Quality and Biodegradation Process of Dissolved Organic Carbon in Typical Fresh-Leaf Leachate in the Wuhan Urban Forest Park
Abstract
1. Introduction
2. Materials and Methods
2.1. Leaves Collection and Preparation of Leachates
2.2. Incubation Experiment of BDOC
2.3. Band Scanning and Spectral Measurements
2.4. Dreem-PARAFAC Analysis
2.5. Water Quality Parameter Determination
2.6. Plotting and Analysis
3. Results
3.1. Water Quality Parameters
3.2. DOC Quantity and BDOC in Leaf Leachates
3.3. Fluorescent and UV Parameters
3.4. Fluorescent Components during the Degradation Process
4. Discussion
4.1. Differences in DOC Leaching and Degradation among Different Tree Leaves
4.2. Kinetic Modeling of the Degradation Process of DOM
4.3. Main Factors Influencing DOM Degradation
4.4. Changes in Fluorescent Components during the Degradation Process
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Duan, S.W.; Delaney-Newcomb, K.; Kaushal, S.S.; Findlay, S.E.G.; Belt, K.T. Potential effects of leaf litter on water quality in urban watersheds. Biogeochemistry 2014, 121, 61–80. [Google Scholar] [CrossRef]
- Hobbie, S.E.; Baker, L.A.; Buyarski, C.; Nidzgorski, D.; Finlay, J.C. Decomposition of tree leaf litter on pavement: Implications for urban water quality. Urban Ecosyst. 2014, 17, 369–385. [Google Scholar] [CrossRef]
- Yusheng, Y.; Ruiyu, L.; Tingbo, L.; Bin, D.; Xiaoguo, W.; Jianfen, G. Ultraviolet-visible spectroscopic characteristics of dissolved organic matter released from forest litterfall. J. Trop. Subtrop. Bot. 2004, 12, 124–128. [Google Scholar]
- Wang, M.; Tian, Q.X.; Liao, C.; Zhao, R.D.; Wang, D.Y.; Wu, Y.; Li, Q.X.; Wang, X.G.; Liu, F. The fate of litter-derived dissolved organic carbon in forest soils: Results from an incubation experiment. Biogeochemistry 2019, 144, 133–147. [Google Scholar] [CrossRef]
- Arellano, C. Production and biodegradability of dissolved organic carbon from dIfferent litter sources. Reg. Stud. Mar. Sci. 2015, 62, 102962. [Google Scholar]
- Hagedorn, F.; Machwitz, M. Controls on dissolved organic matter leaching from forest litter grown under elevated atmospheric CO2. Soil Biol. Biochem. 2007, 39, 1759–1769. [Google Scholar] [CrossRef]
