavy Metal Polluted Environments: A Review. Int. J. Environ. Res. Public Health 2017, 14, 1504. [Google Scholar] [CrossRef]
- Rotthauwe, J.H.; Witzel, K.P.; Liesack, W. The ammonia monooxygenase structural gene amoA as a functional marker: Molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl. Environ. Microb. 1997, 63, 4704–4712. [Google Scholar] [CrossRef]
- Enwall, K.; Philippot, L.; Hallin, S. Activity and composition of the denitrifying bacterial community respond differently to long-term fertilization. Appl. Environ. Microb. 2005, 71, 8335–8343. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Song, C.; Dong, X.Y.; Fan, L.M.; Zhang, C.; Qiu, L.P.; Chen, J.R. Effect of Sulfamethoxazole on Water Bacterial Community Structure in Tilapia (Oreochromis niloticus) Culture Pond. Environ. Sci. Technol. 2019, 42, 1–11. (In Chinese) [Google Scholar]
- Wang, Y.N.; Guo, X.C.; Lu, S.Y.; Liu, X.H.; Wang, X.H. Review of nitrogen removal in low-polluted water by constructed wetlands: Performance, mechanism, and influencing factors. J. Agric. Resour. Environ. 2021, 38, 722–734. (In Chinese) [Google Scholar]
- Naylor, R.L.; Hardy, R.W.; Buschmann, A.H.; Bush, S.R.; Cao, L.; Klinger, D.H.; Little, D.C.; Lubchenco, J.; Shumway, S.E.; Troell, M. A 20-year retrospective review of global aquaculture. Nature 2021, 591, 551–563. [Google Scholar] [CrossRef]
- Pan, Y.; Dong, J.; Wan, L.; Sun, S.; MacIsaac, H.J.; Drouillard, K.G.; Chang, X. Norfloxacin pollution alters species composition and stability of plankton communities. J. Hazard. Mater. 2020, 385, 121625. [Google Scholar] [CrossRef]
- Chen, L.; Huang, F.; Zhang, C.; Zhang, J.; Liu, F.; Guan, X. Effects of norfloxacin on nitrate reduction and dynamic denitrifying enzymes activities in groundwater. Environ. Pollut. 2021, 273, 116492. [Google Scholar] [CrossRef]
- Wei, D.B. Single and Joint Toxicity of Oxytetracycline, Enrofloxacin, Sulfadimidine and Cu on Soil Microorganism. Master’s Thesis, Shandong Agricultural University, Taian, China, 2014. (In Chinese). [Google Scholar]
- Schauss, K.; Focks, A.; Leininger, S.; Kotzerke, A.; Heuer, H.; Thiele-Bruhn, S.; Sharma, S.; Wilke, B.; Matthies, M.; Smalla, K.; et al. Dynamics and functional relevance of ammonia-oxidizing archaea in two agricultural soils. Environ. Microbiol. 2009, 11, 446–456. [Google Scholar] [CrossRef]
- Coombs, K.; Rodriguez-Quijada, C.; Clevenger, J.O.; Sauer-Budge, A.F. Current Understanding of Potential Linkages between Biocide Tolerance and Antibiotic Cross-Resistance. Microorganisms 2023, 11, 2000. [Google Scholar] [CrossRef]
- Orellana, L.H.; Rodriguez-R, L.M.; Higgins, S.; Chee-Sanford, J.C.; Sanford, R.A.; Ritalahti, K.M.; Löffler, F.E.; Konstantinidis, K.T. Detecting nitrous oxide reductase (NosZ) genes in soil metagenomes: Method development and implications for the nitrogen cycle. mBio 2014, 5, e01193-14. [Google Scholar] [CrossRef]
- Li, P.; Chen, C.Z.; Zhao, X.L.; Liu, L.; Li, Z.H. Metagenomics analysis reveals the effects of norfloxacin on the gut microbiota of juvenile common carp (Cyprinus carpio). Chemosphere 2023, 325, 138389. [Google Scholar] [CrossRef]
- Wu, H.; Li, Y.; Zhang, J.; Niu, L.; Zhang, W.; Cai, W.; Zhu, X. Sediment bacterial communities in a eutrophic lake influenced by multiple inflow-rivers. Environ. Sci. Pollut. R. 2017, 24, 19795–19806. [Google Scholar] [CrossRef]