- Yang, J.; Mu, J.; Zhang, Y.; Fu, C.; Dong, Q.; Yang, Y.; Wu, Q. Initial Carbon Quality of Newly Shed Foliar Litter in an Alpine Forest from Proximate Analysis and 13C NMR Spectroscopy Perspectives. Forests 2022, 13, 1886. [Google Scholar] [CrossRef]
- Xu, J.-W.; Ji, J.-H.; Hu, D.-N.; Zheng, Z.; Mao, R. Tree fresh leaf- and twig-leached dissolved organic matter quantity and biodegradability in subtropical plantations in China. Forests 2022, 13, 833. [Google Scholar] [CrossRef]
- Jiang, P.; Li, S.Y. Insights into priming effects of dissolved organic matter degradation in urban lakes with different trophic states. Environ. Res. 2024, 245, 118063. [Google Scholar] [CrossRef]
- Ni, M.F.; Li, S.Y. Ultraviolet humic-like component contributes to riverine dissolved organic matter biodegradation. J. Environ. Sci. 2023, 124, 165–175. [Google Scholar] [CrossRef]
- Ni, M.; Li, S. Optical properties as tracers of riverine dissolved organic matter biodegradation in a headwater tributary of the Yangtze. J. Hydrol. 2020, 582, 124497. [Google Scholar] [CrossRef]
- Kothawala, D.N.; von Wachenfeldt, E.V.; Koehler, B.; Tranvik, L.J. Selective loss and preservation of lake water dissolved organic matter fluorescence during long-term dark incubations. Sci. Total Environ. 2012, 433, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Amado, A.M.; Cotner, J.B.; Suhett, A.L.; Esteves, F.A.; Bozelli, R.; Farjalla, V.F. Contrasting interactions mediate dissolved organic matter decomposition in tropical aquatic ecosystems. Aquat. Microb. Ecol. 2007, 49, 25–34. [Google Scholar] [CrossRef]
- Mao, R.; Li, S.-Y. Temperature sensitivity of biodegradable dissolved organic carbon increases with elevating humification degree in subtropical rivers. Sci. Total Environ. 2018, 635, 1367–1371. [Google Scholar] [CrossRef] [PubMed]
- Mao, R.; Li, S. Temporal controls on dissolved organic carbon biodegradation in subtropical rivers: Initial chemical composition versus stoichiometry. Sci. Total Environ. 2018, 651, 3064–3069. [Google Scholar] [CrossRef] [PubMed]
- Marschner, B.; Kalbitz, K. Controls of bioavailability and biodegradability of dissolved organic matter in soils. Geoderma 2003, 113, 211–235. [Google Scholar] [CrossRef]
- Zhang, X.S. Degradation of Terrigenous Dissolved Organic Matter and Its Association with Phenanthrene in Danjiangkou Reservoir Area. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2022. [Google Scholar]
- Zhang, X.; He, G.; Yan, S.; Long, T.; Peng, X.; Zhang, Z.; Wang, G. Forest status assessment in China with SDG indicators based on high-resolution satellite data. Int. J. Digit. Earth 2023, 16, 1008–1021. [Google Scholar] [CrossRef]
- Cui, Y.; Meng, J.-Q.; Chen, Y.-H.; Shao, F.-F.; Chen, X.-Z.; Jin, Y.; Zhang, M.-X.; Yun-Qian, G.; Luo, F.-L.; Yu, F.-H. The priming effects of plant leachates on dissolved organic matter degradation in water depend on leachate type and water stability. Environ. Res. 2023, 223, 115482. [Google Scholar] [CrossRef]
- Schreeg, L.A.; Mack, M.C.; Turner, B.L. Nutrient--specific solubility patterns of leaf litter across 41 lowland tropical woody species. Ecology 2013, 94, 94–105. [Google Scholar] [CrossRef]
- Zhuang, W.E.; Chen, W.; Cheng, Q.; Yang, L. Assessing the priming effect of dissolved organic matter from typical sources using fluorescence EEMs-PARAFAC. Chemosphere 2021, 264 Pt 2, 128600. [Google Scholar] [CrossRef]
- Wu, P.-P.; Ding, Y.-D.; Li, S.-L.; Sun, X.-X.; Zhang, Y.; Mao, R. Carbon, nitrogen and phosphorus stoichiometry controls interspecific patterns of leaf litter-derived dissolved organic matter biodegradation in subtropical plantations of China. iForest Biogeosci. For. 2021, 14, 80–85. [Google Scholar] [CrossRef]
- Pinsonneault, A.J.; Moore, T.R.; Roulet, N.T.; Lapierre, J.-F. Biodegradability of Vegetation-Derived Dissolved Organic Carbon in a Cool Temperate Ombrotrophic Bog. Ecosystems 2016, 19, 1023–1036. [Google Scholar] [CrossRef]
- Bricaud, A.; Morel, A.; Prieur, L. Absorption by dissolved organic matter of the sea (yellow substance) in the UV and visible domains1. Limnol. Oceanogr. 1981, 26, 43–53. [Google Scholar] [CrossRef]