- Zhou, Z.; Tran, P.Q.; Kieft, K.; Anantharaman, K. Genome diversification in globally distributed novel marine Proteobacteria is linked to environmental adaptation. ISME J. 2020, 14, 2060–2077. [Google Scholar] [CrossRef]
- Zhang, Z.Z.; Chen, D.X.; Liao, M.J.; Wang, S.L.; Li, Z. Spatiotemporal distribution of aerobic ammonia-oxidizing microorganisms in sediments of Lake Donghu, Wuhan. China Environ. Sci. 2021, 41, 1917–1924. (In Chinese) [Google Scholar]
- Abd Aziz, A.; Lee, K.; Park, B.; Park, H.; Park, K.; Choi, I.G.; Chang, I.S. Comparative study of the airborne microbial communities and their functional composition in fine particulate matter (PM 2.5 ) under non-extreme and extreme PM 2.5 conditions. Atmos. Environ. 2018, 194, 82–92. [Google Scholar] [CrossRef]
- Rampelotto, P.H.; Barboza, A.D.M.; Pereira, A.B.; Triplett, E.W.; Schaefer, C.E.G.R.; Camargo, F.A.d.O.; Roesch, L.F.W. Distribution and interaction patterns of bacterial communities in an ornithogenic soil of Seymour Island, Antarctica. Microb. Ecol. 2015, 69, 684–694. [Google Scholar] [CrossRef]
- Lin, X.; Xu, Y.; Han, R.; Luo, W.; Zheng, L. Migration of antibiotic resistance genes and evolution of flora structure in the Xenopus tropicalis intestinal tract with combined exposure to roxithromycin and oxytetracycline. Sci. Total Environ. 2022, 820, 153176. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, C.; Han, R.; Li, S.; Peng, H.; Zhou, X.; Huang, L.; Xu, Y. Oxytetracycline and heavy metals promote the migration of resistance genes in the intestinal microbiome by plasmid transfer. ISME J. 2023, 17, 2003–2013. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, J.; Zhu, K.; Zhang, H. Influence of oxytetracycline on the structure and activity of microbial community in wheat rhizosphere soil. J. Environ. Sci. -China 2009, 21, 954–959. [Google Scholar] [CrossRef] [PubMed]


| Functional Gene | Name of Primers | Primer Sequence (5′ to 3′) | Ref. |
|---|---|---|---|
| AOA amoA | CrenamoA23f | ATGGTCTGGCTWAGACG | [31] |
| CrenamoA616r | GCCATCCABCKRTANGTCCA | ||
| AOB amoA | nxrB-F | TACATGTGGTGGAACA | [32] |
| nxrB-R | CGGTTCTGGTCRATCA | ||
| nxrB | amoA-1F | GGGGTTTCTACTGGTGGT | [33] |
| amoA-2R | CCCCTCKGSAAAGCCTTCTTC | ||
| nosZ | nosZ-F | CGYTGTTCMTCGACAGCCAG | [34] |
| nosZ-1622R | CGSACCTTSTTGCCSTYGCG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; He, H.; Ding, J.; Zhang, Z.; Leng, Y.; Liao, M.; Xiong, W. Effects of Three Antibiotics on Nitrogen-Cycling Bacteria in Sediment of Aquaculture Water. Water 2024, 16, 1256. https://doi.org/10.3390/w16091256
Li Z, He H, Ding J, Zhang Z, Leng Y, Liao M, Xiong W. Effects of Three Antibiotics on Nitrogen-Cycling Bacteria in Sediment of Aquaculture Water. Water. 2024; 16(9):1256. https://doi.org/10.3390/w16091256
Chicago/Turabian StyleLi, Zhu, Huan He, Jianhe Ding, Zhizhong Zhang, Yifei Leng, Mingjun Liao, and Wen Xiong. 2024. "Effects of Three Antibiotics on Nitrogen-Cycling Bacteria in Sediment of Aquaculture Water" Water 16, no. 9: 1256. https://doi.org/10.3390/w16091256
APA StyleLi, Z., He, H., Ding, J., Zhang, Z., Leng, Y., Liao, M., & Xiong, W. (2024). Effects of Three Antibiotics on Nitrogen-Cycling Bacteria in Sediment of Aquaculture Water. Water, 16(9), 1256. https://doi.org/10.3390/w16091256