- Ma, Y.M.; Mao, R.; Li, S.Y. Hydrological seasonality largely contributes to riverine dissolved organic matter chemical composition: Insights from EEM-PARAFAC and optical indicators. J. Hydrol. 2021, 595, 125993. [Google Scholar] [CrossRef]
- Luo, J.C.; Li, S.Y. Optical properties of dissolved organic matter in a monsoonal headwater stream, China: Insights for structure, source and riverine pCO2. J. Clean. Prod. 2021, 282, 124545. [Google Scholar] [CrossRef]
- Chin, Y.-P.; Aiken, G.; O’Loughlin, E. Molecular weight, polydispersity, and spectroscopic properties of aquatic humic substances. Environ. Sci. Technol. 1994, 28, 1853–1858. [Google Scholar] [CrossRef]
- Ma, Y.; Ni, M.; Gu, S.; Zhang, L.; Li, S. Autochthonous sources and biogeochemical processes drive the spatiotemporal variation of DOM composition and optical indicators in a karst river. J. Hydrol. 2023, 623, 129825. [Google Scholar] [CrossRef]
- Lawaetz, A.J.; Stedmon, C.A. Fluorescence Intensity Calibration Using the Raman Scatter Peak of Water. Appl. Spectrosc. 2009, 63, 936–940. [Google Scholar] [CrossRef]
- Parlanti, E.; Wörz, K.; Geoffroy, L.; Lamotte, M. Dissolved organic matter fluorescence spectroscopy as a tool to estimate biological activity in a coastal zone submitted to anthropogenic inputs. Org. Geochem. 2000, 31, 1765–1781. [Google Scholar] [CrossRef]
- Ohno, T. Fluorescence inner-filtering correction for determining the humification index of dissolved organic matter. Environ. Sci. Technol. 2002, 36, 742–746. [Google Scholar] [CrossRef]
- Zhang, L.Q.; Xu, Y.J.; Li, S.Y. Source and quality of dissolved organic matter in streams are reflective to land use/land cover, climate seasonality and pCO2. Environ. Res. 2023, 216, 114608. [Google Scholar] [CrossRef]
- State Environmental Protection Administration of China. Methods for Monitoring and Analysis of Water and Wastewater; China Environmental Science Press: Beijing, China, 2002; pp. 239–284. [Google Scholar]
- He, W.-M.; Sun, Z.-K. Convergent production and tolerance among 107 woody species and divergent production between shrubs and trees. Sci. Rep. 2016, 6, 20485. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Nam, S.-N.; Kim, J.; Oh, J. Photocatalytic degradation of dissolved organic matter under ZnO-catalyzed artificial sunlight irradiation system. Sci. Rep. 2020, 10, 13090. [Google Scholar] [CrossRef]
- Hotchkiss, E.; Hall Jr, R.; Baker, M.; Rosi--Marshall, E.; Tank, J. Modeling priming effects on microbial consumption of dissolved organic carbon in rivers. J. Geophys. Res. Biogeosci. 2014, 119, 982–995. [Google Scholar] [CrossRef]
- Mineau, M.M.; Wollheim, W.M.; Buffam, I.; Findlay, S.E.; Hall, R.O., Jr.; Hotchkiss, E.R.; Koenig, L.E.; McDowell, W.H.; Parr, T.B. Dissolved organic carbon uptake in streams: A review and assessment of reach--scale measurements. J. Geophys. Res. Biogeosci. 2016, 121, 2019–2029. [Google Scholar] [CrossRef]
- Larson, J.H.; Frost, P.C.; Zheng, Z.; Johnston, C.A.; Bridgham, S.D.; Lodge, D.M.; Lamberti, G.A. Effects of upstream lakes on dissolved organic matter in streams. Limnol. Oceanogr. 2007, 52, 60–69. [Google Scholar] [CrossRef]
- Tank, J.L.; Rosi-Marshall, E.J.; Baker, M.A.; Hall, R.O. Are rivers just big streams? a pulse method to quantify nitrogen demand in a large river. Ecology 2008, 89, 2935–2945. [Google Scholar] [CrossRef]
- Holmes, R.M.; McClelland, J.W.; Raymond, P.A.; Frazer, B.B.; Peterson, B.J.; Stieglitz, M. Lability of DOC transported by Alaskan rivers to the Arctic Ocean. Geophys. Res. Lett. 2008, 35, 3. [Google Scholar] [CrossRef]
- Mutschlecner, A.E.; Guerard, J.J.; Jones, J.B.; Harms, T.K. Regional and intra--annual stability of dissolved organic matter composition and biolability in high--latitude Alaskan rivers. Limnol. Oceanogr. 2018, 63, 1605–1621. [Google Scholar] [CrossRef]
- Wickland, K.P.; Aiken, G.R.; Butler, K.; Dornblaser, M.M.; Spencer, R.G.M.; Striegl, R.G. Biodegradability of dissolved organic carbon in the Yukon River and its tributaries: Seasonality and importance of inorganic nitrogen. Glob. Biogeochem. Cycles 2012, 26. [Google Scholar] [CrossRef]
- Morrissey, E.M.; Gillespie, J.L.; Morina, J.C.; Franklin, R.B. Salinity affects microbial activity and soil organic matter content in tidal wetlands. Glob. Change Biol. 2014, 20, 1351–1362. [Google Scholar] [CrossRef]
- Liu, F.; Wang, D. Dissolved organic carbon concentration and biodegradability across the global rivers: A meta-analysis. Sci. Total Environ. 2021, 818, 151828. [Google Scholar] [CrossRef]
- Smith, E.M.; Prairie, Y.T. Bacterial metabolism and growth efficiency in lakes: The importance of phosphorus availability. Limnol. Oceanogr. 2004, 49, 137–147. [Google Scholar] [CrossRef]
- Jian, Q.; Boyer, T.H.; Yang, X.; Xia, B.; Yang, X. Characteristics and DBP formation of dissolved organic matter from leachates of fresh and aged leaf litter. Chemosphere 2016, 152, 335–344. [Google Scholar] [CrossRef]
- Asmala, E.; Autio, R.; Kaartokallio, H.; Stedmon, C.A.; Thomas, D.N. Processing of humic-rich riverine dissolved organic matter by estuarine bacteria: Effects of predegradation and inorganic nutrients. Aquat. Sci. 2014, 76, 451–463. [Google Scholar] [CrossRef]
- Bu, X.; Han, F.; Ruan, H.; Zhu, L. Changes in chemical composition and spectral characteristics of dissolved organic matter from soils induced by biodegradation. Soil Sci. 2014, 179, 197–204. [Google Scholar] [CrossRef]
- Li, X.; Dai, X.; Takahashi, J.; Li, N.; Jin, J.; Dai, L.; Dong, B. New insight into chemical changes of dissolved organic matter during anaerobic digestion of dewatered sewage sludge using EEM-PARAFAC and two-dimensional FTIR correlation spectroscopy. Bioresour. Technol. 2014, 159, 412–420. [Google Scholar] [CrossRef]
- Xu, H.; Guo, L. Intriguing changes in molecular size and composition of dissolved organic matter induced by microbial degradation and self-assembly. Water Res. 2018, 135, 187–194. [Google Scholar] [CrossRef]



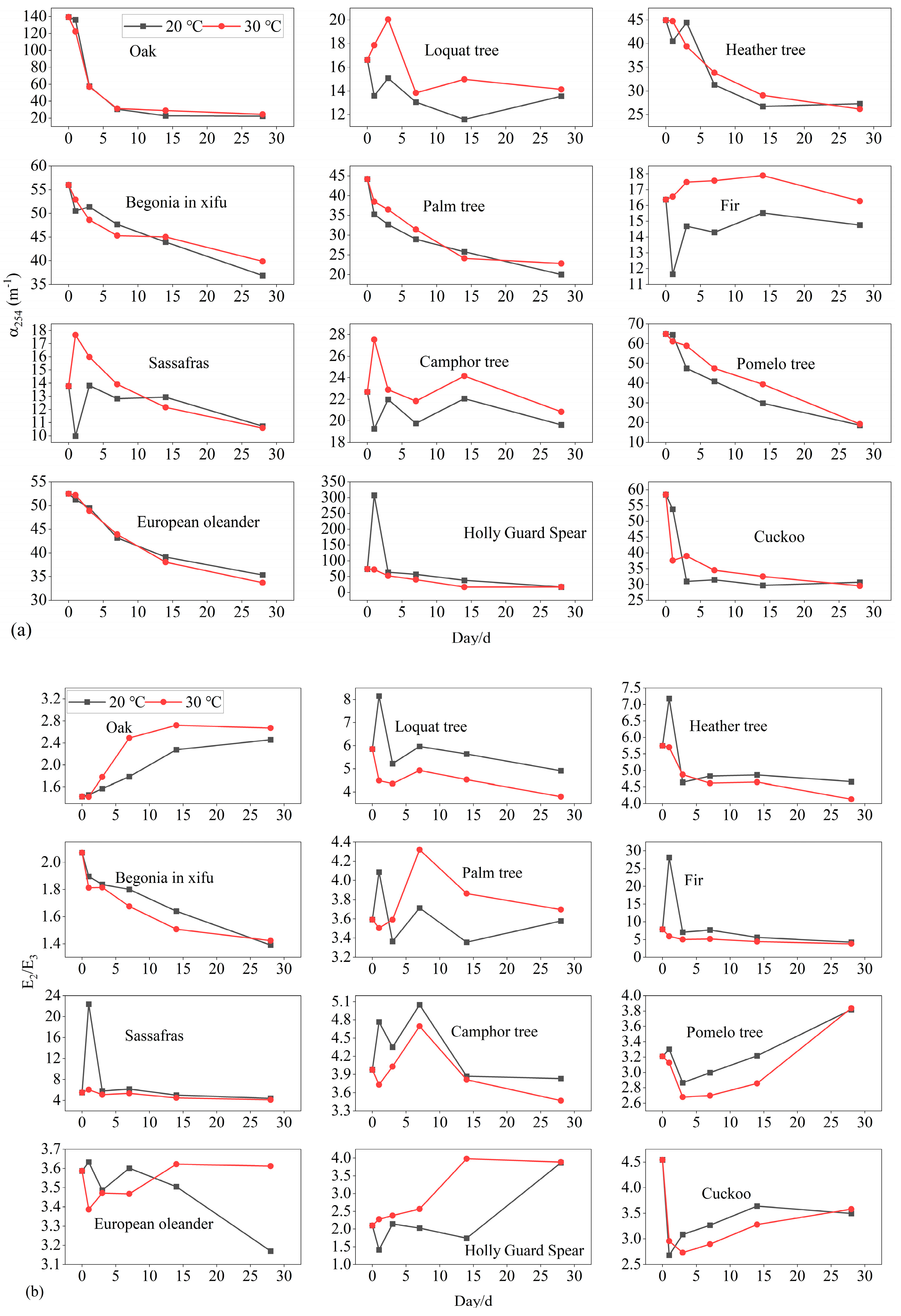
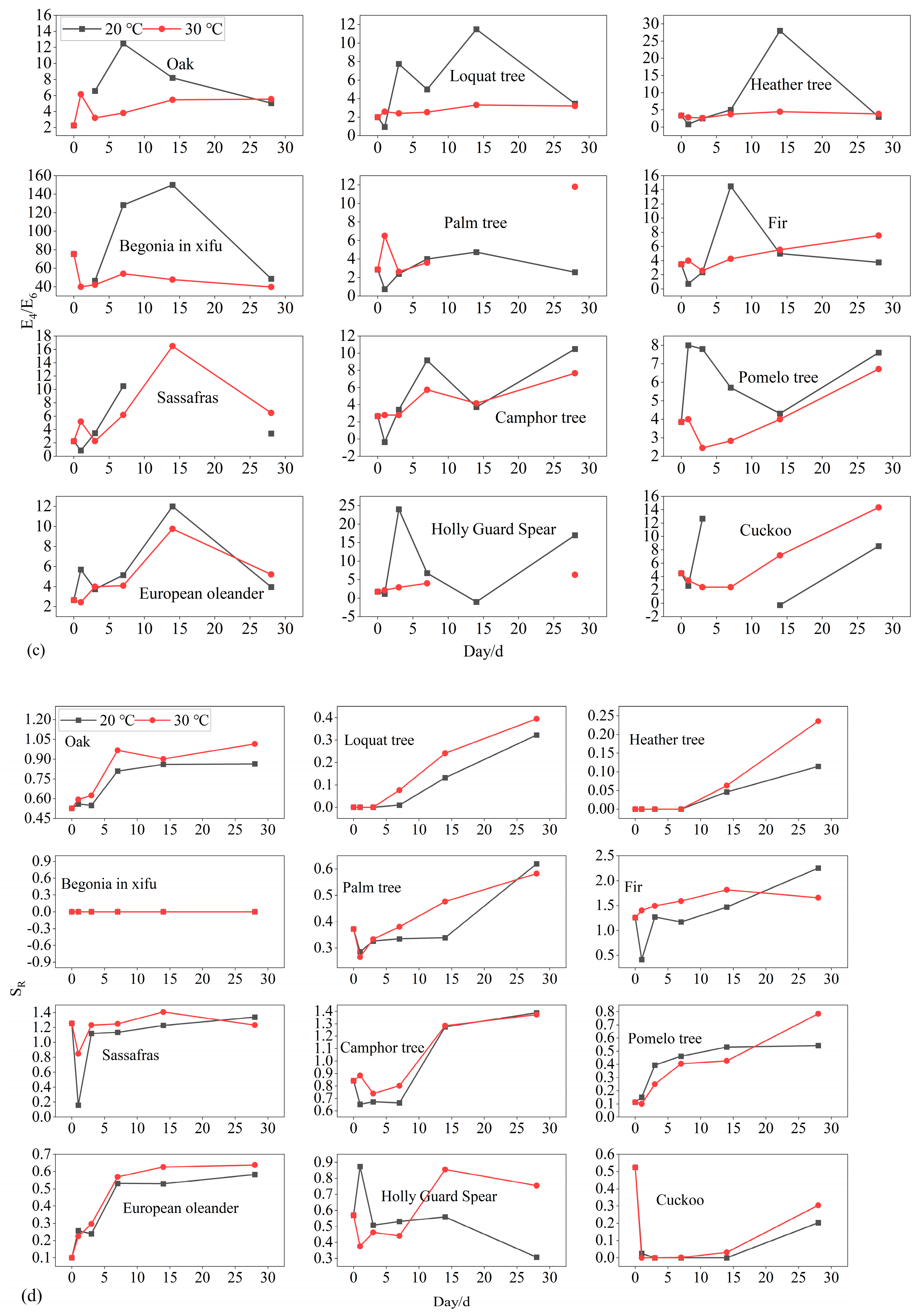
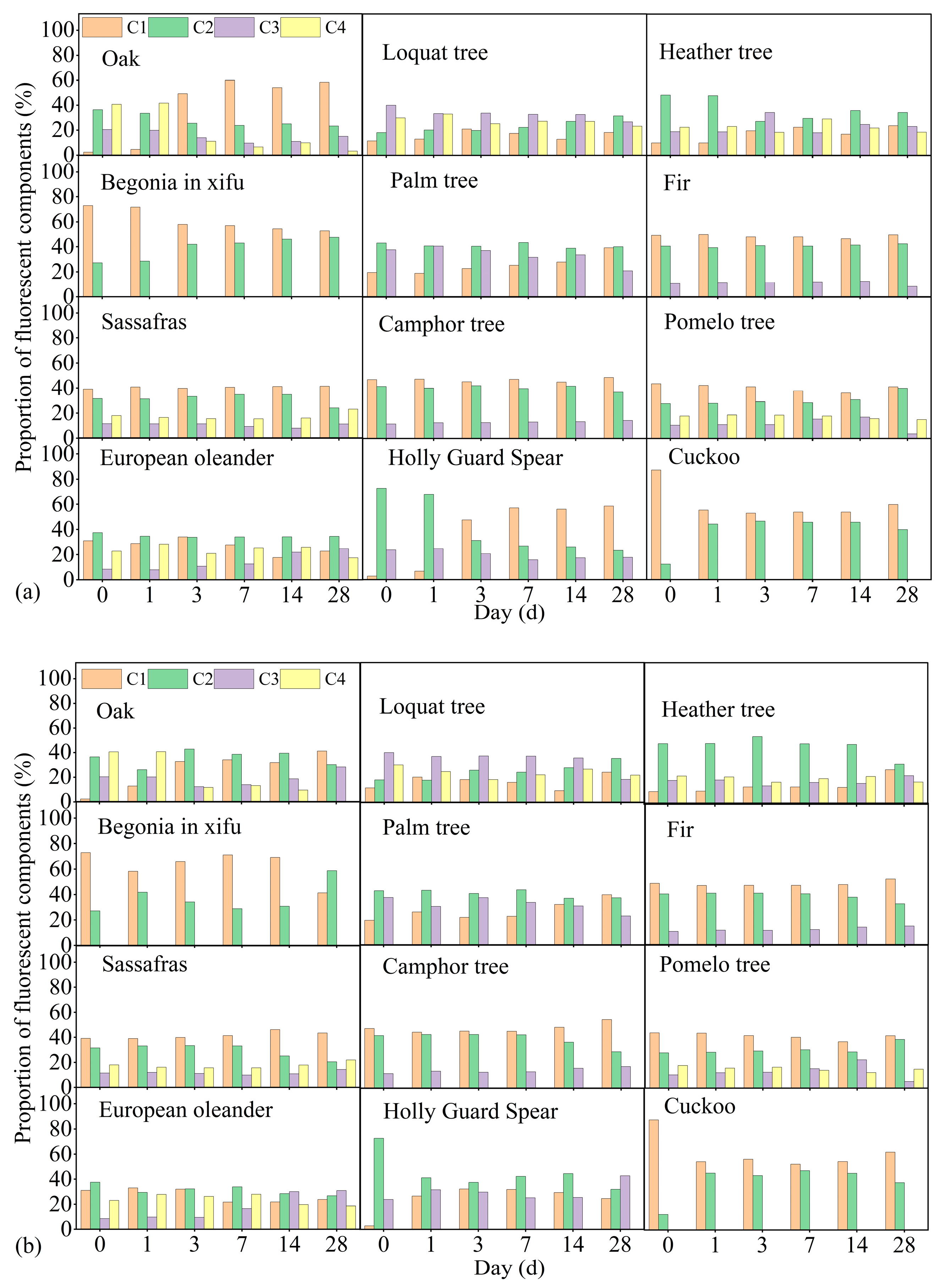
| Leaf Name | Leaf DOC Loss mg/g | Degradation Rate at 20 °C % | Degradation Rate at 30 °C % | Stable DOC at 20 °C mg/g | Stable DOC at 30 °C mg/g |
|---|---|---|---|---|---|
| Oak | 104.25 | 83.08 | 75.60 | 17.64 | 25.44 |
| Loquat tree | 65.61 | 53.68 | 50.16 | 30.39 | 32.7 |
| Heather tree | 112.38 | 53.76 | 57.07 | 51.96 | 48.24 |
| Begonia in xifu | 93.15 | 43.64 | 52.59 | 52.50 | 44.16 |
| Palm tree | 59.73 | 54.14 | 58.11 | 27.39 | 25.02 |
| Fir | 53.52 | 41.09 | 40.13 | 31.53 | 32.04 |
| Sassafras | 60.93 | 50.27 | 59.08 | 30.3 | 24.93 |
| Camphor tree | 74.46 | 51.97 | 52.74 | 35.76 | 35.19 |
| Pomelo tree | 118.38 | 74.15 | 53.47 | 30.6 | 55.08 |
| European oleander | 92.64 | 54.73 | 42.1 | 41.94 | 53.64 |
| Holly Guard Spear | 109.05 | 53.78 | 48.12 | 50.4 | 56.58 |
| Cuckoo | 90.36 | 49.14 | 33.60 | 45.96 | 60.00 |
| Mean value | 86.21 ± 22.65 | 55.29 ± 11.86 | 51.90 ± 10.74 | 37.20 ± 11.18 | 41.09 ± 13.36 |
| p | 0.13 | 0.78 | 0.03 * | 0.17 | 0.02 * |
| p | - | 0.56 | 0.42 | ||
| Project | pH | EC | TDP | TDN | α254 | E2/E3 | E4/E6 | TDN/TDP | DOC/TDN | DOC/TDP | SR | FRI | BIX | HIX | DOC Leaching Rate |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DOC degradation rate at 20 °C | 0.39 | 0.86 ** | 0.54 | 0.86 ** | 0.61 * | −0.55 | −0.37 | −0.02 | −0.70 * | −0.43 | −0.50 | 0.57 | 0.54 | −0.58 * | 0.54 |
| DOC degradation rate at 30 °C | 0.19 | 0.53 | 0.60 * | 0.63 * | 0.22 | −0.46 | −0.08 | −0.30 | −0.67 * | −0.66 * | 0.06 | 0.18 | 0.21 | −0.53 | 0.14 |
| k at 20 °C | 0.21 | 0.12 | 0.22 | −0.20 | 0.09 | −0.08 | 0.30 | −0.49 | 0.48 | −0.13 | −0.50 | 0.11 | 0.31 | −0.03 | 0.38 |
| k at 30 °C | 0.56 | −0.34 | −0.35 | −0.28 | −0.18 | 0.20 | −0.18 | 0.25 | 0.14 | 0.30 | 0.00 | −0.19 | −0.28 | 0.14 | −0.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, X.; Li, S. Quality and Biodegradation Process of Dissolved Organic Carbon in Typical Fresh-Leaf Leachate in the Wuhan Urban Forest Park. Water 2024, 16, 558. https://doi.org/10.3390/w16040558
Tian X, Li S. Quality and Biodegradation Process of Dissolved Organic Carbon in Typical Fresh-Leaf Leachate in the Wuhan Urban Forest Park. Water. 2024; 16(4):558. https://doi.org/10.3390/w16040558
Chicago/Turabian StyleTian, Xiaokang, and Siyue Li. 2024. "Quality and Biodegradation Process of Dissolved Organic Carbon in Typical Fresh-Leaf Leachate in the Wuhan Urban Forest Park" Water 16, no. 4: 558. https://doi.org/10.3390/w16040558
APA StyleTian, X., & Li, S. (2024). Quality and Biodegradation Process of Dissolved Organic Carbon in Typical Fresh-Leaf Leachate in the Wuhan Urban Forest Park. Water, 16(4), 558. https://doi.org/10.3390/w16040558







